Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
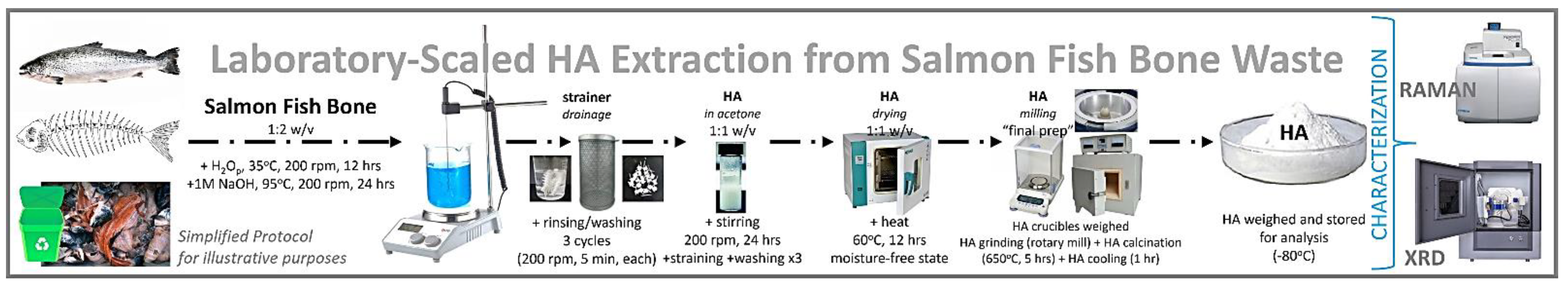
2. Material and Methods
3. Results
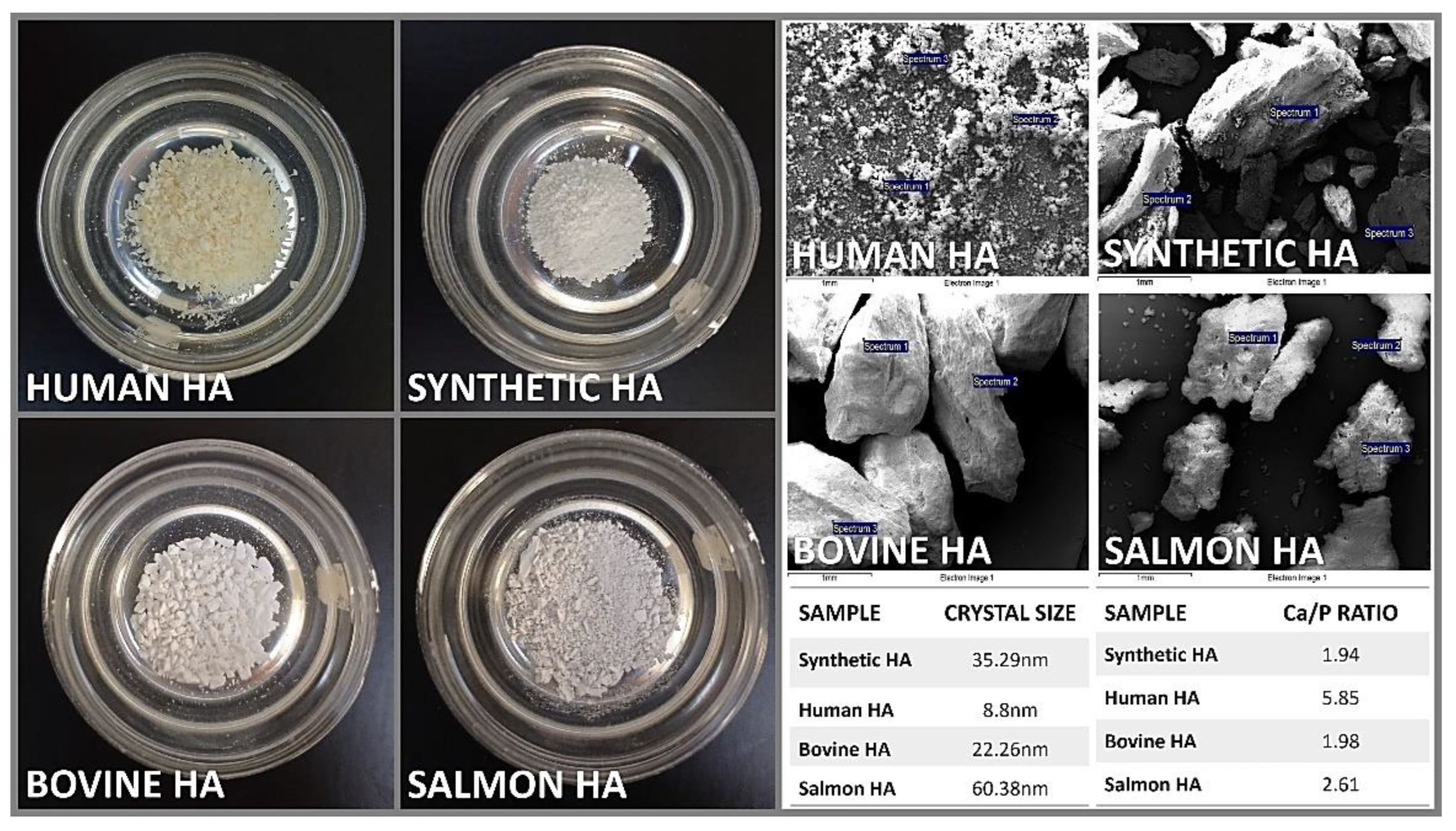
3.1. Characterization
3.2. Raman Spectrometry
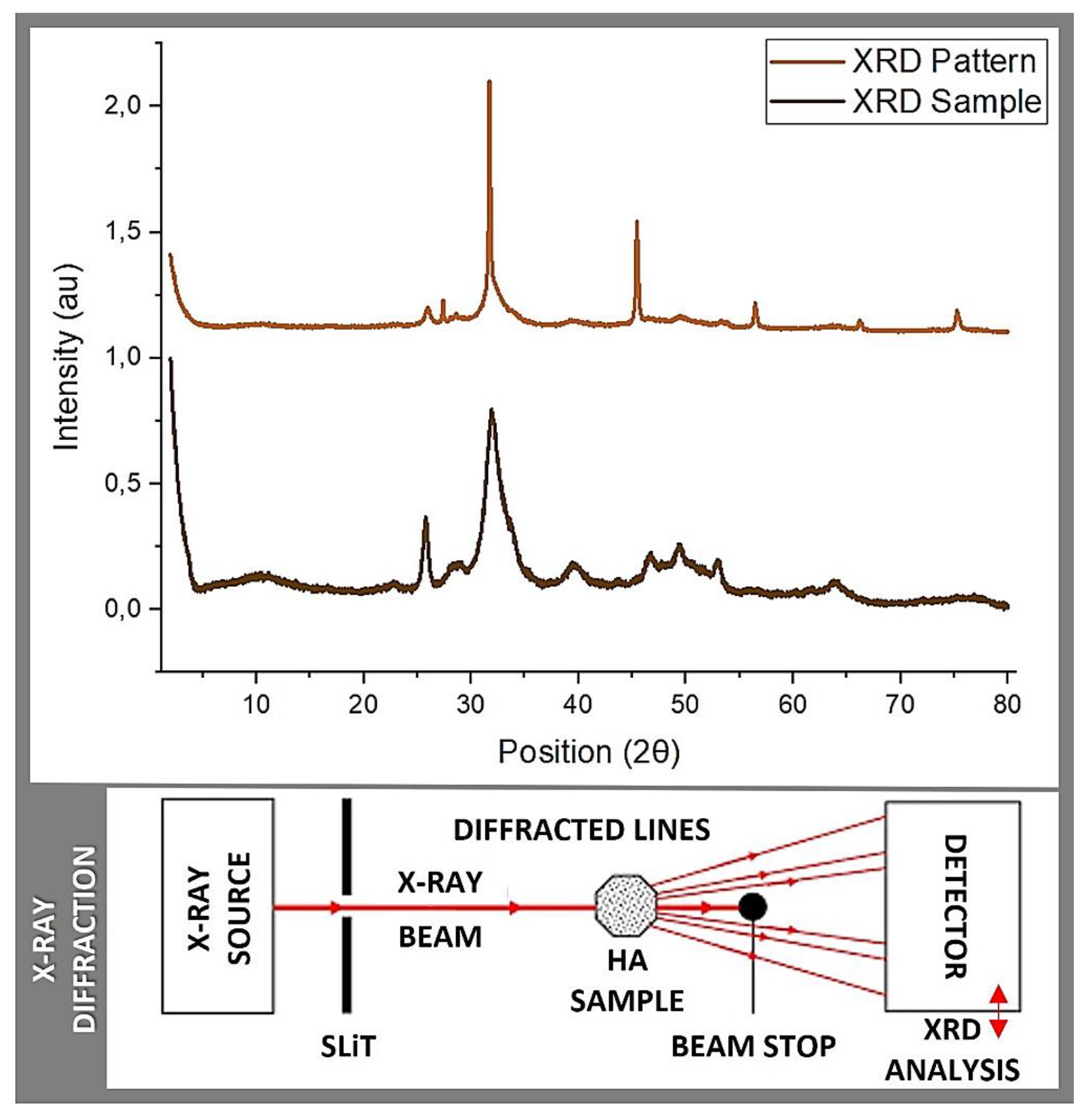
3.3. X-ray Diffraction
4. Discussion
4.1. Production
4.2. Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Patents
References
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Joint J 2016, 98-B (1 Suppl A), 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Lenart, B.A.; Gitelis, S. Function after injection of benign bone lesions with a bioceramic. Clin Orthop Relat Res 2012, 470, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- De Long, W.G.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am 2007, 89, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol Biosci 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Malde, M.K.; Graff, I.E.; Siljander-Rasi, H.; Venäläinen, E.; Julshamn, K.; Pedersen, J.I.; Valaja, J. ORIGINAL ARTICLE: Fish bones – a highly available calcium source for growing pigs. J Anim Physiol Anim Nutr 2010, 94, e66–76. [Google Scholar] [CrossRef]
