Submitted:
17 November 2023
Posted:
21 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Overview of The Experimental Area
2.2. Test Materials
2.3. Experimental Design
2.4. Test Implementation
2.5. Sampling and Physicochemical Analysis
2.6. Statistical Analysis
3. Results
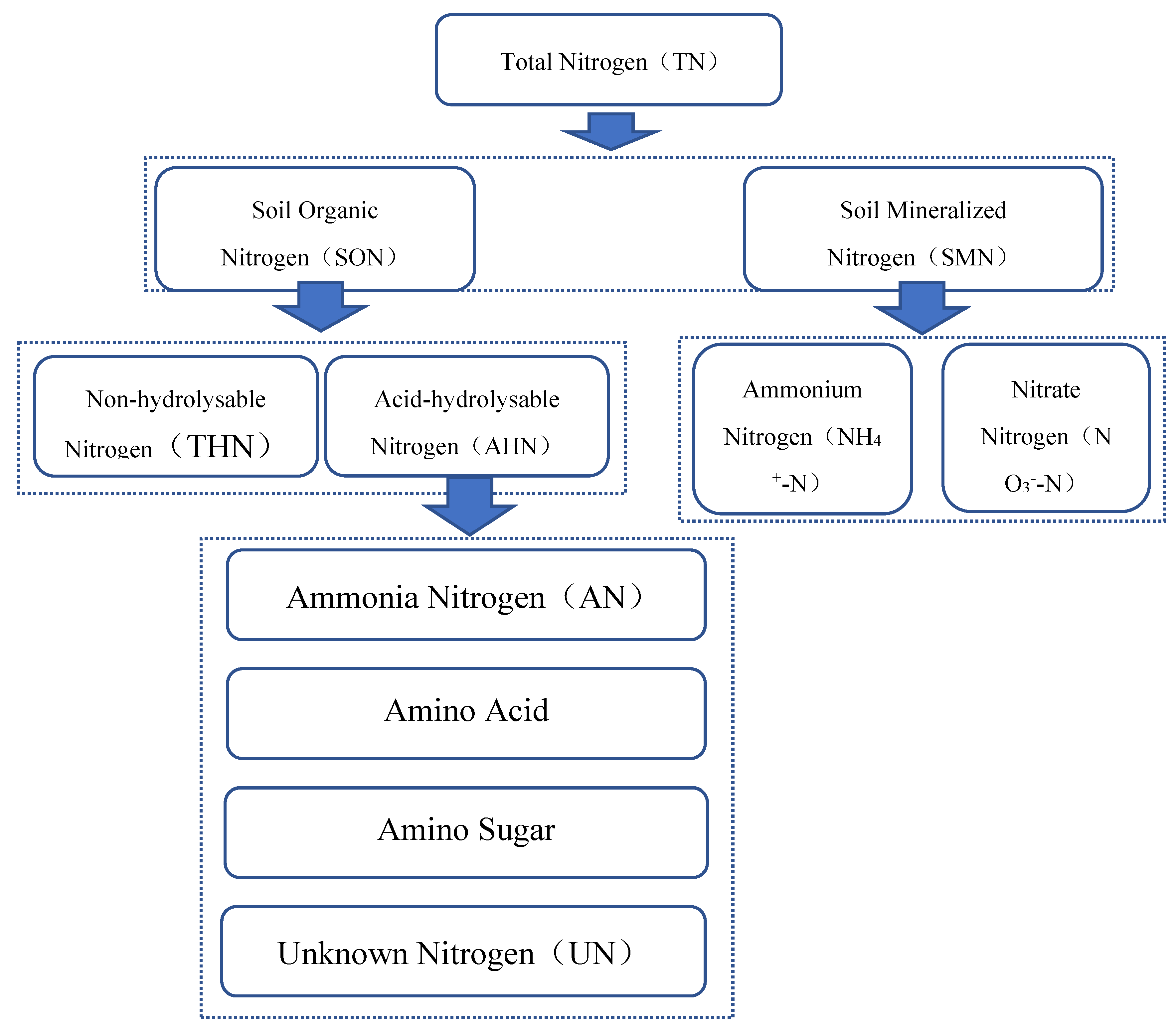
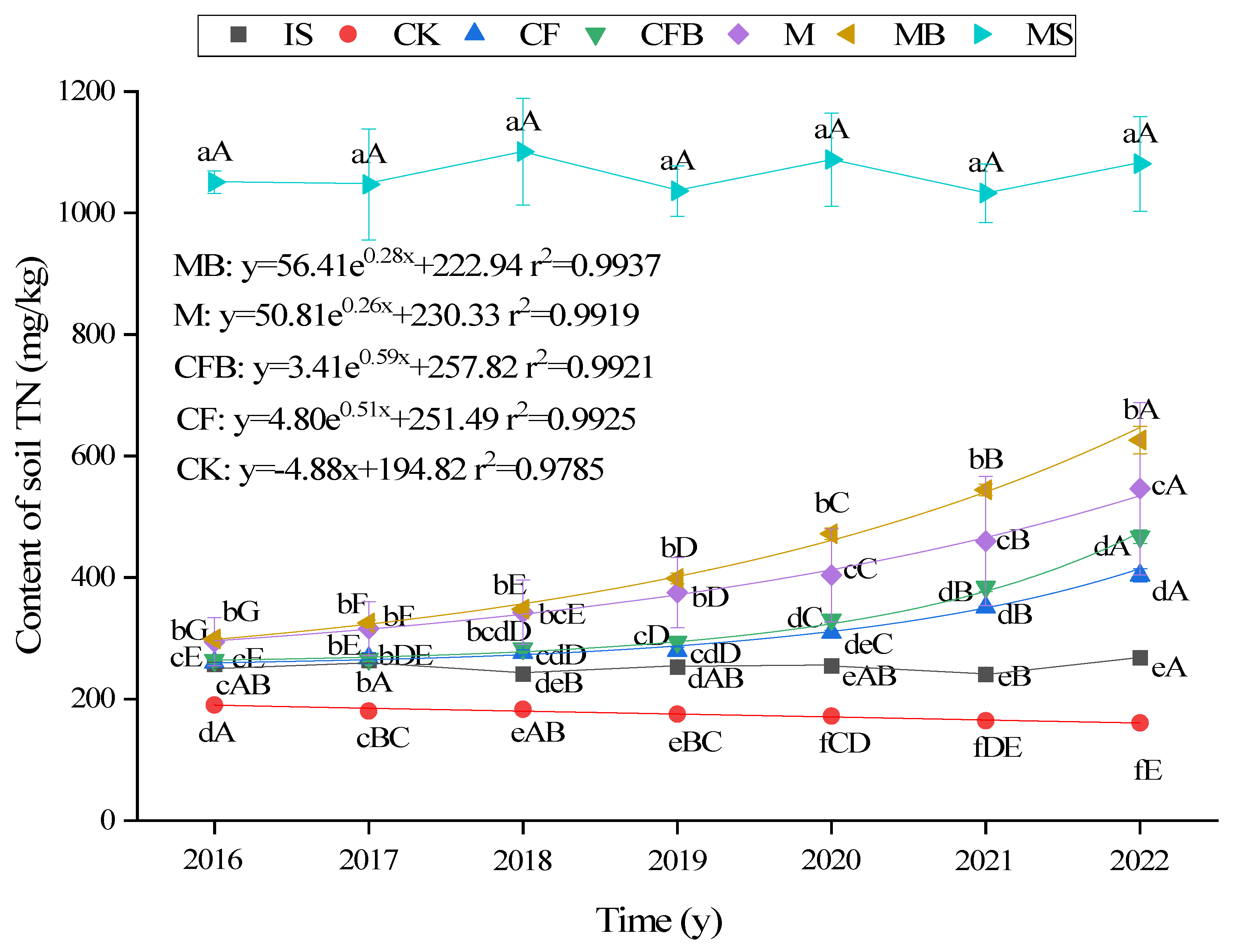
3.1. Changes in TN Content
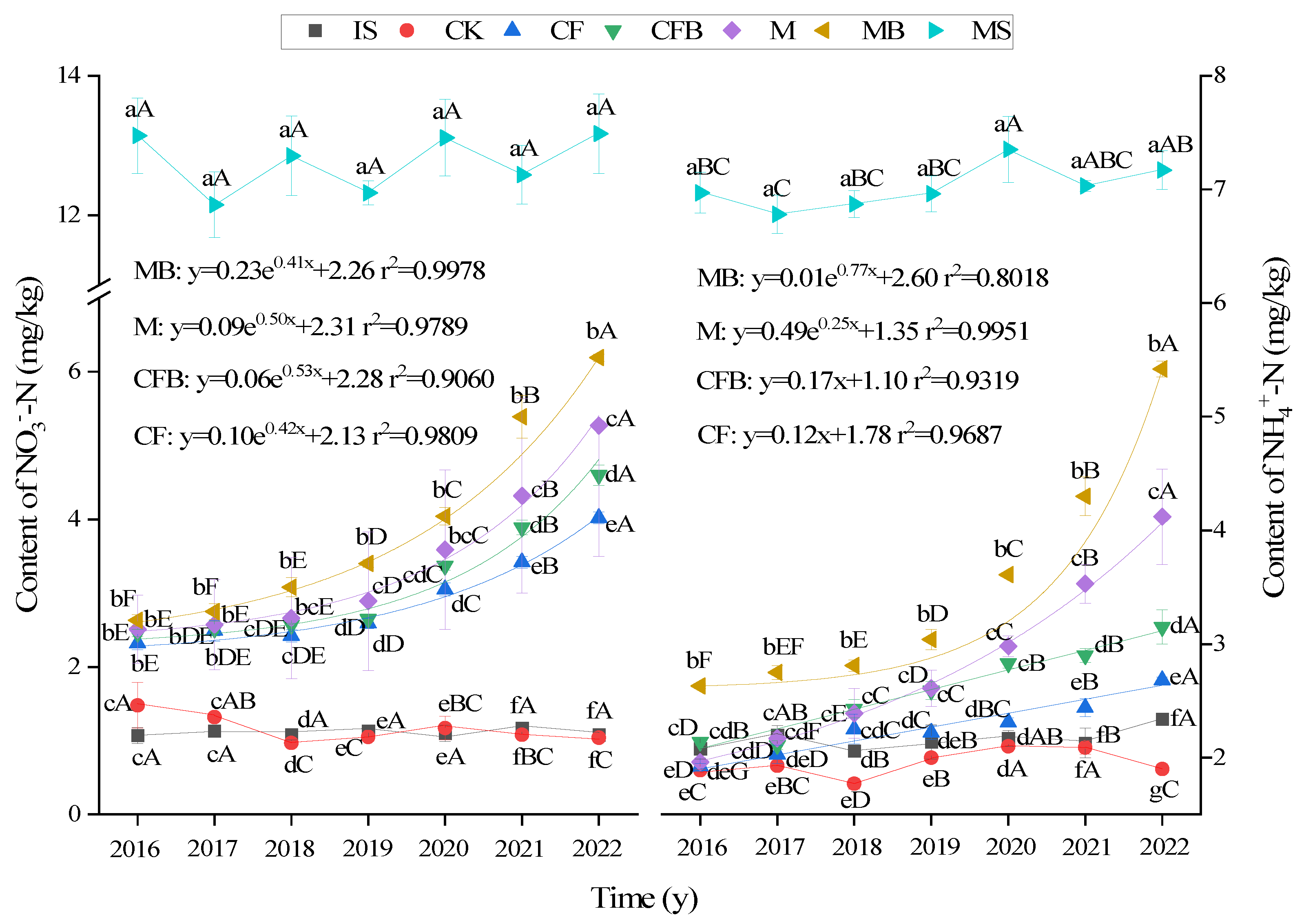
3.2. Changes in SMN Content
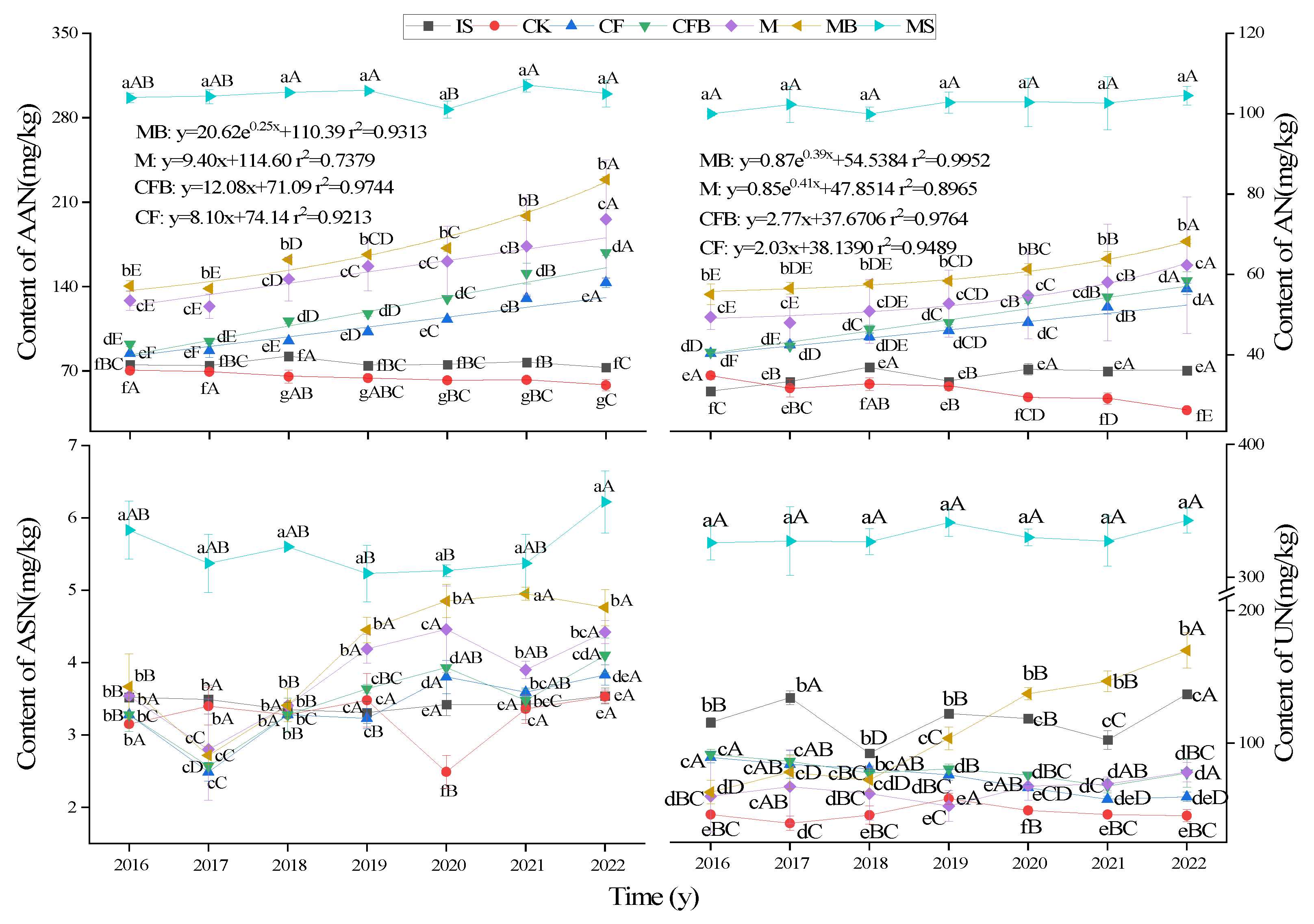
3.3. Changes in AHN Content
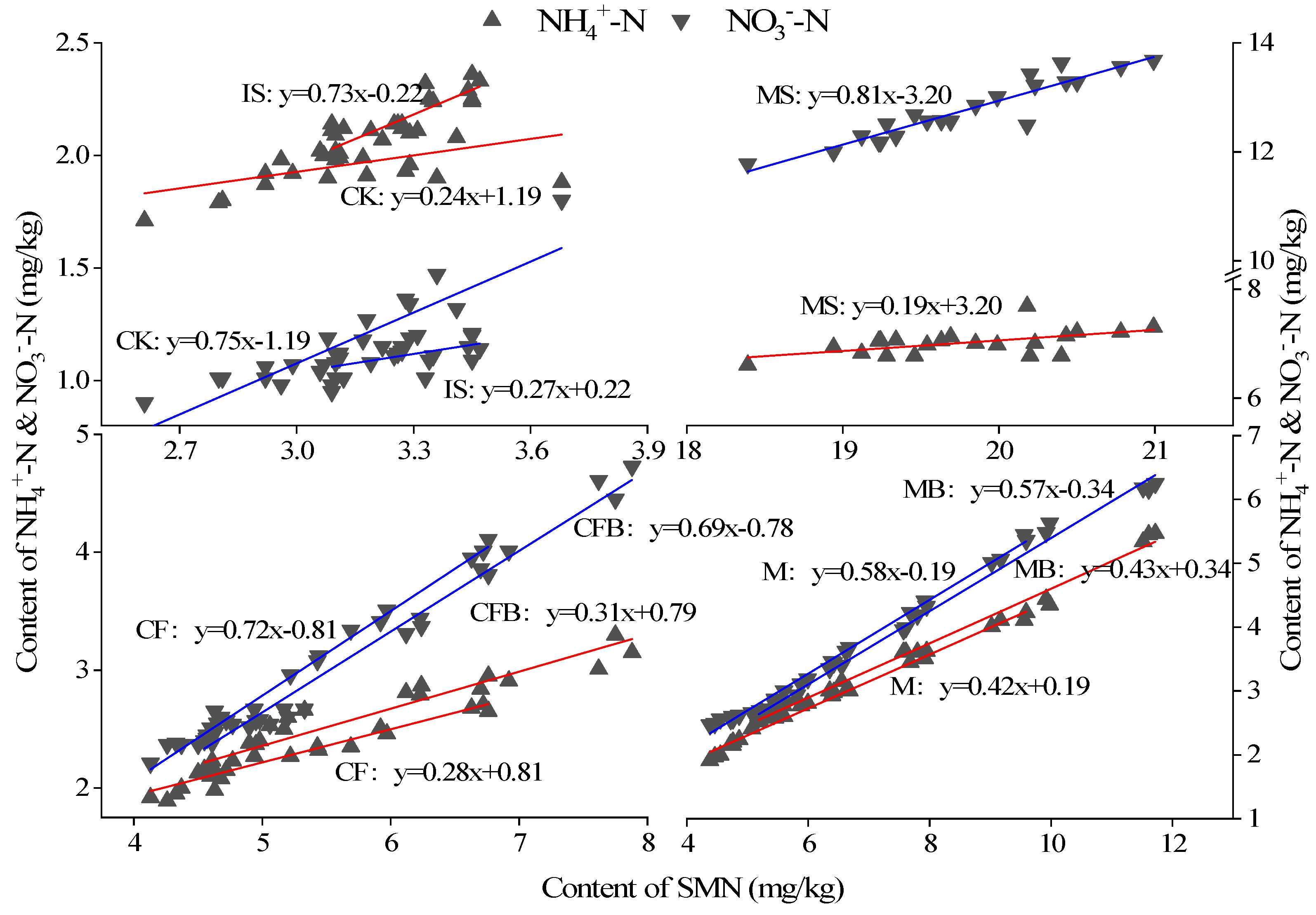
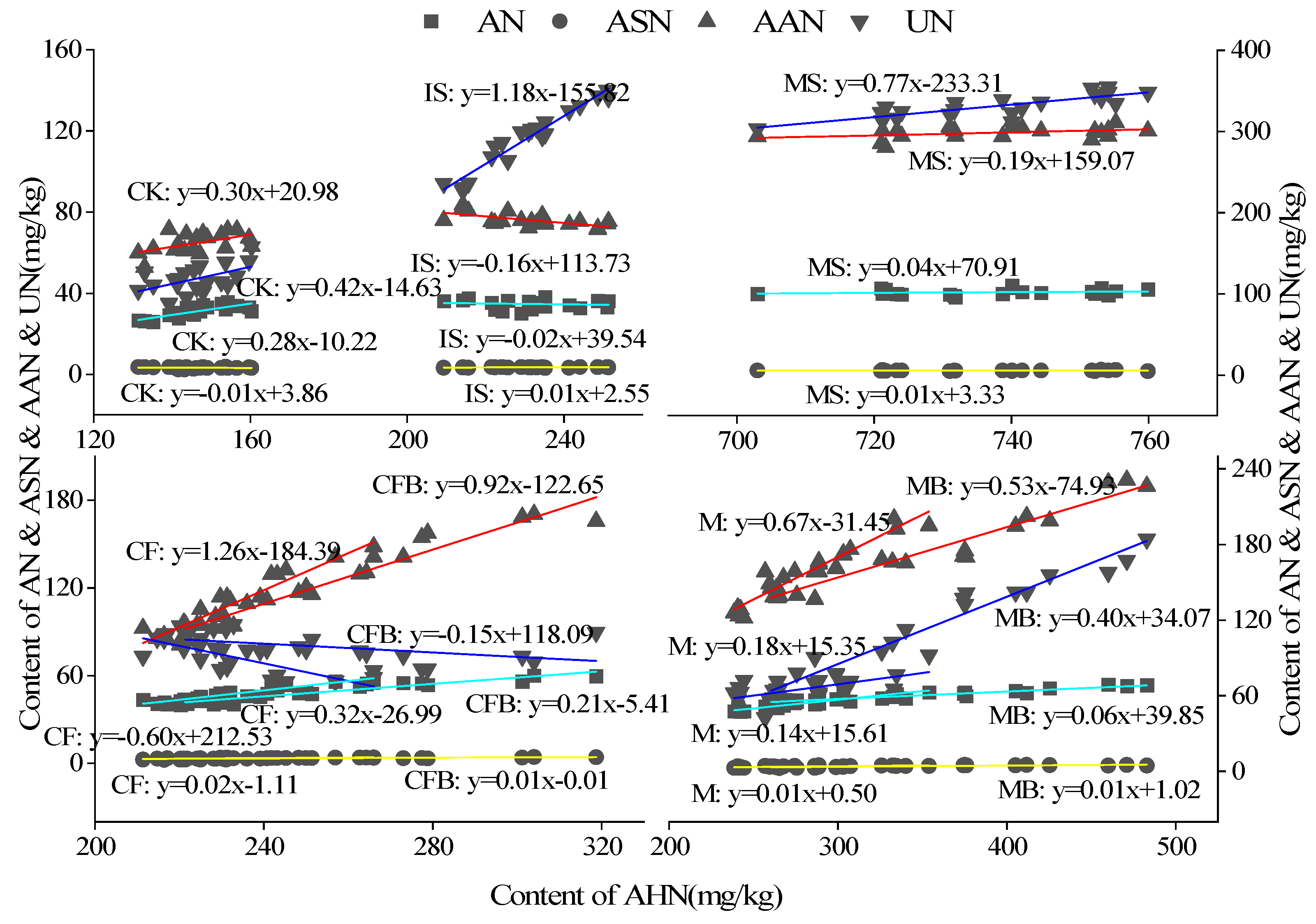
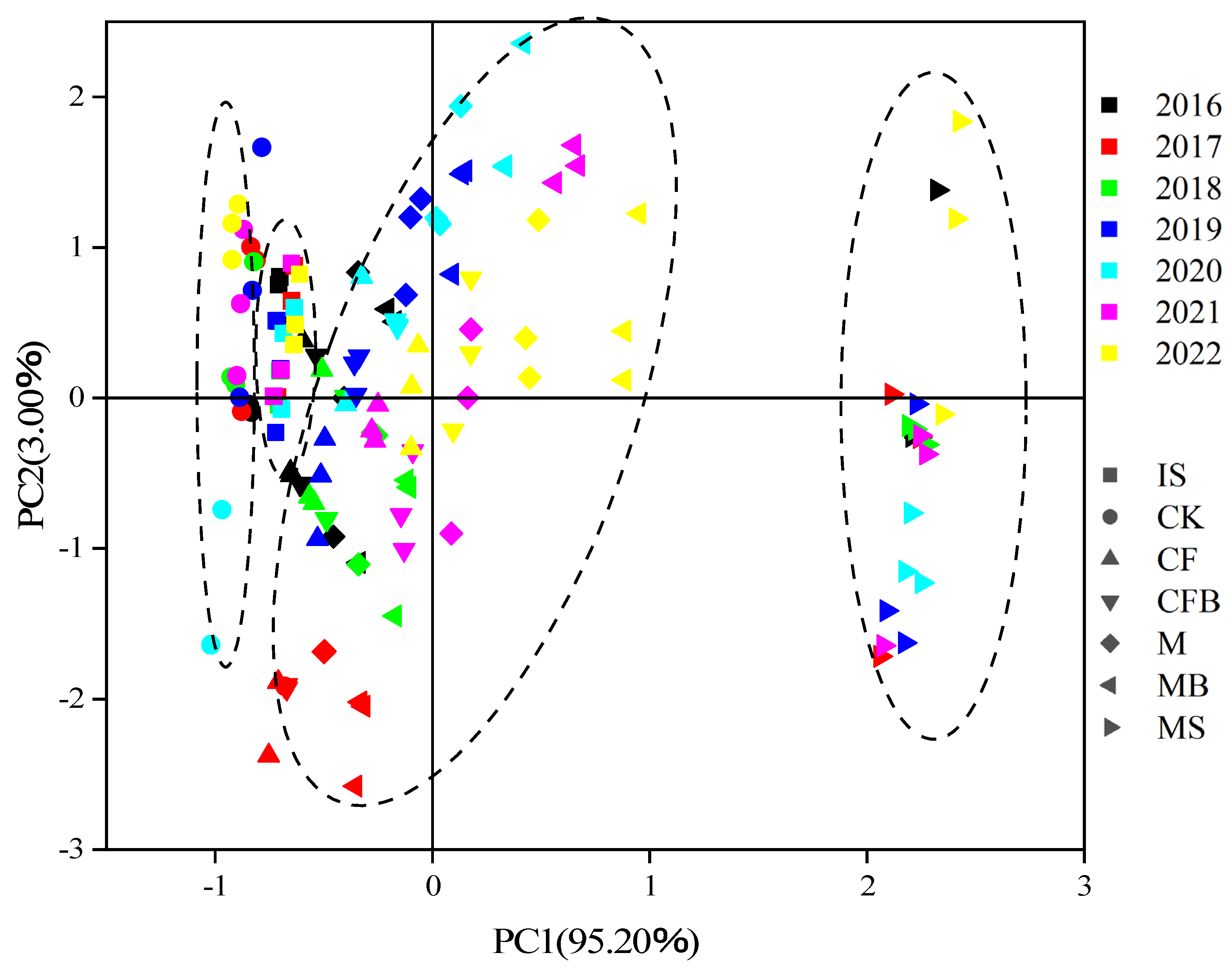
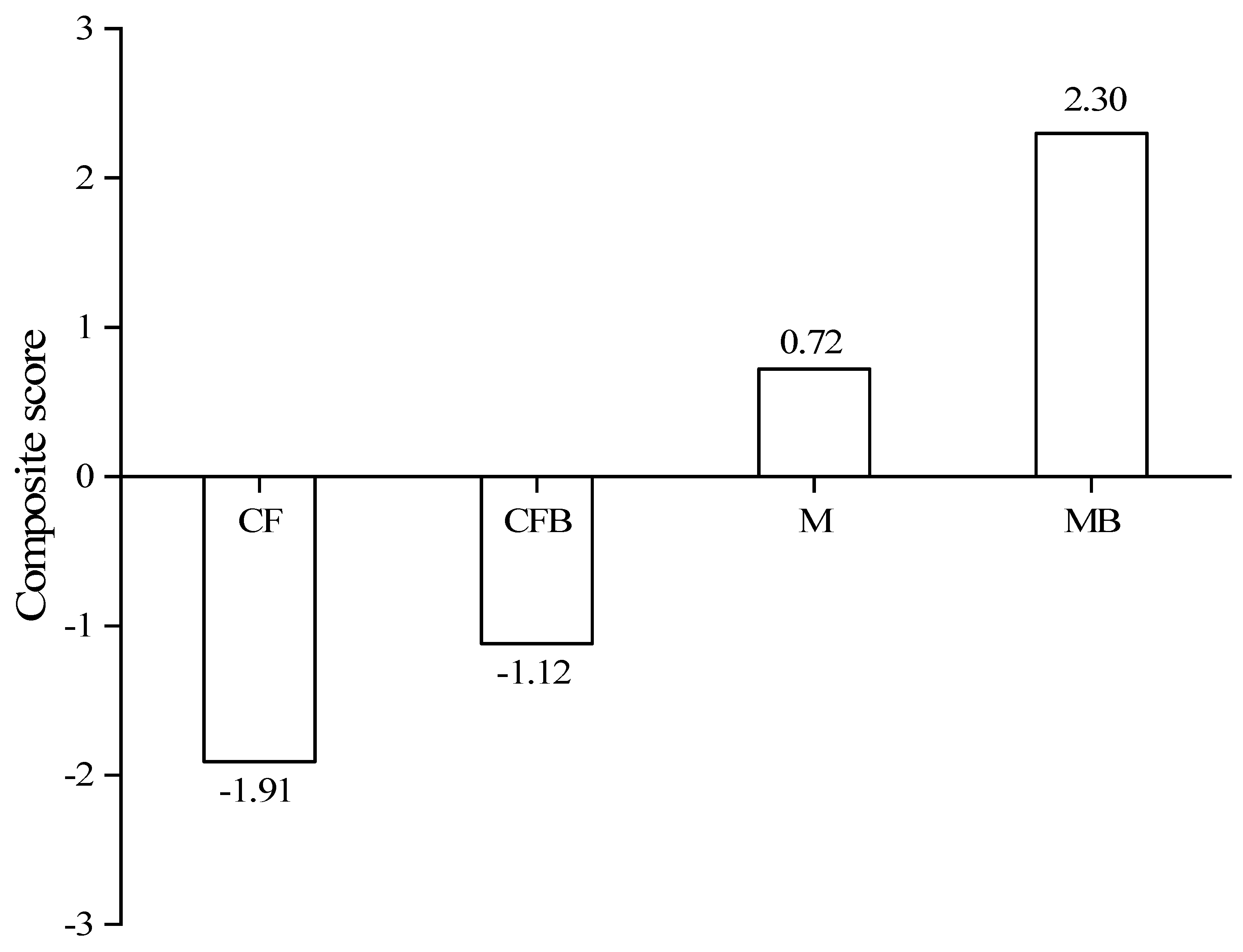
3.4. Principal Component Analysis and Regression Analysis of Nitrogen in Reclaimed Soil
4. Discussion
4.1. Effect of Pseudomonas fluorescens on Soil Nitrogen Accumulation
4.1. Effect of Pseudomonas fluorescens on Soil Nitrogen Accumulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guofa Wang; Shijun Li; Jinhu Zhan; Yihui Wang; Peipei Chen; Guifeng Chen; Yibo Du. Ensuring the safety of coal industry to lay the cornerstone of energy security. China Coal. 2022, 48, 1-9. [CrossRef]
- Zhaorui Jing; Jinman Wang; Yucheng Zhu; Yu Feng. Effects of land subsidence resulted from coal mining on soil nutrient distributions in a loess area of China. Journal of Cleaner Production. 2018, 177, 350-361. [CrossRef]
- Jitendra Ahirwal; Subodh Kumar Maiti. Assessment of soil properties of different land uses generated due to surface coal mining activities in tropical Sal (Shorea robusta) forest, India. Catena. 2016, 140, 155-163. [CrossRef]
