Submitted:
15 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Biofabrication approaches
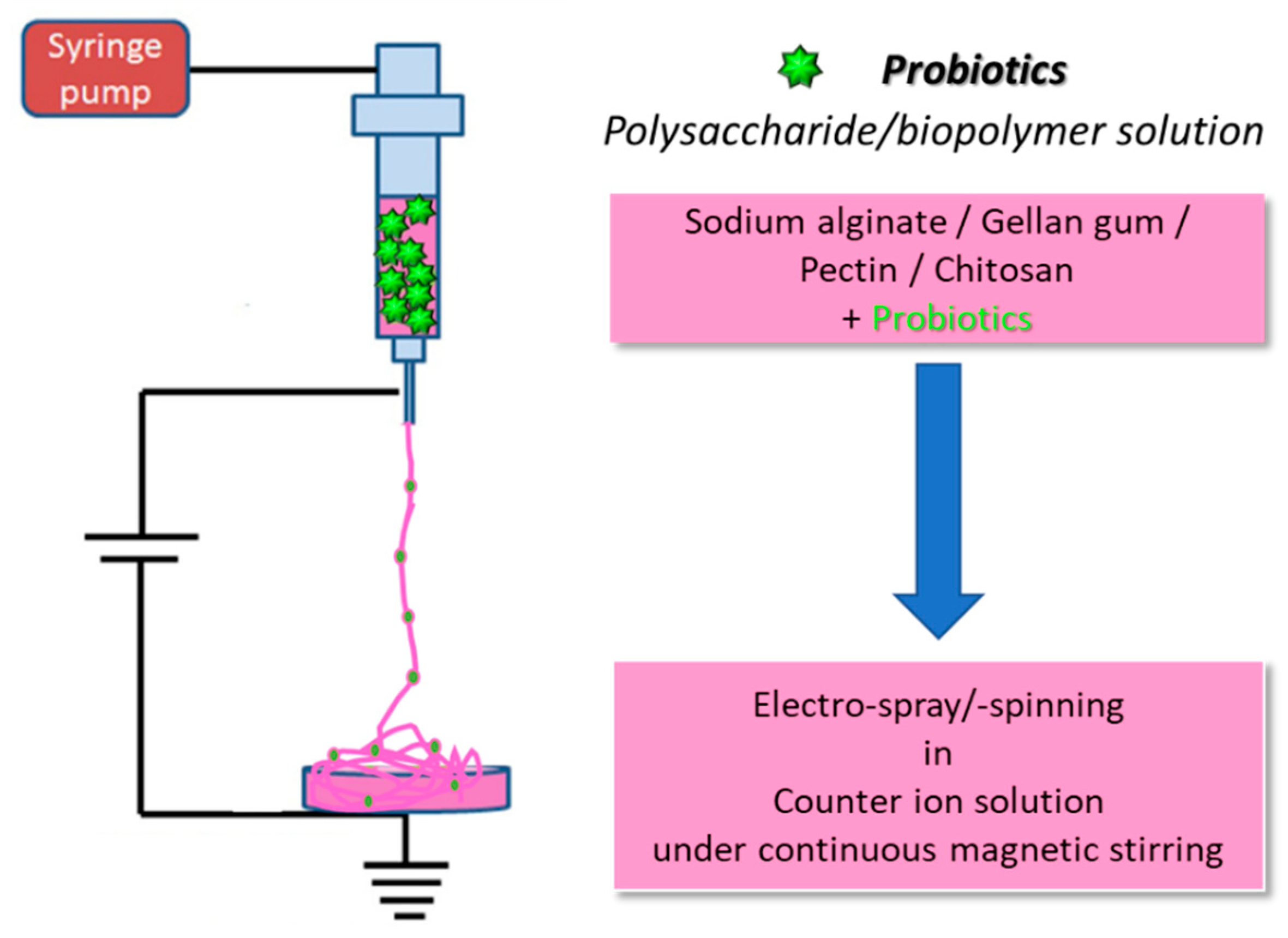
2.1. The electrospinning process
2.1.1. Polymers for bacterial cell electrospinning
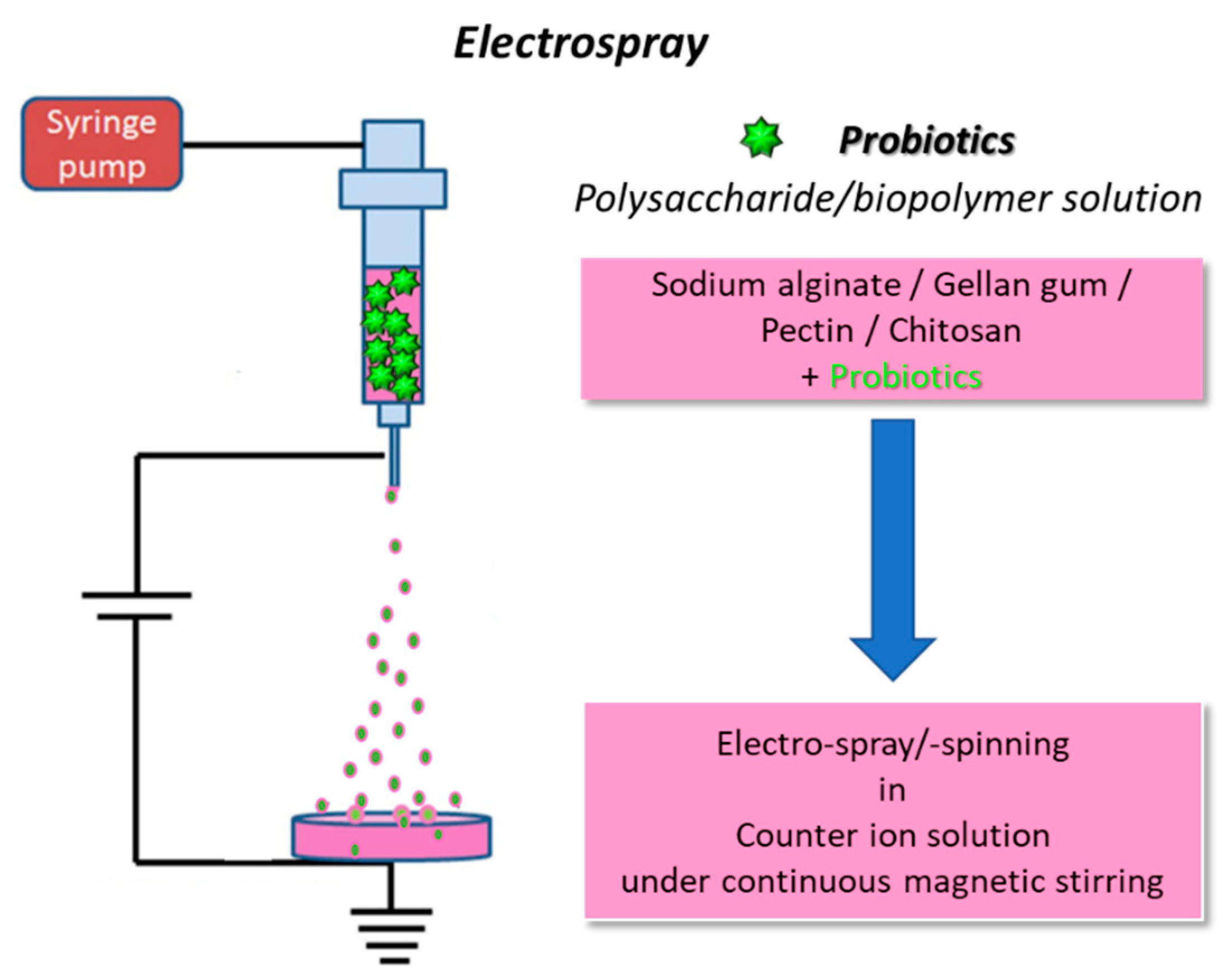
2.2. Electrospray
2.3. The 3D (bio)printing process
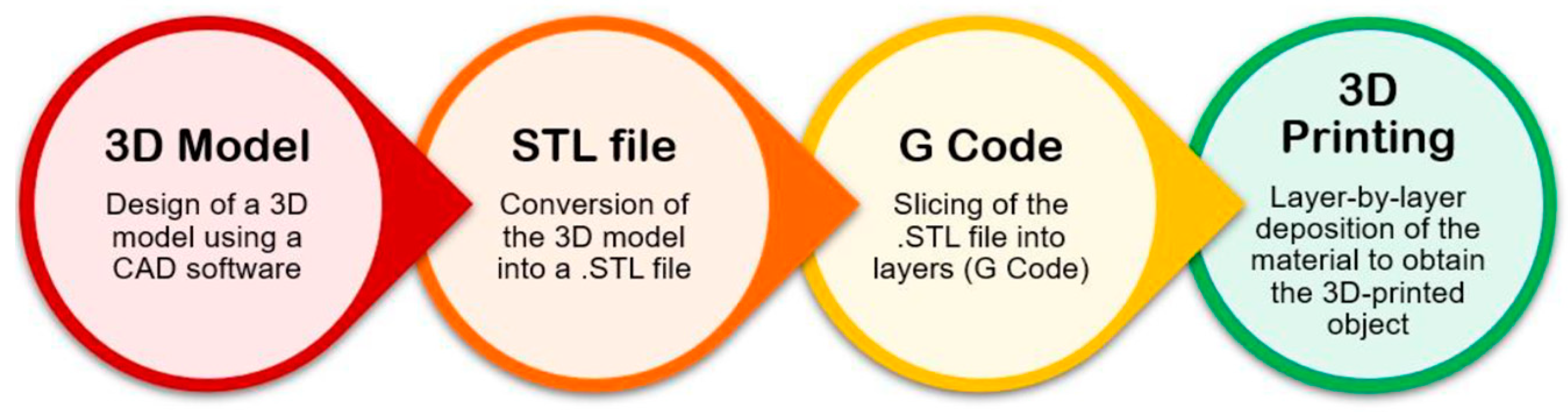
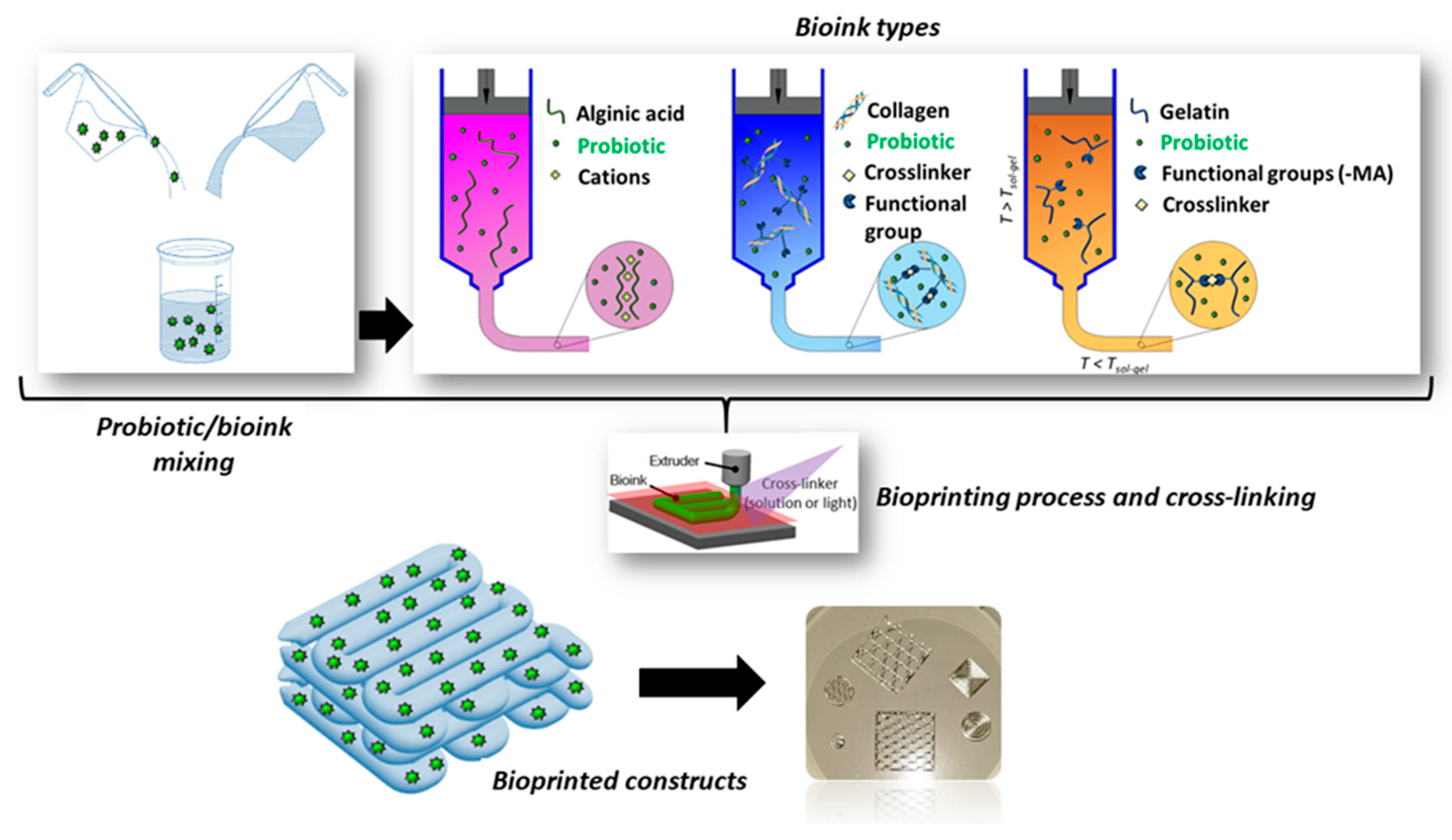
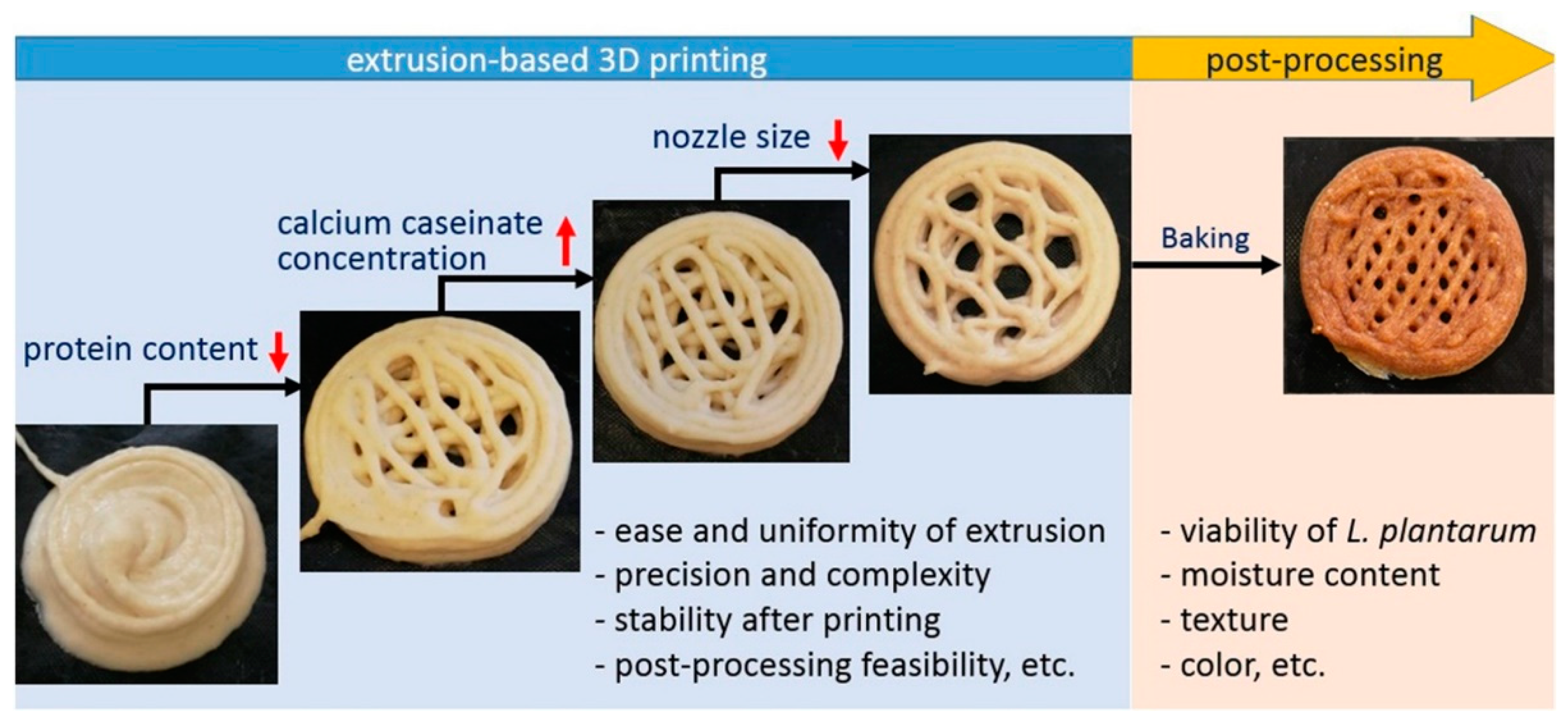
2.3.1. Extrusion-based 3D bioprinting
2.3.2. Bioprinting of probiotic-loaded constructs
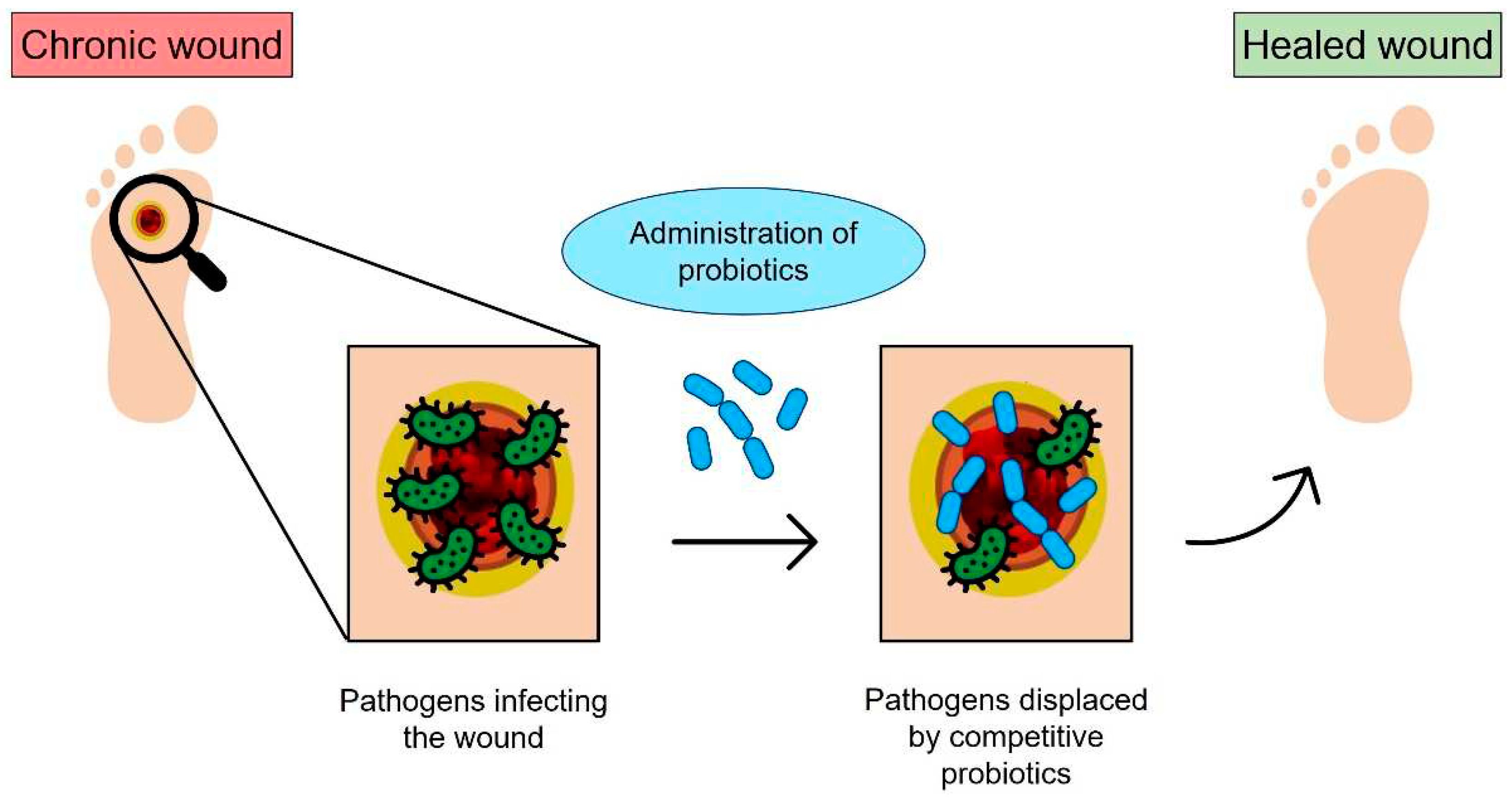
3. Probiotic and post-biotic release
4. Conclusions and future perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batoni, G.; Maisetta, G.; Kaya, E.; Esin, S. Lung-Directed Bacteriotherapy in Cystic Fibrosis: Could It Be an Option? Antibiotics 2022, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.C.; Huaman Martinez, M.A.; Valdez, J.C. Bacteriotherapy with Lactobacillus Plantarum in Burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.G.; Moura, J.; Carvalho, E.; Empadinhas, N. Microbiota of Chronic Diabetic Wounds: Ecology, Impact, and Potential for Innovative Treatment Strategies. Front. Microbiol. 2017, 1791. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; Quilès, F.; Francius, G.; Burgain, J.; Gaiani, C.; Scher, J.; Amara, S.; Lemaitre, C.; Marchal, P.; Alem, H. An Easy and Robust Method of Preparation of Capsules for Delivering Probiotic Bacteria by a 3D Bioprinting. Food Hydrocoll. Heal. 2022, 2, 100088. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics on Wound Healing: A Review of Animal and Human Studies. Int. Wound J. 2020, 17, 1687–1694. [Google Scholar] [CrossRef]
