Introduction
Chronic kidney disease (CKD) patients requiring maintenance hemodialysis (HD) therapy have been widely recognized as the highest-risk population for cardiovascular disease, including peripheral artery disease (PAD).[
1,
2,
3] Although lower limb revascularization has been commonly performed to treat PAD, poorer prognosis such as higher amputation or mortality rate remains a major clinical problem compared to those without CKD after revascularization regardless of bypass surgery[
4,
5] or endovascular therapy (EVT).[
6,
7] In addition, these situations have not improved although the advances in the medical management of HD patients in the last decade yet.[
8,
9] On the other hand, protein-energy wasting (PEW),[
10] a state of decreased body protein mass and energy fuel
, is reportedly prevalent in patients with CKD.[
11,
12] PEW can result not only from a poor diet but also be induced by inflammatory processes,[
13,
14] and inflammatory status itself is associated with higher cardiovascular and all-cause mortality in this population.[
15] Moreover, we previously reported that presence of PEW and inflammatory status was independently associated with lowering ankle brachial index (ABI), and that patients with these factors had a lower chance of survival.[
16] In these contexts, we investigated the association of pre-procedural geriatric nutritional risk index (GNRI),[
17,
18] a surrogate marker of PEW, and C-reactive protein (CRP) levels with limb amputation and/or mortality after lower extremity revascularization in patients with CKD undergoing HD.
Methods
Patients
This is a retrospective study. From January 2009 to April 2018, 800 consecutive HD patients who underwent successful lower extremity revascularization (535 with endovascular therapy [EVT] and 265 with bypass surgery) with measurement of preprocedural GNRI and CRP levels at Matsunami General Hospital (Kasamatsu, Japan) and Nagoya Kyoritsu Hospital (Nagoya, Japan) were enrolled in this study. Patients with acute limb ischemia were excluded. Clinical information such as established risk factors, indications for revascularization, and target lesions was obtained from medical records.
GNRI and CRP Measurements
Blood samples were collected before the procedural day to measure serum albumin and CRP levels. The GNRI was calculated from individually obtained serum albumin levels and body weights, as reported by Yamada et al.[
19]:
The body weight/ideal body weight ratio was set to one when the patient’s body weight exceeded the ideal body weight. Ideal body weight was defined as the value calculated from the height and body mass index of 22.[
19] All patients underwent HD therapy one day prior to the procedure, and body weight after HD therapy was used to calculate the GNRI. Serum CRP levels were measured using a latex-enhanced, highly sensitive CRP immunoassay. Then, patients were divided into tertiles according to their GNRI and CRP levels.
Follow-up
Patients were routinely followed up after discharge at 1, 3, and 6 months during 1 year, thereafter, followed up at yearly intervals using duplex scanning. If patients did not attend a hospital follow-up, they were interviewed by a telephone as possible, and the follow-up ended on the last visit day if we could not contact the patient. The follow-up period ended in January 2019. The primary outcome was amputation-free survival (AFS), officially defined as freedom from above-ankle amputation of the index limb or death from any cause.[
20]
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Matsunami General Hospital (code: 573
) and Nagoya Kyoritsu Hospital (code: K132-02), respectively. Written informed consent was waived to be obtained with Information regarding opt-out of this study on the website in each hospital due to the retrospective manor of this study.
Statistical Analyses
Normal distributed variables were expressed as mean ± SD, and asymmetrically distributed data were given as the median and interquartile range. Differences between the groups were evaluated using one-way analysis of variance or the Kruskal–Wallis test for continuous variables and the chi-square test for categorical variables. The AFS rates among the groups were expressed using the Kaplan–Meier method and the difference was compared using a log-rank test. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each factor using the Cox proportional hazards models. All baseline variables with P < 0.05 by univariable analysis were entered into a multivariate model to determine independent predictors of the outcome. To clarify whether the predictability for amputation and/or mortality would improve after the addition of GNRI alone, CRP alone, and both into a baseline model with established risk factors, we calculated the C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI). The C-index is defined as the area under receiver-operating characteristic curves between individual predictive probabilities for the endpoints and the incidence of the endpoints, and was compared among each predicting model.[
21] The NRI indicates how many rate of patients improved their predicted probabilities for the endpoints, and the IDI expressed the average improvement in predicted probabilities for the endpoints after addition of variables into the baseline model.[
22] Differences were set to be statistically significant at a two-sided P value less than 0.05. All statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The clinical characteristics of the patients are shown in
Table 1.
