1. Introduction
Nowadays, promoting health and healthy habits are of major concern to consumers as functional foods gain in popularity and acceptance. Fermented foods and their advantageous microbiota are anticipated to drive the growth of the entire industry in the coming years, as their health-promoting effects gain recognition [
1]. Among the diverse range of fermented food products, probiotic foods emerge as a prominent category within the functional food industry. Probiotics consist of living microorganisms that, when consumed in sufficient quantities (>10
6 CFU/mL), offer health advantages to the host [
2]. Even though the potential health effects of probiotics depend on multitude parameters, a key characteristic is to retain a sufficient count of live cells during consumption. Moreover, it is considered crucial that probiotic strains survive through the gastrointestinal tract and colonize the intestine to confer their health beneficial effects. Within another perspective, probiotic cells inoculated for food fermentation can produce a wide range of bacterial metabolites depending on the food substrate [
1,
3]. Specifically, probiotic fermented food can pose as a significant source of dietary postbiotics; a new term defined by the ISAPP (International Scientific Association for Probiotics and Prebiotics) as:
a preparation of inanimate [dead] microorganisms and/or their components that confers a health benefit on the host [
4].
Fermentation is a bioprocess applied for controlled microbial growth to preserve food, such as milk. Yogurt is a commonly fermented dairy product, rich in various nutrients and minerals, while its consumption has increased globally due to its nutritional value and ease of digestion. Specifically, yogurt has an increasing compound annual growth rate (6.9%), while its global market value is expected to reach 51
$ billion by year 2024 [
5]. Therefore, innovation comprises one of the most significant challenges of the dairy industry to address adapting consumer demands, while maintaining sustainable development. Likewise, fermented beverages are widely manufactured in the modern food industry as they provide specific organoleptic properties and unique bioactive compounds conferring health benefits [
6].
Starter cultures play a crucial role in the production of fermented foods and beverages, contributing to nutritional value, functionality, flavor, and preservation. The advancements in modern microbiology have allowed the identification and application of specific starter cultures to achieve desirable attributes in food products [
7]. The main application form of starter cultures in food fermentation is the lyophilized form, allowing for direct incorporation into food formulations [
2]. This direct-to-set dried culture form is highly popular from an industrial perspective, as its use in freeze-dried form eliminates in-plant subculturing, reduces costs associated with bulk culture preparation, and lowers the risk of bacteriophage infection [
8].
Yogurt is traditionally produced by milk fermentation with the bacterial strains
Lactobacillus delbrueckii subsp.
bulgaricus and
Streptococcus thermophilus as the starter cultures, and represents one of the major dairy product for probiotic transfer [
2]. Generally, yogurt can be fortified with a variety of bioactive compounds, offering a wide array of possibilities to support and enhance health, including strengthening the immune system, alleviating allergy symptoms, delivering antioxidant properties, averting cardiovascular conditions, increasing energy levels, and promoting cognitive functions [
9,
10]. The combination of probiotics and natural bioactive additives in yogurts is a new trend targeting to enhance its safety and nutritional value [
1,
9,
11]. Such natural bioactive additives can be fresh and dried fruits and fruit juices that are well known for their high nutritional value and bioactive components. They provide essential components for the human diet and their consumption is associated with numerous health and nutritional benefits [
6].
The application of freeze-dried fermented juices as carriers of functional components is a relatively novel concept. The beneficial synergies between fruit juices and bioactive substances, including probiotic bacteria and postbiotics, and their incorporation into dairy products, have the potential to initiate a new era of advancements in functional foods. For instance, probiotic cells added either as starter or adjunct cultures for food fermentation have been reported to produce phenolic compounds and short-chain fatty acids (SCFAs) enhancing product’s nutritional value [
12]. Also, the effect of fruit and fruit juices on the growth of probiotics has been reported to be species as well as strain specific [
6]. However, there is still significant room for further investigation of the impact of dried fermented juices on yogurt starter cultures and yogurt nutritional value.
Therefore, the objective of this study is to assess the potential applications of the probiotic strain Lactobacillus plantarum ATCC 14917, and fermented chokeberry juice with the same strain, as freeze-dried adjuncts in different yogurt formulations. The quality of the resulting yogurts was evaluated based on several parameters, including physicochemical properties (lactic acid, total phenolic content, antioxidant activity), sensory attributes, and microbiological characteristics during storage.
2. Materials and Methods
2.1. Microbial starter cultures
The commercial ready-vat bacterial culture consisting of
S. thermophilus and
L. delbrueckii ssp.
bulgaricus at a 2:1 proportion (Lyofast Y 436 A, Sacco Systems, Cadorago, Italy) was applied as starter culture for yogurt production [
13]. The starter culture was activated by incubation in 10 mL of sterile skim milk at 43 °C for 1 h according to the instructions of the manufacturer. The probiotic strain
L. plantarum ATCC 14917 obtained in lyophilized form (LGC Standards, Middlesex, UK), was activated and grown at 37 °C in de Man-Rogosa-Sharpe (MRS) liquid medium (Merck Darmstadt, Germany) in agitated (180±5 rpm, of atmosphere approx. 5% CO
2) conical flasks for 24 h according to the manufacturer instructions. All media were sterilized prior to use by autoclaving at 121 °C for 15 min (1–1.5 atm). The obtained wet cell mass was harvested by centrifugation (5000 rpm for 10 min, Sigma 3K12 centrifugation system, Bioblock Scientific, France), and subsequently frozen to −44 °C at a cooling rate of 5 °C/min for minimum cell viability loss [
14]. Then the harvested cell mass was freeze-dried for 48 h without cryoprotection (FreeZone 4.5 Freeze-Drying System, Labconco, Kansas City, USA).