- Kurečič, M.; Rijavec, T.; Hribernik, S.; Lapanje, A.; Kleinschek, K.S.; Maver, U. Novel Electrospun Fibers with Incorporated Commensal Bacteria for Potential Preventive Treatment of the Diabetic Foot. Nanomedicine 2018, 13, 1583–1594. [Google Scholar] [CrossRef]
- Broek, M.F.L. Van Den; Boeck, I. De; Kiekens, F.; Boudewyns, A.; Vanderveken, O.M.; Lebeer, S. Translating Recent Microbiome Insights in Otitis Media into Probiotic Strategies. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Jörissen, J.; van den Broek, M.F.L.; De Boeck, I.; Van Beeck, W.; Wittouck, S.; Boudewyns, A.; de Heyning, P.; Topsakal, V.; Van Rompaey, V.; Wouters, I.; et al. Case-Control Microbiome Study of Chronic Otitis Media with Effusion in Children Points at Streptococcus Salivarius as a Pathobiont-Inhibiting Species. MSystems 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Reports 2020, 140. [Google Scholar] [CrossRef]
- Angammana, C.J. A Study of the Effects of Solution and Process Parameters on the Electrospinning Process and Nanofibre Morphology. 2011.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q. Electrospinning and Electrospray for Biomedical Applications. Ref. Modul. Biomed. Sci. Encycl. Biomed. Eng. 2019. [Google Scholar]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of Chain Entanglements on Fiber Formation during Electrospinning of Polymer Solutions: Good Solvent, Non-Specific Polymer--Polymer Interaction Limit. Polymer (Guildf). 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Poshina, D.N.; Tyshkunova, I. V; Petrova, V.A.; Skorik, Y.A. Electrospinning of Polysaccharides for Tissue Engineering Applications. Rev. Adv. Chem. 2021, 11, 112–133. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of Polysaccharides for Regenerative Medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and Electrospray of Bio-Based and Natural Polymers for Biomaterials Development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Stijnman, A.C.; Bodnar, I.; Tromp, R.H. Electrospinning of Food-Grade Polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398. [Google Scholar] [CrossRef]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Venkatraman, S.S. Importance of Viscosity Parameters in Electrospinning: Of Monolithic and Core--Shell Fibers. Mater. Sci. Eng. C 2012, 32, 1037–1042. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of Linear Homopolymers of Poly (Methyl Methacrylate): Exploring Relationships between Fiber Formation, Viscosity, Molecular Weight and Concentration in a Good Solvent. Polymer (Guildf). 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Saquing, C.D.; Tang, C.; Monian, B.; Bonino, C.A.; Manasco, J.L.; Alsberg, E.; Khan, S.A. Alginate-Polyethylene Oxide Blend Nanofibers and the Role of the Carrier Polymer in Electrospinning. Ind. Eng. Chem. Res. 2013, 52, 8692–8704. [Google Scholar] [CrossRef]
- Fareed, F.; Saeed, F.; Afzaal, M.; Imran, A.; Ahmad, A.; Mahmood, K.; Shah, Y.A.; Hussain, M.; Ateeq, H. Fabrication of Electrospun Gum Arabic--Polyvinyl Alcohol Blend Nanofibers for Improved Viability of the Probiotic. J. Food Sci. Technol. 2022, 59, 4812–4821. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Z.; Uslu, E.; İspirli, H.; Meral, R.; Gavgalı, M.; Tahsin’Yilmaz, M.; Dertli, E. A Novel Perspective for Lactobacillus Reuteri: Nanoencapsulation to Obtain Functional Fish Fillets. LWT 2019, 115, 108427. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.; Wu, R.; Wei, Y.; Zong, M.; Linhardt, R.J.; Wu, H. A Novel Route for Double-Layered Encapsulation of Probiotics with Improved Viability under Adverse Conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An Alternative Way to Encapsulate Probiotics within Electrospun Alginate Nanofibers as Monitored under Simulated Gastrointestinal Conditions and in Kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.Y. Psychrophilic Bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in Food Industry with Emerging Electrohydrodynamic Techniques: Electrospinning and Electrospraying--A Review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef]
- Mendes, A.C.; Chronakis, I.S. Electrohydrodynamic Encapsulation of Probiotics: A Review. Food Hydrocoll. 2021, 117, 106688. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus Acidophilus in Zein--Alginate Core--Shell Microcapsules via Electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus Acidophilus in Moist-Heat-Resistant Multilayered Microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Siqueira, N.M.; Pletsch, J.; Soares, R.M.D.; Ayub, M.A.Z. Electrospraying Microencapsulation of Lactobacillus Plantarum Enhances Cell Viability under Refrigeration Storage and Simulated Gastric and Intestinal Fluids. J. Funct. Foods 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H. Electrospray Assisted Fabrication of Hydrogel Microcapsules by Single-and Double-Stage Procedures for Encapsulation of Probiotics. Food Bioprod. Process. 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double Layer Co-Encapsulation of Probiotics and Prebiotics by Electro-Hydrodynamic Atomization. Lwt 2019, 110, 102–109. [Google Scholar] [CrossRef]
