1. Introduction
The peripheral chemoreceptors, especially the carotid body (CB), are major sensors deputed to detect changes in arterial blood oxygen (hypoxemia), carbon dioxide (hypercapnia), and pH (acidosis). The mechanisms by which CB senses the first two are well known, while those of acidosis are unclear [
1,
2,
3].
Recent studies revealed that some membrane proteins, among which are acid-sensing ion channels (ASICs), allow sensory neurons to monitor tissue acidosis [
4]. ASICs form a family of amiloride-sensitive proton-gated channels belonging to the superfamily of degenerin/epithelial sodium channels (DEG/ENa
+C) which are phylogenetically preserved from invertebrates to mammals [
5] and are widely expressed especially in the nervous system [
6]. At present six ASIC isoforms (ASIC1a, 1b, 2a, 2b, 3, and 4) encoded by four genes (
Accn1,
Accn2,
Accn3, and
Accn4) have been identified in vertebrates [
7,
8,
9]. ASICs participate in several physiological roles including mechanosensation [
10], nociception [
11], and chemosensation [
9,
12]. Here we focused on chemosensation although force and pH necessary must act together in inducing ASIC activity [
13].
ASICs can be activated by a drop in extracellular pH below 7.0 and triggered by non-proton ligands during physiological pH levels [
14]. They are sensitive to changes in pH because proton ligands bind to their extracellular domains inducing channel activation that generates currents via sodium transit across membranes in a voltage-insensitive manner [
7,
15]). The pH sensitivity of ASICs is in the order of ASIC3 ≥ ASIC1a > ASIC1b > ASIC2a [
16].
Regarding CB, it has been proposed that extracellular acidosis and a drop in intracellular pH activate chemoreceptors through ASIC channels [
17]. Proton-gated currents with biophysical and pharmacological properties comparable with those of ASICs, as well as expression of ASIC1 and ASIC3, have been reported in rat glomus type I cells mediating the response to pH and hypoxia [
18,
19]. Nevertheless, the roles of ASICs in the CB are unclear since deletion of
Asic3 in mice disrupt the increase in intracellular Ca
2+ evoked in response to extracellular acidification but not to hypoxia [
19], and ASIC1-, ASIC2-, or ASIC3-knockout mice does not significantly alter hypercapnic or hypoxic ventilatory responses [
20]. Independently of the chemosensory cells of the CB, the afferent nerve terminals emanated from the petrosal ganglia neurons they could also directly sense variations in pH as they have been shown to express some ASIC isoforms [
17,
21].
As far as we know there are no data exist on the distribution of ASIC channels in human CB, both in the glomic cells and in the afferent nerve terminals. Therefore, the present research was designed to analyze the occurrence ASIC subunits using immunohistochemistry, and to determine whether they are expressed in nerves, glomus cells or both. The study aims to serve as a baseline in future studies in pathologies in which CB is involved, such as arterial hypertension [
