1. Introduction
The edible pea (
Pisum sativum) is a major grain legume crop with about 7.5 million hectares of planting area worldwide [
1]. Major edible pea cultivars in the market include dry grain pea (DGP), fresh pea (FGP), snow pea (SP), sweet crispy pea (SCP), and pea tip (SEP). In average, the pea grain consists of 55-60% starch, 20-25% protein, and 6-10% crude fiber, making it a healthy food resource of plant proteins, dietary fiber, vitamins, and essential minerals [
2]. Like soybeans [
3] and peanut [
4,
5], pea growth and nutrient uptake require growth-promoting endophytic bacteria [
6], especially those involved in nitrogen fixation [
7].
Endophytic bacteria thrive inside plant tissues and organs with no immediate impact on plant normal growth [
8]. Over time, they may either cause damage to plants or promote plant growth under normal and stressful conditions [
9]. Growth-promoting endophytic bacteria can enhance plant nutrient utilization via multiple approaches. Legume-symbiotic nitrogen-fixing bacteria (NFBs) have nitrogenase activity capable of transforming atmospheric nitrogen into plant-usable inorganic compounds [
10]. Phosphorus-solubilizing bacteria (PSBs) [
11] and potassium-solubilizing bacteria (KSBs) [
12] can solubilize soil inorganic/organic phosphorus and potassium, respectively. These nutrients can be easily utilized by plants in their soluble forms. Endophytic bacteria may also produce auxin [
13], cytokinin [
14], and/or gibberellin [
15] to modulate plant hormonal homeostasis. Nitrogen fixation, nutrient solubilization, and phytohormone production activities are not mutually exclusive in growth-promoting endophytic bacteria, with some strains playing two or more roles in promoting plant nutrient uptake and utilization [
9].
Cultivable endophytic bacteria only account for 0.01-10% of the total bacterial species. They can be studied by morphology observation, Gram staining, biochemical and physiological assays, and sequencing of their 16S ribosomal RNAs [
16,
17]. High throughput 16S RNA sequencing enables an accurate and complete investigation of endophytic bacterial community structure, species diversity and abundances at the microbiome level, with no need for individual strain isolation and culture [
18,
19,
20].
Endophytic bacteria can be found in plant seeds, leaves, flowers, roots, and root nodules [
21]. Seed endophytic bacteria can be horizontally transferred through multiple generations of plants and help to keep seed dormancy status, protect seed from pathogen infection, promote seed germination in the soil, and improve seedling tolerance to abiotic stress [
22,
23,
24]. Leaf endophytic bacterial microbiomes have high variation associated with plant species, leaf sub-locations, and growth stages. They can suppress pathogen attachment on leaves and enhance plant growth and biomass accumulation [
25,
26,
27]. Flower endophytic bacteria reside in stigmas, pollens, petals, and nectars to function in pollination [
28]. Roots harbor far more endophytic bacterial species and abundance than any other organs. These bacteria are highly diverse in both community structure and functions [
29]. Both rhizobia and nonrhizobial endophytic bacteria in root nodules are critical for legume growth and resilience [
30].
Most endophytic bacterial studies in pea cultivars focus on roots and root nodules due to their legume and symbiotic nitrogen fixation nature [
31,
32,
33]. In contrast, little is known about endophytic bacteria in plant embryos [
34], which may harbor bacteria transmitted from flowers and seeds, and facilitate plant nutrient utilization after seed germination. The embryo bacterial microbiome also shapes the blueprint of bacterial communities in the next generation of plant body. We previously reported the community structure and associated networks of endophytic bacteria in pea roots throughout plant life cycle [
35]. Here, we collected and investigate the endophytic bacterial microbiomes in edible pea embryos using five representative cultivars and a pea sprout control. Twenty-six NFBs were isolated from pea embryos. Some of the NFB isolates are also KSBs or PSBs. All 26 NFBs showed variable levels of indole-3-acetic acid (IAA) secretion. High throughput 16S RNA sequencing of 40 bacterial collections from pea embryos revealed similar community structures, species abundances and diversities in most samples. Our work identified nutrient-solubilizing NFBs with biofertilizer potential and established an embryo-derived endophytic bacterial pool for seed dormancy and germination research of edible peas.
2. Materials and Methods
2.1. Plant materials and sampling
Edible pea seeds were obtained from Qingdao Academy of Agricultural Sciences. Five pea cultivars with different fiber and sugar contents were used in this research, including dry grain pea (DGP), fresh pea (FGP), snow pea (SP), sweet crispy pea (SCP), and pea tip (SEP). Twenty pea accessions were sown with three to four accessions included in each cultivar and a pea sprout (SHP) accession used as the control. Pea plants were grown from March to June 2018. Samplings were carried out at seedling, flowering, grain-filling, and seed maturity stages. All collected samples were stored at -20℃.
2.2. Isolation and purification of cultivable endophytic bacteria
Pea embryos were isolated from de-coated seeds, sterilized with 70% ethanol for 3 min, and rinsed/washed with sterilized water. Adequate sterilization was tested by spreading 100 µl water from last rinse to Trypticase Soy Agar (TSA) plates (15 g/l tryptone, 5.0 g/l soybean peptone, 30 g/l NaCl, 15 g/l agar). Embryo sterilization was considered successful if no microbe grew after 72-h incubation of the TSA plates at 28℃. Plastic-wrapped sterilized embryos were crushed and transferred to 1.5-ml Eppendorf tubes. After adding 1 ml saline solution to each tube, embryo samples were sonicated for 1 min. After 10-fold dilution, 100-µl suspension was spread on either TSA or Ashby’s nitrogen-free (20 g/l mannitol, 0.2 g/l K2HPO4, 0.2 g/l MgSO₄, 0.2 g/l NaCl, 0.1 g/l K2SO4, 5.0 g/l CaCO3, 15 g/l agar, pH 7.4±0.2) plate. After incubation at 28℃ for 72 h, single bacterial colonies were picked for multiple rounds of streak cultivation. Purified isolates were cultured and stored at -80℃. Bacteria strains isolated from the last round of streak were classified based on colony sizes, colors, transparency, and morphology.
2.3. Nitrogenase activity and nutrient solubilization assays of plant growth-promoting bacteria
Nitrogenase activity was measured by adding 1 ml NFB culture to a 13-ml glass vial and replacing 1 ml air with the same volume of acetylene (99.99% purity). After cultured at 28℃ for 24 h, the bacterial sample was mixed with 2 ml of saturated NaCl solution. 200-μl mixture (10% gas concentration) was then extracted and added to the absorption buffer (5.0 g/l CuSO4, 5.3 g/l ammonia, 23 g/l NH2OH·HCl, 0.9 g/l gelatin, 31.6% ethanol). The optical density (OD) was measured at 548 nm after shaking the mixture for 5 min.