2.2. Black chokeberry juice production and fermentation
Black chokeberries (
Aronia melanocarpa) were sourced fresh from a local organic farming producer in Nea Orestiada, located in the northeastern part of Evros, Thrace, Greece. The black chokeberries were carefully washed using a sterile ringer 1/4 solution, and the juice was subsequently extracted. The pulp was removed by a strainer and centrifuged (30,000 rpm, 10 min). Sterilized, deionized water was added to adjust the initial sugar concentration (approximately 40 g/L) and the juice was pasteurized (80 °C, 10 min). Subsequently, the black chokeberry juice was left to cool at room temperature and 1 g (dry weight) of freeze-dried
L. plantarum was suspended per 100 mL of fermentation substrate. The juice underwent fermentation at 30 °C for 48 h, with simultaneous pH maintenance at 4.0±0.2 achieved by adding Na
2CO
3 solution at various time intervals. Subsequently, each fermented juice was subjected to freeze-drying [
14].
2.3. Novel yogurt-style production
Yogurt-style products were prepared from pasteurized homogenized cow’s milk of Greek origin (pH 6.8±0.1, 3.7% fat, 13.0% total solids) as a fermentation media. The milk was initially heated at 90 °C for 5 min, cooled at 40 °C and subsequently divided into 5 equal portions of 100 mL each in sterile glass containers [
13]. Five different formulations were prepared, namely: CY (commercial yogurt used as control sample) inoculated with yogurt starter culture (5% inoculum,
S. thermophilus and
L. bulgaricus); LPCY inoculated with yogurt starter culture and
L. plantarum (5% inoculum, 1:1); LPY inoculated only with
L. plantarum (5% inoculum); PDCY inoculated with yogurt starter culture and chokeberry juice fermented with
L. plantarum (5% inoculum); and PDY inoculated with chokeberry juice fermented with
L. plantarum (5% inoculum). The samples were incubated (40±1 °C for approx. 5-5.5 h) and the acidification was monitored (Consort D130 system, Turnhout, Belgium) until a pH drop at 4.6±0.1. When pH drop was achieved each fermented sample was placed for cold storage at 4 °C for 28 days.
2.4. Physicochemical analysis
Compositional analysis and pH were performed for each fermented milk samples after the 1st day of cold storage. Specifically, the samples were analyzed for total solids (method 990.19), ash (method 945.46) and fat (method 989.05) and using the methods of AOAC (2005). Fat and protein contents were determined by the Soxhlet and Kjeldahl methods, respectively. The pH values of samples were determined by direct immersion of the electrode using a digital pH meter (Hanna HI99161).
High performance liquid chromatography (HPLC) was used for sugar and organic acid quantification. In brief, lactose was determined on a HPLC system (Shimadzu, Kyoto, Japan) with a SCR-101N stainless steel column, a LC-9A pump, a CTO-10A oven (60 °C) and a RID-6A index detector. Lactic acid was determined on a HPLC system (Shimadzu, Kyoto, Japan) with a Shim-pack IC-A1 stainless-steel column, a LC-10A pump, a CTO-10A oven (40 °C) and a CDD-6A detector. For quantitative analysis, standard solutions of sugars and acids (Sigma-Aldrich Ltd) were prepared in ultrapure water (Milli-Q, Merk) at various concentrations [
13].
Syneresis (S%) was assessed on the first day of production to analyze the potential effects of supplements on the yogurt-style products during refrigerated storage. Samples weighing 10 g were placed in 15 mL Falcon tubes, and were then centrifuged (350×g, 20 min., 4 °C) and the separated serum was weighed. The level of syneresis was calculated using the following equation:
2.5. Microbiological assessment and viable probiotic cell counts
Microbiological analysis was conducted at various time intervals during 28 days of cold storage (4 °C) according to the literature with small modifications [
15]. Specifically, 10 g of each yogurt-style product was thoroughly homogenized in 90 mL of sterile Ringer solution (LABM, UK) and serially diluted in sterile 0.1% (wt/vol) peptone water (Oxoid Ltd.). The microbial loads of each sample were determined by plating on selective media. Specifically,
S. thermophilus was plated on M17 agar containing 1% lactose and incubated aerobically (40 °C, 72 h);
L. bulgaricus was plated on de Man, Rogosa, Sharpe (MRS) agar with 10% sorbitol and incubated anaerobically (37 °C, 48 h); yeasts and molds were plated on Potato Dextrose Agar and incubated aerobically (30 °C for 72 h); coliforms were plated on Violet Red Bile agar after and incubated anaerobically (30 °C, 24 h); staphylococci were plated on Baird Parker agar after and incubated anaerobically (37 °C, 48 h) according to the manufacturer instructions (LABM, UK).