3,
22,
23] or sleep apnea syndrome [
24,
25].
2. Results
The glomus lobes were arranged in the vicinity of, and around, the blood vessels and are separated from each other by connective tissue septa of different thicknesses. Each lobe, in turn, it consists of a variable number of glomeruli made up of type I and type II cells. The identification of the two main types of glomic cells was established in serial sections for the immunohistochemical detection of NSE or SYN, which mark type I, and S100P which selectively and type II cells, respectively [
26,
27] (
Supplementary material 1 and 2). Antibodies against NFP (phosphorylated 200 kDa subunit) and NSE were used to demonstrate the innervation of carotid body. The anti-NFP antibody used strongly immunostained peripheral nerve fibers but has the disadvantage that it does not allow to differentiate between afferent and efferent nerve fibers [
28]. But as already mentioned, the profile of the innervation of the CB is completed with the results with anti-SYN and anti-NSE antibodies (
Supplementary material 3 and 4).
To our knowledge there are no studies on the distribution of ASIC subunits in the human carotid body. Here we used immunohistochemistry in serial sections, as well as double immunofluorescence associated with laser confocal microscopy, to accurately identify the glomic structural elements, especially glomic cells and nerves, expressing ASICs.
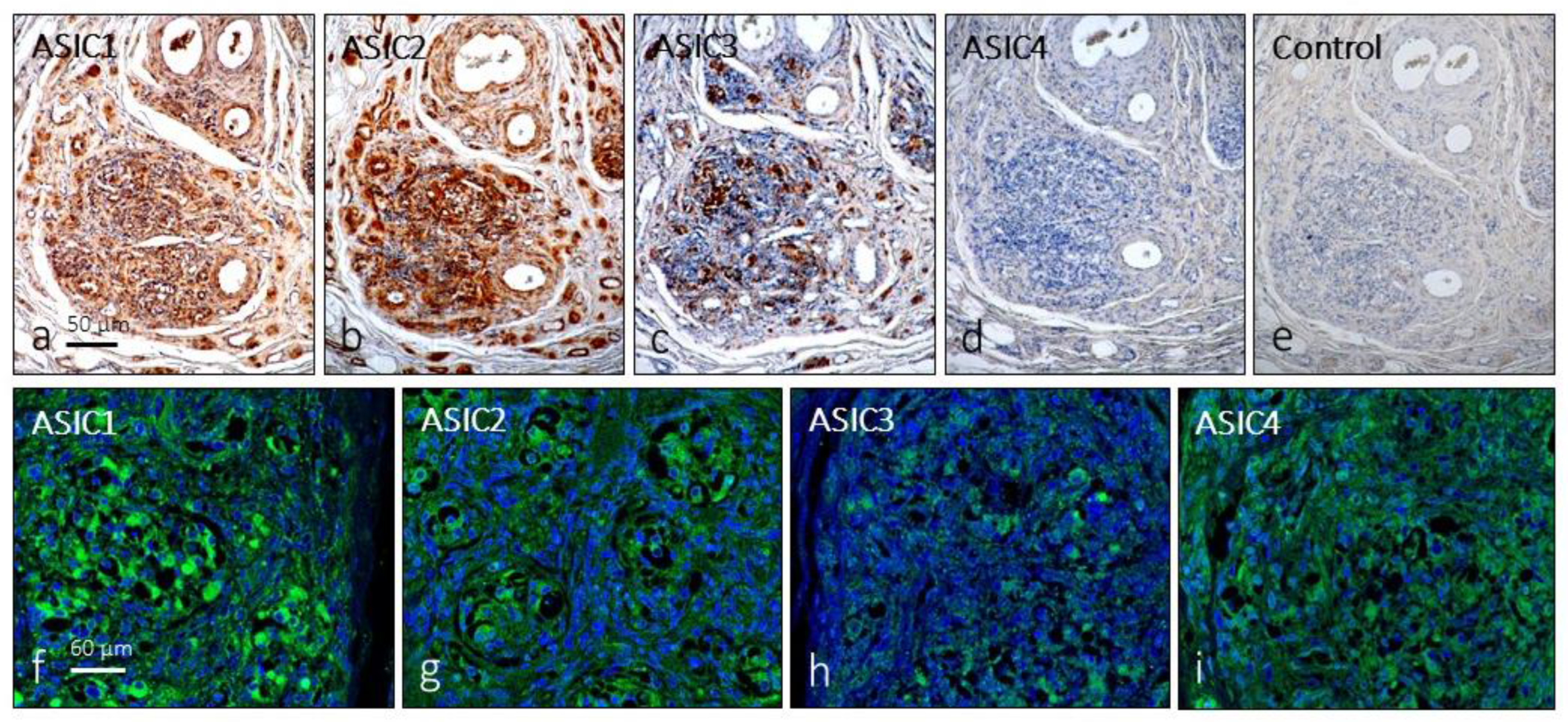
The first approach, in serial sections, showed that distinct cell populations displayed immunoreactivity for all ASIC subunits, although for ASIC4 immunostaining was very weak or not detectable in some cases; negative controls omitting the primary antibody at incubation resulted in total absence of immunoreactivity (
Figure 1a-e). Furthermore, ASIC1 and ASIC2, were detected in blood vessels and in some nerve profiles; however, in the glomus glomeruli it is not possible to establish precisely which cells express these proteins. The results of immunofluorescence confirmed the results (
Figure 1f-m).
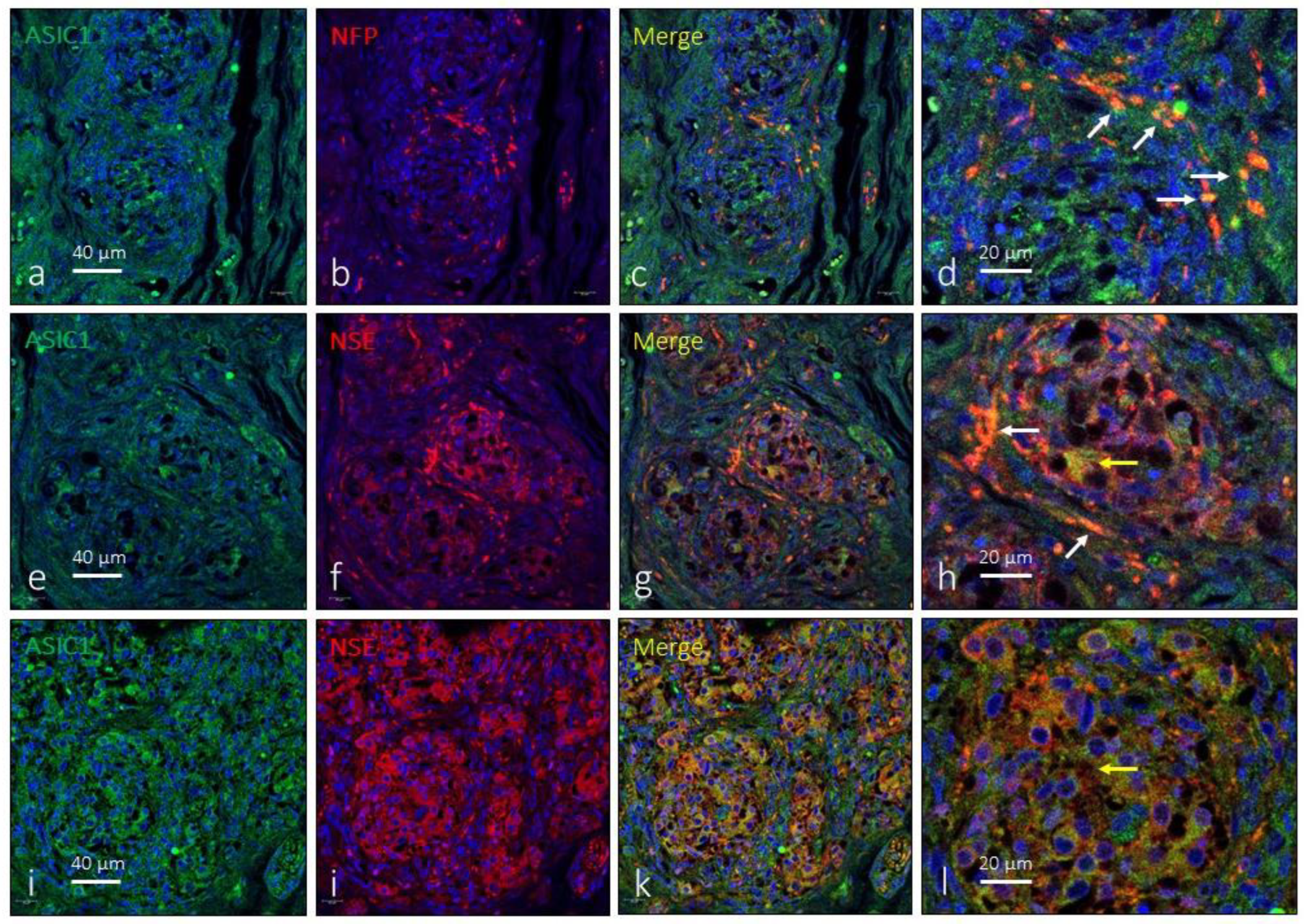
As the antibody against ASIC1 used here recognizes the two isoforms of this protein (suppliers notice). ASIC1 immunoreactivity was detected broadly in the CB apparently in subpopulations of type I glomus cells and nerve terminals (
Figure 2). However, the immunoreaction was not the same in all glomeruli nor was it present in all cells. Regarding ASIC1-positive nerve profiles, they were observed both in the peripheral part of the organ as well as in nerve localized in the connective septa between lobules and/or glomeruli. At the level of light-microscope synaptic contacts between nerve and type I glomus cells were not observed. The area occupied by the ASIC1-specific immunoreaction was 43.2 ± 3.6% of the total section, and the area occupied by the emergence of both glomic cells and nerves was 2.6 ± 0.3% of the total section (
Table 1).