To measure inorganic phosphorus solubilization, bacterial strains were inoculated to inorganic phosphorus screen medium (10 g/l glucose, 0.5 g/l (NH4)2SO4, 0.2 g/l NaCl, 0.2 g/l KCl, 0.03 g/l MgSO4·7H2O, 0.03 g/l MnSO4, 0.003 g/L FeSO4, 0.5 g/l yeast extract, 5.0 g/l Ca3(PO4)2, 15 g/l agar, pH 6.8-7.0) and cultured at 30℃ for 3 d. Inorganic phosphorus solubilization was indicated by the emergence of clear halos on the plates, with halo sizes positively correlated to solubilization activity. Similarly, organic phosphorus solubilization was tested by bacterial inoculation to egg yolk medium (10 g/l tryptone, 3.0 g/l beef extract, 5.0 g/l NaCl, 15 g/l agar, pH 7.0-7.5; add 3 ml of fresh egg yolk to 50-ml medium plate before use), incubation at 30℃ for 3 d, and subsequent formation of turbid halos.
To measure potassium solubilization, bacterial strains were inoculated to silicate medium (10 g/l sucrose, 0.5 g/l yeast extract, 1.0 g/l (NH4)2SO4, 2.0 g/l Na2HPO4, 0.5 g/l MgSO4·7H2O, 1.0 g/l CaCO3, 1.0 g/l potash feldspar powder, 15 g/l agar) and cultured at 30℃ for 3 d. The emergence of clear halos on the plates indicated solubilization of inorganic potassium, with halo sizes positively correlated to bacterial solubilization activity.
Bacterial IAA production was quantified by using the Salkowski reagent (fresh mixture of 0.5 M FeCl
3 and 35% HClO
4) to generate a standard curve [
36]. 50-μl bacterial culture at the logarithmic growth phase was inoculated to beef extract liquid medium containing 0.5 g/l tryptophan. After shaking at 140 rpm and 28℃ for 36 h, 50-μl activated bacterial culture at the logarithmic growth phase were mixed with the Salkowski reagent and incubated (avoid light) at 25℃ for 30 min. Bacterial IAA production was indicated by the emergence of pink color. IAA-producing bacterial cultures were then centrifugated at 8000 rpm for 5 min. 2-ml supernatant from each strain was mixed with 4-ml Salkowski reagent and incubated (avoid light) at 25℃ for 30 min. IAA production was quantified by measuring ODs at 530 nm with three biological replicates for each sample. The standard curve was generated by using 8 IAA standards of gradient concentrations for Salkowski reactions and OD measurements.
2.4. Bacterial DNA extraction and sequencing
Genomic DNA samples were extracted from endophytic bacterial collections from pea embryos. Their 16S rDNA V3/V4-specific fragments (16S) were amplified using the high-fidelity TaKaRa Ex Taq DNA Polymerase (TakaRa, Dalian, China) and two specific barcoded primers 343F (5'-TACGGRAGGCAGCAG-3') and 798R (5'-AGGGTATCTAATCCT-3'). PCR products were sequenced by OE Biotech (Shanghai, China).
2.5. Bioinformatic analysis of high-throughput DNA sequencing data
The raw bacterial DNA sequences in FASTQ format were processed by Trimmomatic [
37] to remove low-quality reads. Optimized paired-end reads were assembled using FLASH [
38]. Shorter-than-normal reads and reads containing ambiguous or single repetitive bases were discarded to improve assembly accuracy. At the same time, UCHIME [
39] was used to detect and remove chimeric sequences. The high-quality sequences generated afterwards were processed by VSEARCH [
40] to define operational taxonomic units (OTUs), with sequences sharing at least 97% identities being clustered as an OTU. Representative 16S OTU sequences were selected using QIIME [
41] for blast searches against Greengenes [
42] and SILVA [
43] databases. Blast results with confidence intervals larger than 0.7 were used for species annotation using Ribosomal Database Project (RDP) Classifier [
44].
3. Results
3.1. Morphological identification of cultivable endophytic bacteria in pea embryos
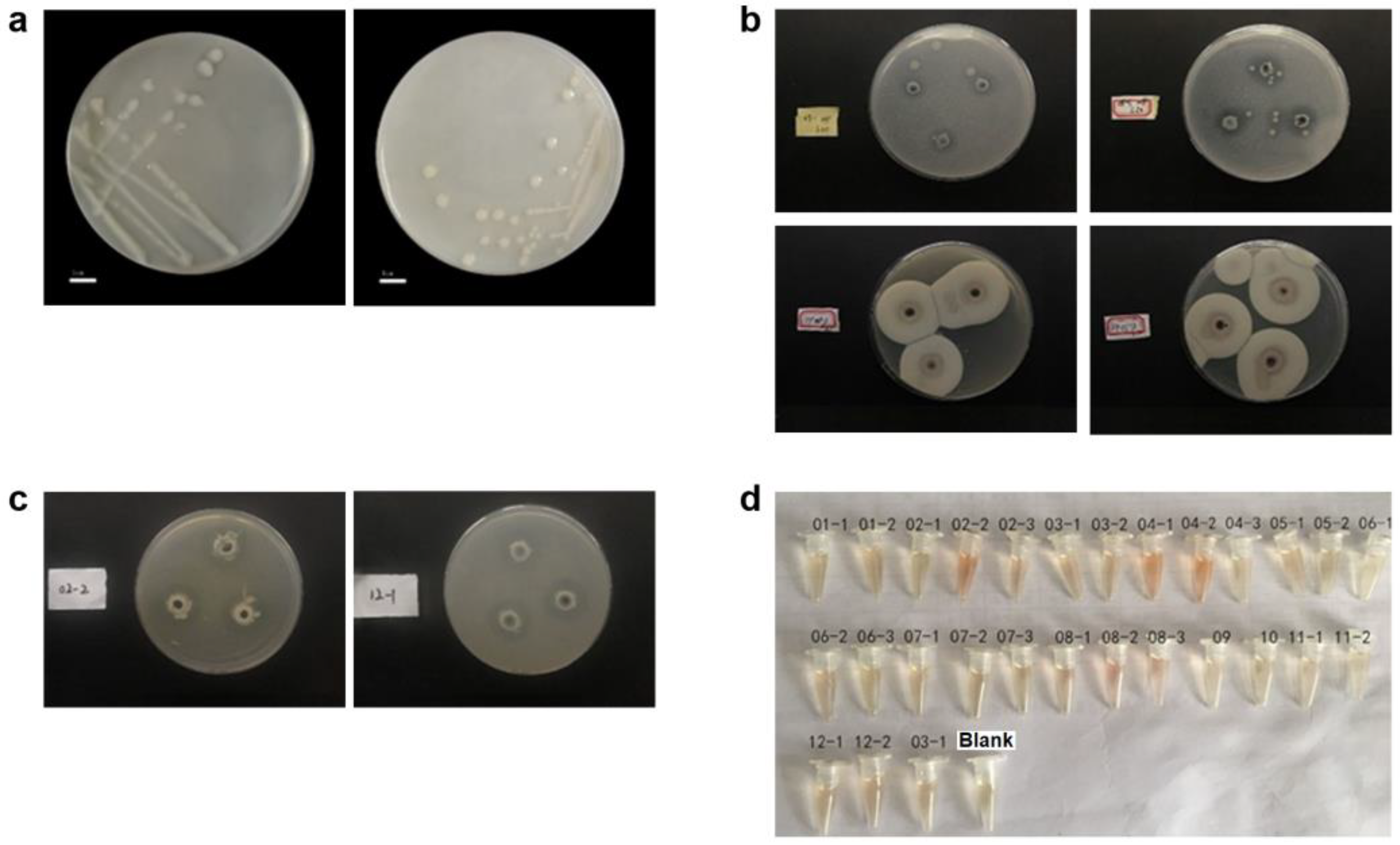
Twenty pea accessions were divided into six cultivar groups, including DGPS (dry grain pea samples S18P01-04), FGPS (fresh pea samples S18P05-08), SPS (snow pea samples S18P09-12), SCPS (sweet crispy pea samples S18P13-16), SEPS (pea tip samples S18P17-19), and SHPS (pea sprout sample S18P20) used as a control. Twenty-seven putative NFB isolates were identified from pea embryos based on their sizes, shapes, texture, and colors (
Table S1;
Figure 1a). These strains were somewhat evenly distributed in DGPS (S18P01-1, S18P01-2, S18P02-1, S18P02-2, S18P02-3, S18P03-1, S18P03-2, S18P04-1, S18P04-2, and S18P04-3), FGPS (S18P05-1, S18P05-2, S18P06-1, S18P06-2, S18P06-3, S18P07-1, S18P07-2, S18P07-3, S18P08-1, S18P08-2, and S18P08-3), and SPS (S18P09, S18P10, S18P11-1, S18P11-2, S18P12-1, and S18P12-2), with no NFB isolates being identified from SCPS, SEPS, or SHPS.