Patients were divided into tertiles according to GNRI levels; tertile 1 (T1): <88.1, T2:88.1-96.7and T3: >96.7, and CRP levels; T1: <2.0 mg/l, T2:2.0-12.6 mg/l and T3: >12.6 mg/l. Patients with lower GNRI had higher CRP levels [11.3 (2.9-44.5) mg/l, 4.0 (1.0-14.0) mg/l and 3.0 (1.0-12.0) mg/l in T1, T2, and T3, respectively; p<0.0001], and a higher prevalence of ulcer/gangrene (49.6%, 44.6%, and 27.7% in T1, T2, and T3, respectively; p<0.0001). Similarly, patients with higher CRP also had lower GNRI (94.3±9.4, 93.1±9.7 and 89.1±10.1 in T1, T2 and T3, respectively, p<0.0001) and higher prevalence of ulcer/gangrene (23.5%, 34.5% and 63.9% in T1, T2 and T3, respectively, p<0.0001).
Predictive value of the GNRI and CRP
During the follow-up period (median, 43 months), 56 (7.0%) patients required major amputation, and 183 (22.9%) patients died. Kaplan-Meier analysis showed that the AFS rates for 8 years were 47.0%, 56.9%, and 69.5% in T1, T2, and T3 of the GNRI, and 65.8%, 58.7%, and 33.2% in T1, T2, and T3 of CRP, respectively (p<0.0001 for both) (Figure 1).
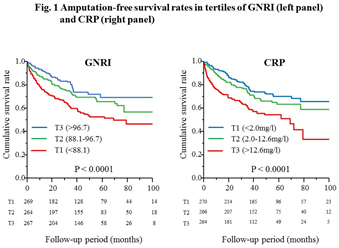
After adjustment for male sex, age, previous coronary artery disease, procedure (EVT vs. bypass surgery), below-knee artery disease, and ulcer/gangrene as covariates with p<0.05 by univariate analysis, decreased GNRI [adjusted HR 1.78, 95% CI 1.24-2.59, p=0.0016 for T1 vs. T3], and elevated CRP (adjusted HR 1.86, 95%CI 1.30-2.70, p=0.0007 for T3 vs. T1) were identified as independent predictors of amputation and/or mortality (
Table 2). Similar results were obtained for the amputation and mortality rates.
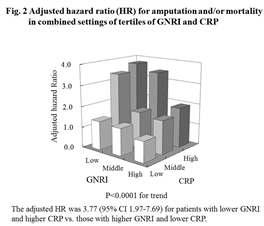
Combined predictive value of the GNRI and CRP
The combined setting of both variables could stratify the risk of amputation and/or mortality, and the risk was 3.77-fold higher (95% CI 1.97-7.69, p<0.0001) in GNRI T1 with CRP T3 than in GNRI T3 with CRP T1 (Figure 2).
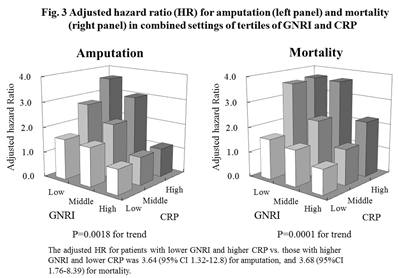
Similar results were also obtained for amputation and mortality (adjusted HR 3.64, 95% CI 1.32-12.8, p=0.0018 for amputation, and adjusted HR 3.68, 95% CI 1.76-8.39, p<0.0001 for mortality for GNRI T1 with CRP T3 vs. GNRI T3 with CRP T1, respectively) (Figure 3).