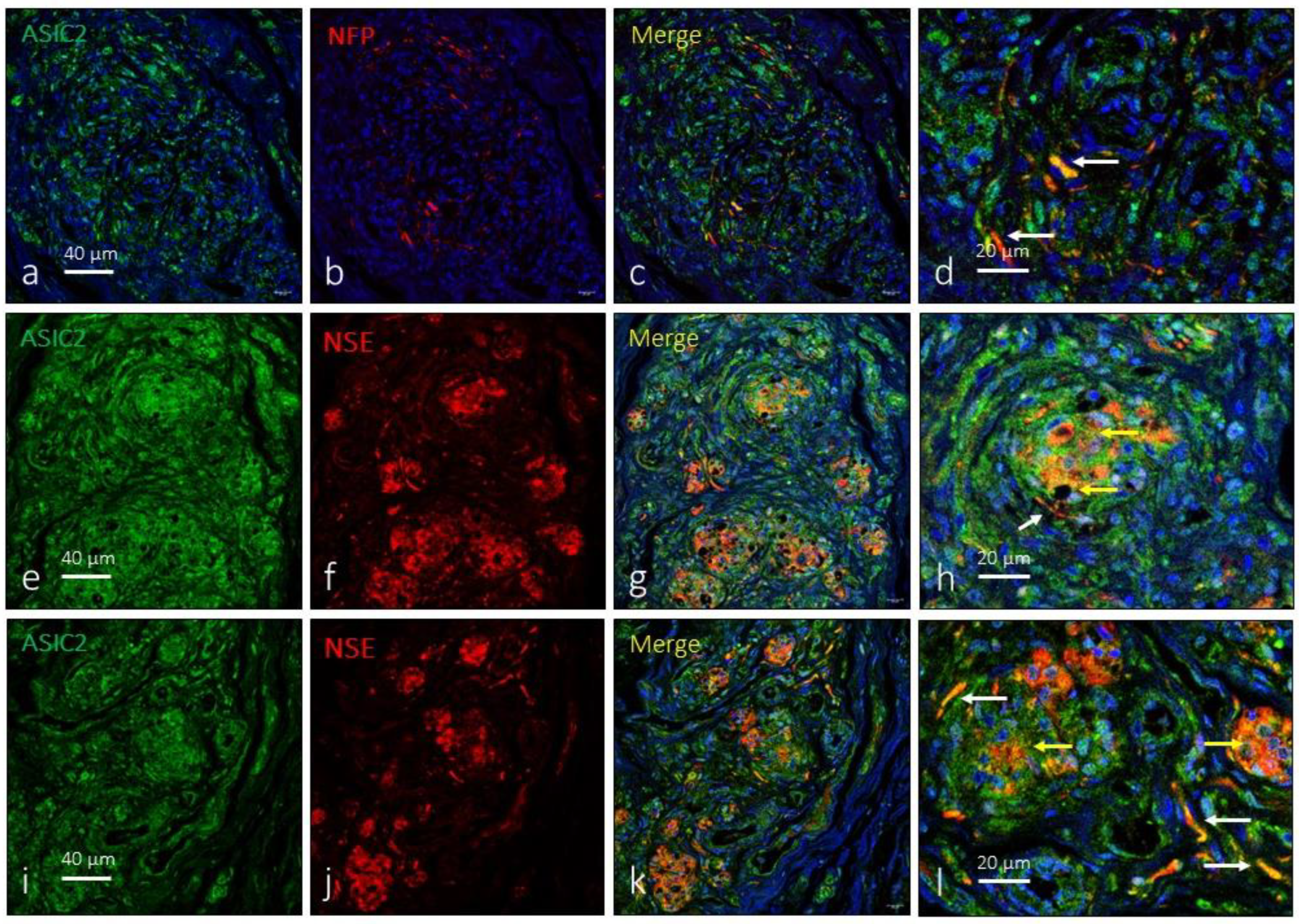
As for ASIC1, the anti-ASIC2 antibody used recognizes the two subunits of the protein. ASIC2 are also widely expressed in the carotid body. A large proportion of type I glomus cells showed ASIC2-immunoreactivity and positivity was also detected in nerve terminals (
Figure 3) located, preferably, on the surface of the organ and not between the glomeruli. The area occupied by the ASIC2-specific immunoreaction was 61.1 ± 2.8% of the total section, and the area occupied by the emergence of both glomic cells and nerves was 1.9 ± 0.2% of the total section (
Table 1).