Viable bacterial counts of
L. plantarum were determined by plating on MRS agar with 10 mg/L of vancomycin antibiotic that promotes its growth against
L. bulgaricus [
16] and incubating aerobically (37 °C, 48 h).
All above media were sterilized (135 °C, 15 min) before use. Cell counts were expressed as log cfu/g.
2.6. Antioxidant capacity
Free radical-scavenging activity was determined using the free radical DPPH (2,2 diphenyl-1-picrylhydrazyl) method [
17]. The DPPH radical scavenging activity was determined according to the following equation (Ac: the absorbance of the control solution, As: the absorbance of the test solution):
Total phenolic content (TPC) was determined by the Folin–Ciocalteu method assessed accordingly to the literature [
18]. Concentrations are expressed in Gallic Acid Equivalents (GAE).
2.7. Sensory attributes
All yogurt-style samples (LPCY, LPY, PDCY, PDY) underwent sensory evaluations and were compared to yogurt made using the commercial yogurt culture (CY). Approximately 50 g of yogurt produced the day before the evaluation were provided to the assessors. The assessors, both males and females aged 25 to 45 years who were non-smokers and familiar with consuming fermented milk products, were involved in the evaluation. The samples were served in 50 mL transparent plastic cups, each numbered randomly with 3-digit codes. The sensory evaluation session took place in individual booths, and assessors were instructed to assess each product using a preference scale ranging from 0 to 10. They were provided with bread and low-mineral content water to cleanse their palate after each tasting. The panel was asked to give scores on a 0–10 scale (0 = unacceptable, 10 = exceptional) for attributes based into three main categories: aroma, taste, and flavor.
2.9. Statistical analysis
All fermentation experiments were carried out in triplicate. The results were analyzed using one-way analysis of variance (ANOVA). The different treatments were compared at the same storage period, and samples from the same treatment were compared during the same time. Duncan’s multiple range tests were applied in order to determine significant differences (coefficients, ANOVA tables and significance). P value <0.05 was considered statistically significant for all analyses.
3. Results and discussion
3.1. Impact of incorporated enriched materials on the physicochemical characteristics of the products
The results regarding the pH, lactic acid and lactose content of the yogurts during cold storage (4 °C) for 28 days are presented in
Table 1. In general, a decline in pH values was observed throughout storage in all samples. The pH reduction during lactic acid milk fermentation is the result of the breakdown of lactose into lactic acid (the higher the production of lactic acid the lower the pH value). The pH of all yogurt samples that contained the probiotic culture exhibited lower pH values as compared to the control sample. The incorporation of the probiotic strain in all the respective produced yogurts (LPCY, LPY, PDCY and PDY) enhanced the lactic acid production leading to lower pH values after fermentation and during cold storage, compared to the control (CY). This is in accordance with previous studies reporting yogurt production with adjunct probiotic cultures. Consequently, an inclination of lower pH is generally observed as a result of an enhanced lactic acid and possibly other minor acids production [
13].
The decrease in pH in all samples during storage, most likely due to the production of organic acids, is a result of the activity of lactic acid bacteria (LAB), which seem to remain active even at low temperatures. Notably, the samples containing the adjunct probiotic culture exhibited lower pH values, while the samples with the dried supplements displayed even lower pH levels and the highest lactic acid production. Specifically, by the end of storage period, the sample PDY recorded the lowest pH (4.24) followed by PDCY sample (pH 4.27) with the other samples had pH in the range 4.48–4.35. The distinct acidification observed in the yogurt produced with the addition of fermented chokeberry juice with
L. plantarum may be attributed to the stimulating effect of the phenolic compounds found in chokeberries on the metabolic activity of LAB. However, it should be noted that the pH of these samples remained within acceptable values. This result aligns with recent studies that have confirmed the positive influence of phenolic compounds on lactic acid fermentation [
19,
20].
The protein, fat, total solids, and ash content from the 1
st day of production is presented in
Table 2 and ranged between 3.3–3.6 (%), 3.5–3.6 (%), 16.6–16.9 (%), and 0.5–0.7 (%), respectively. The slight reduction of fat content in all samples may be attributed to the lipolytic activity of microorganisms. The protein content varied among samples. Specifically, the control sample (CY) showed a similar protein content with LPCY and LDY (3.5%), while sample PDY had a lower protein content (3.3%). On the other hand, PDCY sample had a protein content of 3.0%; the lowest among samples. The reduction of protein content could be attributed to the proteolytic activity of microorganisms [
21]. Another significant effect on the structural, nutritional and functional properties of the products may arise from hydrophilic or hydrophobic interactions between proteins and phenolic compounds during milk fermentation. For instance, polyphenols, being abundant in chokeberry, are known to form complexes with proteins resulting in alterations to the properties of both compounds [
22]. Hence, the decrease in protein content noticed in samples with powdered chokeberry supplements also may be due to the formation of complexes between chokeberry phenolic compounds and milk proteins [
23].
