Submitted:
15 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Inoculum preparation
2.2. Honey bee inoculation
2.3. Stimulus preparation
2.4. Olfactometric bioassays
2.5. RNA extraction and cDNA synthesis
2.6. Real-Time PCR quantification for viral load and gene expression
2.7. Data analysis
3. Results
3.1. Viral load in antenna and head
3.2. Olfactory tests
3.3. Gene expression
3.2. Figures, Tables and Schemes
| Primers Names | Sequence | Reference |
|---|---|---|
| AmNrx-1 | F- CTGCTTCGAGCGACGACTAT | Morfin et al., 2019 |
| R- ACGACCGGATGGATGATTGG | ||
| AmNlg-1 | F- ATGTCGAGGATGCTGCGACTGGA | Morfin et al., 2008 |
| R-TACCTGTGCACTATCTCCTGTTGTA | ||
| DWV-A | F-TATCTTCATTAAAGCCACCTGGAA | Yang et al., 2005 |
| R- TTTCCTCATTAACTGTGTCGTTGAT | ||
| AmelOBP5 | F- ATGCGGAAATCGTGCTTGCA | Li et al., 2015 |
| R- TGCCATTACTCACGGGAAGA | ||
| AmelOBP1 | F- TGAGGATGTCGAAGCTACGGAA | Li et al., 2015 |
| R- CACGGAGCAATAAACGCTATGG | ||
| β -actin | F- ATGCCAACACTGTCCTTTCTGG | Yang et al., 2005 |
| R- GACCCACCAATCCATACGGA |
| Bee age (days) | Pheromone (benzyl alcohol) vs. Air | |||||||||
| Status | Pheromone | χ2 | p-value | Air | χ2 | p-value | No preference | χ2 | p-value | |
| 5 | I-DWV | 0.33 | 1.36 | 0.243 | 0.30 | 3.75 | 0.053 | 0.37 | 6.70 | 0.010 |
| N-DWV | 0.20 | 0.10 | 0.70 | |||||||
| 10 | I-DWV | 0.10 | 13.02 | <0.001 | 0.30 | 3.75 | 0.053 | 0.60 | 3.27 | 0.071 |
| N-DWV | 0.53 | 0.10 | 0.37 | |||||||