- Yanjun Guan; Wei Zhou; Zhongke Bai; Yingui Cao; Yuhan Huang; Hongyuan Huang. Soil nutrient variations among different land use types after reclamation in the Pingshuo opencast coal mine on the Loess Plateau, China. Catena. 2020, 188, 104427. [CrossRef]
- Daniel L. Mummey; Peter D. Stahl; Jeffrey S. Buyer. Soil microbiological properties 20 years after surface mine reclamation: spatial analysis of reclaimed and undisturbed sites. Soil Biology and Biochemistry. 2002, 11, 1717-1725. [CrossRef]
- Xiangying Wang; Yi Li; Ying Wei; Huisheng Meng; Yanzhuan Cao; J.R. Lead; Jianping Hong. Effects of fertilization and reclamation time on soil bacterial communities in coal mining subsidence areas. Science of The Total Environment. 2020. 739, 139882. [CrossRef]
- G. K. Ganjegunte; A. F. Wick; P. D. Stahl; G. F. Vance. Accumulation and composition of total organic carbon in reclaimed coal mine lands. Land Degradation and Development. 2009. 20, 156-175. [CrossRef]
- Ye Yuan; Zhongqiu Zhao; Shuye Niu; Xuezhen Li; Yangyang Wang; Zhongke Bai. Reclamation promotes the succession of the soil and vegetation in opencast coal mine: A case study from Robinia pseudoacacia reclaimed forests, Pingshuo mine, China. Catena. 2018. 165, 72-79. [CrossRef]
- Pengfei Dang; Congfeng Li; Tiantian Huang; Chen Lu; Yajun Li; Xiaoliang Qin; Kadambot H.M.Diddique. Effects of different continuous fertilizer managements on soil total nitrogen stocks in China: A meta-analysis. Pedosphere. 2022. 32(1), 39-48. [CrossRef]