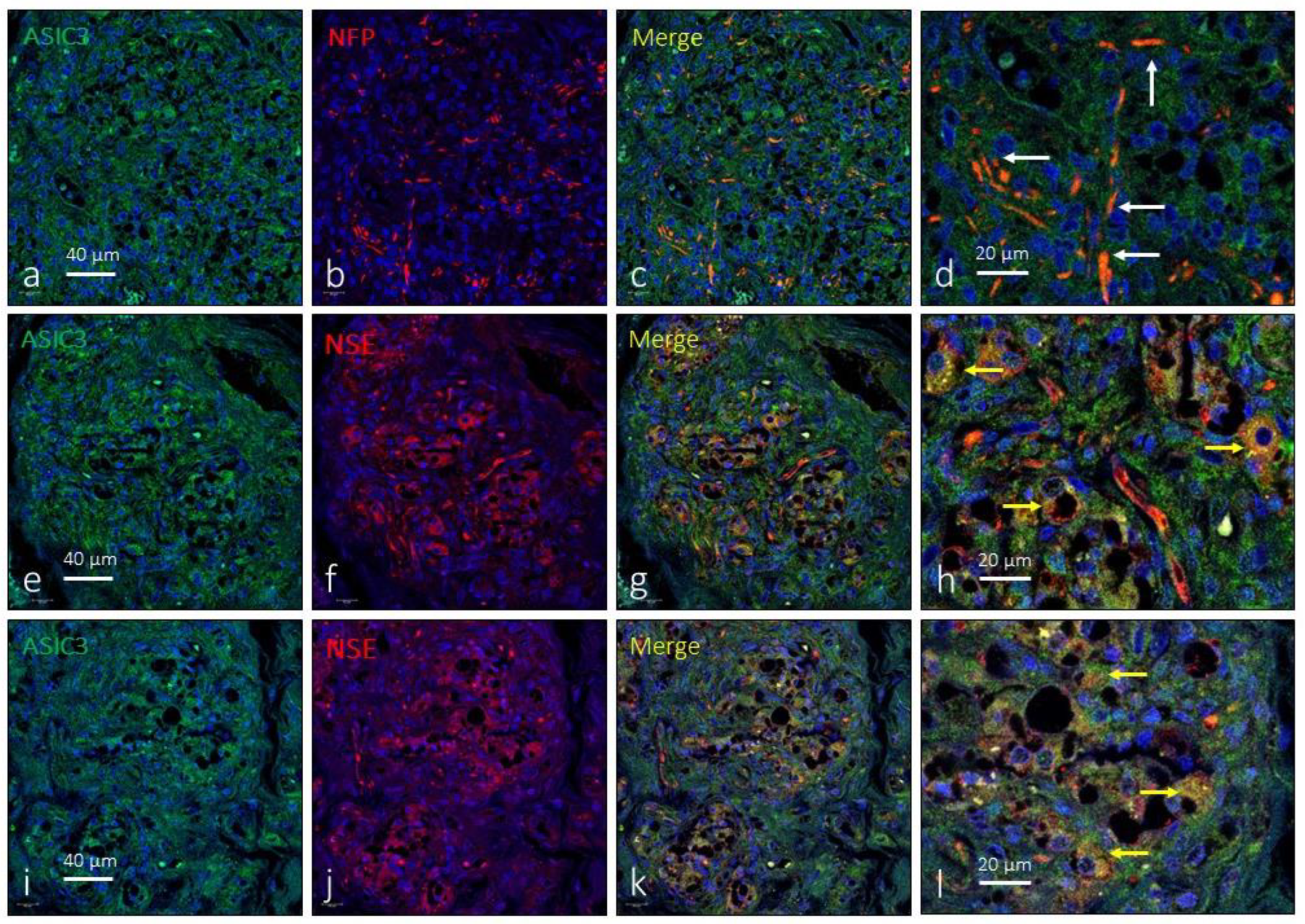
The expression of ASIC3 in the human CB was low and was only detected in small groups of a type I glomus cells. Occasionally images suggestive of the presence of ASIC3 were observed in the interlobular nerve profiles (
Figure 4). The area occupied by the ASIC3-specific immunoreaction was 9.7 ± 2.4% of the total section, and the area occupied by the emergence of both glomic cells and nerves was almost identical, from 2.3 ± 0.3% of the total section (
Table 1).
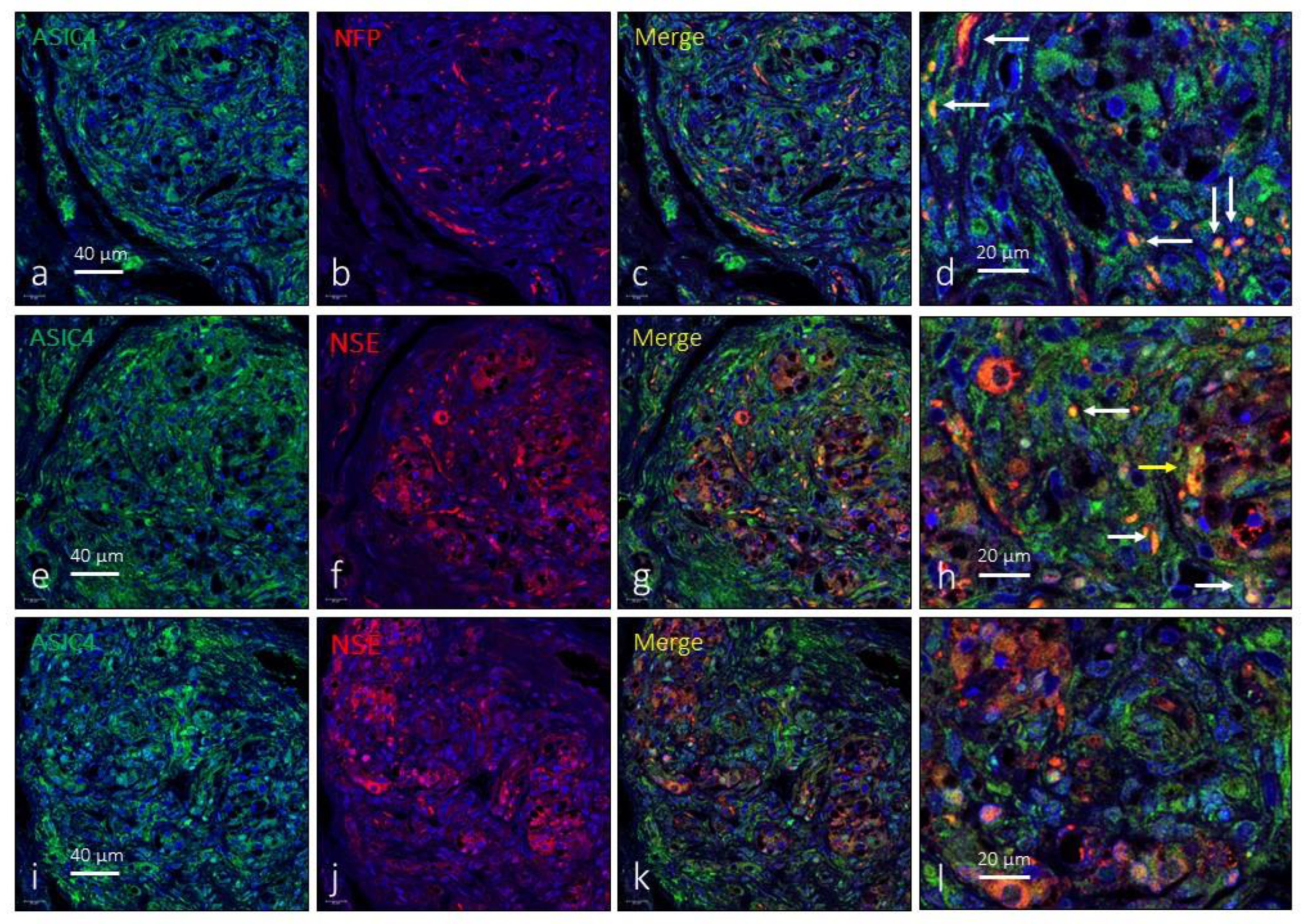
Finally, the immunoexpression of ASIC4 in the human CB was limited to some isolated type I glomus cells in isolated glomeruli as well as to nerve profiles (
Figure 5). The area occupied by the ASIC4-specific immunoreaction was 2.4 ± 2.1% of the total section, and the area occupied by the emergence of both glomic cells and nerves was 2.3 ± 0.9% of the total section.
