Submitted:
16 November 2023
Posted:
16 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
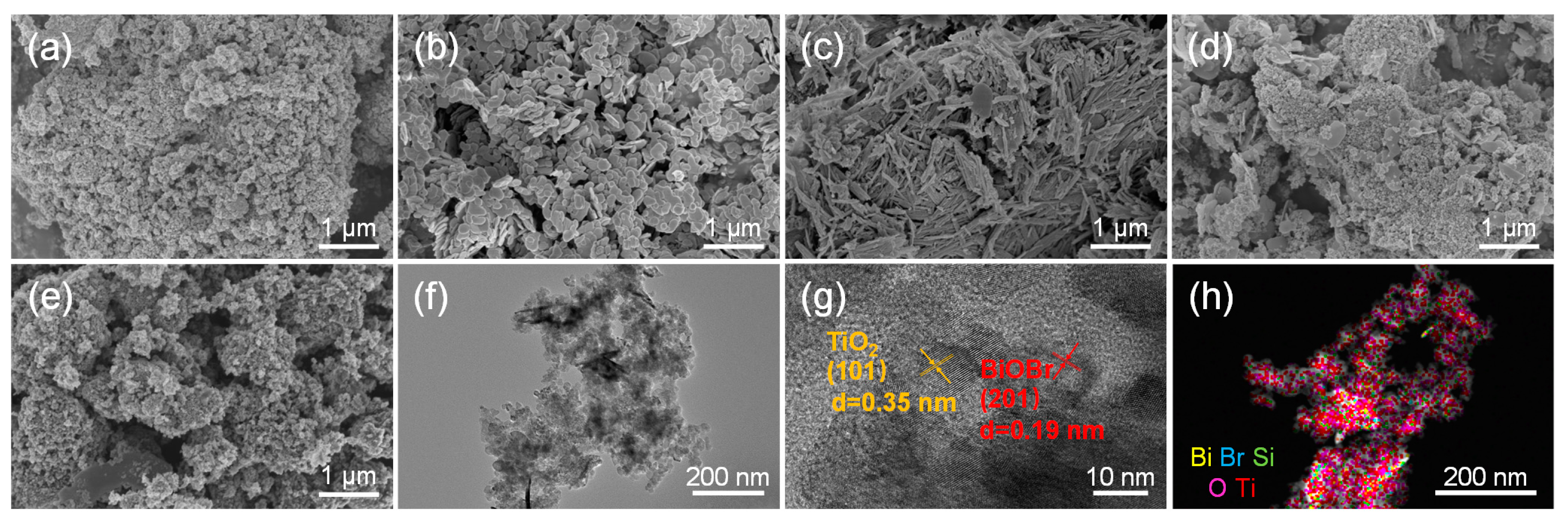
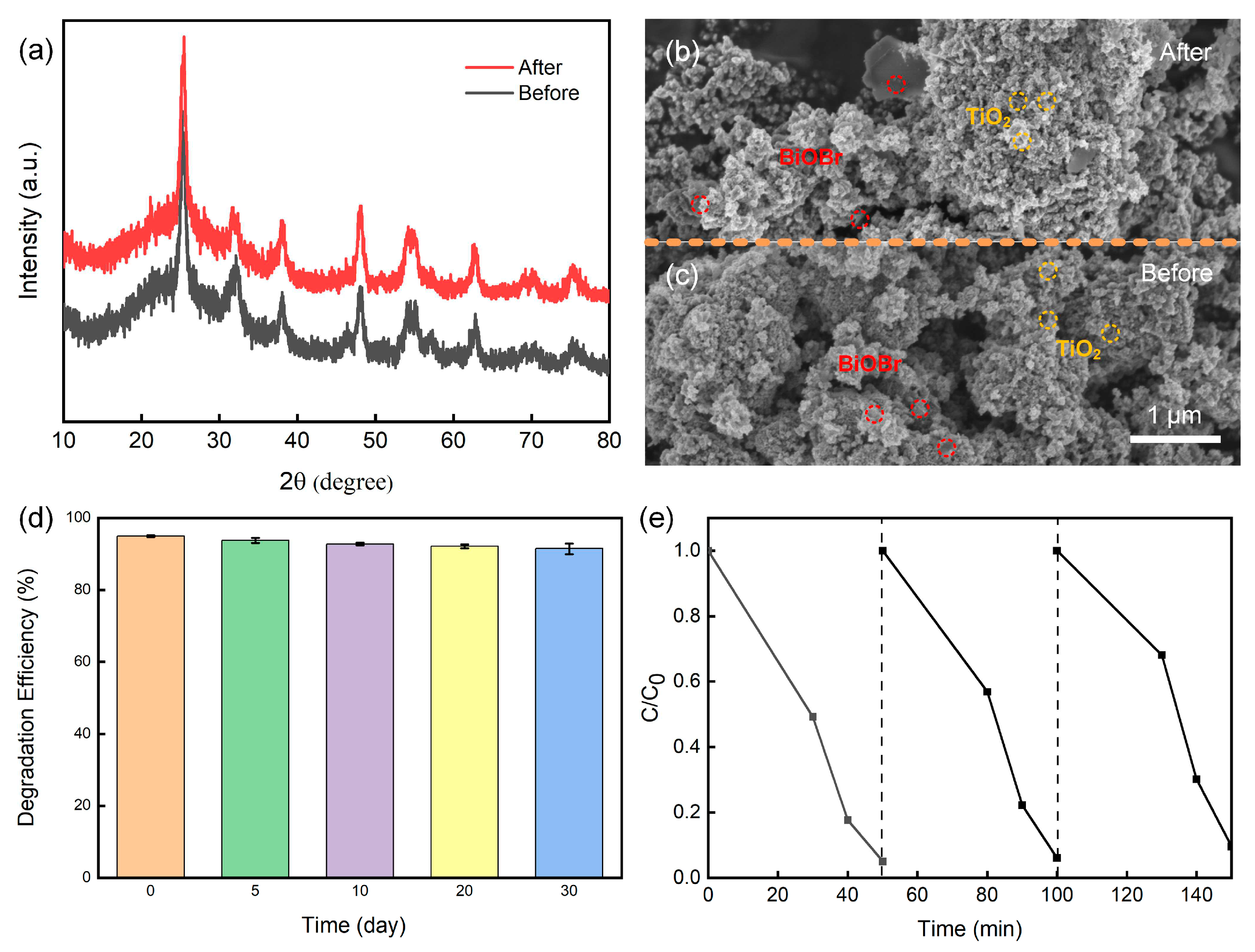
2.1. Characterization of materials
2.1.1. Phase analysis
2.1.2. Phase analysis
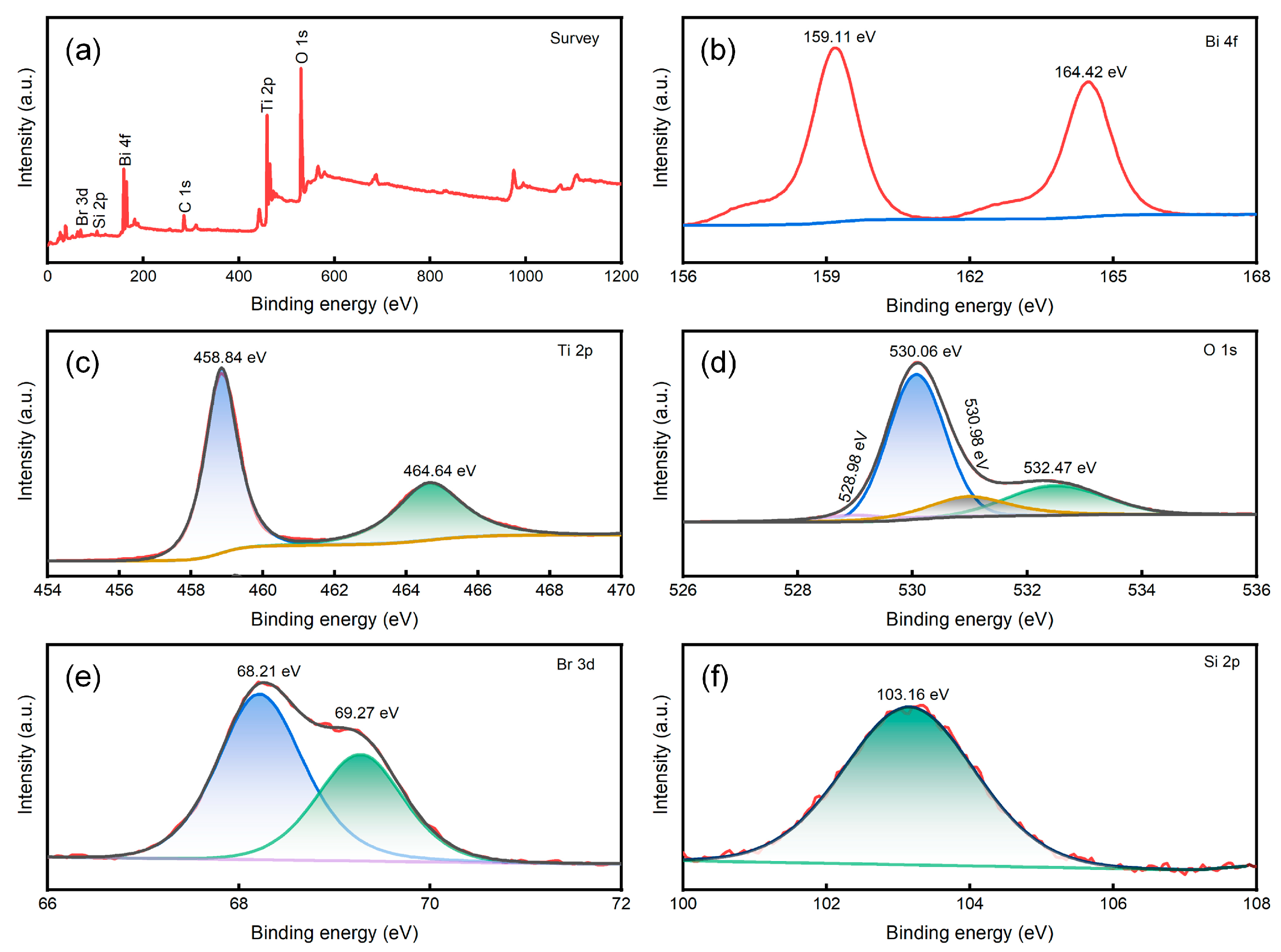
2.1.3. Surface chemical state analysis
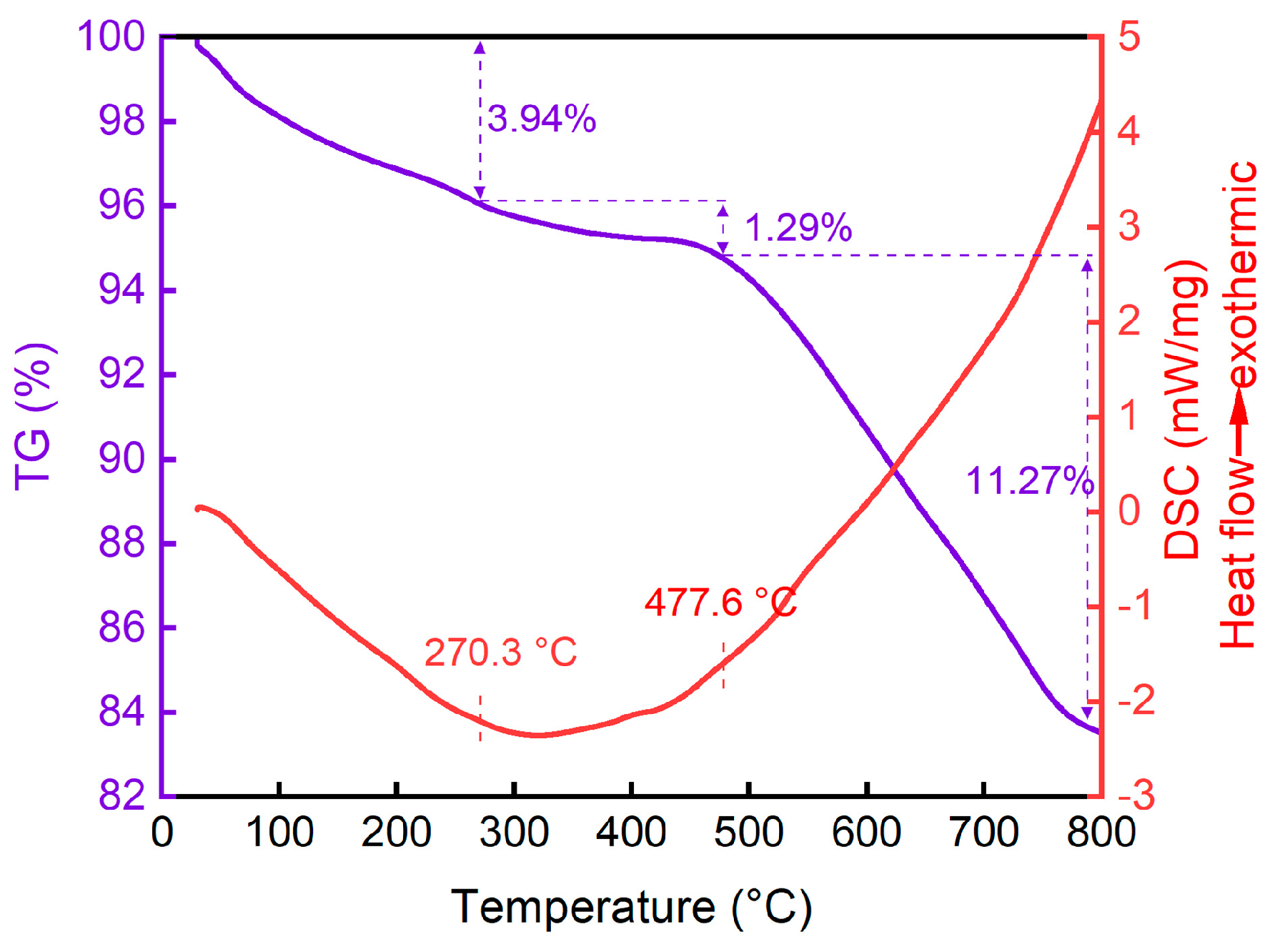
2.1.4. TG-DSC analysis
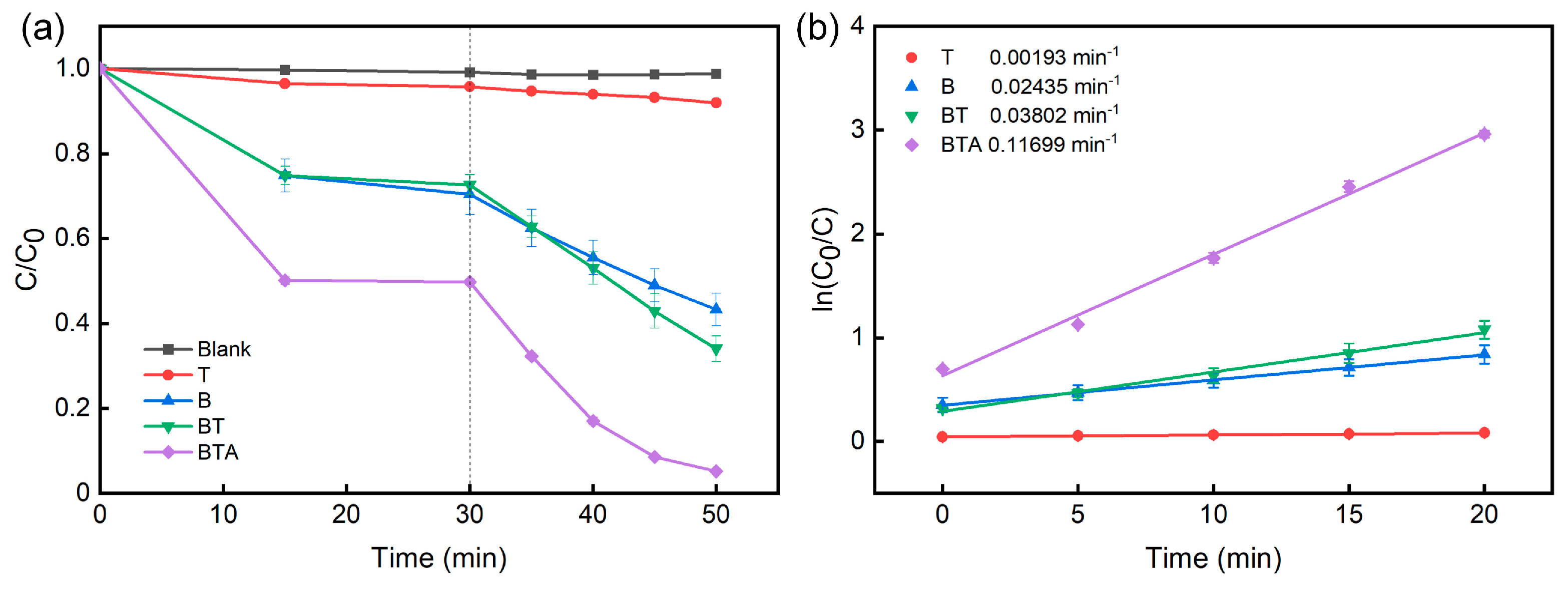
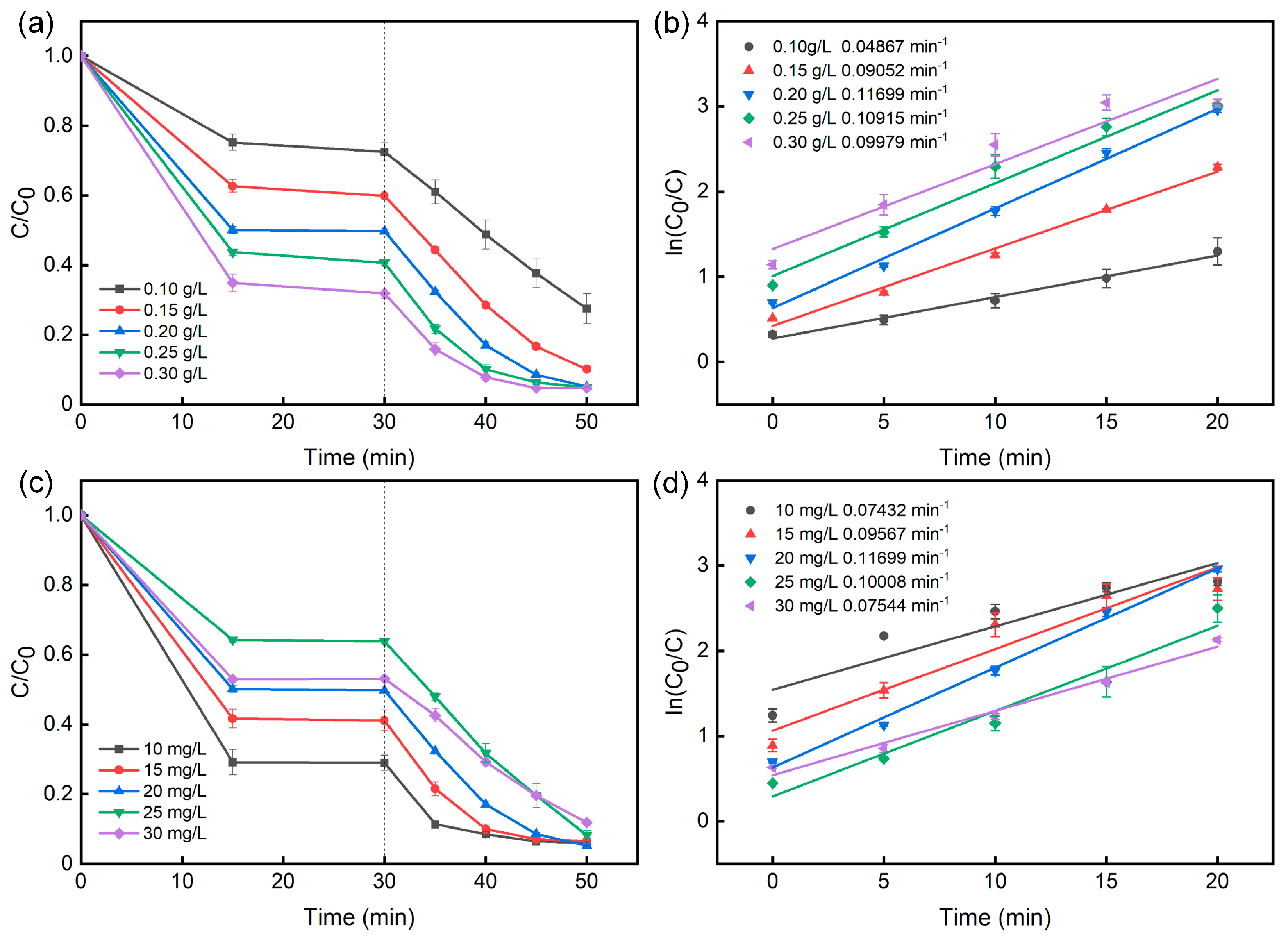
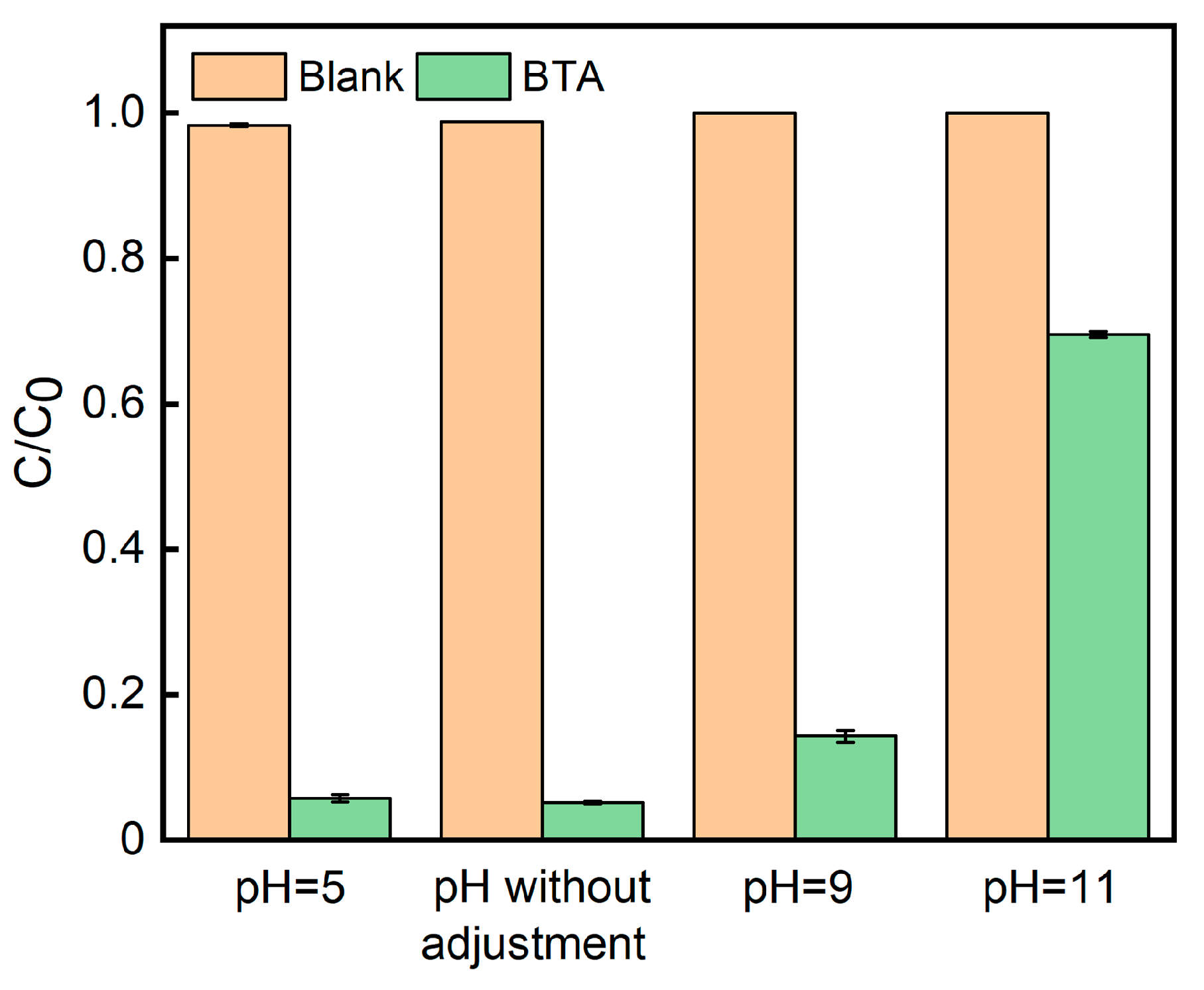
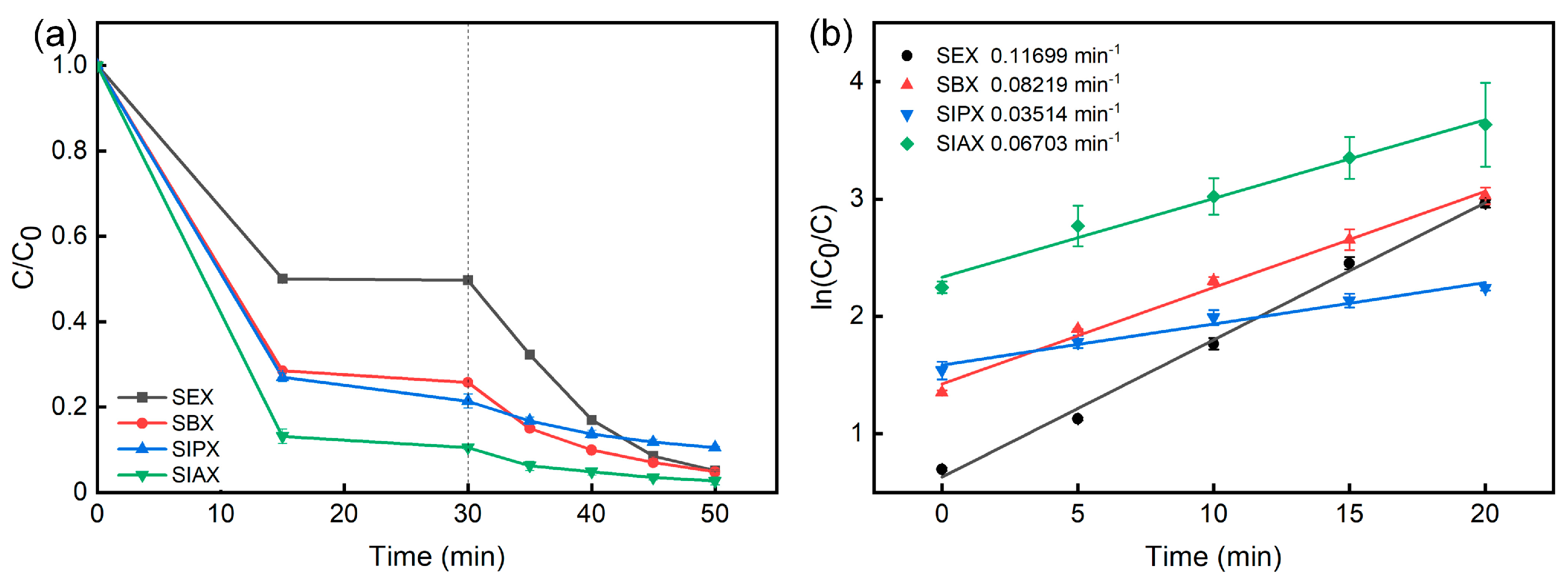
2.2. Photocatalytic activity
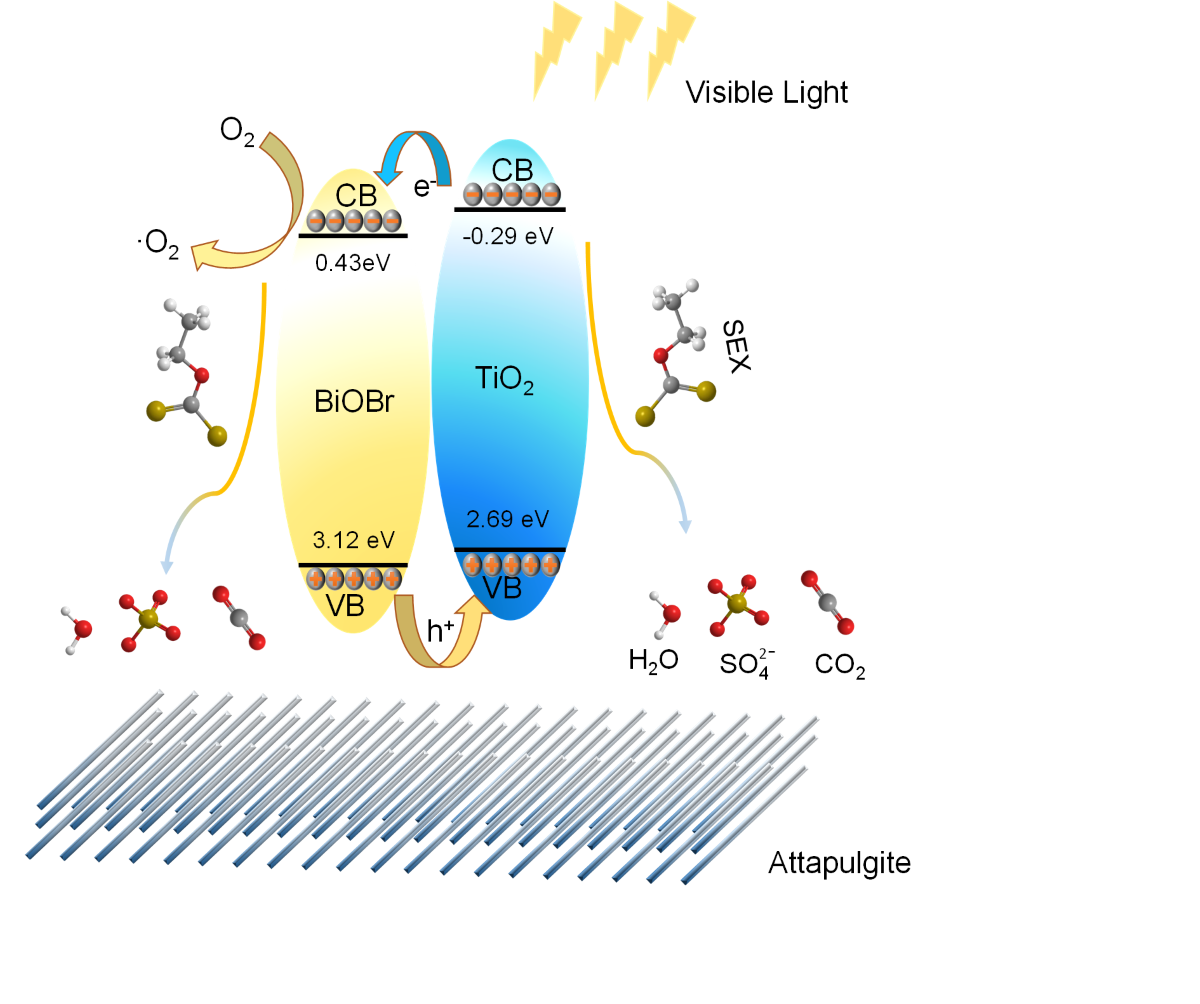
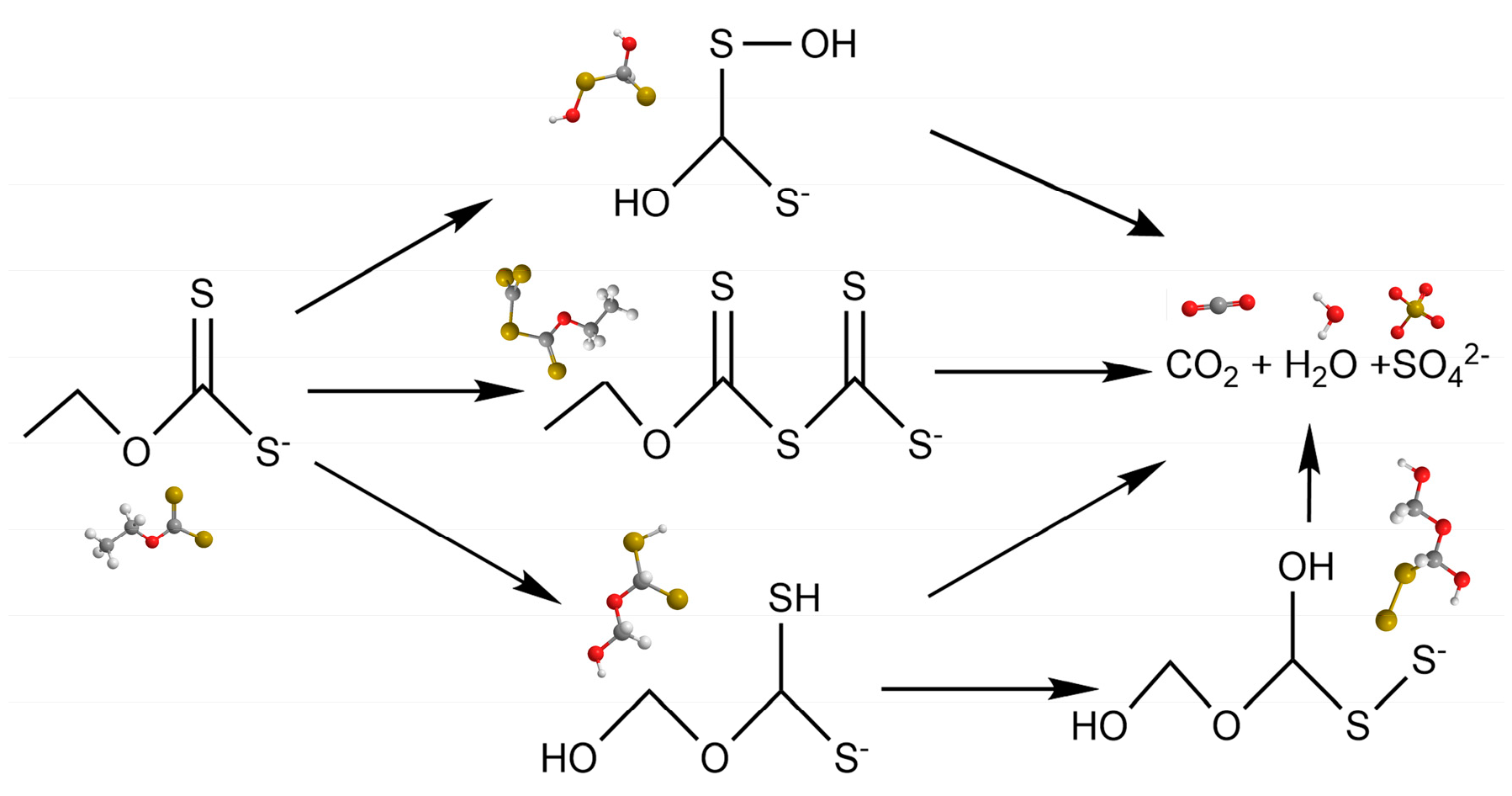
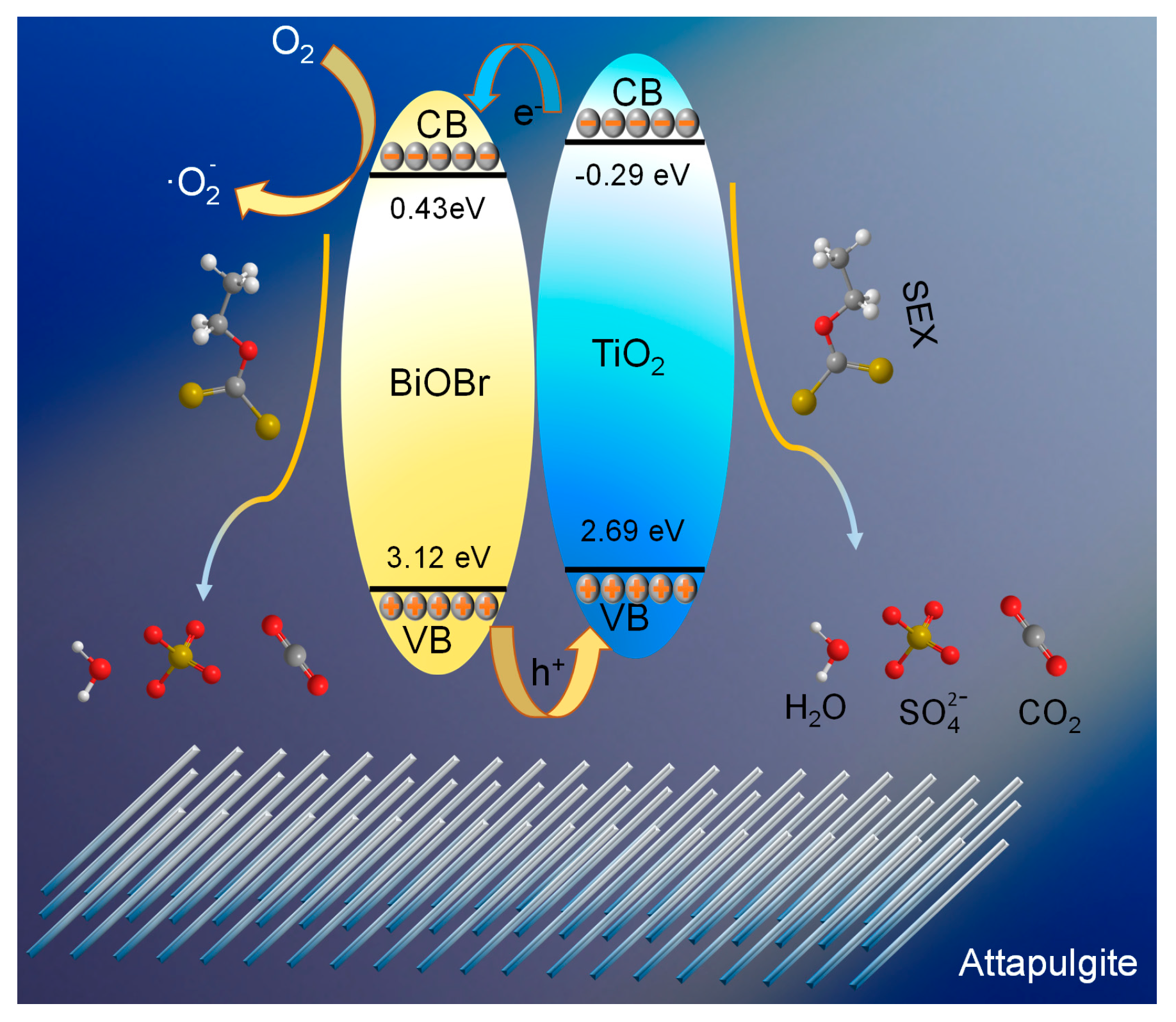
2.3. Possible degradation mechanism
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, K.; Chuaicham, C.; Noguchi, Y.; Xu, L.; Sasaki, K. In-situ hydrothermal synthesis of Fe-doped hydroxyapatite photocatalyst derived from converter slag toward xanthate photodegradation and Cr(VI) reduction under visible-light irradiation. Chemical Engineering Journal 2023, 459, 141474. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, G.; Liu, J.; Liu, Y.; Zhai, J.; Zhang, H.; Yu, H. Visible-light-driven photodegradation of xanthate in a continuous fixed-bed photoreactor: Experimental study and modeling. Chemical Engineering Journal 2023, 461, 141833. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Li, G.; Cui, B.; Wei, D.; Shen, Y. Synthesis of clinoptilolite-supported BiOCl/TiO2 heterojunction nanocomposites with highly-enhanced photocatalytic activity for the complete degradation of xanthates under visible light. Chemical Engineering Journal 2021, 407, 126697. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, T.; Zheng, S.; Sun, Z.; Li, C. Adsorptive and photocatalytic behaviour of PANI/TiO2/metakaolin composites for the removal of xanthate from aqueous solution. Minerals Engineering 2021, 171, 107129. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Zhang, X.; Cheng, J.; Xie, Y.; Zhang, Y.; Yin, X.; Song, F.; Cui, H. Novel CdS/PANI/MWCNTs photocatalysts for photocatalytic degradation of xanthate in wastewater. Separation and Purification Technology 2023, 309, 123022. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, P.; Zhao, S.; Li, A.; Chen, Y.; Bai, J.; Han, C.; Wei, D.; Ao, Y. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Minerals Engineering 2020, 159, 106640. [Google Scholar] [CrossRef]
- Fallahpour, M.; Poursalehi, R.; Yourdkhani, A. Highly efficient photocatalytic removal of concentrated PEX, PAX and SIPX xanthates collectors by an immobilized nanostructured g-C3N4/ZnO low power backlighted module. Minerals Engineering 2023, 204, 108416. [Google Scholar] [CrossRef]
- Yuan, F.; Zheng, Y.; Gao, D.; Wang, L.; Hu, X. Facile assembly and enhanced visible-light-driven photocatalytic activity of S-scheme BiOBr/g-C3N4 heterojunction for degrading xanthate in wastewater. Journal of Molecular Liquids 2022, 366, 120279. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Shen, X.; Zhang, Y.; Lou, Y.; Pan, C.; Zhu, Y.; Xu, J. A plasmonic Z-scheme Ag@AgCl/PDI photocatalyst for the efficient elimination of organic pollutants, antibiotic resistant bacteria and antibiotic resistance genes. Applied Catalysis B: Environmental 2023, 324, 122220. [Google Scholar]
- Zhou, P.; Shen, Y.; Zhao, S.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Hydrothermal synthesis of novel ternary hierarchical MoS2/TiO2/clinoptilolite nanocomposites with remarkably enhanced visible light response towards xanthates. Applied Surface Science 2021, 542, 148578. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Bai, J.; Han, C.; Liu, W.; Wei, D. Facile synthesis of clinoptilolite-supported Ag/TiO2 nanocomposites for visible-light degradation of xanthates. Journal of the Taiwan Institute of Chemical Engineers 2021, 122, 231–240. [Google Scholar] [CrossRef]
- Shu, K.; Chuaicham, C.; Noguchi, Y.; Xu, L.; Sasaki, K. Charge transfer mechanism through S-scheme heterojunction in in-situ synthesized TiO2/Fe-doped hydroxyapatite for improved photodegradation of xanthate. Jounral of Hazardous Materials 2023, 460, 132337. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Teng, Q.; Sun, B.; Yang, Z.; Liu, S.; Zhu, X. Synthesis scaly Ag-TiO2 loaded fly ash magnetic bead particles for treatment of xanthate wastewater. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 624, 126795. [Google Scholar]
