Submitted:
17 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
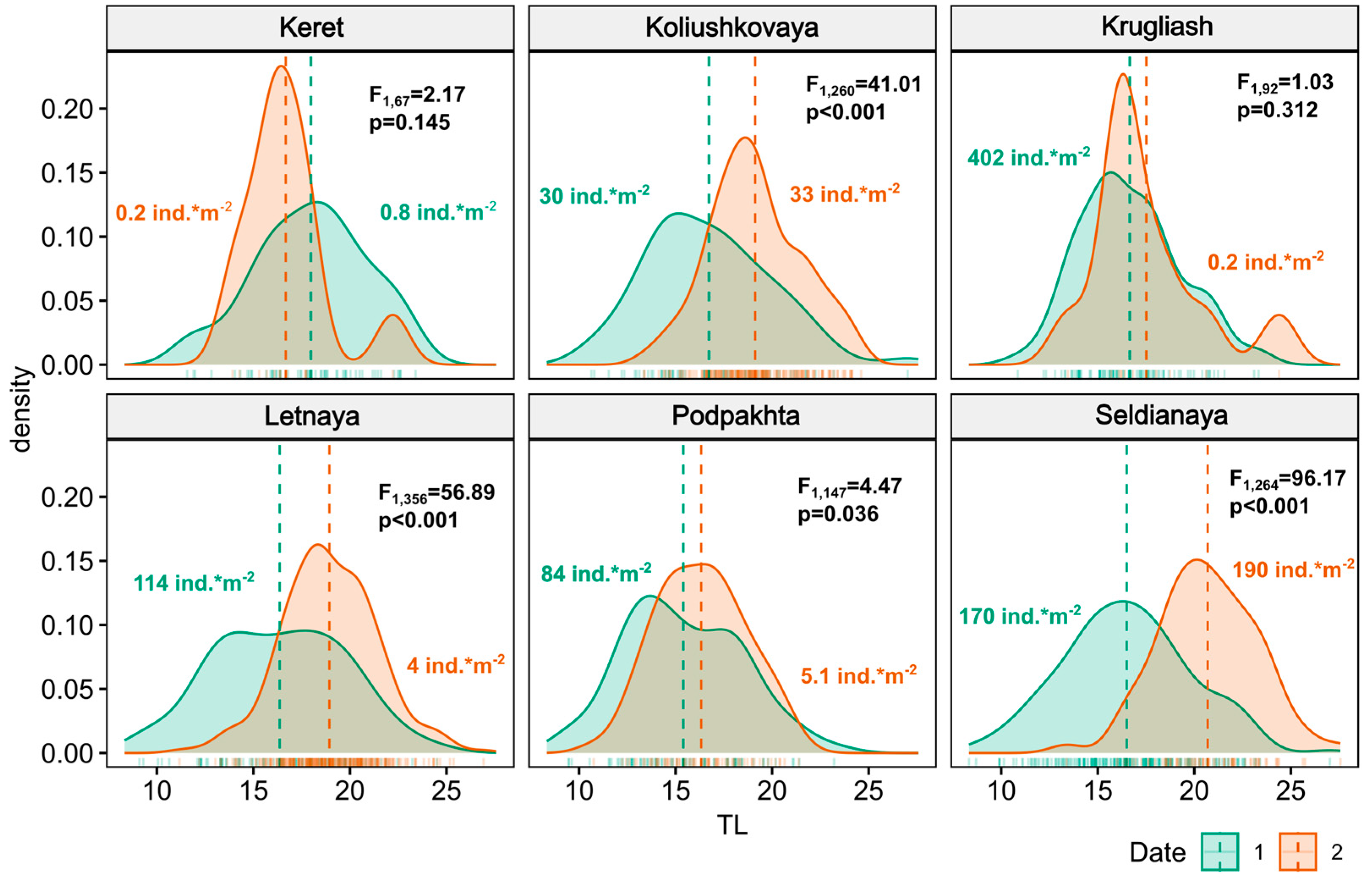
3.1. Size Structure and Abundance of G. aculeatus Juveniles
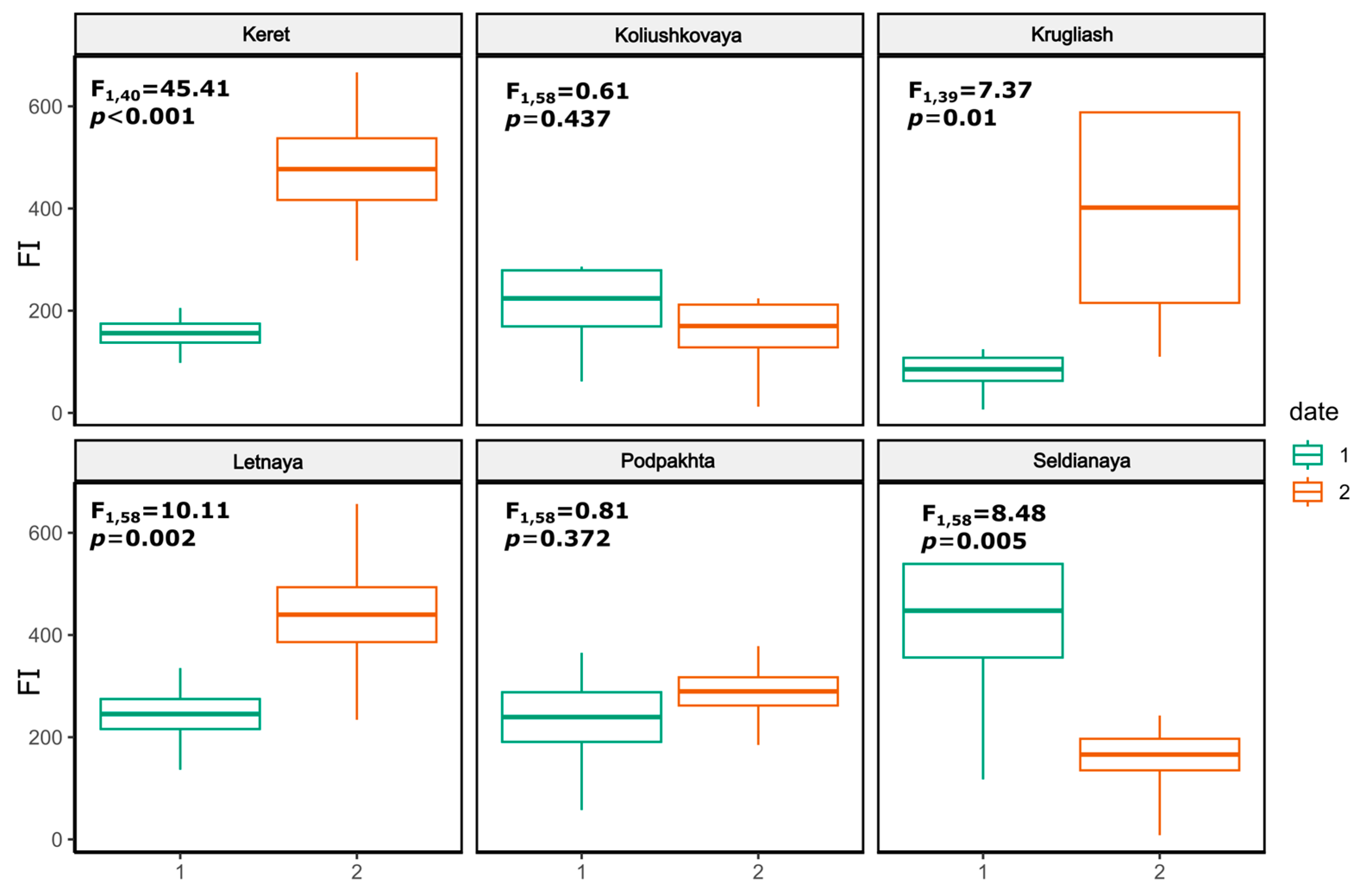
3.2. Feeding Intensity of G. aculeatus
3.3. Stomach Content Analysis
3.4. Food Resources: Zooplankton
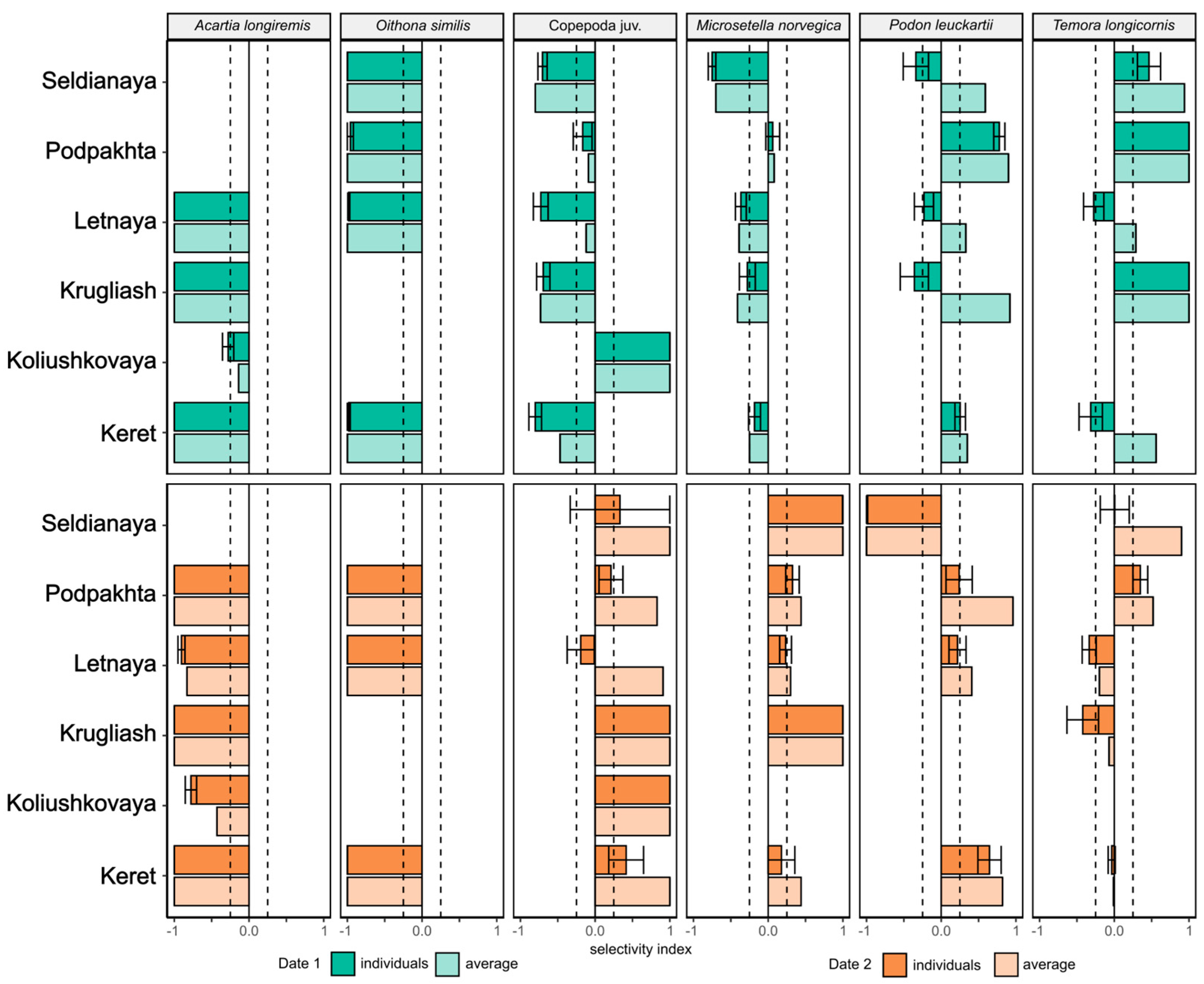
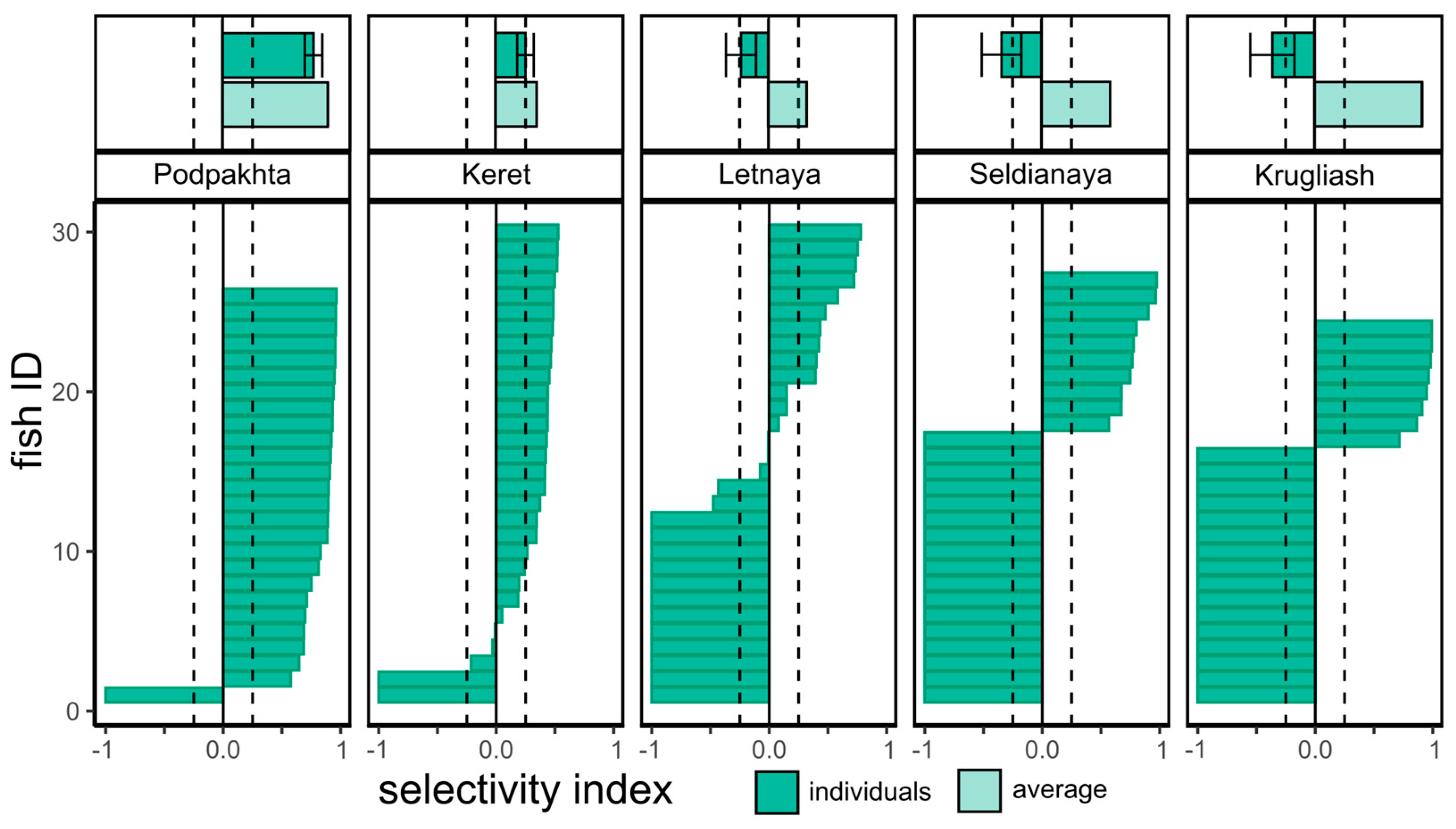
3.5. Food Selectivity
4. Discussion
4.1. August Planktonic Food Resources of Juvenile Sticklebacks
4.2. Size Structure and Abundance of Juvenile Sticklebacks at Nursery Grounds
4.3. Feeding Intensity in G. aculeatus Juveniles
4.4. Stomach Content in Juvenile G. aculeatus
4.5. Prey Selectivity by Juvenile Sticklebacks in the White Sea
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van De Wolfshaar, K.E.; Barbut, L.; Lacroix, G. From Spawning to First-Year Recruitment: The Fate of Juvenile Sole Growth and Survival under Future Climate Conditions in the North Sea. ICES J. Mar. Sci. 2022, 79, 495–505. [Google Scholar] [CrossRef]