| 15 | I-DWV | 0.13 | 10.80 | 0.001 | 0.13 | 0.00 | 0.992 | 0.73 | 9.64 | 0.002 |
| N-DWV | 0.53 | 0.13 | 0.33 | |||||||
| 20 | I-DWV | 0.23 | 8.30 | 0.004 | 0.23 | 1.92 | 0.166 | 0.53 | 3.36 | 0.067 |
| N-DWV | 0.60 | 0.10 | 0.30 | |||||||
| Bee age (days) | Pheromone (benzyl alcohol) vs. essential oil | |||||||||
| Status | Pheromone | χ2 | p-value | Essential oil | χ2 | p-value | No preference | χ2 | p-value | |
| 5 | I-DWV | 0.23 | 0.09 | 0.766 | 0.07 | 1.46 | 0.228 | 0.70 | 1.15 | 0.284 |
| N-DWV | 0.27 | 0.17 | 0.57 | |||||||
| 10 | I-DWV | 0.20 | 7.18 | 0.007 | 0.07 | 1.46 | 0.228 | 0.73 | 11.28 | 0.001 |
| N-DWV | 0.53 | 0.17 | 0.30 | |||||||
| 15 | I-DWV | 0.10 | 11.43 | 0.001 | 0.27 | 0.32 | 0.573 | 0.63 | 13.61 | <0.001 |
| N-DWV | 0.50 | 0.33 | 0.17 | |||||||
| 20 | I-DWV | 0.07 | 1.46 | 0.228 | 0.27 | 11.28 | 0.001 | 0.67 | 17.78 | <0.001 |
| N-DWV | 0.17 | 0.70 | 0.13 | |||||||
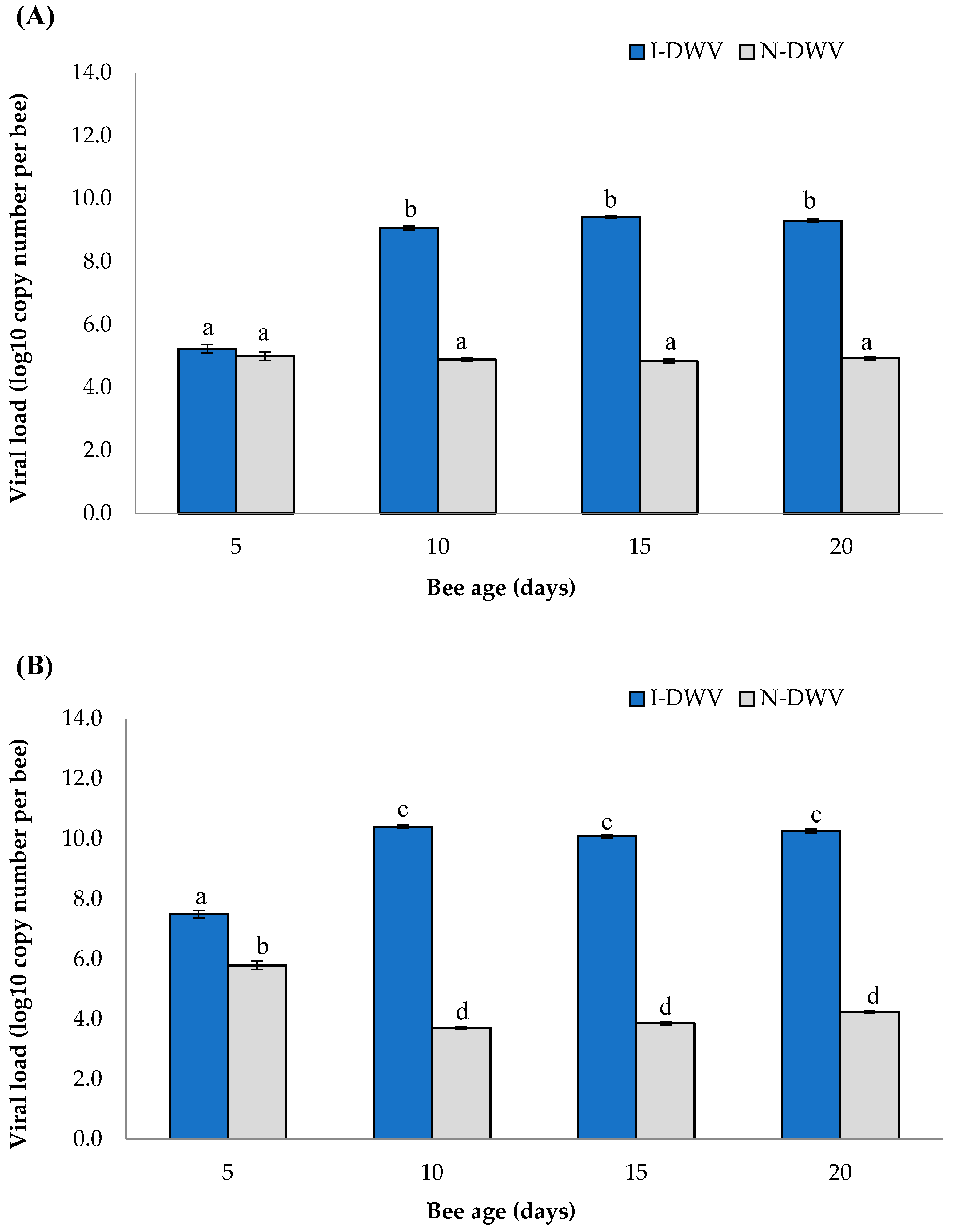
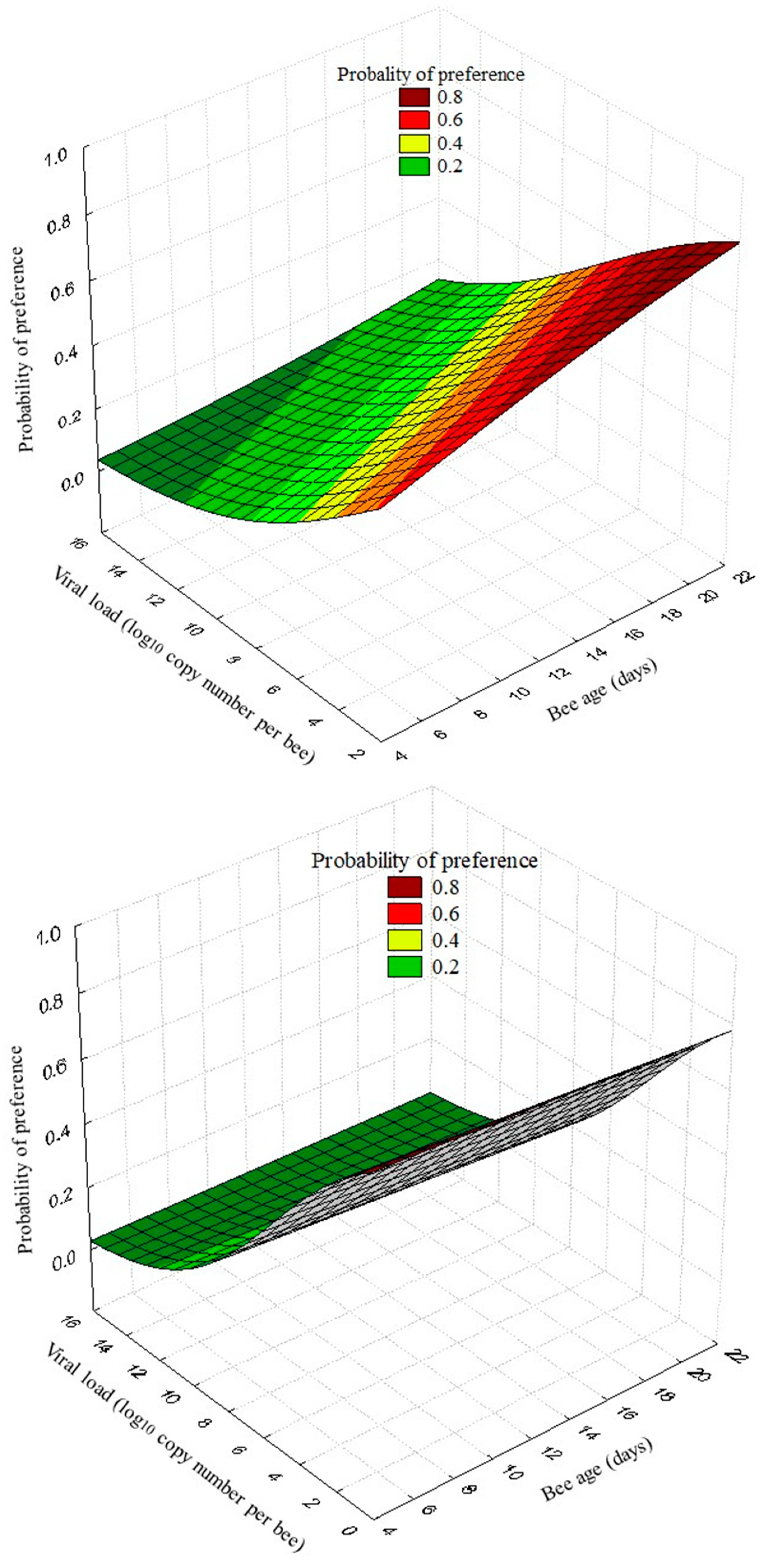
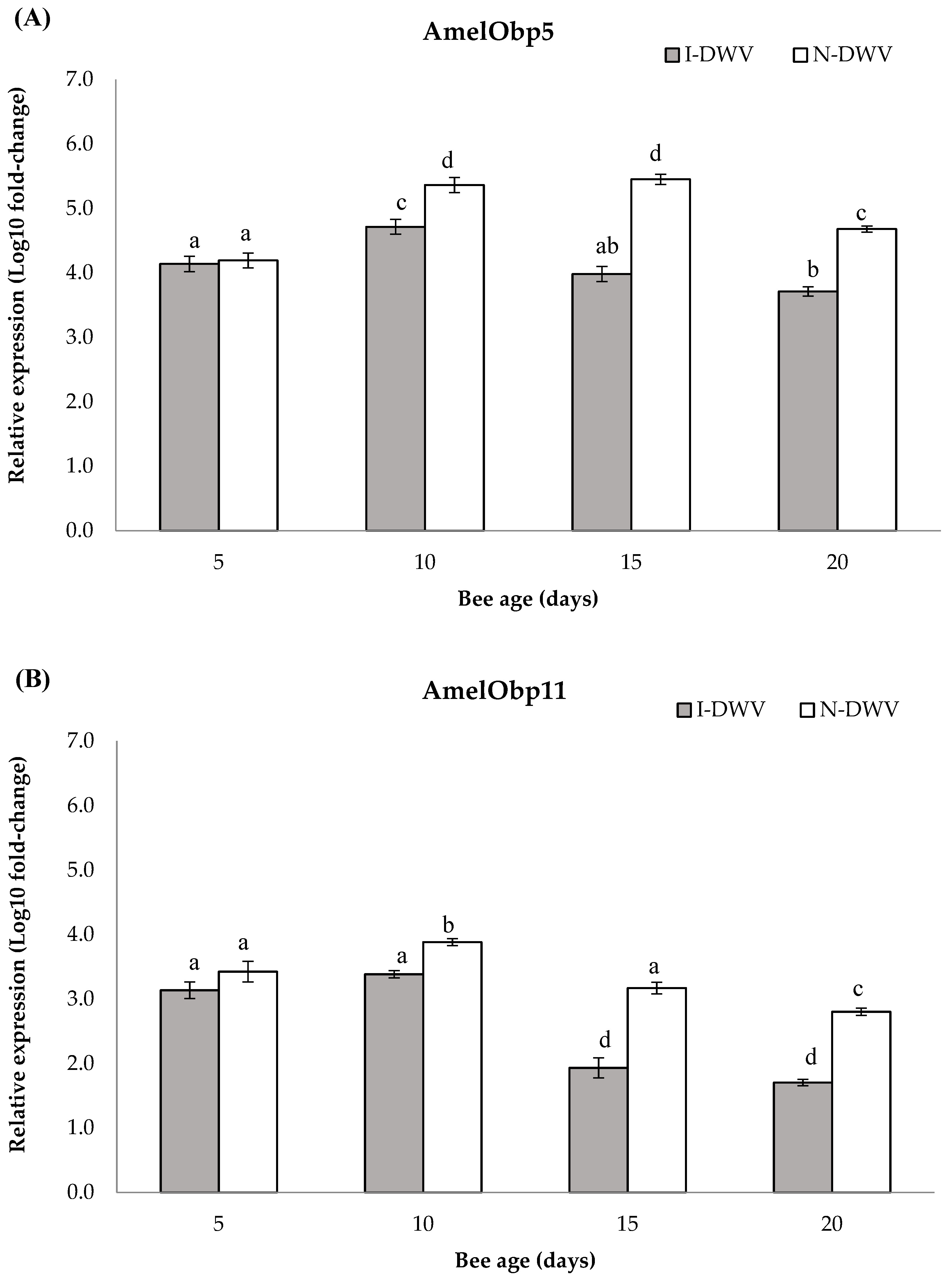
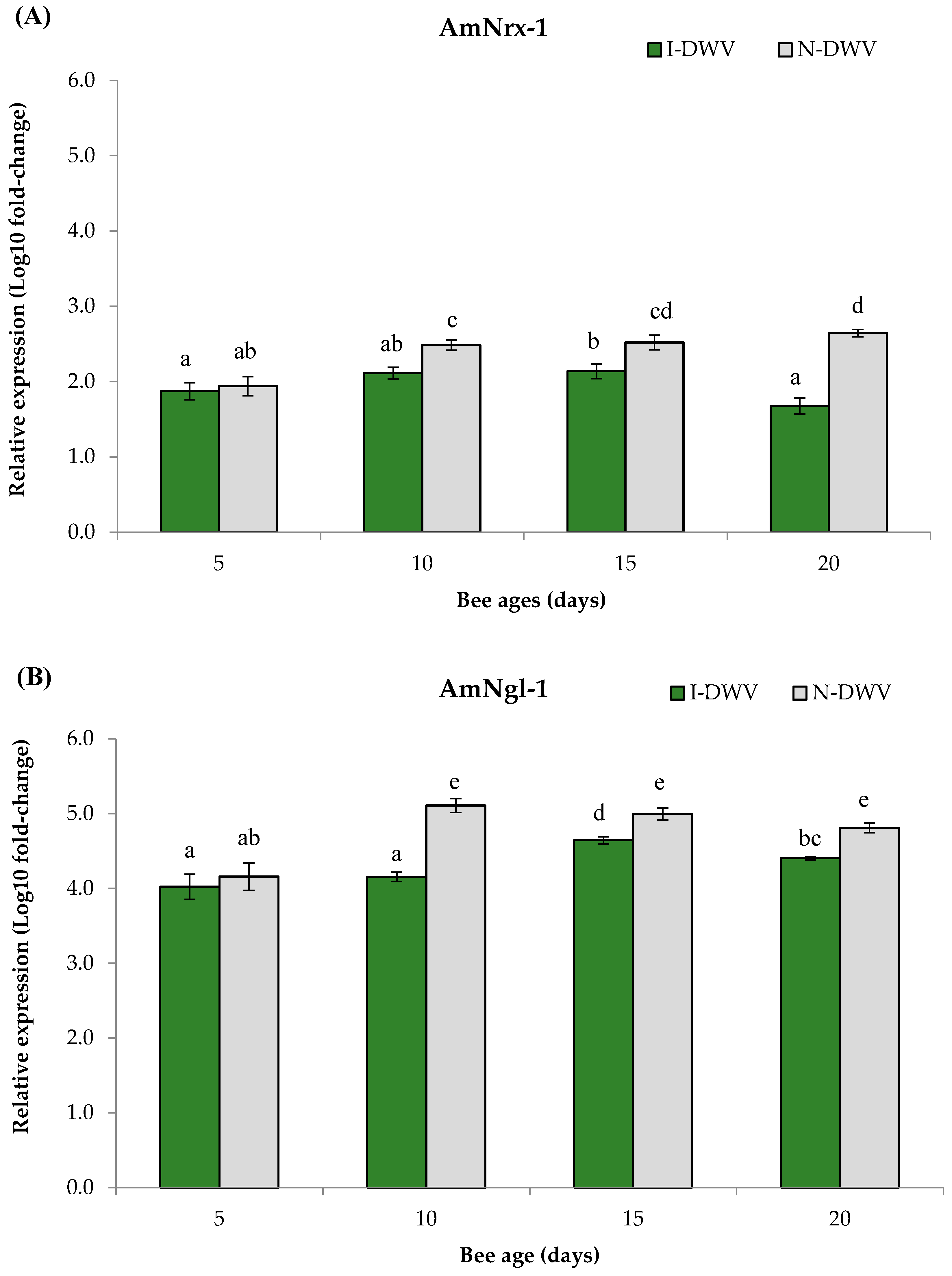
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groot, A. T.; Dekker, T.; Heckel, D. The Genetic Basis of Pheromone Evolution in Moths. Annu. Rev. Entomol 2016, 61(1): 99–117. [CrossRef]
- Li, Q and Liberles, S.D. Aversion and attraction through olfaction. Curr. Biol. 2015, 25(3):120-129. [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [CrossRef]