3.2. Nutrient utilization activities of nitrogen-fixing bacteria (NFB) from pea embryos
Nitrogenase activities of the 27 putative NFB cultures ranged from 10.67 to 89.45 nmol C2H4/mL/h and averaged at 71.95. They were classified into four nitrogenase activity categories of less than 40 (S18P02-1 [10.67]), 40-60 (S18P11-1 [54.37]), 60-80 (21 isolates), and above 80 (S18P05-1 [85.02], S18P08-2 [89.45], and S18P12-1 [85.86]). The three strongest NFBs came from FGPS (S18P05-1 and S18P08-02) and SPS (S18P12-1). Overall, 26 out of 27 isolates, excluding S18P02-1, were verified as NFBs with considerable nitrogen fixation capability.
PSBs solubilize soil phosphorus for easy absorption by plants [
45,
46]. Eleven out of 26 NFB isolates generated clear halos on inorganic phosphorus screen plates, demonstrating their ability to solubilize inorganic phosphorus. On the other hand, only 3 out of 26 isolates were organic phosphorus solubilizers, as revealed by their ability to generate turbid halos on egg yolk plates. Two overlappers of the inorganic and organic PSB groups, S18P05-2 and S18P08-3, could solubilize both inorganic and organic phosphorus (
Figure 1b).
KSBs catabolize potash feldspar powder in the silicate medium. They also degrade apatite and aluminosilicate minerals in the soil [
47]. These soluble nutrients can then be utilized by plants. Thirteen out of 26 NFB isolates generated clear halos on silicate plates, demonstrating that they are also KSBs. Among them, S18P02-2 and S18P12-1 were the two strongest potassium solubilizers whose clear halos diameters were as large as 2.2 cm (
Figure 1c).
Tests using the Salkowski reagent showed that all NFB isolates secreted IAA (
Figure 1d). They were classified into four categories based on IAA production capacity (mg/ml) [
48], including “below 2” (S18P11-1 [0.41] and S18P12-2 [1.97]), “2-4” (18 isolates), “4-6” (5 isolates), and “above 6” (S18P02-2 [7.10]). Isolates with the highest (S18P02-2) and the lowest (S18P11-1) IAA production levels came from FGPS and SPS, respectively.
3.3. Quality control of bacterial DNA sequencing data and operational taxonomic unit (OTU) clustering
23449 to 37104 (33793 in average) effective DNA sequencing reads were obtained from 20 pea embryo bacterial collections (two replicates per collection) after removing low-quality sequences (