- Wang, C.; Zhang, B.; Zhao, Z.; Zhang, Z.; Zhang, S.; Bala, H.; Zhang, Z. Enhanced n-butanol sensing properties of Au modified TiO2 nanorod arrays: A combined experimental and first-principle study. Applied Surface Science 2023, 641, 158458. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Martins, T.S.; Oliveira Jr, O.N.; Marken, F. Controlled electrodeposition of brookite TiO2 for photoelectroanalysis at printed carbon electrodes. Applied Surface Science 2023, 640, 158316. [Google Scholar] [CrossRef]
- Wang, S.-q.; Li, X.-x.; Wu, J.-j. Preparation of TiO2/graphene oxide and their photocatalytic properties at room temperature. Journal of Fuel Chemistry and Technology 2022, 50, 1307–1316. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, Y.; Liu, H.; Yan, Y.; Yan, F. Reinforced UHMWPE composites by grafting TiO2 on ATP nanofibers for improving thermal and tribological properties. Tribology International 2022, 172, 107585. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, Y.; Mao, H.; Zhou, S.; Hui, J.; Li, H.; Li, M.; Zhao, Y.; Zhang, Q.; Xia, S. Development of attapulgite based catalytic membrane for activation of peroxymonosulfate: A singlet oxygen-dominated catalytic oxidation process for sulfamethoxazole degradation. Separation and Purification Technology 2023, 312, 123382. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.; Zhang, X.; Lin, D.; Geng, H.; Han, B.; Zhang, M.; Wang, H. A mechanically robust slippery surface with ‘corn-like’ structures fabricated by in-situ growth of TiO2 on attapulgite. Chemical Engineering Journal 2021, 415, 128953. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zhang, W.; Liu, J.; Zhong, H.; Zhao, Y. Photocatalytic activity of attapulgite–BiOCl–TiO2 toward degradation of methyl orange under UV and visible light irradiation. Materials Research Bulletin 2015, 66, 109–114. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, S.; Yue, X.; Zhao, F.; Xi, F.; Yan, D.; Ling, H.; Zhang, R.; Tang, F.; You, K.; Luo, H.a.; Zhang, X. Attapulgite as a cost-effective catalyst for low-energy consumption amine-based CO2 capture. Separation and Purification Technology 2022, 298, 121577. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhou, S.; Chen, H.; Zhong, H.; Zhao, Y.; Wang, X. Magnetically separable attapulgite−TiO2−Fe O composites with superior activity towards photodegradation of methyl orange under visible light radiation. Journal of Industrial and Engineering Chemistry 2014, 20, 3884–3889. [Google Scholar] [CrossRef]
- Cao, W.; Wang, W.; Yang, Z.; Wang, W.; Chen, W.; Wu, K. Enhancing photocathodic protection performance by controlled synthesis of Bi/BiOBr/TiO2 NTAs Z-scheme heterojunction films. Journal of Alloys and Compounds 2023, 960, 170675. [Google Scholar] [CrossRef]
- Yin, Y.; Kang, X.; Han, B. Two-dimensional materials: synthesis and applications in the electro-reduction of carbon dioxide. Chemical Synthesis 2022, 2, 19. [Google Scholar] [CrossRef]