- Xue Tian; Chong Zhang; Xiaotang Ju. Crop responses to application of optimum nitrogen fertilizers on soils of various fertilities formed from long-term fertilization regimes. European Journal of Agronomy. 2023. 148, 126857. [CrossRef]
- S. S. Malhi; R. Lemke. Tillage, crop residue and N fertilizer effects on crop yield, nutrient uptake, soil quality and nitrous oxide gas emissions in a second 4-yr rotation cycle. Soil and Tillage Research. 2007. 96, 269-283. [CrossRef]
- Kinfe Tekulu; Gebeyehu Taye; Dereje Assefa. Effect of starter nitrogen and phosphorus fertilizer rates on yield and yield components, grain protein content of groundnut (Arachis Hypogaea L.) and residual soil nitrogen content in a semiarid north Ethiopia. Heliyon. 2020. 10, e05101. [CrossRef]
- Harald Cederlund; Ella Wessén; Karin Enwall; Christopher M. Jones; Jaanis Juhanson; Mikael Pell; Laurent Philippot; Sara Hallin. Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Applied Soil Ecology. 2014. 84, 62-68. [CrossRef]
- Koen Willekens; Bart Vandecasteele; David Buchan; Stefaan De Neve. Soil quality is positively affected by reduced tillage and compost in an intensive vegetable cropping system. Applied Soil Ecology. 2014. 82, 61-71. [CrossRef]
- Julien Verzeaux; Abdelrahman Alahmad; Hazzar Habbib; Elodie Nivelle; David Roger; Jérôme Lacoux; Guillaume Decocq; Bertrand Hirel; Manuella Catterou; Fabien Spicher; Frédéric Dubois; Jérôme Duclercq; Thierry Tetu. Cover crops prevent the deleterious effect of nitrogen fertilisation on bacterial diversity by maintaining the carbon content of ploughed soil. Geoderma. 2016. 281, 49-57. [CrossRef]