For model discrimination, the addition of both GNRI and CRP in a predicting model with established risk factors improved the C-statistics (from 0.661 to 0.716, p=0.0021), NRI (0.508, p<0.0001), and IDI (0.042, p<0.0001), even greater than that of each individual (NRI 0.145, p=0.047 and IDI 0.006, p=0.035 vs. GNRI alone, and NRI 0.427, P<0.0001 and IDI 0.029, p<0.0001 vs. CRP alone, respectively) (
Table 3).
Discussion
Our results clearly demonstrate that a preprocedural decline in GNRI and elevated CRP levels, which reflect PEW and chronic inflammation status, affect poor AFS after lower limb revascularization in patients undergoing HD, and that the combination of both variables could not only stratify the risk of poor AFS but also improve predictability.
Numerous studies have reported consistently poorer prognosis after lower limb revascularization in patients undergoing HD compared to the general population in spite of advances in medical management of HD.[
4,
5,
6,
7,
8,
9] In previous studies, we have reported the following findings: 1) severe/moderate nutritional risk (GNRI<92) was higher in patients undergoing HD (53%) than in the elderly general population (21%-43%) despite HD patients (average of 64 years) being younger than the elderly general population (80-85 years).[
12] 2) In patients who underwent bypass surgery, pre-procedural CRP levels was markedly higher in HD patients than in non-HD patients (median of 11 mg/l vs. 4 mg/l).[
23] 3) Interestingly, pre-procedural elevated CRP levels could predict poor AFS in only HD patients but not non-HD patients who underwent infrapopliteal bypass surgery.[
24] Thus, our results in the present study might be reasonably explained, and the PEW and chronic inflammation status, a CKD-specific morbidity, might be considered to be one of cause of poor AFS after lower limb revascularization in this population.
In addition, we previously reported that the limb salvage rate after bypass surgery was comparable between HD and non-HD patients when performing propensity score matching with unfavorable factors, including pre-procedural CRP levels.[
24] This suggests the possibility of improved prognosis if inflammation status is adequately managed even in patients undergoing HD. In this context, the recently developed Wound, Ischemia, and foot infection (WIfI) scoring system is considered important for assessing the risk of poor AFS. Unfortunately, WIfI scores were not measured in the present study. The association between WIfI score and prognosis in this high-risk population should be clarified in the future.
The condition of PEW was previously referred to as malnutrition, inflammation, and atherosclerosis (MIA) syndrome before it was officially defined by the International Society of Renal Nutrition and Metabolism (ISRNM).[
13,
14] We have previously reported the close association of both declined GNRI and elevated CRP with abnormal ABI.[
16] Abnormal ABI also reportedly reflects not only PAD but also systemic atherosclerosis,[
25,
26] thus, these previous findings might manifest as MIA syndrome. In this context, patients with decreased preprocedural GNRI and elevated CRP levels were considered to have advanced atherosclerosis and poor prognosis in the present study. Thus, physicians should pay more attention to these unfavorable conditions in this population.
Finally, the addition of both preprocedural GNRI and CRP levels in a predictive model with established risk factors such as age, infrapopoliteal disease, or ulcer/gangrene significantly improved the predictability for poor AFS after revascularization to a greater extent than the addition of GNRI and CRP alone. Thus, measurement of both variables before procedures might be clinically beneficial to predict prognosis more accurately because these variables are also easily obtained in daily practice.
The present study has several limitations. First, all the study participants were Japanese, who reportedly have a lower atherosclerotic risk compared to patients in the United States and Europe.[
27] Second, the study participants were from two centers only. Third, we could not assess the WIfI scores. The lack of data regarding wound or infection status in the limbs might be the most important limitation of this study.