- Venkatesan, J.; Ryu, B.; Sudha, P.N.; Kim, S.-K. Preparation and characterization of chitosan-carbon nanotube scaffolds for bone tissue engineering. Int J Biol Macromol 2012, 50, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liu, M.; Fan, F.; Yu, C.; Lu, W.; Du, M. Characterization of natural hydroxyapatite originated from fish bone and its biocompatibility with osteoblasts. Mater Sci Eng C Mater Biol Appl 2018, 90, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.; Haidar, Z.S.; Puigdollers, A.; Guerra, I.; Padilla, M.C.; Ortega, N.; García, M.J. A novel Chilean salmon fish backbone-based nanoHydroxyApatite functional biomaterial for potential use in bone tissue engineering. Frontiers in Medicine 2024, 11, 1330482. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Lowe, B.; Manivasagan, P.; Kang, K.-H.; Chalisserry, E.P.; Anil, S.; et al. Isolation and Characterization of Nano-Hydroxyapatite from Salmon Fish Bone. Materials (Basel) 2015, 8, 5426–5439. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sae-leaw, T.; Suzuki, N.; Kitani, Y.; et al. Effect of Alkaline Treatment on Characteristics of Bio-Calcium and Hydroxyapatite Powders Derived from Salmon Bone. Applied Sciences 2020, 10, 4141. [Google Scholar] [CrossRef]
- Kotak, D.J.; Devarajan, P.V. Bone targeted delivery of salmon calcitonin hydroxyapatite nanoparticles for sublingual osteoporosis therapy (SLOT). Nanomedicine 2020, 24, 102153. [Google Scholar] [CrossRef]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; et al. Mechanical and Biocompatibility Properties of Calcium Phosphate Bioceramics Derived from Salmon Fish Bone Wastes. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J Biomed Mater Res 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.-C.; Chen, S.-Y.; Lee, H.-Y.; Bow, J.-S. Structural characterization of nano-sized calcium deficient apatite powders. Biomaterials 2004, 25, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Khor, K.A.; Dong, Z.L.; Gu, Y.W.; Kumar, R.; Cheang, P. Preparation and characterization of nano-sized hydroxyapatite powders produced in a radio frequency (rf) thermal plasma. Materials Science and Engineering: A 2004, 374, 101–108. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; et al. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Haidar, Z.S. Biomechanics and Functional Tissue Engineering; IntechOpen: London, United Kingdom, 2021; pp. 1–268. [Google Scholar]
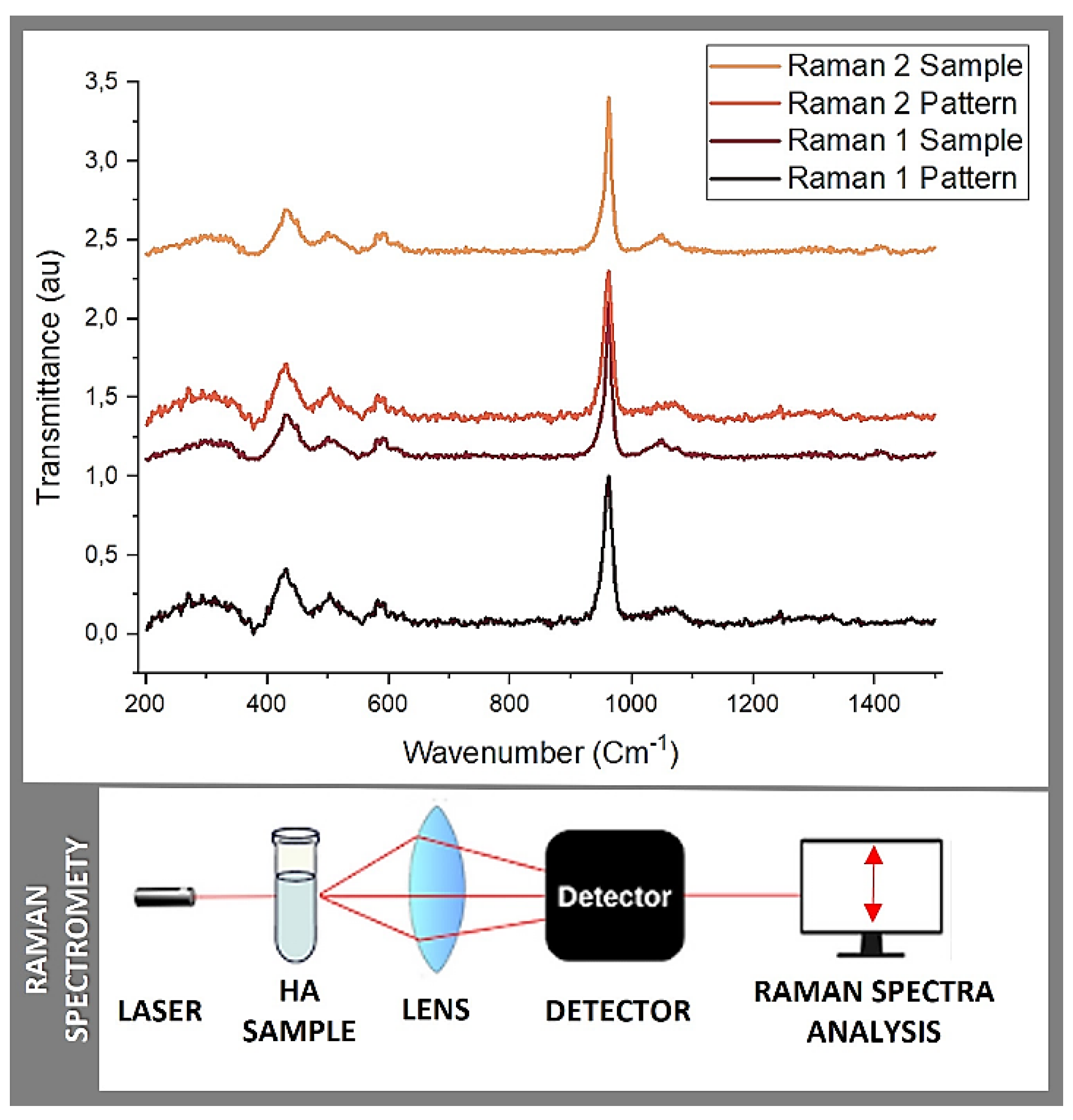
| Raman 1 Pattern |
Raman 1 Sample |
Raman 2 Pattern |
Raman 2 Sample |
HORIBA Scientific Raman Database* |
|---|---|---|---|---|
| 307,572 | - | 307,572 | - | δ(CC) aliphatic chains |
| - | 269,532 | - | 269,532 | δ(CC) aliphatic chains |
| - | 292,969 | - | 292,969 | υ(Se-Se) |
| - | 313,404 | - | 313,404 | υ(Se-Se) |
| 428,881 | - | 428,881 | - | υ(S-S) |
| - | 431,74 | - | 431,74 | υ(S-S) |
| 448,8075 | - | 448,8075 | - | υ(Si-O-Si) |
| 499,951 | - | 499,951 | - | υ(Si-O-Si) |
| - | 502,777 | - | 502,77 | υ(Si-O-Si) |
| 580,40886 | 581,35 | 589,707 | 581,35 | υ(C-Cl) |
| 614,708 | - | - | 623,019 | υ(C-I) |
| 727,278 | - | - | 759,819 | υ(C-S) aliphatic |
| 961,748 | 961,748 | 961,748 | 961,748 | ν 1 (PO4 3−) /(A/E2 ) |
| 1049,81 | - | 1049,81 | - | υ(C=S) |
| 1074,87223 | 1071,03918 | 1074,87223 | 1070,33 | υ(C=S) |