In relation to type II cells, the presence of ASIC proteins in them cannot be ruled out, since the immunoreaction area of ASICs was apparently larger than that of type I cells. However, it has not been taken into consideration because of the difficulty of isolating type II cells from connective tissue and because it could also be a case of non-specific background.
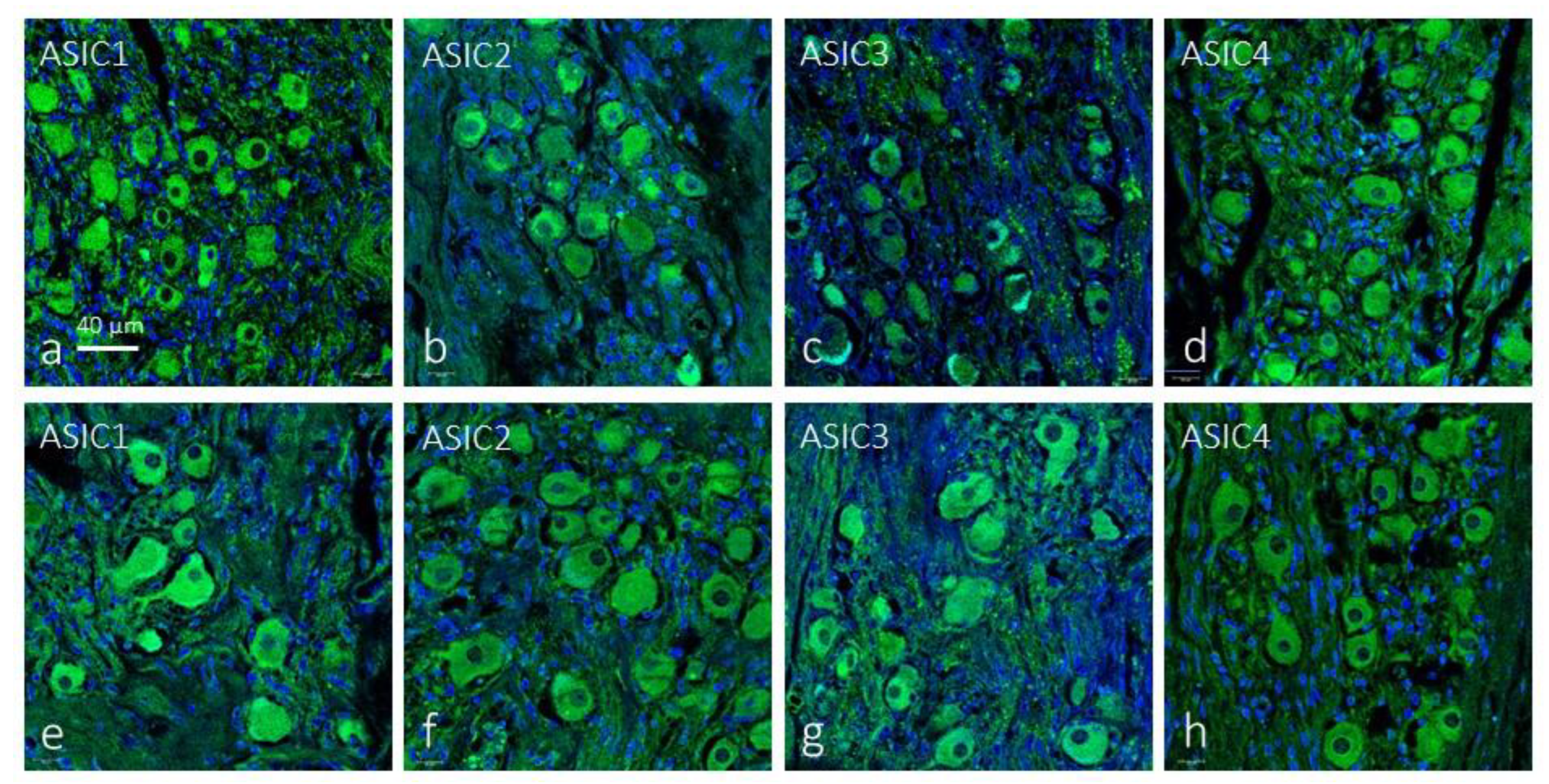
In the sections of both the petrosal ganglia and the superior cervical sympathetic ganglia analyzed immunoreactivity has been found for all ASIC isoforms. The immunoreaction was restricted to the neuronal bodies while the satellite cells were unreactive. In the petrosal ganglia de percentage and size of the immunoreactive neurons was variable for each ASIC protein, whereas apparently all neurons were immunoreactive for each ASIC protein in the sympathetic ganglia (
Figure 6 and
Table 3).
3. Discussion
It is now accepted that the CB is a multimodal sensor that detect variations in oxygen, carbon dioxide and pH, but low glucose levels, and additionally participates in the regulation of metabolism [
29,
30]. The detection of blood pH and systemic acidosis is important for the regulation of respiratory feedback and cardiovascular function.
The present research study was designed to contribute to the knowledge of how the human CB contributes to the detection of variations in pH, to analyze whether it expresses ASIC ion channels, and to know if these channels are in the cells of the CB itself or in the afferent nerves. We used samples of the CB from subjects free of disease.
Structurally, the CB consists of two main cell types: chemoreceptor cells, or type I cells, organized into tight clusters surrounded by supportive glial cells, or type II cells. Type I cells are innervated by afferent fibres whose soma are placed at the petrosal ganglion of the glossopharyngeal nerve, and by efferent fibres emanated from the superior sympathetic cervical ganglion [
31,
32]. The activation of chemoreceptors in type I cells leads to depolarization, an increase in intracellular Ca
2+ concentration, followed by the release of multiple neurotransmitters, and the synaptic activation of afferent nerve endings [
19,
33,
34,
35]. Although type I glomus cells are regarded to be chemosensory, the real chemoreceptors are subtypes of sensory neurons in the petrosal ganglion responsible for detecting tissue acidosis. In this context, type I glomus cells can be regarded as accessory cells acting as intermediaries between the blood/extracellular medium/intracellular medium and chemoreceptor neurons [
36].