- Benjamin Leon Bodirsky; Alexander Popp; Hermann Lotze-Campen; Jan Philipp Dietrich; Susanne Rolinski; Isabelle Weindl; Christoph Schmitz; Christoph Müller; Markus Bonsch; Florian Humpenöder; Anne Biewald; Miodrag Stevanovic. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nature Communications. 2014. 5, 3858. [CrossRef]
- William Battye; Viney P. Aneja; Willaim H. Schlesinger. Is nitrogen the next carbon? Earth’s Future. 2017. 9, 894-904. [CrossRef]
- Huiqiao Wang; Weifeng Chen; Fupeng Song; Yuanjie Dong. Effects of Nitrogen Fixing Bacteria on Growth of Maize and Nutrient Characteristics of Coastal Saline Soil. Chinese Journal of Soil Science, 2018. 49, 1341-1347. [CrossRef]
- Sharon Nagpal; Poonam Sharma; Asmita Sirari; K.C. Kumawat; Leela Wati; S.C. Gupta; Kamalpreet Singh Mandahal. Chickpea (Cicer arietinum L.) as model legume for decoding the co-existence of Pseudomonas fluorescens and Mesorhizobium sp. as bio-fertilizer under diverse agro-climatic zones. Microbiological Research. 2021. 247, 126720. [CrossRef]
- Ashutosh Upadhyay; Sheela Srivastava. Phenazine-1-carboxylic acid is a more important contributor to biocontrol Fusarium oxysporum than pyrrolnitrin in Pseudomonas fluorescens strain Psd. Microbiological Research. 2011. 166, 323-335. [CrossRef]
- Maurhofer M.; Keel C.; Haas D.; Défago G.. Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. European Journal of Plant Pathology. 1994. 100, 221-232. [CrossRef]