Serum separation in yogurt and yogurt-style products is a critical factor that impacts the appearance and physical characteristics [
24]. Yogurt syneresis denotes the separation of whey on the surface of the product, which can happen either upon opening a yogurt container or in a sealed container. This separation is primarily attributed to (i) the higher concentration of whey protein compared to casein, and (ii) the lower concentrations of total solids, as well as (iii) changes in organic acids produced by viable LAB during storage [
25]. According to the results of the current study, syneresis was influenced by the initial starter culture and acidification method, as well as the added powdered supplements (
Table 1). Addition of chokeberry juice fermented with
L. plantarum in yogurt (PDCY, PDY) can enhance the total solid content providing higher consistency. Furthermore, as indicated by previous studies, the addition of fruits or supplements with high antioxidant activity has the potential to reduce serum separation and enhance the storage stability of fermented milk products [
26,
27].
3.2. Antioxidant activity and phenolic content
As stated in previous studies lactic acid fermentation enhances the antioxidant activity [
20,
28]. Likewise, the results of this study indicate that the addition of powdered supplements combined either with commercial starter culture or fermented milk (PDY, PDCY) boosted the levels of the antioxidant activity (approximately (69 µmol TE/100 g). In general, all samples fermented with the adjunct probiotic
L. paracasei strain showed higher antioxidant capacity in contrast to plain yogurt, which reached DPPH radical scavenging activity of 49 µmol TE/100 g. The results indicated that the probiotic strain increased the antioxidant capacity of the fermented milk products, while chokeberry components can also significantly enhance the antioxidant content either by direct contribution of antioxidant compounds or thought bioconversion derived from LAB activity.
The TPC appeared also enhanced in the samples produced with the chokeberry juice fermented with
L. plantarum (PDY, PDCY) compared to all other samples. According to the literature, in terms of the contribution sources of bioactive polyphenols (phenolic acids, flavonols, anthocyanins, proanthocyanidins) included in chokeberry, the total antioxidant activity mainly depends on the contribution of free polyphenols [
22,
29]. As a result, it is evident that incorporating chokeberry as a supplement can contribute to the development of functional food by providing a natural source of polyphenols.
3.3. Microbial Stability of yogurts-style products during cold storage
Microbial stability is crucial in preserving the physicochemical and organoleptic characteristics, as well as the safety of foods during storage, especially dairy products [
30]. Microbial counts (log cfu/g) of possible spoilage microorganisms in the fermented milk samples were monitored throughout cold storage for 28 days. As noted, no spoilage or possible pathogenic microorganisms such as staphylococci, coliforms, enterobacteria (data not shown), yeasts or molds were detected during cold storage for 28 days in samples with the adjunct probiotic strain (
Table 3). On the contrary, yeasts and molds were detected in the control yogurt samples after the 21
st day of storage. This result is most likely due to the slightly higher pH values of the control yogurt, as well as due to the antagonistic effect for
L. plantarum in all other samples (LPCY, LPY, PDCY, PDY).
Microbial counts of
S. thermophilus,
L. bulgaricus (
Table 3), and
L. plantarum (
Figure 1) were also monitored. The counts of LAB during cold storage are closely related to nutritional and environmental factors. As indicated in
Table 3, the addition of chokeberry juice fermented with
L. plantarum led to an increase in LAB counts of the yogurt samples during cold storage (PDCY), in contrast to the control (CY). More specifically, this resulted in higher counts of
S. thermophilus and
L. bulgaricus, exceeding 8 log cfu/mL on the 28
th day of storage, whereas the control yogurt exhibited significantly lower viability at 7 log cfu/mL. This result can be attributed to the enrichment of nutrients and prebiotic oligosaccharides provided by the adjunct fermented chokeberry juice. This finding is consistent with previous studies suggesting that prebiotic ingredients can enhance LAB viability in dairy products through cold storage [
2,
13]. Moreover, the
S. thermophilus and
L. bulgaricus viable cell counts were found at significantly lower concentration in sample LPCY, compared to the yogurts produced with the commercial starter culture (
Table 3). This could be attributed to the antagonistic effect of the probiotic
L. plantarum culture against other bacteria during milk fermentation [
13,
31]. In addition, the effect of low temperature on
S. thermophilus and
L. bulgaricus can affect their viability as they belong to thermophilic bacteria (optimum temperature 40−45 °C).
It has been reported that phenolic compounds at relatively high concentrations can inhibit the growth of bacteria [
32]. In this study, higher viable counts of both
S. thermophilus and
L. bulgaricus were found in PDCY yogurt from the 1
st till the last days of storage, although these samples were enriched in phenolic compounds from the fermented chokeberry juice. On the other hand, samples produced with the commercial starter culture (CY and LPCY) showed significantly lower viability after the 1
st days of production compared to PDCY samples. This may be attributed to the high phenolic content of PDCY samples, which, in line with previous studies, can promote the growth of
S. thermophilus and
Lactobacillus spp. during milk fermentation [
28].