Table S2). Most of these high-quality reads are 410-450 base pairs (bp) in lengths (
Figure S1). A total of 4234 OTUs were obtained using 97%-identity as the threshold for clustering high-quality effective reads (valid tags). OTUs per sample varied from 271 (S18P02.1) to 690 (S18P01.1), with 396 OTUs being annotated on average.
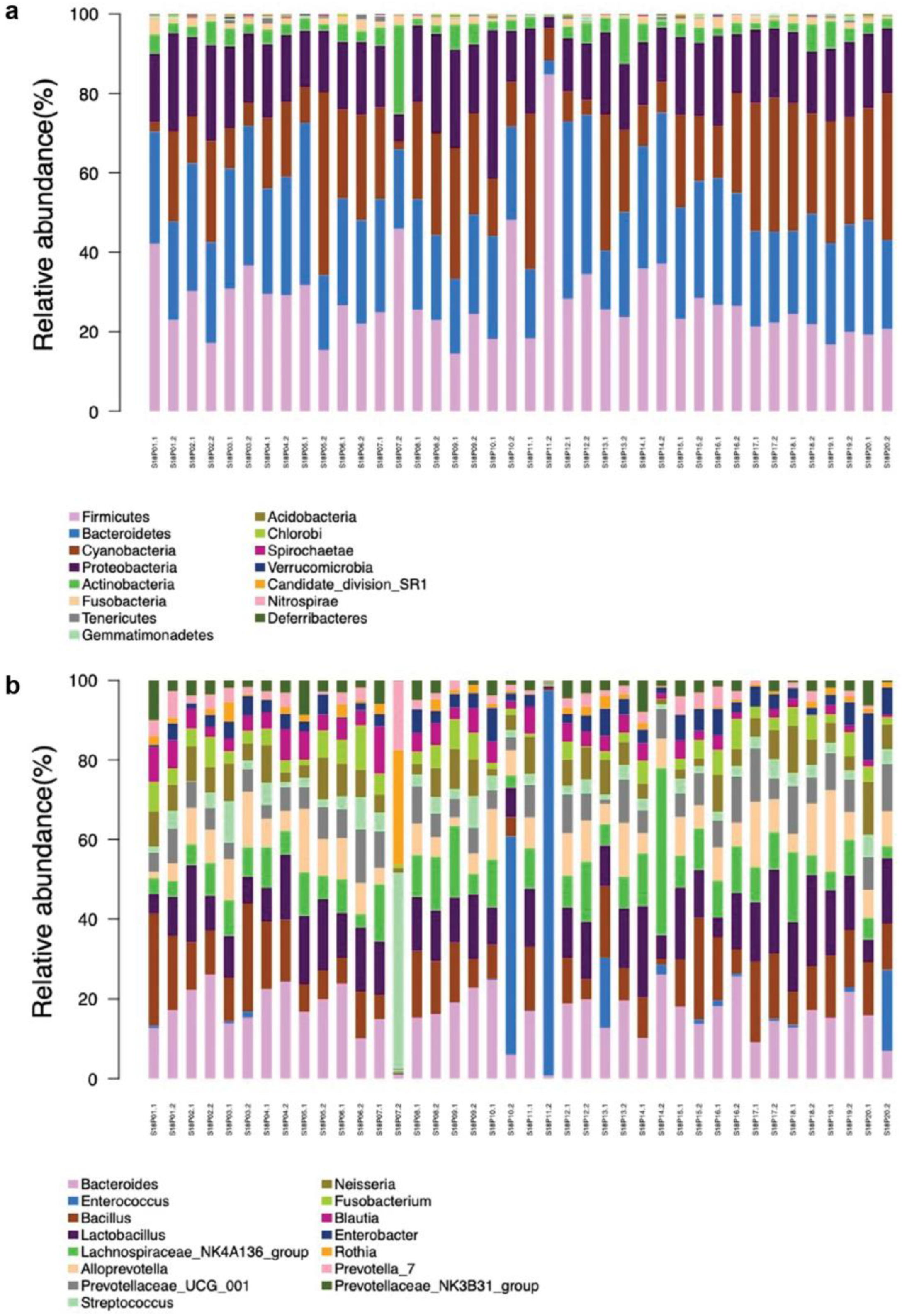
3.4. The community structure of endophytic bacteria in pea embryos
Based on OTU annotation results, endophytic bacteria isolated from the embryos of 20 pea accessions were clustered into 28 phyla, with
Bacteroidetes (27.82%),
Firmicutes (26.60%),
Proteobacteria (17.92%), and
Actinobacteria (4.10) exhibiting the highest abundances in average (
Figure 2a). These OTUs were further clustered into 517 genera (
Figure 2b). Overall, dominant genera include
Bacteroides,
Enterococcus,
Bacillus,
Lactobacillus,
Lachnospiraceae_NK4A136_group,
Alloprevotella,
Prevotellaceae_UCG_001,
Streptococcus,
Neisseria, and
Fusobacterium. Different from most samples, S18P07.2 and S18P10.2/S18P20.2/S18P11.2 harbored the most dominant genus of
Streptococcus and
Enterococcus, respectively. In the heat map of bacterial genus abundance (
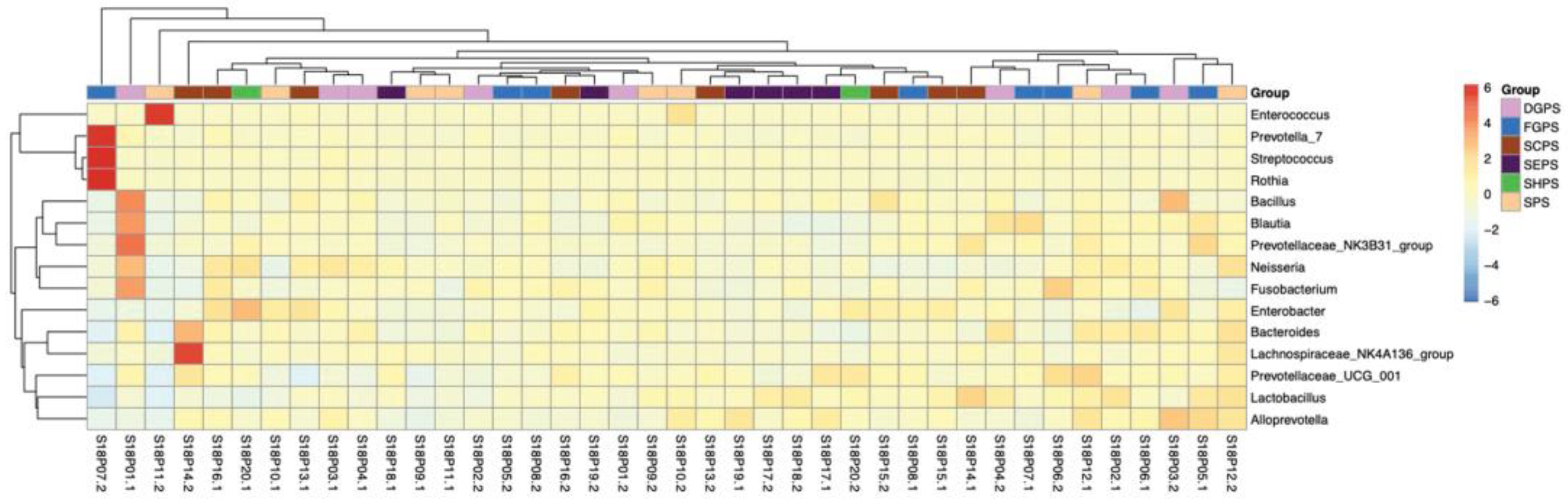
Figure 3), OTUs were somewhat evenly distributed across different genera in most samples except for S18P03.2 (low abundances of
Lactobacillus and
Bacteroides and high abundances of
Prevotella_7,
Streptococcus, and
Rothia), S18P01.1 (high abundances of
Bacillus,
Blautia,
Prevotellaceae_NK3B31_group,
Neisseria, and
Fusobacterium), S18P011.2 (very high abundance of
Enterococcus and low abundances of all other genera), and S18P14.2 (high abundances of
Bacteroides and
Lachnospiraceae_NK4A136_group).
3.5. Alpha diversity analysis of endophytic bacteria in pea embryos
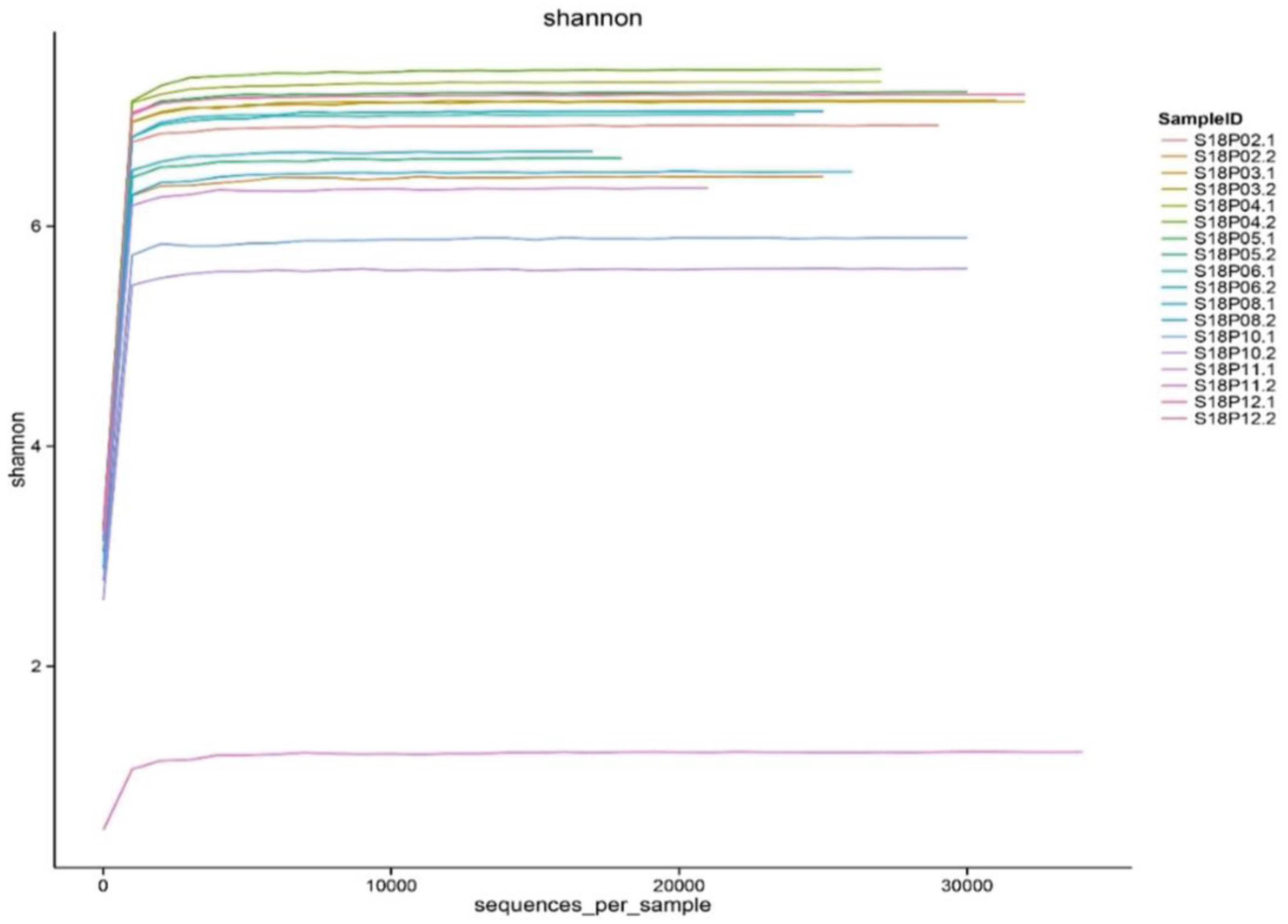
Meaningful microbial community diversity and abundance analysis requires sufficient sequencing depth to cover most bacterial species in the samples. Constructed from the diversity indexes of different sequencing volumes, the Shannon-Wiener dilution curves [
49] all went flat (
Figure 4) with sequencing depths above 99% (
Table S3), indicating that our sequencing data sizes are large enough to accurately reflect the endophytic bacterial microbiome in pea embryos.