- Mohamad Sharifazizi; Behrouz Harighi; Amin Sadeghi. Evaluation of biological control of Erwinia amylovora, causal agent of fire blight disease of pear by antagonistic bacteria. Biological Control. 2017. 104, 28-34. [CrossRef]
- Fatemeh Jamali; Abbas Sharifi-Tehrani; Matthias P. Lutz; Monika Maurhofer. Influence of Host Plant Genotype, Presence of a Pathogen, and Coinoculation with Pseudomonas fluorescens Strains on the Rhizosphere Expression of Hydrogen Cyanide- and 2,4-Diacetylphloroglucinol Biosynthetic Genes in P. fluorescens Biocontrol Strain CHA0. Microbial Ecology, 2009, 57, 267-275. [CrossRef]
- Cheryl L. Patten; Bernard R. Glick. Bacterial biosynthesis of indole-3-acetic acid. Canadian Journal of Microbiology. 1996. 42, 207-220. [CrossRef]
- Yu Feng; Jinman Wang; Zhongke Bai; Lucy Reading. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Science Reviews. 2019. 191, 12-25. [CrossRef]
- Wenyao Zhang; Yihang Chen; Keyang Huang; Feng Wang, Ziqing Mei. Molecular Mechanism and Agricultural Application of the NifA-NifL System for Nitrogen Fixation. International Journal of Molecular Sciences. 2023. 24, 907. [CrossRef]
- S. Sivasakthi; G. Usharani; P. Saranraj. Biocontrol potentiality of Plant growth promoting rhizobacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A Review. African journal of microbiology research. 2014. 9, 1265-1277. [CrossRef]