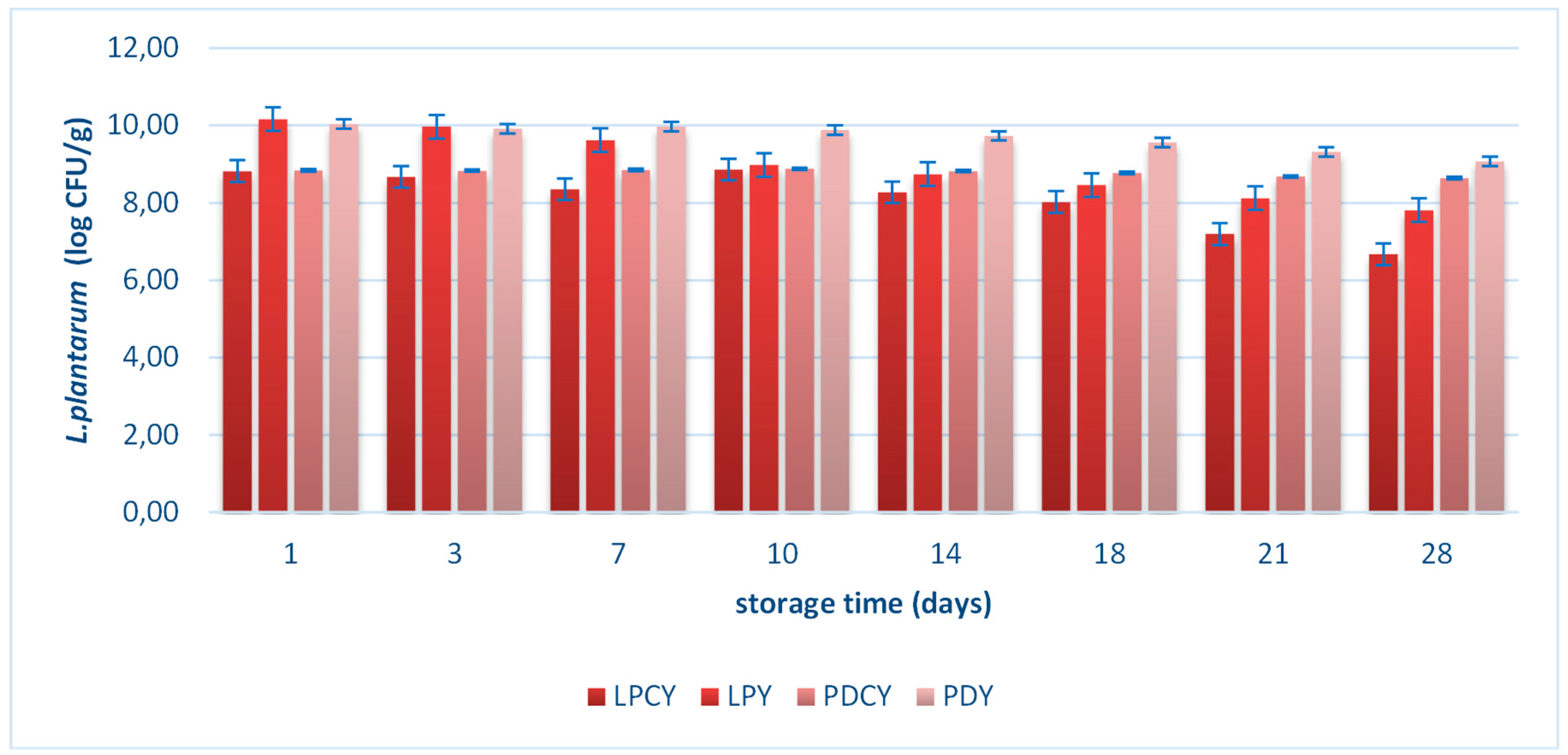
3.4. Viability of L. plantarum and possible beneficial effects
Figure 1 illustrates the population of
L. plantarum in samples LPCY, LPY, PDCY, PDY during cold storage (4 °C) at various time intervals (days 1, 3, 7, 10, 14, 18, 21, 28). The probiotic
L. plantarum strain consistently maintained levels above 8 log cfu/g throughout the storage period in all samples.
All yogurt-style products meet the criteria to be considered probiotic, aligning with the recommended levels of viable probiotic cell counts at the time of consumption [
2]. A significant decline (P<0.05) in probiotic cell counts was observed at the end of the storage period in samples produced with
L. plantarum as the starter culture (LPY) and in samples produced with both
L. plantarum and the commercial yogurt culture (LPCY), compared to yogurt produced with chokeberry juice fermented with
L. plantarum (PDCY). This decrease can be attributed to the acidic environment of fermented milk samples, which adversely affects the viability of free bacterial cells, leading to their reduction [
13]. In contrast, yogurts produced with chokeberry juice and
L. plantarum (PDCY), as well as milk fermented with chokeberry juice and
L. plantarum (PDY), demonstrated remarkable stability in viable probiotic cell counts throughout the entire storage period (4 °C, 28 days). This may be attributed to the enhanced phenolic content of samples produced with the powdered supplements. Anthocyanins, in particular, may promote the growth of LAB due to their prebiotic activities [
33].