- Liu, C.; Xu, N.; Zong, Q.; Yu, J.; Zhang, P. Hydrogel Prepared by 3D Printing Technology and Its Applications in the Medical Field. Colloid Interface Sci. Commun. 2021, 44, 100498. [Google Scholar] [CrossRef]
- Taneja, H.; Salodkar, S.M.; Parmar, A.S.; Chaudhary, S. Hydrogel Based 3D Printing: Bio Ink for Tissue Engineering. J. Mol. Liq. 2022, 120390. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. Part B Eng. 2022, 238. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Truby, R.L.; Lewis, J.A. Printing Soft Matter in Three Dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Karthikeyan, C.; Yallapu, M.M.; Sadiku, R. The Significance of Biomacromolecule Alginate for the 3D Printing of Hydrogels for Biomedical Applications. Int. J. Biol. Macromol. 2022, 212, 561–578. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Aubin-Tam, M.-E.; Meyer, A.S. 3D Printing for the Fabrication of Biofilm-Based Functional Living Materials. ACS Synth. Biol. 2019, 8, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Lehner, B.A.E.; Schmieden, D.T.; Meyer, A.S. A Straightforward Approach for 3D Bacterial Printing. ACS Synth. Biol. 2017, 6, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Schmieden, D.T.; Basalo Vázquez, S.J.; Sangüesa, H.; Van Der Does, M.; Idema, T.; Meyer, A.S. Printing of Patterned, Engineered E. Coli Biofilms with a Low-Cost 3D Printer. ACS Synth. Biol. 2018, 7, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-Art of 3D Printing Technology of Alginate-Based Hydrogels—An Emerging Technique for Industrial Applications. Adv. Colloid Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef] [PubMed]