- Lixiu Zhou; Wei Liu; Huijie Duan; Haiwen Dong; Jingchao Li; Shuxi Zhang; Jing Zhang; Shigang Ding; Tongtong Xu; Beibei Guo. Improved effects of combined application of nitrogen-fixing bacteria Azotobacter beijerinckii and microalgae Chlorella pyrenoidosa on wheat growth and saline-alkali soil quality. Chemosphere. 2023. 313, 137409. [CrossRef]
- Kainan Wang; Jinju Hou; Shudong Zhang; Wenjin Hu; Guanwen Yi; Wenjie Chen; Lei Cheng; Qiuzhuo Zhang. Preparation of a new biochar-based microbial fertilizer: Nutrient release patterns and synergistic mechanisms to improve soil fertility. Science of The Total Environment. 2023. 860, 160478. [CrossRef]
- Baizhao Ren; Zhentao Ma; Yanqing Guo; Peng Liu; Bin Zhao; Jiwang Zhang. Applying urea ammonium nitrate solution saves nitrogen resources by changing soil microbial composition under micro sprinkling fertigation: an effective nitrogen management practice. Field Crops Research. 2023. 302, 109087. [CrossRef]
- M. J. Glendining; D. S. Powlson; P. R. Poulton; N. J. Bradbury; D. Palazzo; X. Li. The effects of long-term applications of inorganic nitrogen fertilizer on soil nitrogen in the Broadbalk Wheat Experiment. The Journal of Agricultural Science. 1996. 127, 347-363. [CrossRef]
- G. Bengtsson; P. Bengtson; Katarina F; Månsson. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biology and Biochemistry. 2003. 35, 143-154. [CrossRef]
- Ho-Young Kwon; Robert J. M. Hudson; Richard L. Mulvaney. Characterization of the Organic Nitrogen Fraction Determined by the Illinois Soil Nitrogen Test. Soil Science Society of America Journal. 2009. 73, 1033-1043. [CrossRef]