- Doyle, M.J.; Strom, S.L.; Coyle, K.O.; Hermann, A.J.; Ladd, C.; Matarese, A.C.; Shotwell, S.K.; Hopcroft, R.R. Early Life History Phenology among Gulf of Alaska Fish Species: Strategies, Synchronies, and Sensitivities. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2019, 165, 41–73. [Google Scholar] [CrossRef]
- Robert, D.; Shoji, J.; Sirois, P.; Takasuka, A.; Catalán, I.A.; Folkvord, A.; Ludsin, S.A.; Peck, M.A.; Sponaugle, S.; Ayón, P.M.; et al. Life in the Fast Lane: Revisiting the Fast Growth—High Survival Paradigm during the Early Life Stages of Fishes. Fish Fish. 2023, 24, 863–888. [Google Scholar] [CrossRef]
- Westrelin, S.; Balzani, P.; Haubrock, P.J.; Santoul, F. Interannual Variability in the Trophic Niche of Young-of-year Fish Belonging to Four Piscivorous Species Coexisting in a Natural Lake. Freshw. Biol. 2023, 68, 487–501. [Google Scholar] [CrossRef]
- Wells, R.A.; McNeil, W.J. Effect of Quality of the Spawning Bed on Growth and Development of Pink Salmon Embryos and Alevins; US Department of the Interior, Bureau of Commercial Fisheries, 1970;