In recent literature it is also suggested that prebiotics may contribute to the protection of probiotic cells against acidic and harsh environmental conditions during production and storage of dairy products [
2]. Likewise, in this study the viability of
L. plantarum could be enhanced by prebiotic ingredients present in the dried chokeberry juice providing a synbiotic supplement [
34]. Importantly, prior studies have proposed a synergistic interaction between phenolic compounds and probiotic bacteria, resulting in an improved antioxidant capacity and the colonization of probiotic cells within the gastrointestinal tract [
35]. These synergistic interactions may also be associated with postbiotic effects, which involve the production of small metabolites by bacteria during their life cycles. These metabolites play a crucial role in regulating bacterial growth and cell communication. Additionally, they contribute to the growth of beneficial bacteria and offer protection against various stresses [
36].
Based on these findings, the current study proposed a combination of probiotic bacteria and chokeberry juice, which is rich in phenolic compounds, as a powdered adjunct for yogurt production. This approach, utilizing the ingredients of chokeberry juice, can play a role in maintaining the observed high probiotic survival rates in the resulting dairy products.
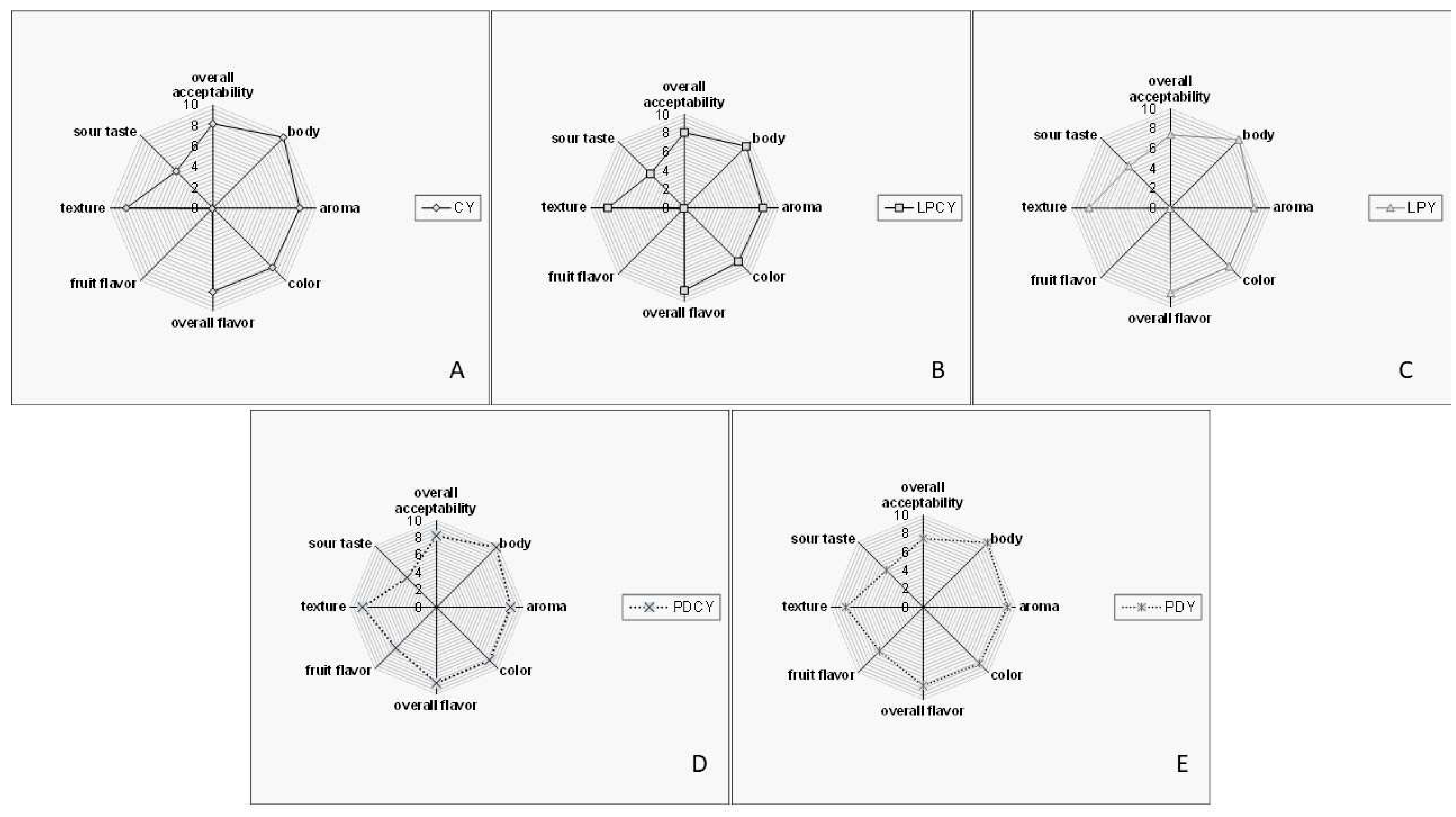
3.5. Sensory evaluation
Overall, all samples garnered high acceptance scores and were marked by a favorable total impression, with no off-flavors detected (
Figure 2). The yogurt made with fermented chokeberry juice containing
L. plantarum stood out for its exceptional aroma and fruit flavor. This characteristic could be attributed to the abundance of phenolic compounds, recognized as significant constituents in plant-origin food products. Phenolic compounds are related to significant sensory characteristics of foods such as flavor, astringency, and color [
37]. The abundance of phenolic compounds (
Table 2) in samples PDCY (
Figure 2D) and PDY (
Figure 2E), provided by the initial juice content, can contribute to the flavor of the produced yogurt highlighting its unique aromatic characteristics. Similarly, volatile phenols generated through the metabolic processes of probiotic or yogurt cultures can contribute to an enriched flavor and aromatic profile, aligning with findings from prior studies [
37].