- Peng Gao; Tuo Zhang; Xingyu Lei; Xinwei Cui; Yaoxiong Lu; Pengfei Fan; Shiping Long; Jing Huang; Jusheng Gao; Zhenhua Zhang; Huimin Zhang. Improvement of soil fertility and rice yield after long-term application of cow manure combined with inorganic fertilizers. Journal of Integrative Agriculture. 2023. 22, 2221-2232. [CrossRef]
- Georgia Ntatsi, Anestis Karkanis, Dionisios Yfantopoulos, Valentini Pappa, Inara Helena Konosonoka, Ilias Travlos, Dimitrios Bilalis, Penelope Bebeli, Dimitrios Savvas. Evaluation of the field performance, nitrogen fixation efficiency and competitive ability of pea landraces grown under organic and conventional farming systems. Archives of Agronomy and Soil Science. 2019. 65, 294-307. [CrossRef]
- Peng Wang; Lan Yang; Xichao Sun; Wenjun Shi; Rui Dong; Yuanhua Wu; Guohua Mi. Lateral root elongation in maize is related to auxin synthesis and transportation mediated by N metabolism under a mixed NO3- and NH4+ supply. Journal of Integrative Agriculture. 2023. [CrossRef]
- R. K. Cunningham; G. W. Cooke. Soil nitrogen. II.—Changes in levels of inorganic nitrogen in a clay-loam soil caused by fertilizer additions, by leaching and uptake by grass. Journal of the Science of Food & Agriculture. 2010. 9. [CrossRef]
- Yu Xie, Cheng Yang, Erdeng Ma, Hao Tan, Tongbin Zhu, Christoph Müller. Biochar stimulates NH4+ turnover while decreasing NO3− production and N2O emissions in soils under long-term vegetable cultivation. Science of The Total Environment. 2020. 737, 140266. [CrossRef]