- Freyman, M.C.; Kou, T.; Wang, S.; Li, Y. 3D Printing of Living Bacteria Electrode. Nano Res. 2020, 13, 1318–1323. [Google Scholar] [CrossRef]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D Printing of Bacteria into Functional Complex Materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lou, Y.; Schutyser, M.A.I. 3D Printing of Cereal-Based Food Structures Containing Probiotics. Food Struct. 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Kyser, A.J.; Masigol, M.; Mahmoud, M.Y.; Ryan, M.; Lewis, W.G.; Lewis, A.L.; Frieboes, H.B.; Steinbach-Rankins, J.M. Fabrication and Characterization of Bioprints with Lactobacillus Crispatus for Vaginal Application. J. Control. Release 2023, 357, 545–560. [Google Scholar] [CrossRef]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The Impacts of Antimicrobial and Antifungal Activity of Cell-Free Supernatants from Lactic Acid Bacteria in Vitro and Foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu Y Abreu, A.T.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The Clinical Evidence for Postbiotics as Microbial Therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Hajipour, N.; Hasannezhad, P.; Baghbanzadeh, A.; Aghebati-Maleki, L. Potential in Vivo Delivery Routes of Postbiotics. Crit. Rev. Food Sci. Nutr. 2022, 62, 3345–3369. [Google Scholar] [CrossRef] [PubMed]