| - | 1244,80409 | - | 1244,23 | υ(C=S) |
| *KnowItAll® Informatics System; a spectra database covering many applications, available for data mining, analytical and comparative studies, from HORIBA Scientific, Japan. | ||||
| JCPDS 74-0565* | Shi et al. Natural HA |
HA Sigma Aldrich |
Salmon Fish Bone Bio-Ceramic |
|---|---|---|---|
| - | - | 10,8 | 10,43 |
| 25,882 | 25,845 | 25,81 | 25,9 |
| - | - | 28,08 | 28,37 |
| - | - | 28,89 | 28,5 |
| - | - | 29,64 | 29,16 |
| 31,765 | 31,792 | 31,73 | 31,6 |
| 32,194 | 32,142 | 32,13 | - |
| 32,896 | 32,935 | 32,86 | - |
| 34,062 | 34,055 | 34 | - |
| 39,79 | 39,816 | 39,74 | 39,46 |
| - | - | 45,25 | 45,41 |
| 46,693 | 46,698 | 46,61 | 46,72 |
| - | - | 48,01 | 48,04 |
| 49,489 | 49,496 | 49,39 | 49,42 |
| 50,474 | 50,568 | 50,42 | - |
| - | - | 51,21 | 51,41 |
| 53,218 | 53,183 | 53,1 | 53,27 |
| - | - | 57,8 | 56,43 |
| - | - | 62,93 | 63,84 |
| - | - | 66,26 | 66,17 |
| - | - | 75,49 | 75,25 |
| * JCPDS 74-0565: Joint Committee on Powder Diffraction Standards | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