The texture of all samples received high evaluation scores, likely because the syneresis values remained within acceptable ranges. This could be attributed to the presence of LAB metabolites, which may enhance the firmness of the product's texture. For example, probiotic and LAB strains are recognized for their exopolysaccharide production, which can contribute to the texture of fermented milk, reducing syneresis and improving the overall mouthfeel and body of the product [
38]. Similarly, it has been indicated that yogurt texture can be enhanced through the production of exopolysaccharides, which interact with the water in the products, creating a gel-like structure [
38]. The elevated texture scores observed in the samples of this study may also be attributed to other constituents, such as total solids. Notably, the slightly higher texture scores of PDCY and PDY (yogurts with fermented chokeberry juice with
L. plantarum) compared to other samples could be associated with carbohydrates and fibers derived from the fermented juice powder. These components might play a significant role in enhancing yogurt texture. Such quality characteristics hold considerable importance for the food industry, as they can exert substantial effects on the viscosity and firmness of fermented milk products. Consumers are increasingly open to exploring new flavor combinations, especially those incorporating ingredients that may impart beneficial health effects.
4. Conclusions
Freeze-dried fermented chokeberry juice with the probiotic strain L. plantarum ATCC 14917 was effectively integrated in the production of functional fermented milk products. The resulting innovative yogurt-style products maintained robust probiotic culture viabilities and an enhanced phenolic profile throughout a 4-week storage period. Notably, in all cases where L. plantarum was incorporated, the achieved viabilities consistently surpassed the threshold (106–107 cfu/mL) required to confer the health benefits of a specific probiotic food. Furthermore, the novel yogurt containing the incorporated fermented chokeberry juice exhibited exceptional sensory attributes, a firm texture, improved mouthfeel, and microbiological safety. This ready-to-use culture, represented by freeze-dried fermented chokeberry juice, not only fulfils the demand as a probiotic product but also combines bioactive phenolic components from the initial juice with those produced by LAB metabolism. Ready-to-use functional supplements hold significant appeal to the food industry, particularly in the post-pandemic era. Consequently, the novel functional yogurts developed in this study present high commercialization potential in the dairy industry, offering a blend of advantageous antioxidant, prebiotic, probiotic, and postbiotic characteristics.
Author Contributions
Conceptualization, S.P. and A.T.; methodology, I.M.; software, I.M.; validation, A.T. and I.M.; formal analysis, A.T.; investigation, I.M.; resources, A.B. and S.P.; data curation, A.T.; writing—original draft preparation, S.P.; writing—review and editing, A.B. and A.T.; visualization, S.P.; supervision, S.P.; project administration, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Terpou, A.; Rai, A.K. Microbial transformation for improving food functionality. In Current Developments in Biotechnology and Bioengineering: Technologies for Production of Nutraceuticals and Functional Food Products; 2021; pp. 31-45.
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591.
- Gill, P.; Staudacher, H.M. Are postbiotics key to the potential benefits of fermented foods? The Lancet Gastroenterology & Hepatology 2023, 8, 509. [CrossRef]
- ISAPP. 2023. https://isappscience.org/.
- Wang, F.; Wang, H.; Cho, J.H. Consumer Preference for Yogurt Packaging Design Using Conjoint Analysis. Sustainability 2022, 14, 3463.
- Plessas, S. Advancements in the Use of Fermented Fruit Juices by Lactic Acid Bacteria as Functional Foods: Prospects and Challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum Application. Fermentation 2022, 8, 6.
- Sahu, S.; Parija, T.; Panda, S.K. Chapter 25 - Starter cultures: an insight into specific applications in flavoring and health promotion. In Indigenous Fermented Foods for the Tropics, Adebo, O.A., Chinma, C.E., Obadina, A.O., Soares, A.G., Panda, S.K., Gan, R.-Y., Eds.; Academic Press: 2023; pp. 409-418.
- Peighambardoust, S.H.; Golshan Tafti, A.; Hesari, J. Application of spray drying for preservation of lactic acid starter cultures: a review. Trends in Food Science & Technology 2011, 22, 215-224. [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology 2017, 44, 94-102. [CrossRef]
- Baspinar, B.; Güldaş, M. Traditional plain yogurt: a therapeutic food for metabolic syndrome? Critical Reviews in Food Science and Nutrition 2021, 61, 3129-3143. [CrossRef]
- Abdelhamid, S.M.; Edris, A.E.; Sadek, Z. Novel approach for the inhibition of Helicobacter pylori contamination in yogurt using selected probiotics combined with eugenol and cinnamaldehyde nanoemulsions. Food Chemistry 2023, 417, 135877. [CrossRef]
- Bond, J. Gut reactions. New Scientist 2022, 256, 46-49. [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochemistry 2017, 55, 1-10. [CrossRef]
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chemistry 2020, 308. [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242-249. [CrossRef]
- Tharmaraj, N.; Shah, N.P. Selective Enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and Propionibacteria. Journal of Dairy Science 2003, 86, 2288-2296. [CrossRef]
- Sihag, S.; Pal, A.; Ravikant; Saharan, V. Antioxidant properties and free radicals scavenging activities of pomegranate (Punica granatum L.) peels: An in-vitro study. Biocatalysis and Agricultural Biotechnology 2022, 42, 102368. [CrossRef]
- Malta, L.G.; Liu, R.H. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. In Encyclopedia of Agriculture and Food Systems, Van Alfen, N.K., Ed.; Academic Press: Oxford, 2014; pp. 305-314.