Evidence accumulated over the past 20 years has demonstrate that some membrane proteins allow sensory neurons, and sensory cells like type I glomus cells, to monitor tissue acidosis; among those proteins are the different isoforms of ASIC channels. Iturriaga et al. [
3] in a general hypothesis about the possible localization of ASIC channels in the carotid body, assume that they may be present in both type I glomus cells and afferent nerve terminals. Our findings are in total agreement with the opinion of these authors since we have detected them in both locations. We observed that mainly ASIC1 and ASIC2, to a lesser extent ASIC3, and at almost residual levels ASIC4, are expressed in type I glomus cells of the human carotid body. The expression of ASIC1, ASIC2 and ASIC3 in the rat CB was demonstrated earlier by of Tan et al. [
28] who found that all these ASIC proteins are expressed in groups of type I glomus cells, and some of them are co-expressed in the same glomic cells. We did not confirm these results in the human CB because no co-localization studies were performed, however, when the expression of these proteins was observed in serial sections it seems that it is highly possible.
Regarding the occurrence of ASICs immunoreactivity in nerves, we have regularly observed their presence in the nerves of the carotid body, with very few differences in the innervation density for the positive nerves for each of the 4 subunits. However, we cannot identify whether they are afferent and/or efferent since the presence of all ASIC proteins has been detected in neurons of the petrosal ganglion and the upper cervical sympathetic ganglion. The presence of ASIC isoforms in human sensory ganglia, both spinal and cranial ganglia, has been little studied. Only Fukuda et al. [
21] have reported the presence of ASIC channels in the rat petrosal ganglion, specifically ASIC3. Cabo et al. [
37] demonstrated ASIC2 in mechanosensory neurons, as well as small subpopulations of proprioceptive and nociceptive neurons in human lumbar dorsal root ganglia. Nevertheless, the presence of ASIC proteins in the human petrosal ganglion neurons is reported here for the first time. On the contrary, there is ample functional, physiological, and pharmacological evidence for the presence of all ASIC subunits in rat and mouse spinal ganglia [
38,
39,
40,
41]. Thus, present results suggest that the sensory neurons of the human petrosal ganglion can detect wide ranges of drops in pH, and therefore acidosis, since it expresses all ASIC subunits. Obviously, based on the techniques used, it cannot be said whether they capture variations in intracellular or extracellular pH. Further studies are necessary to try to clarify this issue. Regarding the sympathetic ganglia, there is no reference in the scientific literature that addresses this topic. Only the study by Liu et al. [
17] makes specific reference to the upper cervical sympathetic ganglion of the rat and affirm that under the conditions of their experiment the ASICs are not modified in the neurons of this ganglion, indirectly suggesting that they do detect some isoforms. However, our work is the first to demonstrate the presence of all ASIC isoforms in sympathetic efferent neurons. The functional significance of these findings if any remains to be elucidated.
As a summary our results that demonstrate for the first-time expression the occurrence of ASIC1, ASIC2, ASIC3 and ASIC4 in the human carotid body, both in the type I cells and the nerves supplying it. Therefore, both chemosensory cells and nerves can sense acidosis, and ASICs can generate acid-evoked responses.
4. Materials and Methods
4.1. Materials
Tissue samples containing the common artery bifurcation and they were obtained from 8 subjects (5 males and 3 females), with ages ranging between 38 and 68 years, during removal of organs for transplantation in subjects that died in traffic accidents (Hospital Universitario Central de Asturias, Oviedo, Spain). Furthermore, the petrosal ganglion (n = 6) and the sympathetic superior ganglion (n = 4) were dissected out and included in the study. The material was obtained in compliance with the Spanish Law and the guidelines of the Helsinki Declaration II.
The tissue samples were cleaned in 4°C saline solution, fixed in 10% formaldehyde in 0.1 M PBS, pH 7.4 for 24 h at 4°C, dehydrated and routinely embedded in paraffin. For detection of ASIC1 and ASIC3 deparaffined and rehydrated sections were heated in Envision FLEX target retrieval solution high pH (Dako) at 65°C for 20 min and then for 20 min at room temperature in the same solution. For detection of ASIC2 this step was omitted.
4.2. Immunohistochemistry
To detect expression of ASIC proteins in CB indirect peroxidase-anti-peroxidase immunohistochemistry was used. Briefly, 10 μm thick sections of paraffin-embedded tissues were mounted on gelatin-coated microscope slides and processed for light microscopy. Rehydrated sections were rinsed in 0.05 M HCl Tris buffer (pH 7.5) containing 0.1% bovine serum albumin and 0.1% Triton X-100. Endogenous peroxidase activity (3% H
2O
2) and non-specific binding (10% fetal calf serum) were blocked, and the sections incubated overnight in a humid chamber at 4°C with primary antibodies (
Table 3). The antibodies were diluted in a solution of Tris-HCl buffer (0.05 M, pH 7.5) containing 0.1% bovine serum albumin, 0.2% fetal calf serum and 0.1% Triton X-100. After incubation with the primary antibodies, the sections were rinsed in the same buffer and incubated with Dako EnVision System labeled polymer-HR anti-rabbit IgG or anti-mouse IgG (DakoCytomation, Denmark) for 30 minutes at room temperature.
ASIC1: raised against a synthetic peptide to an extracellular domain epitope of human ASIC1, conjugated to immunogenic carrier protein; recognizes both subtype1 and subtype2; ASIC2: raised against a synthetic peptide, specific human:; ASIC3: raised against a synthetic peptide to an internal sequence of human ASIC3 conjugated to immunogenic carrier protein; ASIC4: raised against a synthetic peptide from the cytoplasmic domain of human ASIC4 conjugated to immunogenic carrier protein used as an immunogen.
Finally, sections were washed, and the immunoreaction visualized using 3-3′-diaminobenzidine as a chromogen. For control purposes representative sections were processed as above using non-immune rabbit or mouse sera instead of the primary antibodies or omitting the primary antibodies in the incubation. To visualize structural details sections were slightly counterstained with hematoxylin & eosin.