- Zhou, J.-.-J., Vieira, F.G., He, X.-.-L., Smadja, C., Liu, R., Rozas, J. and Field, L.M. Genome annotation and comparative analyses of the odorant-binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum. Insect Molecular Biology 2010, 19: 113-122. [CrossRef]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93: 184-200. [CrossRef]
- Forêt, S.; Maleszka, R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 2006, 16(11): 1404–1413. [CrossRef]
- Zhao, H.; Luo, Y.; Lee, J.; Zhang, X.; Liang, Q.; Zeng, X. The Odorant-binding protein gene obp11 shows different spatiotemporal roles in the olfactory system of Apis mellifera ligustica and Apis cerana cerana. Sociobiology. 2013, 60, 429–435. [CrossRef]
- Zhao, H.X.; Zeng, X.N.; Liang, Q.; Zhang, X.F.; Huang, W.Z.; Chen, H.S.; Luo, Y.X. Study of the obp5 gene in Apis mellifera ligustica and Apis cerana cerana. Genet. Mol. Res. 2015, 14 (2), 6482–6494. [CrossRef]
- Plettner, E., Eliash, N., Singh, N.K. The chemical ecology of host-parasite interaction as a target of Varroa destructor control agents. Apidologie 2017, 48, 78–92. [CrossRef]
- Potts, S.G.; Biesmeier, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: trends, impacts and drivers. Trend. Ecol. Evol. 2010, 25, 345-353. [CrossRef]
- Hall, D.M.; Steiner, R. Insect pollinator conservation policy innovations at subnational levels: Lessons for lawmakers. Environ. Sci. Policy 2019, 93, 118-128. [CrossRef]
- vanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLOS ONE 2009, 4(8): e6481. [CrossRef]