Conclusions
A preprocedural decline in GNRI and elevated CRP level, which reflect PEW and chronic inflammation status, are closely associated with poor AFS after lower limb revascularization in chronic HD patients. Furthermore, the combination of both variables could not only stratify the risk of amputation and/or mortality, but also improve predictability when added to established risk factors.
Author Contributions
Conceptualization; Y.K., H.T., H.I., T.M., H.I. Methodology; Y.K., H.T., H.I., T.M., H.I. Software; H.T. Validation; Y.K., H.T., H.I., T.M., H.I. Formal Analysis; H.T. Investigation; Y.K., N.K., N.I., Y.N., H.T., S.O., R.I., H.I., T.M., H.I. Resources; Y.K., N.K., N.I., Y.N., S.O., R.I. Data Curation; Y.K., N.K., N.I., Y.N., S.O., R.I. Writing – Original Draft Preparation; Y.K., H.T., H.I. Writing – Review & Editing; Y.K., N.K., N.I., Y.N., H.T., S.O., R.I., H.I., T.M., H.I. Visualization; H.T. Supervision; Y.K., H.T., H.I., T.M., H.I. Project Administration; Y.K., H.T., H.I., T.M., H.I. Funding Acquisition; No available.
Funding
H. Izawa received grant support through his institution from Bayer, Sumitomo Pharma, PDR Pharma, Biotronik Japan, Abbott Japan, Boston Scientific Japan, Japan Lifeline, and Medtronic Japan, and honoraria for lectures from Otsuka, Novartis, Eli Lilly Japan, Bayer, Nippon Boehringer Ingelheim and Daiichi Sankyo. T. M. received lecture fees from Bayer Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Sanofi-aventis K.K., and Takeda Pharmaceutical Co., Ltd. T.M. received unrestricted research grant for Department of Cardiology, Nagoya University Graduate School of Medicine from Astellas Pharma Inc, Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Otsuka Pharma Ltd., Pfizer Japan Inc., Sanofi-aventis K.K., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd. H. Ishii received lecture fees from Astellas Pharma Inc., Astrazeneca Inc., Bayer Pharmaceutical Co., Ltd., Bristol-Myers Squibb Inc., Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo Pharma Inc., and MSD K. KH
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Matsunami General Hospital (code: 573) and Nagoya Kyoritsu Hospital (code: K132-02), respectively.
Informed Consent Statement
Written informed consent was waived to be obtained with Information regarding opt-out of this study on the website in each hospital due to the retrospective manor of this study.
Data Availability Statements
The data presented in this study are available on request from the corresponding author.
Acknowledgements
Part of this study was presented at the European Society of Cardiology Congress in 2023.
Conflicts of Interest
No found was available in the present study.
References
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Rajagopalan S, Dellegrottaglie S, Furniss AL, Gillespie BW, Satayathum S, Lameire N; et al. Peripheral arterial disease in patients with end-stage renal disease: Observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation 2006, 114, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- O’Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol 2001, 12, 2838–2847. [Google Scholar] [CrossRef]
- Owens CD, Ho KJ, Kim S, Schanzer A, Lin J, Matros E; et al. Refinement of survival prediction in patients undergoing lower extremity bypass surgery: Stratification by chronic kidney disease classification. J Vasc Surg 2007, 45, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: Derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg 2008, 48, 1464–1471. [Google Scholar] [CrossRef]
- Conrad MF, Kang J, Cambria RP, Brewster DC, Watkins MT, Kwolek CJ; et al. Infrapopliteal balloon angioplasty for the treatment of chronic occlusive disease. J Vasc Surg 2009, 50, 799–805.e4. [Google Scholar] [CrossRef] [PubMed]