Chao1, Shannon, and Simpson indexes [
50] were calculated (
Table S3) to estimate the Alpha (within-sample) diversity [
51] of endophytic bacteria in pea embryos. The Chao1 index is the estimation of total OTUs in a sample by counting OTUs that appear only one to two times. High Shannon index values indicate high diversity and relatively even distribution of bacterial species. The Simpson index represents the possibility that two randomly selected bacterial isolates are different species. Simpson values are positively correlated to species diversities in samples. To facilitate diversity comparison among samples, an OTU matrix was constructed by randomly retrieving sequences from all samples with identical sampling depth. This new matrix was used to normalize the deviation caused by sequencing depth difference so that all diversity indexes of different samples can be calculated with uniform depth. Sample Chao1 values varied from 226.26 to 394.01 (
Table S3), which are comparable to the actual annotated OTU numbers (
Table S2). Shannon and Simpson index values are also consistent for each sample (
Table S3). Unlike most samples, endophytic bacteria from S18P05.2, S18P11.1, and S18P11.2 exhibited much lower diversity and uneven distributions (Shannon index < 5; Simpson index < 0.9), with S18P11.2 showing the lowest values for both indexes (
Table S3).
3.6. Principal component analysis (PCA) of endophytic bacteria in pea embryos
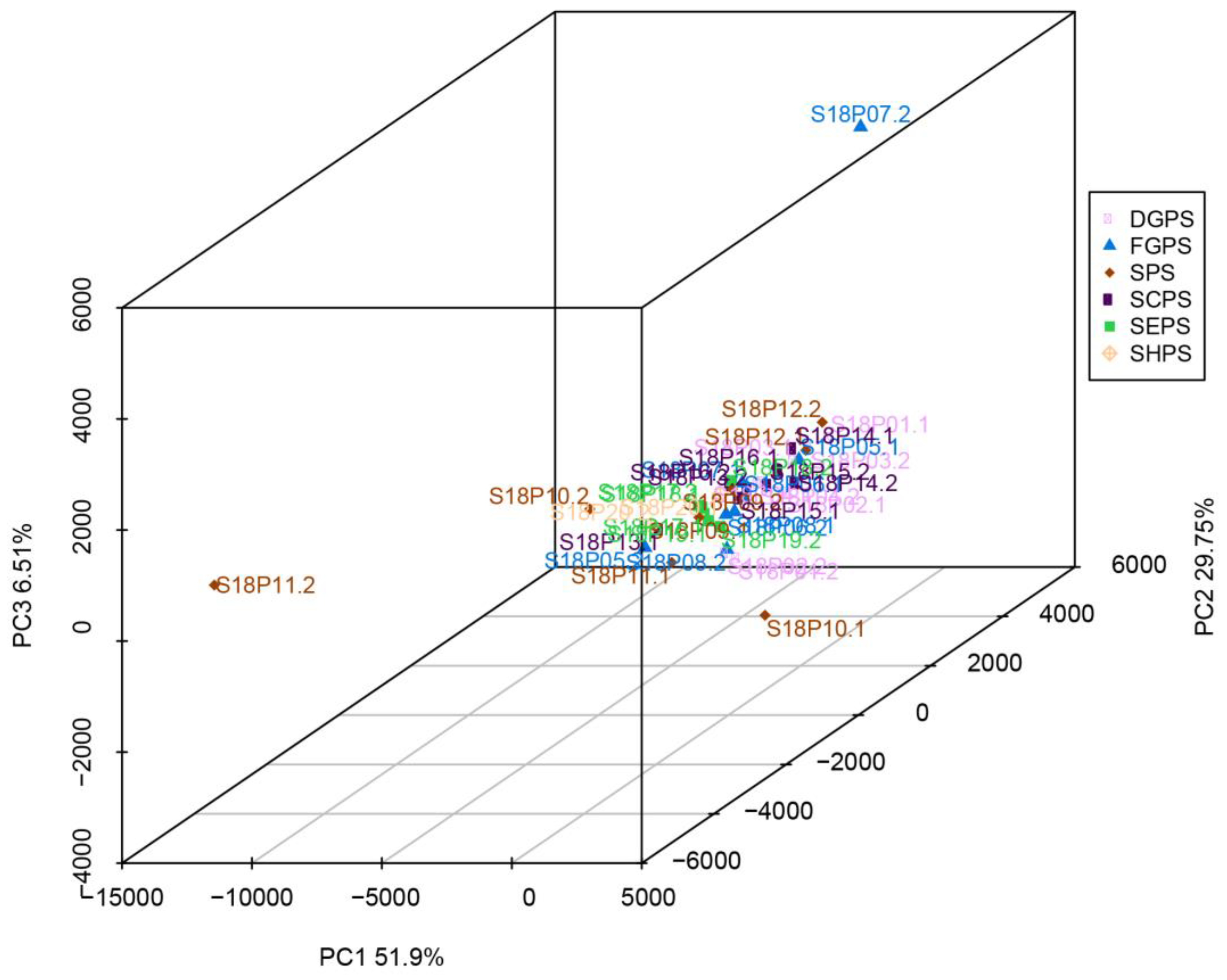
Principal component analysis (PCA) was carried out on endophytic bacterial samples from the embryos of all six pea cultivar groups, including DGPS, FGPS, SPS, SCPS, SEPS, and SHPS. Principal components 1 and 2 (PC1 and PC2) contributed 51.9% and 29.7% of the total variation, respectively (
Figure 5). S18P10.2 and S18P11.2 in the snow pea sample (SPS) group showed long distances and low similarities to all the other samples in the PCA map (
Figure 5). Except for the two outliers, all bacterial collections, especially those from SEPS, exhibited short distances, good sample parallelism, and similar components and community structures (
Figure 5). Most samples aggregated in both PC1 and PC2 (
Figure 5), indicating that there is no significant structural difference in endophytic bacterial communities from pea embryos of different cultivars/accessions.