- Martin, S.J.; Hardy, J.; Villalobos, E.; Martin-Hernández, R.; Nikaido, S.; Higes, M. Do the honeybee pathogens Nosema ceranae and deformed wing virus act synergistically?. Environ Microbiol Rep. 2013, 5-4.
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226.
- Brettell, LE; Mardoqueo, GJ; Schroeder, DC; Jones, IM; da Silva, JR; Vicente-Rubiano, M.; Martin, SJ. A Comparison of Deformed Wing Virus in Deformed and Asymptomatic Honey Bees. Insects, 2017 , 8 , 28.
- Benaets, K.; Van Geystelen, A.; Cardoen, D.; De Smet, L.; de Graaf, DC; Schoofs, L.; Larmuseau, MH; Brettell, LE; Martín, SJ; Wenseleers, T. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. Biol. Science, 2017 , 284. [CrossRef]
- Kevill, J. L.; de Souza, F.S.; Sharples, C.; Oliver, R.; Schroeder, D.C.; Martin, S.J. DWV-A Lethal to Honey Bees (Apis mellifera): A Colony Level Survey of DWV Variants (A, B, and C) in England, Wales, and 32 States across the US. Viruses 2019, 11(5): 426-426. [CrossRef]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet,V.; Furst, M.; Weging, S.; Brown, M.J.; Gogol-Doring, A.; Paxton, R.J.; Mallinger, R.E.; Gratton, C. Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. Journal of Applied Ecology 2016, 52 (2): 323-330.
- Riveros, G.; Arismendi, N.; Zapata, N.; Evans, D.; Pérez, I.; Aldea, P.; Vargas, M. Occurrence, prevalence and viral load of deformed wing virus variants in Apis mellifera colonies in Chile. J. Apic. Res. 2019, 59, 63–68. [CrossRef]