- Aulivola B, Gargiulo M, Bessoni M, Rumolo A, Stella A. Infrapopliteal angioplasty for limb salvage in the setting of renal failure: Do results justify its use? Ann Vasc Surg 2005, 19, 762–768. [Google Scholar] [CrossRef]
- Rao A, Baldwin M, Cornwall J, Marin M, Faries P, Vouyouka A. Contemporary outcomes of surgical revascularization of the lower extremity in patients on dialysis. J Vasc Surg 2017, 66, 167–177. [Google Scholar] [CrossRef]
- Ambur V, Park P, Gaughan JP, Golarz S, Schmieder F, Van Bemmelen P; et al. The impact of chronic kidney disease on lower extremity bypass outcomes in patients with critical limb ischemia. J Vasc Surg 2019, 69, 491–496. [Google Scholar] [CrossRef]
- Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Kopple, JD. McCollum Award Lecture, 1996: Protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr 1997, 65, 1544–1557. [Google Scholar] [CrossRef]
- Takahashi H, Ito Y, Ishii H, Aoyama T, Kamoi D, Kasuga H; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol 2014, 64, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis 2001, 38, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L; et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 1999, 55, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Takahashi R, Ito Y, Takahashi H, Ishii H, Kasuga H, Mizuno M; et al. Combined values of serum albumin, C-reactive protein and body mass index at dialysis initiation accurately predicts long-term mortality. Am J Nephrol 2012, 36, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ishii H, Takahashi H, Ito Y, Aoyama T, Kamoi D, Sakakibara T; et al. The association of ankle brachial index, protein-energy wasting, and inflammation status with cardiovascular mortality in patients on chronic hemodialysis. Nutrients 2017, 9, 416. [Google Scholar] [CrossRef]
- Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I; et al. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Takahashi H, Inoue K, Shimizu K, Hiraga K, Takahashi E, Otaki K; et al. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J Ren Nutr 2017, 27, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S; et al. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr 2008, 87, 106–113. [Google Scholar] [CrossRef]
- Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL; et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg 2009, 50, 1462–1473.e1. [Google Scholar] [CrossRef]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008, 27, 157–172; discussion 207. [Google Scholar] [CrossRef] [PubMed]
- Kumada Y, Kawai N, Ishida N, Mori A, Ishii H, Ohshima S et al. Impact of hemodialysis on clinical outcomes in patients undergoing lower extremity bypass surgery for peripheral artery Disease-10-year follow-up study. Angiology 2022, 73, 744–752. [Google Scholar] [CrossRef]
- Kumada Y, Nogaki H, Ishii H, Aoyama T, Kamoi D, Takahashi H et al. Clinical outcome after infrapopliteal bypass surgery in chronic hemodialysis patients with critical limb ischemia. J Vasc Surg 2015, 61, 400–404. [Google Scholar] [CrossRef]
- Tanaka M, Ishii H, Aoyama T, Takahashi H, Toriyama T, Kasuga H; et al. Ankle brachial pressure index but not brachial-ankle pulse wave velocity is a strong predictor of systemic atherosclerotic morbidity and mortality in patients on maintenance hemodialysis. Atherosclerosis 2011, 219, 643–647. [Google Scholar] [CrossRef]
- Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A; et al. Ankle-brachial blood pressure index pre- dicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol 2003, 14, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa A, Ueshima H, Kadowaki T, El-Saed A, Okamura T, Takamiya T; et al. Less subclinical atherosclerosis in Japanese men in Japan than in White men in the United States in the post-World War II birth cohort. Am J Epidemiol 2007, 165, 617–624. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Patient clinical characteristics.
Table 1.
Patient clinical characteristics.