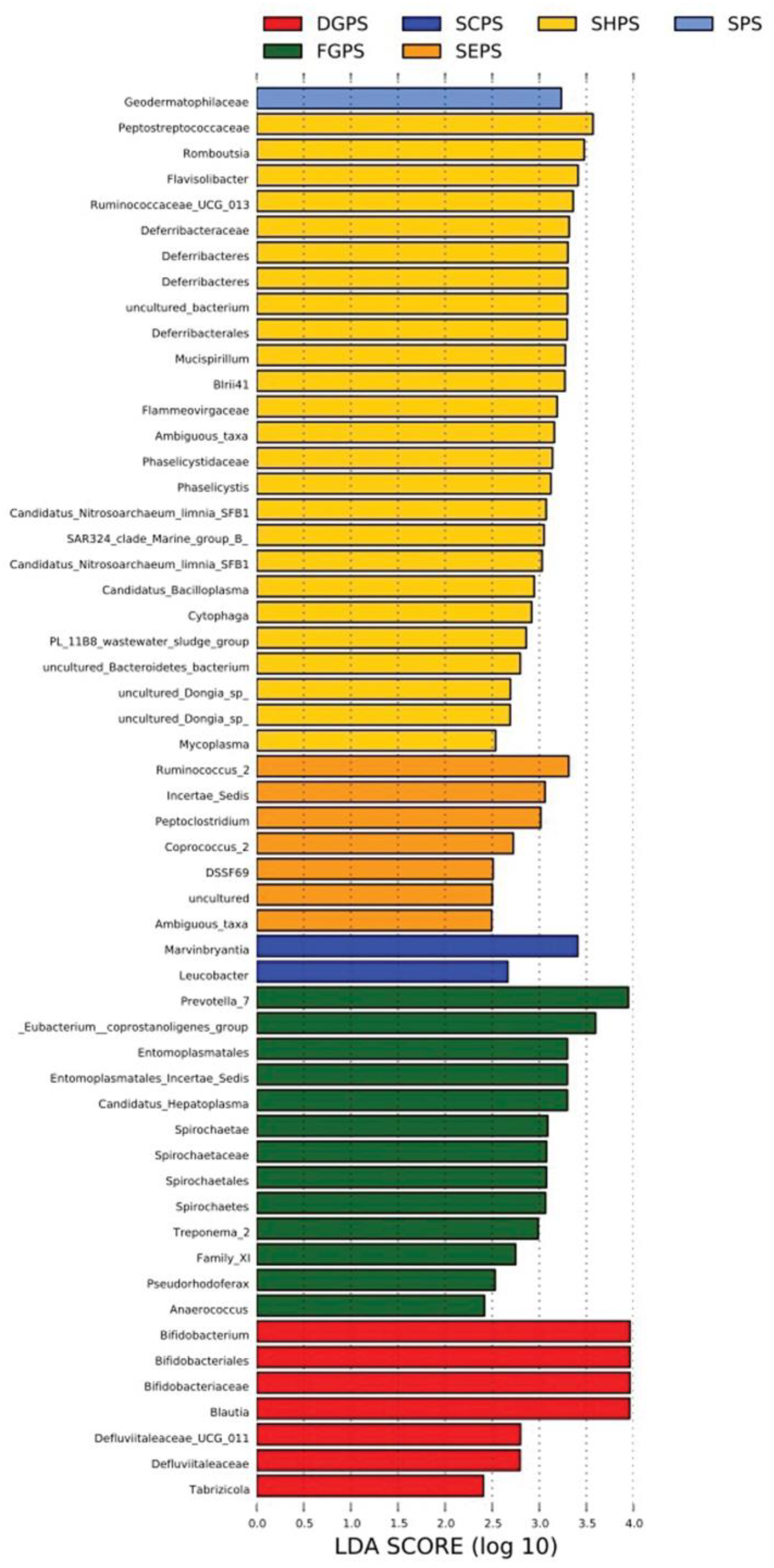
3.7. Linear discriminant analysis (LDA) coupled with effect size measurement (LEfSe) analysis of endophytic bacteria in pea embryos
Linear discriminant analysis (LDA) coupled with effect size measurement (LEfSe) analysis was carried out to analyze endophytic bacterial difference in pea embryos. Relatively abundant endophytic bacterial species (LDA score > 3) were identified from all six cultivar groups of DGPS (
Bifidobacterium,
Bifidobacteriales,
Bifidobacteriaceae, and
Blautia), FGPS (
Prevotella_7, Eubacterium_coprostanoligenes_group,
Entomoplasmatales,
Entomoplasmatales_Incertae_Sedis,
Candidatus_Hepatoplasma,
Spirochaetae, Spirochaetaceae,
Spirochaetales,
Spirochaetes, and
Treponema_2), SPS (
Geodermatophilaceae), SCPS (
Marvinbryantia), SEPS (
Ruminococcus_2,
Incertae_Sedis, and
Peptoclostridium), and SHPS (
Peptostreptococcaceae,
Romboutsia,
Flavisolibacter,
Ruminococcaceae_UCG_013,
Deferribacteraceae,
Deferribacteres,
uncultured_bacterium,
Deferribacterales,
Mucispirillum,
BIrii41,
Flammeovirgaceae,
Ambiguous_taxa,
Phaselicystidaceae,
Phaselicystis,
Candidatus_Nitrosoarchaeum_limnia_SFB1, and
SAR324_clade_Marine_group_B) (
Figure 6).
4. Discussion
In our research, the three NFB isolates with strongest nitrogenase activity are from FGPS (S18P05-1 and S18P08-02) and SPS (S18P12-1). Although they are embryo-localized and may not actually participate in nitrogen fixation at the current generation of peas, their transmission and subsequent symbiosis with the root nodules of offspring pea plants can contribute to nitrogen fixation. Pea lines with improved nitrogen fixation capacity can often be selected from nodulation mutants [
52]. Crossing of the selected mutant lines with popular pea cultivars generate hybrid lines pertaining the trait of higher nitrogen fixation potential [
53]. Alternatively, the strong NFB isolates isolated in this research can be inoculated to pea root nodules as biofertilizer. Their high nitrogenase activity is expected to improve the nitrogen fixation potential of host pea plants.