- Vargas, M.; Arismendi, N.; Riveros, G.; Zapata, N.; Bruna, A.; Vidal, M.; Rodríguez, M.; Gerding, M. Viral and intestinal diseases detected in Apis mellifera in Central and Southern Chile. Chil. J. Agric. Res. 2017, 77, 243–249. [CrossRef]
- Kim, S. H.; Mercer, A.; Mitchell, A.; de Miranda, J.R.; Ward, V.; Mondet, F.; Bostina, M. Viral infections alter antennal epithelium ultrastructure in honey bees. J. Invertebr. Pathol. 2019, 168, 107252. [CrossRef]
- Silva, D.; Ceballos, R.; Arismendi, N.; Dalmon, A.; Vargas, M. Variant A of the Deformed Wings Virus Alters the Olfactory Sensitivity and the Expression of Odorant Binding Proteins on Antennas of Apis mellifera. Insects 2021, 12, 895. [CrossRef]
- Shah, K.S.; Evans, E.C.; Pizzorno, M.C. Localization of deformed wing virus (DWV) in the brains of the honeybee, Apis mellifera Linnaeus. Virol. 2009, 6, 182. [CrossRef]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. The Combined Effects of Varroa destructor Parasitism and Exposure to Neonicotinoids Affects Honey Bee (Apis mellifera L.) Memory and Gene Expression. Biology 2019, 9(9):237. [CrossRef]
- Gusachenko, O.N.; Woodford, L.; Balbirnie-Cumming, K.; Ryabov, E.V.; Evans, D.J. Evidence for and against deformed wing virus spillover from honey bees to bumble bees: A reverse genetic analysis. Sci. Rep. 2020, 10, 16847. [CrossRef]