- K. M. Goh; D. C. Edmeades. Distribution and partial characterisation of acid hydrolysable organic nitrogen in six New Zealand soils. Soil Biology & Biochemistry. 1997. 11, 127-132. [CrossRef]
- Huijie Lü; Hongbo He; Jinsong Zhao; Wei Zhang; Hongtu Xie; Guoqing Hu; Xiao Liu; Yeye Wu; Xudong Zhang. Dynamics of fertilizer-derived organic nitrogen fractions in an arable soil during a growing season. Plant Soil. 2013. 373, 595-607. [CrossRef]
- W. Amelung, X. Zhang. Determination of amino acid enantiomers in soils. Soil Biology & Biochemistry. 2010. 33, 553-562. [CrossRef]
- JingShu Wang; J. Ryan Stewart; Saeed A. Khan; Jeffrey O. Dawson. Elevated amino sugar nitrogen concentrations in soils: a potential method for assessing N fertility enhancement by actinorhizal plants. Symbiosis. 2010. 50, 71-76. [CrossRef]
- Shengxiu Li; Zhaohui Wang; Yanfang Miao; Shiqing Li. Soil Organic Nitrogen and Its Contribution to Crop Production. Journal of Integrative Agriculture. 2014. 13, 2061-2080. [CrossRef]
- Jin Wang, Shunyao Zhuang, Zhaoliang Zhu. Soil organic nitrogen composition and mineralization of paddy soils in a cultivation chronosequence in China. Journal of soil & sediments. 2017. 17, 1588-1598. [CrossRef]
| Treatment | Urea | Superphosphate | potassium chloride | organic fertilizer | Pseudomonas fluorescens | LB culture medium | corn |
|---|---|---|---|---|---|---|---|
| IS | × | ||||||
| CK | √ | ||||||
| MS | 8000 | √ | |||||
| CF | 378 | 855 | 170 | 750 | √ | ||
| CFB | 378 | 855 | 170 | 750 | √ | ||
| M | 12000 | 750 | √ | ||||
| MB | 12000 | 750 | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