| |
|
GNRI |
|
| |
All patients
(n = 800) |
< 88.1
(n = 269) |
88.1-96.7
(n = 264) |
> 96.7
(n = 267) |
P value |
| Male gender (%) |
66.9 |
62.5 |
67.4 |
70.7 |
0.12 |
| Age (years) |
67±10 |
69±10 |
67±9 |
66±10 |
0.0024 |
| Diabetes (%) |
63.3 |
63.2 |
64.7 |
61.8 |
0.78 |
| Hypertension (%) |
62.1 |
58.0 |
61.7 |
66.7 |
0.12 |
| Dyslipidemia (%) |
24.5 |
18.2 |
25.8 |
29.6 |
0.0076 |
| Smoking (%) |
25.7 |
18.6 |
31.4 |
27.2 |
0.0078 |
| Body mass index (Kg/m2) |
21.2±3.3 |
19.2±2.9 |
21.0±2.6 |
23.3±3.0 |
<0.0001 |
| Coronary artery disease (%) |
63.5 |
58.7 |
63.9 |
67.8 |
0.092 |
| Stroke (%) |
16.9 |
18.6 |
15.5 |
16.5 |
0.63 |
| Indications (%) |
|
|
|
|
<0.0001 |
| Claudication |
47.1 |
36.8 |
43.9 |
60.4 |
|
| Rest pain |
12.3 |
13.6 |
11.5 |
11.9 |
|
| Ulcer / gangrene |
40.6 |
49.6 |
44.6 |
27.7 |
|
| GNRI |
92.0±9.8 |
81.4±5.8 |
92.3±2.4 |
102.4±5.3 |
<0.0001 |
| CRP (mg/l) |
5.1 (2.0-20.0) |
11.3 (2.9-44.5) |
4.0 (1.0-14.0) |
3.0 (1.0-12.0) |
<0.0001 |
| Pre-procedural ABI |
0.62 (0.45-0.79) |
0.65 (0.41-0.87) |
0.57 (0.44-0.79) |
0.64 (0.49-0.77) |
0.35 |
| Procedure (%) |
|
|
|
|
<0.0001 |
| Bypass surgery |
33.1 |
38.7 |
39.8 |
21.0 |
|
| Endovascular therapy |
66.9 |
61.3 |
60.2 |
79.0 |
|
| Number of lesions |
825 |
282 |
271 |
272 |
|
| Target artery (%) |
|
|
|
|
<0.0001 |
| Iliac |
18.1 |
22.7 |
17.6 |
14.3 |
|
| Femoropopliteal |
62.1 |
52.8 |
59.1 |
72.8 |
|
| Below-knee |
21.3 |
24.5 |
23.3 |
12.9 |
|
Table 1.
Clinical characteristics (continued).
Table 1.
Clinical characteristics (continued).
| |
Serum CRP |
|
| |
< 2.0 mg/l
(n = 270) |
2.0–12.6 mg/l
(n = 266) |
> 12.6 mg/l
(n = 264) |
P value |
| Male gender (%) |
62.6 |
69.2 |
68.9 |
0.19 |
| Age (years) |
66±10 |
67±10 |
69±10 |
0.046 |
| Diabetes (%) |
60.4 |
60.9 |
63.3 |
0.091 |
| Hypertension (%) |
63.7 |
61.7 |
61.0 |
0.80 |
| Dyslipidemia (%) |
25.9 |
24.8 |
22.7 |
0.68 |
| Smoking (%) |
27.5 |
24.8 |
24.9 |
0.75 |
| Body mass index (Kg/m2) |
20.9±3.1 |
21.2±3.0 |
21.5±3.7 |
0.15 |
| Coronary artery disease (%) |
63.2 |
65.0 |
63.5 |
0.78 |
| Stroke (%) |
18.6 |
18.4 |
13.6 |
0.22 |
| Indications (%) |
|
|
|
<0.0001 |
| Claudication |
61.9 |
49.6 |
29.6 |
|
| Rest pain |
14.6 |
15.9 |
6.5 |
|
| Ulcer / gangrene |
23.5 |
34.5 |
63.9 |
|
| GNRI |
94.3±9.4 |
93.1±9.7 |
89.1±10.1 |
<0.0001 |
| CRP (mg/l) |
1.0 (1.0-2.0) |
5.9 (3.9-8.0) |
39.5 (20.0-70.0) |
<0.0001 |
| Pre-procedural ABI |
0.65 (0.47-0.79) |
0.63 (0.44-0.82) |
0.57 (0.43-0.76) |
0.23 |
| Procedure (%) |
|
|
|
<0.0001 |
| Bypass surgery |
22.2 |
30.5 |
47.0 |
|
| Endovascular therapy |
77.8 |
69.5 |
53.0 |
|
| Number of lesions |
274 |
271 |
280 |
|
| Target artery (%) |
|
|
|
<0.0001 |
| Iliac |
22.3 |
18.5 |
13.6 |
|
| Femoropopliteal |
69.7 |
64.6 |
52.1 |
|
| Below-knee |
8.0 |
17.0 |
34.3 |
|
Table 2.