- Van Deurs, M.; Persson, A.; Nilsson, P.A.; Jørgensen, C. Fish Resist Temptation from Junk Food: State-dependent Diet Choice in Reproductive Atlantic Cod Gadus Morhua Facing Seasonal Fluxes of Lipid-rich Prey. Oikos 2022, 2022, oik–08739. [Google Scholar] [CrossRef]
- Byström, P.; Persson, L.; Wahlström, E. COMPETING PREDATORS AND PREY: JUVENILE BOTTLENECKS IN WHOLE-LAKE EXPERIMENTS. Ecology 1998, 79, 2153–2167. [Google Scholar] [CrossRef]
- Okun, N.; Mehner, T. Distribution and Feeding of Juvenile Fish on Invertebrates in Littoral Reed (Phragmites) Stands. Ecol. Freshw. Fish 2005, 14, 139–149. [Google Scholar] [CrossRef]
- Lajus, D.L.; Alekseeva, Y.I.; Lajus, J.A. Herring Fisheries in the White Sea in the 18th–Beginning of the 20th Centuries: Spatial and Temporal Patterns and Factors Affecting the Catch Fluctuations. Fish. Res. 2007, 87, 255–259. [Google Scholar] [CrossRef]
- Evseenko, S.A.; Mishin, A.V. On the Distribution of Larvae and Localization of Spawning Stocks of White Sea Herring Clupea Pallasii Marisalbi. J. Ichthyol. 2011, 51, 776–787. [Google Scholar] [CrossRef]