In our NFB collection, the number of inorganic PSB isolates are almost three folds more than that of organic PSBs. This observation is consistent with the soil origination of most endophytic bacteria in embryos. Most soil PSBs tend to solubilize and utilize inorganic phosphorus [
54,
55]. Since organic PSBs are scarce, they can be valuable biofertilizer resources supplementing with the more common inorganic PSBs that are already possessed by plants. Particularly, we obtained two dual-role PSB isolates (S18P05-2 and S18P08-3) capable of solubilizing both inorganic and organic phosphorus. When used as biofertilizer, they may help plants utilize broad types of soil phosphorus. Their potential to solubilize other forms of inorganic and organic phosphorus should be tested in the future.
Most soil potassium exists in mineral forms such as aluminosilicate, which cannot be directly utilized by plants. Endophytic KSBs play a critical role in the generation of soluble potassium for plant utilization [
56]. Bacteria from the
Klebsiella genus have been previously reported to be KSBs [
57]. Consistently, one of the strongest KSB we obtained, S18P02-2, is a
Klebsiella bacterium.
Members from the
Pseudomonas genus are well known for their growth promotion and IAA production activities [
58,
59]. No surprise, A
Pseudomonas isolate (S18P02-2) is the strongest IAA producer of all 26 NFBs in our collection.
Data analysis of high-throughput 16S sequencing results found little variation in endophytic bacterial abundance and diversity in most embryo samples, indicating that the bacterial community structure is not significantly affected by pea cultivars and accessions. The abundant endophytic bacteria identified in this research will be the targets for further biochemical, physiological, and functional investigations due to their strong presence in the pea embryo bacterial microbiome.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Morphological description of the 27 putative nitrogen-fixing bacterial (NFB) strains identified from pea embryos; Table S1: Morphological description of the 27 putative nitrogen-fixing bacterial (NFB) strains identified from pea embryos; Table S2: Summary of effective DNA sequencing reads from endophytic bacteria in pea embryos and derived operational taxonomic units (OTUs); Table S3: Sequencing depths and diversity indexes of endophytic bacterial samples in pea embryos.
Author Contributions
Conceptualization, J.H., H.P. and Y.M.; methodology, X.Z., S.Q., F.S. and X.L.; validation, X.Z., S.Q., F.S. and X.L.; formal analysis, X.Z., S.Q., F.S. and X.L.; investigation, X.Z., S.Q., F.S. and X.L.; resources, J.H., H.P. and Y.M.; data curation, X.Z., S.Q., F.S. and X.L.; writing—original draft preparation, H.P., X.Z., Y.M. and J.H.; writing—review and editing, H.P., X.Z., Y.M. and J.H.; visualization, X.Z., S.Q., F.S. and X.L.; supervision, J.H., H.P. and Y.M.; project administration, J.H., H.P. and Y.M.; funding acquisition, J.H. and H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the internal grant of Qingdao Academy of Agricultural Sciences (J.H.) and the USDA-ARS appropriated project 2034-22000-015-000D (H.P.).
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not constitute endorsement by USDA. USDA is an equal opportunity provider and employer.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
DGP: Dry grain pea; DGPS: Dry grain pea samples; FGP: Fresh pea; FGPS: Fresh pea samples; IAA: Indole-3-acetic acid; KSB: Potassium-solubilizing bacteria; LDA: Linear discriminant analysis; LEfSe: Linear discriminant analysis (LDA) coupled with effect size measurement; NFB: Nitrogen-fixing bacteria; OTU: Operational taxonomic unit; PCA: Principal component analysis; PSB: Phosphorus-solubilizing bacteria; SCP: Sweet crispy pea; SCPS: Sweet crispy pea samples; SEP: Pea tip; SEPS: Pea tip samples; SHP: Pea sprout; SHPS: Pea sprout samples; SP: Snow pea; SPS: Snow pea samples; TSA: Trypticase Soy Agar.
References
- Powers, S.; Mirsky, E.; Bandaranayake, A.; Thavarajah, P.; Shipe, E.; Bridges, W.; Thavarajah, D. Field pea (Pisum sativum L.) shows genetic variation in phosphorus use efficiency in different P environments. Sci. Rep. 2020, 10, 18940. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Li, Y. Pea protein composition, functionality, modification, and food applications: A review. Adv. Food. Nutr. Res. 2022, 101, 71–127. [Google Scholar]
- Ayilara, M.S.; Adeleke, B.S.; Babalola, O.O. Bioprospecting and Challenges of Plant Microbiome Research for Sustainable Agriculture, a Review on Soybean Endophytic Bacteria. Microb. Ecol. 2023, 85, 1113–1135. [Google Scholar] [CrossRef]
- Lucero, C.T.; Lorda, G.S.; Anzuay, M.S.; Ludueña, L.M.; Taurian, T. Peanut Endophytic Phosphate Solubilizing Bacteria Increase Growth and P Content of Soybean and Maize Plants. Curr. Microbiol. 2021, 8, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal; Mangalassery, S. ; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; Thomas, M.; Patel, M.B.; Maida, P.; Nawade, B.D.; Ahmad, S.; Dash, P.; Radhakrishnan, T. Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria. Front. Microbiol. 2021, 12, 650771. [Google Scholar]
- Maheshwari, R.; Kumar, P.; Bhutani, N.; Suneja, P. Exploration of plant growth-promoting endophytic bacteria from Pisum sativum and Cicer arietinum from South-West Haryana. J. Basic Microbiol. 2022, 62, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Hameed, S.; Yasmeen, T.; Zahid, M.; Zafar, M. Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.). World J. Microbiol. Biotechnol. 2014, 30, 719–725. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume-rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and Characterization of Phosphorus Solubilizing Bacteria with Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Shen, Z.; Ye, J. Whole-Genome Sequencing and Potassium-Solubilizing Mechanism of Bacillus aryabhattai SK1-7. Front. Microbiol. 2022, 12, 722379. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Poulev, A.; Chrisler, W.; Acosta, K.; Orr, G.; Lebeis, S.; Lam, E. Auxin-Producing Bacteria from Duckweeds Have Different Colonization Patterns and Effects on Plant Morphology. Plants 2022, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Bhore, S.J.; Ravichantar, N.; Loh, C.Y. Screening of endophytic bacteria isolated from leaves of Sambung Nyawa [Gynura procumbens (Lour.) Merr.] for cytokinin-like compounds. Bioinformation 2010, 5, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef]
- Hazarika, S.N.; Saikia, K.; Borah, A.; Thakur, D. Prospecting Endophytic Bacteria Endowed with Plant Growth Promoting Potential Isolated from Camellia sinensis. Front. Microbiol. 2021, 12, 738058. [Google Scholar] [CrossRef] [PubMed]