Predictive value of GNRI and CRP for amputation and mortality.
Table 2.
Predictive value of GNRI and CRP for amputation and mortality.
| |
Non-Adjusted |
Adjusted** |
| |
HR (95%CI) |
P value |
HR (95%CI) |
P value |
| Amputation or death
|
|
|
|
|
| GNRI (vs. T3) |
|
<0.0001* |
|
0.0070* |
| T2 |
1.46 (1.03-2.09) |
0.031 |
1.42 (0.97-2.09) |
0.070 |
| T1 |
2.18 (1.57-3.07) |
<0.0001 |
1.78 (1.24-2.59) |
0.0016 |
| CRP (vs. T1) |
|
<0.0001* |
|
0.0026* |
| T2 |
1.32 (0.93-1.89) |
0.11 |
130 (0.90-1.91) |
0.15 |
| T3 |
2.33 (1.67-3.27) |
<0.0001 |
1.86 (1.30-2.70) |
0.0007 |
| Amputation
|
|
|
|
|
| GNRI (vs. T3) |
|
<0.0001* |
|
0.032* |
| T2 |
1.11 (0.78-2.44) |
0.79 |
1.05 (0.46-2.39) |
0.89 |
| T1 |
3.17 (1.70-6.37) |
0.0002 |
2.01 (1.04-4.12) |
0.034 |
| CRP (vs. T1) |
|
0.0003* |
|
0.045* |
| T2 |
1.26 (0.58-2.79) |
0.54 |
1.01 (0.45-2.23) |
0.98 |
| T3 |
3.35 (1.75-6.85) |
0.0001 |
2.02 (1.02-4.25) |
0.042 |
| Mortality
|
|
|
|
|
| GNRI (vs. T3) |
|
0.0002* |
|
0.0083* |
| T2 |
1.51 (1.03-2.23) |
0.032 |
1.51 (0.99-2.33) |
0.052 |
| T1 |
2.12 (1.48-3.09) |
<0.0001 |
1.87 (1.25-2.84) |
0.0020 |
| CRP (vs. T1) |
|
0.0004* |
|
0.043* |
| T2 |
1.30 (0.89-1.90) |
0.17 |
1.29 (0.86-1.94) |
0.20 |
| T3 |
2.03 (1.42-2.93) |
<0.0001 |
1.64 (1.11-2.45) |
0.012 |
Table 3.
Discrimination of each prediction model for amputation or mortality using the C-index, net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Table 3.
Discrimination of each prediction model for amputation or mortality using the C-index, net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
| |
C-index (95%CI) |
P value |
NRI |
P value |
IDI |
P value |
| Established risk factors* |
0.661 |
reference |
|
reference |
|
reference |
| +GNRI |
0.710 |
0.0060 |
0.456 |
<0.0001 |
0.037 |
<0.0001 |
| +CRP |
0.681 |
0.0034 |
0.217 |
0.0063 |
0.014 |
0.0001 |
| +GNRI and CRP |
0.716 |
0.0021 |
0.508 |
<0.0001 |
0.042 |
<0.0001 |
| +GNRI and CRP vs. +GNRI |
0.006** |
0.047 |
0.145 |
0.047 |
0.006 |
0.035 |
| +GNRI and CRP vs. +CRP |
0.035** |
0.038 |
0.427 |
<0.0001 |
0.029 |
<0.0001 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).