4.3. Double immunofluorescence
Sections were processed for simultaneous detection of ASICs with specific axonal (neurofilament protein -NFP- or neuron-specific enolase -NSE-) markers, supportive type II glial cells and Schwann cells (S100 protein - S100P-) markers, or type I glomus cells (synaptophysin -Syn) [
32,
35]. Non-specific binding was reduced by incubation with a solution of 25% calf bovine serum in tris buffer solution (TBS) for 30 min. Sections were incubated with a 1:1 v/v mixture of polyclonal antibody against ASIC1, ASIC2, ASIC3 and ASIC4 and monoclonal antibodies against NFP, NSE, S100P, or Syn in a humid chamber overnight at 4ᵒ C. After rinsing with TBS, sections were incubated for one hour with CFL488-conjugated bovine anti-rabbit IgG (diluted 1:200 in TBS; sc-362260, Santa Cruz Biotechnology, Heidelberg, Germany) and then, rinsed and incubated again for another hour with CyTM3-conjugated donkey anti-mouse antibody (diluted 1:100 in TBS; Jackson-ImmunoResearch, Baltimore, MD, USA). Both steps were performed in a dark humid chamber at room temperature. Sections were finally washed, and cell nuclei were stained with DAPI (10 ng/mL). Triple fluorescence was detected by using a Leica DMR-XA automatic fluorescence microscope (Microscopía fotónica y Proceso de imágen, Servicios científico-técnicos, Universidad de Oviedo) coupled with a Leica Confocal Software (version 2.5; Leica Microsystems, Heidelberg GmbH, Germany), and captured images were processed using the software Image J (version 1.43 g; Master Biophotonics Facility, Mac Master University Ontario;
www.macbiophotonics.ca, access on 11 January 2021).
For control purposes, representative sections were processed in the same way as described but either not using immune rabbit or mouse sera instead of primary antibodies or omitting primary antibodies when incubation. Furthermore, when available, additional controls were conducted using specifically preabsorbed antisera. Under these conditions, no positive immunostaining was found.
4.4. Quantitative analysis
A quantitative image analysis was carried out in carotid body, petrosal ganglia and upper cervical sympathetic ganglia reacted for all ASIC isoforms detection using an automatic image analysis system (Quantimet 550, Leica, QWIN Program). The percentage of immunoreactive area for SYN (which identify type I cells; regarded as 100%) and the merge SYN + each individual ASIC was determined in 5 sections for sample, 100 µm apart, measuring 10 randomly selected fields per section (5 mm2). Results are expressed values of mean ± standard error. Furthermore, in the same fields and in 5 additional ones the density of nerve profiles displaying merge for NFP + each individual ASIC was evaluated. Results are expressed as number of nerve profiles/mm2.
On the other hand, the percentage and size (mean diameter in μm) of the immunoreactive neurons for each ASIC isoform were evaluated. Measurements were made on three sections per specimen, 200 μm apart to avoid measuring the same neuron twice, evaluating five randomly selected fields per section (2.5 mm2). For evaluation of the cell body size, only the neuronal profiles with apparent nuclei were considered, and the neurons were divided into three size classes, measured in 20 μm steps of diameter (≤ 20 μm, 21-40 μm, ≥ 41 μm). The results are expressed as mean ± standard deviation and refer the variation among specimens.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
GM-B, YG-M, TC and PC performed the experiments. JF, TC and OG-S collected the material in compliance with ethical guidelines and performed part of the experiments. RC and OG-S conducted the quantitative analysis. GM-B and JAV designed the study, analyzed the data, and wrote the manuscript.
Funding
This research received no external funding. P.C. was supported by a Grant “Severo Ochoa Program” from the Govern of the Principality of Asturias (PA-21-PF-BP20-122).
Institutional Review Board Statement
These materials were obtained from our laboratory (Registro Nacional de Biobancos, Sección Colecciones, Ref. C-0001627) of the Peripheral Nervous System and Sense Organs Research Group (SINPOs) at the University of Oviedo. The biological material was obtained in compliance with the Spanish Legislation (RD 1301/2006; Law 14/2007; RD 1716/2011; Order ECC/1404/2013) and in agreement with the guidelines of the Declaration of Helsinki II. This study was approved by the Ethical Committee for Biomedical Research of the Principality of Asturias, Spain (Cod. Celm. Past: Proyecto 266/18).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Authors thank Dr. Marta Alonso-Guervos for technical assistance with confocal laser microcopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cummins, E.P.; Strowitzki, M.J.; Taylor, C.T. Mechanisms and Consequences of Oxygen and Carbon Dioxide Sensing in Mammals. Physiol. Rev. 2020, 100, 463–488. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Sáenz, P.; López-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. Annu. Rev. Physiol. 2020, 82, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Alcayaga, J.; Chapleau, M.W.; Somers, V.K. Carotid body chemoreceptors: physiology, pathology, and implications for health and disease. Physiol. Rev. 2021, 101, 1177–1235. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.R.; Buck, J. Physiological roles of acid-base sensors. Annu. Rev. Physiol. 2015, 77, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nat. 1997, 386, 173–177. [Google Scholar] [CrossRef]
- Sivils, A.; Yang, F.; Wang, J.Q.; Chu, X.P. Acid-Sensing Ion Channel 2: Function and Modulation. Memb. 2022, 12, 113. [Google Scholar] [CrossRef]
- Krishtal, O. The ASICs: Signaling molecules? Modulators? Trends. Neurosci. 2003, 26, 477–483. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. International Union of Basic and Clinical Pharmacology. XCI. Structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 2015, 67, 1–35. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiang, B.; Chen, C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46. [Google Scholar] [CrossRef]
- Ruan, N.; Tribble, J.; Peterson, A.M.; Jiang, Q.; Wang, J.Q.; Chu, X.P. Acid-sensing ion channels and mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810. [Google Scholar] [CrossRef]