- Arismendi, N.; Caro, S.; Castro, M.P.; Vargas, M.; Riveros, G.; Venegas, T. Impact of mixed infections of gut parasites Lotmaria passim and Nosema ceranae on the lifespan and immune-related biomarkers in Apis mellifera. Insects 2020, 11, 420. [CrossRef]
- Liu, J.; Chen, M.; Ma, W.; Zheng, L.; Zhang, B.; Zhao, H.; Jiang, Y. Composition of Strawberry Flower Volatiles and Their Effects on Behavior of Strawberry Pollinators, Bombus terrestris and Apis mellifera. Agronomy 2023, 13, 339. [CrossRef]
- Mondet. F.; Alaux, C.; Severac, D.; Rohmer, M.; Mercer, A.; Le Conte, Y. Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 2015 ,5: 10454. [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2003–2007. [CrossRef]
- Wu, Y.; Dong, X.; Kadowaki, T. Characterization of the copy number and variants of deformed wing virus (DWV) in the pairs of honey bee pupa and infesting Varroa destructor or Tropilaelaps mercedesae. Front. Microbiol. 2017, 8, 1558. [CrossRef]
- Yang, X.; Cox-Foster, D. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [CrossRef]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Current Opinion in Insect Science 2020, 42. 1-7. [CrossRef]
- Nakamura, A.; Chahad-Ehlers, S.; Lima, A. et al. Reference genes for accessing differential expression among developmental stages and analysis of differential expression of OBP genes in Anastrepha obliqua. Sci Rep 2016, 6, 17480. [CrossRef]