- da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food Applications of Liposome-Encapsulated Antimicrobial Peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Huang, R.; Wang, H.; Lan, P.; Zhao, Y. Prebiotics and Postbiotics Synergistic Delivery Microcapsules from Microfluidics for Treating Colitis. Adv. Sci. 2022, 9, 2104089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-Propionic Acid Inhibits Gut Dysbiosis and Endotoxin Leakage to Attenuate Steatohepatitis in Rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, 2102545. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly (Lactic Acid)-Based Electrospun Fibrous Structures for Biomedical Applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Ricci, C.; Azimi, B.; Panariello, L.; Antognoli, B.; Cecchini, B.; Rovelli, R.; Rustembek, M.; Cinelli, P.; Milazzo, M.; Danti, S.; et al. Assessment of Electrospun Poly (ε-Caprolactone) and Poly (Lactic Acid) Fiber Scaffolds to Generate 3D In Vitro Models of Colorectal Adenocarcinoma: A Preliminary Study. Int. J. Mol. Sci. 2023, 24, 9443. [Google Scholar] [CrossRef]
- Azimi, B.; Sorayani Bafqi, M.S.; Fusco, A.; Ricci, C.; Gallone, G.; Bagherzadeh, R.; Donnarumma, G.; Uddin, M.J.; Latifi, M.; Lazzeri, A.; Danti, S. Electrospun ZnO/Poly(Vinylidene Fluoride-Trifluoroethylene) Scaffolds for Lung Tissue Engineering, Tissue Eng. Part A. 2020, 26(23-24), 1312-1331. [CrossRef]
- Hasbum, A.; Karabulut, O.; Reyes, R.E.; Ricci, C.; Franchi, A.; Danti, S.; Chew, S.A. Combined Application of Patient-Derived Cells and Biomaterials as 3D In Vitro Tumor Models. Cancers (Basel). 2022, 14, 2503. [Google Scholar] [CrossRef]
| Probiotic | Polymer | Mechanical properties |
Targeted application |
Morphological parameters |
Ref. |
|---|---|---|---|---|---|
| Lactobacillus acidophilus | Arabic gum/ PVA | Tensile strength = 14.21 ± 0.7 MPa Elongation at break = 27.8 ± 0.3 % |