- Giniatullin, R. Ion Channels of Nociception. Int. J. Mol. Sci. 2020, 21, 3553. [Google Scholar] [CrossRef] [PubMed]
- Carattino, M.D.; Montalbetti, N. Acid-sensing ion channels in sensory signaling. Am. J. Physiol. Renal. Physiol. 2020, 318, F531–F543. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Fronius, M. Shear force modulates the activity of acid-sensing ion channels at low pH or in the presence of non-proton ligands. Sci. Rep. 2019, 9, 6781. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Z.; Li, W.G.; Cao, H.; Feng, E.G.; Yu, F.; Liu, H.; Jiang, H.; Xu, T.L. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010, 68, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Gründer, S.; Pusch, M. Biophysical properties of acid-sensing ion channels (ASICs). Neuropharm. 2015, 94, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, L.; Dinger, B.; Fidone, S.J. Chronic hypoxia-induced acid-sensitive ion channel expression in chemoafferent neurons contributes to chemoreceptor hypersensitivity. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2011, 301, L985–L992. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.Y.; Lu, Y.; Whiteis, C.A.; Benson, C.J.; Chapleau, M.W.; Abboud, F.M. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ. Res. 2007, 101, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Whiteis, C.A.; Sluka, K.A.; Chapleau, M.W.; Abboud, F.M. Responses of glomus cells to hypoxia and acidosis are uncoupled, reciprocal and linked to ASIC3 expression: selectivity of chemosensory transduction. J. Physiol. 2013, 591, 919–932. [Google Scholar] [CrossRef]
- Detweiler, N.D.; Vigil, K.G.; Resta, T.C.; Walker, B.R.; Jernigan, N.L. Role of acid-sensing ion channels in hypoxia- and hypercapnia-induced ventilatory responses. PLoS. One. 2018, 13, e0192724. [Google Scholar] [CrossRef]
- Fukuda, T.; Ichikawa, H.; Terayama, R.; Yamaai, T.; Kuboki, T.; Sugimoto, T. ASIC3-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain. Res. 2006, 1081, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.; Ratcliffe, L.; Hering, D.; Wolf, J.; Sobotka, P.A.; Narkiewicz, K. Revelations about carotid body function through its pathological role in resistant hypertension. Curr. Hypertens. Rep. 2013, 15, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R. Translating carotid body function into clinical medicine. J. Physiol. 2018, 596, 3067–3077. [Google Scholar] [CrossRef]
- Fung, M.L. Pathogenic roles of the carotid body inflammation in sleep apnea. Mediators. Inflamm. 2014, 2014, 354279. [Google Scholar] [CrossRef]
- Iturriaga, R.; Del Rio, R.; Idiaquez, J.; Somers, V.K. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol. Res. 2016, 49, 13. [Google Scholar] [CrossRef]
- Navarro-Guerrero, E.; Platero-Luengo, A.; Linares-Clemente, P.; Cases, I.; López-Barneo, J.; Pardal, R. Gene Expression Profiling Supports the Neural Crest Origin of Adult Rodent Carotid Body Stem Cells and Identifies CD10 as a Marker for Mesectoderm-Committed Progenitors. Stem. Cells. 2016, 34, 1637–1650. [Google Scholar] [CrossRef]
- Annese, V.; Navarro-Guerrero, E.; Rodríguez-Prieto, I.; Pardal, R. Physiological Plasticity of Neural-Crest-Derived Stem Cells in the Adult Mammalian Carotid Body. Cell. Rep. 2017, 19, 471–478. [Google Scholar] [CrossRef]
- Vega, J.A.; Humara, J.M.; Naves, F.J.; Esteban, I.; Del Valle, M.E. Immunoreactivity for phosphorylated 200-kDa neurofilament subunit is heterogeneously expressed in human sympathetic and primary sensory neurons. Anat. Embryol. (Berl). 1994, 190, 453–459. [Google Scholar] [CrossRef]
- Pardal, R.; López-Barneo, J. Low glucose-sensing cells in the carotid body. Nat. Neurosci 2002, 5, 197–198. [Google Scholar] [CrossRef]
- Zhang, M.; Buttigieg, J.; Nurse, C.A. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J. Physiol. 2007, 578 Pt 3, 735–350. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J. Peripheral chemoreceptors in health and disease. J. Appl. Physiol. 2004, 96, 359366. [Google Scholar] [CrossRef] [PubMed]
- Otlyga, D.; Tsvetkova, E.; Junemann, O.; Saveliev, S. Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development. Int. J. Mol. Sci. 2021, 22, 8222. [Google Scholar] [CrossRef]
- Eyzaguirre, C.; Fidone, S.J. Transduction mechanisms in carotid body: glomus cells, putative neurotransmitters, and nerve endings. Am. J. Physiol. Cell. Physiol. 1980, 239, C135–C152. [Google Scholar] [CrossRef]
- Nurse, C.A. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton. Neurosci. 2005, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Macchi, V.; Stecco, C.; De Caro, R. The Carotid Sinus Nerve-Structure, Function, and Clinical Implications. Anat. Rec. (Hoboken). 2019, 302, 575–587. [Google Scholar] [CrossRef]
- López-López, J.R.; Pérez-García, M.T. An ASIC channel for acid chemotransduction. Circ. Res. 2007, 101, 965–967. [Google Scholar] [CrossRef]
- Cabo, R.; Alonso, P.; Viña, E.; Vázquez, G.; Gago, A.; Feito, J.; Pérez-Moltó, F.J.; García-Suárez, O.; Vega, J.A. ASIC2 is present in human mechanosensory neurons of the dorsal root ganglia and in mechanoreceptors of the glabrous skin. Histochem. Cell. Biol. 2015, 143, 267–276. [Google Scholar] [CrossRef]
- Liu, T.T.; Wei, S.; Jin, Y.; Qiu, C.Y.; Hu, W.P. Inhibition of ASIC-Mediated Currents by Activation of Somatostatin 2 Receptors in Rat Dorsal Root Ganglion Neurons. Mol. Neurobiol. 2021, 58, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Hao, J.W.; Qiao, W.L.; Li, Q.; Liu, T.T.; Qiu, C.Y.; Hu, W.P. Suppression of ASIC activity by the activation of A1 adenosine receptors in rat primary sensory neurons. Neuropharm. 2022, 205, 108924. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, W.L.; Hao, J.W.; Wei, S.; Li, X.M.; Liu, T.T.; Qiu, C.Y.; Hu, W.P. Potentiation of ASIC currents by lysophosphatidic acid in rat dorsal root ganglion neurons. J. Neurochem. 2022, 163, 327–337. [Google Scholar] [CrossRef]
- Li, Q.; Qin, L.; Li, J. Characteristics of acid-sensing ion channel currents in male rat muscle dorsal root ganglion neurons following ischemia/reperfusion. Physiol. Rep. 2023, 11, e15654. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell. Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).