- Semenova, A.V.; Stroganov, A.N.; Ponomareva, E.V.; Afanas’ev, K.I.; Vilkina, O.V. Large-Scale Genetic Structure and Diversity of Arctic Rainbow Smelt Osmerus Dentex Steindachner et Kner, 1870 throughout Its Distributional Range Based on Microsatellites. Polar Biol 2021, 44, 927–940. [Google Scholar] [CrossRef]
- Lajus, D.; Ivanova, T.; Rybkina, E.; Lajus, J.; Ivanov, M. Multidecadal Fluctuations of Threespine Stickleback in the White Sea and Their Correlation with Temperature. ICES J. Mar. Sci. 2021, 78, 653–665. [Google Scholar] [CrossRef]
- Cusa, M.; Berge, J.; Varpe, Ø. Seasonal Shifts in Feeding Patterns: Individual and Population Realized Specialization in a High Arctic Fish. Ecol. Evol. 2019, 9, 11112–11121. [Google Scholar] [CrossRef] [PubMed]
- Hure, M.; Mustać, B. Feeding Ecology of Sardina Pilchardus Considering Co-Occurring Small Pelagic Fish in the Eastern Adriatic Sea. Mar. Biodivers. 2020, 50, 40. [Google Scholar] [CrossRef]
- Kingsford, M.J. Drift Algae and Small Fish in Coastal Waters of Northeastern New Zealand. Mar. Ecol. Prog. Ser. 1992, 41–55. [Google Scholar] [CrossRef]