- Yarte, M.E.; Gismondi, M.I.; Llorente, B.E.; Larraburu, E.E. Isolation of endophytic bacteria from the medicinal, forestal and ornamental tree Handroanthus impetiginosus. Environ. Technol. 2022, 43, 1129–1139. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; Mohanraju, P.; Arifah, A.; van der Oost, J.; Paulson, J.N.; Mendes, R.; van Wezel, G.P.; Medema, M.H.; Raaijmakers, J.M. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef]
- Riva, V.; Mapelli, F.; Bagnasco, A.; Mengoni, A.; Borin, S. A Meta-Analysis Approach to Defining the Culturable Core of Plant Endophytic Bacterial Communities. Appl. Environ. Microbiol. 2022, 88, e0253721. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, A.; Agarwal, T.; Kotamreddy, J.N.R.; Bhattacharya, R.; Mitra, A.; Maiti, T.K.; Maiti, M.K. Impact of seed-transmitted endophytic bacteria on intra- and inter-cultivar plant growth promotion modulated by certain sets of metabolites in rice crop. Microbiol. Res. 2020, 241, 126582. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, B.; Agarwal, H.; Krishnatreya, D.B.; Sharma, P.L.; Kalita, N.; Agarwala, N. Evaluation of seed associated endophytic bacteria from tolerant chilli cv. Firingi Jolokia for their biocontrol potential against bacterial wilt disease. Microbiol. Res. 2021, 248, 126751. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, S.; Liu, N. Diversity of Endophytic Bacteria in Cardamine hupingshanensis and Potential of Culturable Selenium-Resistant Endophytes to Enhance Seed Germination Under Selenate Stress. Curr. Microbiol. 2021, 78, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Carbó, M.; Gademann, K.; Eberl, L.; Carlier, A. Leaf nodule symbiosis: function and transmission of obligate bacterial endophytes. Curr. Opin. Plant Biol. 2018, 44, 23–31. [Google Scholar] [CrossRef]
- Sun, J.; Chang, M.; Li, H.; Zhang, Z.; Chen, Q.; Chen, Y.; Yao, Y.; Pan, A.; Shi, C.; Wang, C.; Zhao, J.; Wan, X. Endophytic Bacteria as Contributors to Theanine Production in Camellia sinensis. J. Agric. Food Chem. 2019, 67, 10685–10693. [Google Scholar] [CrossRef]
- Acar, T.; Moreau, S.; Coen, O.; De Meyer, F.; Leroux, O.; Beaumel, M.; Wilkin, P.; Carlier, A. Motility-Independent Vertical Transmission of Bacteria in Leaf Symbiosis. mBio 2022, 13, e0103322. [Google Scholar] [CrossRef] [PubMed]
- Cullen, N.P.; Fetters, A.M.; Ashman, T.L. Integrating microbes into pollination. Curr. Opin. Insect Sci. 2021, 44, 48–54. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef]
- Mayhood, P.; Mirza, B.S. Soybean Root Nodule and Rhizosphere Microbiome: Distribution of Rhizobial and Nonrhizobial Endophytes. Appl. Environ. Microbiol. 2021, 87, e02884–20. [Google Scholar] [CrossRef]
- Terpolilli, J.J.; Masakapalli, S.K.; Karunakaran, R.; Webb, I.U.; Green, R.; Watmough, N.J.; Kruger, N.J.; Ratcliffe, R.G.; Poole, P.S. Lipogenesis and Redox Balance in Nitrogen-Fixing Pea Bacteroids. J. Bacteriol. 2016, 198, 2864–2875. [Google Scholar] [CrossRef]
- Susniak, K.; Krysa, M.; Kidaj, D.; Szymanska-Chargot, M.; Komaniecka, I.; Zamlynska, K.; Choma, A.; Wielbo, J.; Ilag, L.L.; Sroka-Bartnicka, A. Multimodal Spectroscopic Imaging of Pea Root Nodules to Assess the Nitrogen Fixation in the Presence of Biofertilizer Based on Nod-Factors. Int. J. Mol. Sci. 2021, 22, 12991. [Google Scholar] [CrossRef] [PubMed]
- Westhoek, A.; Clark, L.J.; Culbert, M.; Dalchau, N.; Griffiths, M.; Jorrin, B.; Karunakaran, R.; Ledermann, R.; Tkacz, A.; Webb, I.; James, E.K.; Poole, P.S.; Turnbull, L.A. Conditional sanctioning in a legume-Rhizobium mutualism. Proc. Natl. Acad. Sci. USA 2021, 118, e2025760118. [Google Scholar] [CrossRef]
- Thomas, P.; Sahu, P.K. Vertical Transmission of Diverse Cultivation-Recalcitrant Endophytic Bacteria Elucidated Using Watermelon Seed Embryos. Front. Microbiol. 2021, 12, 635810. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, Q.; Zhang, X.; Hao, J.; Li, L.; Chen, W.; Li, H.; Wang, Y.; Ma, C.; Wang, J.; Liu, Q. Community structure and associated networks of endophytic bacteria in pea roots throughout plant life cycle. Plant Soil 2021, 468, 225–238. [Google Scholar] [CrossRef]
- Kamnev, A.; Shchelochkov, A.; Perfiliev, Y.D.; Tarantilis, P.A.; Polissiou, M.G. Spectroscopic investigation of indole-3-acetic acid interaction with iron(III). J. Mol. Struct. 2001, 563, 565–572. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; Huttley, G.A.; Kelley, S.T.; Knights, D.; Koenig, J.E.; Ley, R.E.; Lozupone, C.A.; McDonald, D.; Muegge, B.D.; Pirrung, M.; Reeder, J.; Sevinsky, J.R.; Turnbaugh, P.J.; Walters, W.A.; Widmann, J.; Yatsunenko, T.; Zaneveld, J.; Knight, R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and "All-species Living Tree Project (LTP)" taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: applications for agriculture and environment. Chemosphere 2018, 194, 682–691. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K⁺ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Çakmakçı, R.; Mosber, G.; Milton, A.H.; Alatürk, F.; Ali, B. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr. Microbiol. 2020, 77, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Konopiński, M.K. Shannon diversity index: a call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ 2020, 8, e9391. [Google Scholar] [CrossRef]
- Hughes, J.B.; Hellmann, J.J.; Ricketts, T.H.; Bohannan, B.J. Counting the Uncountable: Statistical Approaches to Estimating Microbial Diversity. Appl. Environ. Microbiol. 2001, 67, 4399–4406. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Bueckert, R.; Schoenau, J.; Diederichsen, A.; Zakeri, H.; Warkentin, T.D. Evaluation of growth and nitrogen fixation of pea nodulation mutants in western Canada. Can. J. Plant Sci. 2017, 97, 1121–1129. [Google Scholar] [CrossRef]
- Dhillona, L.K.; Lindsay, D.; Yang, T.; Zakeri, H.; Tar’an, B.; Knight, J.D.; Warkentin, T.D. Biological nitrogen fixation potential of pea lines derived from crosses with nodulation mutants. Field Crops Res. 2022, 289, 108731. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Hixson, K.K.; Ahkami, A.H.; Sher, A.W.; Barnes, M.E.; Chu, R.K.; Battu, A.K.; Nicora, C.D.; Winkler, T.E.; Reno, L.R.; Fakra, S.C.; Antipova, O.; Parkinson, D.Y.; Hall, J.R.; Doty, S.L. Endophyte-Promoted Phosphorus Solubilization in Populus. Front. Plant Sci. 2020, 11, 567918. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, H.; Wei, G.; Li, Z. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 2020, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Laird, T.S.; Flores, N.; Leveau, J.H.J. Bacterial catabolism of indole-3-acetic acid. Appl. Microbiol. Biotechnol. 2020, 104, 9535–9550. [Google Scholar] [CrossRef]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr. Res. Microb. Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).