- Liao, X.; Ren, H.-T.; Yang, T.; Shen, B.; Lin, J.-H.; Lou, C.-W.; Li, T.-T. A flexible, highly adaptive, self-standing photoelectrochemical aptasensor based on 3D pinecone-like structure BiOBr/TiO2 hierarchical nanofiber membranes. Ceramics International 2023, 49, 27912–27921. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, P.; Zhan, S. Shedding light on the role of interfacial chemical bond in heterojunction photocatalysis. Nano Research 2022, 15, 10158–10170. [Google Scholar] [CrossRef]
- Zhao, S.-Z.; Lu, Y.; Lu, R.; Hu, Y.-D.; Rodriguez, R.D.; Chen, J.-J. Constructing BiOBr/TiO2 heterostructure nanotubes for enhanced adsorption/photocatalytic performance. Journal of Water Process Engineering 2023, 54, 103972. [Google Scholar] [CrossRef]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Zamani, M.R.; Aminabhavi, T.M. Photocatalytic activity of visible-light-driven L-Proline-TiO2/BiOBr nanostructured materials for dyes degradation: The role of generated reactive species. Journal of Environmental Management 2023, 326, 116691. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shen, Y.; Zhao, S.; Bai, J.; Gao, S.; Liu, W.; Wei, D.; Meng, D.; San, X. Construction of rGO-SnO2 heterojunction for enhanced hydrogen detection. Applied Surface Science 2022, 585, 152623. [Google Scholar] [CrossRef]
- Swathi, K.S.; Gopalakrishna Naik, K. Structural, morphological, and optical studies of sol–gel spin coated TiO2 thin films. Materials Today: Proceedings 2023, 960, 170335. [Google Scholar] [CrossRef]