- Zhao, X.; Tang, F.; Cai, W.; Peng, B.; Zhang, P.; Shan, C. Effect of fermentation by lactic acid bacteria on the phenolic composition, antioxidant activity, and flavor substances of jujube–wolfberry composite juice. LWT 2023, 114884. [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chemistry: X 2023, 17, 100535. [CrossRef]
- Abd El-Fattah, A.; Sakr, S.; El-Dieb, S.; Elkashef, H. Developing functional yogurt rich in bioactive peptides and gamma-aminobutyric acid related to cardiovascular health. LWT 2018, 98, 390-397. [CrossRef]
- Gao, N.; Si, X.; Han, W.; Gong, E.; Shu, C.; Tian, J.; Wang, Y.; Zhang, J.; Li, B.; Li, B. The contribution of different polyphenol compositions from chokeberry produced in China to cellular antioxidant and antiproliferative activities. Food Science and Human Wellness 2023, 12, 1590-1600. [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Research International 2013, 51, 954-970. [CrossRef]
- Ahmed, J.; Barua, S.; Roy, S. Chapter 12 - Rheology and microstructure of yogurt. In Advances in Food Rheology and Its Applications (Second Edition), Ahmed, J., Basu, S., Eds.; Woodhead Publishing: 2023; pp. 335-363.
- Gilbert, A.; Rioux, L.E.; St-Gelais, D.; Turgeon, S.L. Studying stirred yogurt microstructure using optical microscopy: How smoothing temperature and storage time affect microgel size related to syneresis. Journal of Dairy Science 2020, 103, 2139-2152. [CrossRef]
- Durmus, N.; Capanoglu, E.; Kilic-Akyilmaz, M. Activity and bioaccessibility of antioxidants in yoghurt enriched with black mulberry as affected by fermentation and stage of fruit addition. International Dairy Journal 2021, 117, 105018. [CrossRef]
- Molina, C.V.; Lima, J.G.; Moraes, I.C.F.; Pinho, S.C. Physicochemical characterization and sensory evaluation of yogurts incorporated with beta-carotene-loaded solid lipid microparticles stabilized with hydrolyzed soy protein isolate. Food Science and Biotechnology 2019, 28, 59-66. [CrossRef]
- Amirdivani, S.; Baba, A.S.H. Green tea yogurt: major phenolic compounds and microbial growth. J Food Sci Technol 2015, 52, 4652-4660. [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustainable Food Technology 2023. [CrossRef]
- Cocolin, L.; Dolci, P.; Alessandria, V.; Rantsiou, K. Microbiology of Fermented Dairy Products. In Reference Module in Life Sciences; Elsevier: 2018.
- Ng, E.W.; Yeung, M.; Tong, P.S. Effects of yogurt starter cultures on the survival of Lactobacillus acidophilus. International journal of food microbiology 2011, 145, 169-175. [CrossRef]
- Salim, A.; Deiana, P.; Fancello, F.; Molinu, M.G.; Santona, M.; Zara, S. Antimicrobial and antibiofilm activities of pomegranate peel phenolic compounds: Varietal screening through a multivariate approach. Journal of Bioresources and Bioproducts 2023, 8, 146-161. [CrossRef]
- Wang, M.; Zhang, Z.; Sun, H.; He, S.; Liu, S.; Zhang, T.; Wang, L.; Ma, G. Research progress of anthocyanin prebiotic activity: A review. Phytomedicine 2022, 102, 154145. [CrossRef]
- Ferrer-Sierra, M.; Rodríguez-López, P.; Leyva-Jiménez, F.J.; Borras-Linares, I.; Giacomazza, D.; Fredes, C.; Canales, P.S.R.; Segura-Carretero, A.; Lozano-Sánchez, J. Chapter 22 - Encapsulation technologies applied to bioactive phenolic compounds and probiotics with potential application on chronic inflammation. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress, Hernández-Ledesma, B., Martínez-Villaluenga, C., Eds.; Academic Press: 2022; pp. 447-476.
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. Journal of Functional Foods 2020, 66, 103829. [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. Journal of Agriculture and Food Research 2023, 14, 100708. [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. International Journal of Food Microbiology 2009, 132, 79-90. [CrossRef]
- Guzel-Seydim, Z.B.; Sezgin, E.; Seydim, A.C. Influences of exopolysaccharide producing cultures on the quality of plain set type yogurt. Food Control 2005, 16, 205-209. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).