- Hoch, J.M.; Cabanelas Bermudez, A.; Coury, O.S.; Donahou, A.S.; Jeffers, C.N.; LaMartina, M.; Ramsaran, D.; Spadafore, S. The Influence of Personality on Small Fish Migration and Dispersal in the Everglades. Wetlands 2019, 39, 991–1002. [Google Scholar] [CrossRef]
- Lajus, D.L.; Golovin, P.V.; Zelenskaia, A.E.; Demchuk, A.S.; Dorgham, A.S.; Ivanov, M.V.; Ivanova, T.S.; Murzina, S.A.; Polyakova, N.V.; Rybkina, E.V. Threespine Stickleback of the White Sea: Population Characteristics and Role in the Ecosystem. Contemp. Probl. Ecol. 2020, 13, 132–145. [Google Scholar] [CrossRef]
- Genelt-Yanovskaya, A.S.; Polyakova, N.V.; Ivanov, M.V.; Nadtochii, E.V.; Ivanova, T.S.; Genelt-Yanovskiy, E.A.; Tiunov, A.V.; Lajus, D.L. Tracing the Food Web of Changing Arctic Ocean: Trophic Status of Highly Abundant Fish, Gasterosteus Aculeatus (L.), in the White Sea Recovered Using Stomach Content and Stable Isotope Analyses. Diversity 2022, 14, 955. [Google Scholar] [CrossRef]
- Jakubavičiūtė, E.; Casini, M.; Ložys, L.; Olsson, J. Seasonal Dynamics in the Diet of Pelagic Fish Species in the Southwest Baltic Proper. ICES J. Mar. Sci. 2016, 74, 750–758. [Google Scholar] [CrossRef]