- Zhou, J.-J.; Zhang, G.-A.; Huang, W.; Birkett, M.A.; Field, L.M.; Pickett, J.A.; Pelosi, P. Revisiting the odorant-binding protein LUSH of Drosophila melanogaster: evidence for odour recognition and discrimination, FEBS Lett. 2004, 558 (1) 23–26. [CrossRef]
- Zhu, G.; Zheng, M.-Y.; Sun, J.-B.; Khuhro, S.A.; Yan, Q.; Huang, Y.; Syed, Z.; Dong, S.-L. CRISPR/Cas9 mediated gene knockout reveals a more important role of PBP1 than PBP2 in the perception of female sex pheromone components in Spodoptera litura. Insect Biochem. Mol. Biol. 2019, 115, 103244. [CrossRef]
- Dukas, R. Mortality rates of honey bees in the wild. Insect. Soc. 2008, 55, 252–255. [CrossRef]
- Iqbal J. and Mueller U. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B. 2007, 274:1517-1521.
- Chen, P.; Lu, Y.-H.; Lin, Y.-H.; Wu, C.-P.; Tang, C.-K.; Wei, S.-C.; Wu, Y.-L. Deformed Wing Virus Infection Affects the Neurological Function of Apis mellifera by Altering Extracellular Adenosine Signaling. Insect Biochem. Mol. Biol. 2021, 139, 103674. [CrossRef]
- Robinson, G.E. Genomics and integrative analyses of division of labor in honeybee colonies. Am. Nat. 2002, 160:S160–S172.
- Seid, M.A.; Traniello, J.F.A. Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav. Ecol. Sociobiol. 2006, 60, 631–644. [CrossRef]
- Seeley, T.D. Division of labor among worker honey bees. Ethology 1986, 71:249–251.
- Johnson, B.R. Within-nest temporal polyethism in the honey bee. Behav. Ecol. Sociobiol. 2008, 62, 777–784. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