- Zhao, M.; Qin, J.; Wang, N.; Zhang, Y.; Cui, H. Reconstruction of surface oxygen vacancy for boosting CO2 photoreduction mediated by BiOBr/CdS heterojunction. Separation and Purification Technology 2024, 329, 125179. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Zhang, Q.; Weng, W.; Wan, H. Attapulgite as natural catalyst for glucose isomerization to fructose in water. Catalysis Communications 2017, 99, 20–24. [Google Scholar] [CrossRef]
- Dong, L.; Wang, H.; Huang, Y.; Zha, J.; Cheng, H.; Liu, L.; Zhu, Z.; Chen, H.; Ding, S.; Wang, S. γ-Fe2O3 decorated attapulgite composite modified with CuCl2 as magnetically separable sorbents for Hg0 removal from coal combustion flue gas. Chemical Engineering Journal 2021, 408, 127888. [Google Scholar] [CrossRef]
- Deng, J.; Xu, D.; Zhang, J.; Xu, Q.; Yang, Y.; Wei, Z.; Su, Z. Cs3Bi2Br9/BiOBr S-scheme heterojunction for selective oxidation of benzylic C−H bonds. Journal of Materials Science & Technology 2023, 924, 166608. [Google Scholar]
- Wu, M.; Zhang, B.; Wang, H.; Chen, Y.; Fan, M.; Dong, L.; Li, B.; Chen, G. Exposed 110 facets of BiOBr anchored to marigold-like MnCo2O4 with abundant interfacial electron transfer bridges and efficient activation of peroxymonosulfate. Journal of Colloid and Interface Science 2023, 653, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, L.; Fan, Z.; Sun, S.; Feng, Z.; Li, W.; Ding, H. Construction of R-TiO2/n-TiO2 heterophase photocatalysts for efficient degradation of organic pollutants. Journal of Alloys and Compounds 2023, 968, 172127. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zha, X.; Luo, Y.; Hu, Y.; Chen, G.; He, X. Interfacial chemical bond and oxygen vacancies modulated Mo2S3/BiOBr high-low junctions for enhanced photocatalysis gatifloxacin degradation. Applied Surface Science 2023, 641, 158548. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, C.; Zheng, S.; Di, Y.; Sun, Z.; Li, C. Design and controllable preparation of Bi2MoO6/attapulgite photocatalyst for the removal of tetracycline and formaldehyde. Applied Clay Science 2021, 215, 106319. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Liu, W.; Hu, X.; Zhang, C.; Zhou, Z.; Lang, J.; Wu, G.; Zhang, Y.; Yang, J.; Ni, Z.; Zhao, G. Regulating mechanical performance of poly (l-lactide acid) stent by the combined effects of heat and aqueous media. International Journal of Biological Macromolecules 2023, 242, 124987. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, T.; Zhang, Y.; Peng, X.; Song, W.; Yang, J.; Yuan, C. Oxidation behaviors of Hongqian heavy crude oil characterized by TG-DSC-FTIR-MS within full temperature regions. Fuel 2023, 353, 129242. [Google Scholar] [CrossRef]
- Zhu, S.-R.; Wu, M.-K.; Zhao, W.-N.; Yi, F.-Y.; Tao, K.; Han, L. Fabrication of heterostructured BiOBr/Bi24O31Br10/TiO2 photocatalyst by pyrolysis of MOF composite for dye degradation. Journal of Solid State Chemistry 2017, 255, 17–26. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chemical Engineering Journal 2020, 386, 124010. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