- Demchuk, A.S.; Uspenskiy, A.A.; Golubkov, S.M. Abundance and Feeding of Fish in the Coastal Zone of the Neva Estuary, Eastern Gulf of Finland. Boreal Environ. Res. 2021, 26, 1. [Google Scholar]
- Demchuk, A.; Ivanov, M.; Ivanova, T.; Polyakova, N.; Mas-Martí, E.; Lajus, D. Feeding Patterns in Seagrass Beds of Three-Spined Stickleback Gasterosteus Aculeatus Juveniles at Different Growth Stages. J. Mar. Biol. Assoc. United Kingd. 2015, 95, 1635–1643. [Google Scholar] [CrossRef]
- Ivanova, T.S.; Ivanov, M.V.; Golovin, P.V.; Polyakova, N.V.; Lajus, D.L. The White Sea Threespine Stickleback Population: Spawning Habitats, Mortality, and Abundance. Evol. Ecol. Res. 2016, 17, 301–315. [Google Scholar]
- Amundsen, P.; Sánchez-Hernández, J. Feeding Studies Take Guts – Critical Review and Recommendations of Methods for Stomach Contents Analysis in Fish. J. Fish Biol. 2019, 95, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, E.J. Stomach Contents Analysis—a Review of Methods and Their Application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Ivlev, V.S.