GI | Diameter > 617 nm Thickness = 0.12 ± 0.01 mm |
[23] |
| Lactobacillus reuteri | PVA | - | Functional fish fillets | Diameter = 381.83 ± 130.69 nm | [24] |
| Lactobacillus plantarum | SA/PVA | - | Food industry | Diameter = 270 ± 64 nm | [25] |
| Lactobacillus paracasei | SA/PVA | - | GI applications |
Diameter = 842 ± 72 nm | [26] |
| Probiotic | Polymer | Average diameter | Ref. |
|---|---|---|---|
| Lactobacillus acidophilus | Alginate–glycerol/Zein | < 550 μm | [30] |
| Lactobacillus acidophilus | EA-SA | < 700 μm | [31] |
| Lactobacillus plantarum | Pectin | - | [32] |
| Lactobacillus plantarum | Ca-alginate-Chitosan | < 500 μm | [33] |
| Lactobacillus plantarum/ Bifidobacterium lactis | Ca-alginate-Chitosan | = 710 μm | [34] |
| Probiotic | Polymer | Targeted application |
Mechanical Properties |
Ref. |
|---|---|---|---|---|
| Lactobacillus rhamnosus | SA-gelatin |
GI |
Elasticity = 3.56 ± 1.53 at day 0, 1.07 ± 0.69 at day 7 | [4] |
| Escherichia coli/ Bacillus subtilis | Alginate | Prevention device for infections caused by pathogenic bacteria | - | [42] |
| Escherichia coli | SA | Artificial biofilm | - | [43] |
| Escherichia coli | Alginate | Water filtration, metal ion sequestration, or civil engineering | - | [44] |
| Shewanella Oneidensis MR-1 | Alginate-Cellulose | Fabrication of microbial devices | - | [46] |
| P. putida, X. xylinum | Hyaluronic acid (HA)- κ-carrageenan (κ-CA) - fumed silica (FS) | Biomedical and biotechnological applications. Biologically generated functional materials. |
G’’> G’ at strains > 10%; Viscosity < 108 mPa·s Yield stress < 350 Pa |
[47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