- Wootton, R.J. A Functional Biology of Sticklebacks; Calow, P., Ed.; 1st ed.; Croom Helm, 1984;
- Zeng, H.; Tang, F.; Li, Z.; Lu, W.; Zheng, Y. Feeding Selectivity and Diet Shift of Protosalanx Chinensis during Spring in Lake Dalong, Northeastern China. Water 2023, 15, 1847. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. Past: Paleontological Statistics Software Package for Educaton and Data Anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Кoсoбoкoва, К.Н.; Перцoва, Н.М. Зooпланктoн Белoгo Мoря: Структура, Динамика И Экoлoгия Сooбществ. Система Белoгo Мoря. Вoдная Тoлща И Взаимoдействующие С Ней Атмoсфера, Криoсфера, Речнoй Стoк И Биoсфера 2012, 640–675.
- Бек, Т.А. Прибрежная Зoна в Экoсистеме Белoгo Мoря. Журн. oбщ. биoл. 1990, 51, 116–124. [Google Scholar]
- Бек, Т.А.; Калякина, Н.М. Общая Характеристика Литoрали. Серия Исследoвание фауны мoрей. Белoе мoре. Биoлoгические ресурсы и прoблемы их рациoнальнoгo испoльзoвания. Ч.1. 1995, 42, 197–212. [Google Scholar]
- Прыгункoва, Р.В. Различия в Смене Биoлoгических Сезoнoв в Планктoне Губы Чупа (Белoе Мoре) в Разные Гoды. Исследoвания фауны мoрей 1985, 31, 99–108. [Google Scholar]
- Lazzari, M.A.; Sherman, S.; Kanwit, J.K. Nursery Use of Shallow Habitats by Epibenthic Fishes in Maine Nearshore Waters. Estuar. Coast. Shelf Sci. 2003, 56, 73–84. [Google Scholar] [CrossRef]
- Lazzari, M.A.; Stone, B.Z. Use of Submerged Aquatic Vegetation as Habitat by Young-of-the-Year Epibenthic Fishes in Shallow Maine Nearshore Waters. Estuar. Coast. Shelf Sci. 2006, 69, 591–606. [Google Scholar] [CrossRef]
- Зарубина, Е.Ю.; Ермoлаева, Н.И. Сезoнная Динамика Макрoфитoв и Зooпланктoна Литoральнoй Зoны Нoвoсибирскoгo Вoдoхранилища в 2013 г. Междунарoдный журнал прикладных и фундаментальных исследoваний 2014.
- Семенченкo, В.П.; Разлуцкий, В.И.; Бусева, Ж.Ф.; Палаш, А.Л. Зooпланктoн Литoральнoй Зoны Озер Разнoгo Типа; Беларус. наука, 2013;
- Rybkina, E.V.; Ivanova, T.S.; Ivanov, M.V.; Kucheryavyy, A.V.; Lajus, D.L. Habitat Preference of Three-Spined Stickleback Juveniles in Experimental Conditions and in Wild Eelgrass. J. Mar. Biol. Assoc. United Kingd. 2017, 97, 1437–1445. [Google Scholar] [CrossRef]
- Jackson, E.L.; Attrill, M.J.; Rowden, A.A.; Jones, M.B. Seagrass Complexity Hierarchies: Influence on Fish Groups around the Coast of Jersey (English Channel). J. Exp. Mar. Biol. Ecol. 2006, 330, 38–54. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fong, C.W.; Wu, R.S.S. The Effects of Seagrass (Zostera Japonica) Canopy Structure on Associated Fauna: A Study Using Artificial Seagrass Units and Sampling of Natural Beds. J. Exp. Mar. Biol. Ecol. 2001, 259, 23–50. [Google Scholar] [CrossRef]
- Lovell, T. Nutrition and Feeding of Fish; Van Nostrand Reinhold, 1989;
- Neverman, D.; Wurtsbaugh, W.A. The Thermoregulatory Function of Diel Vertical Migration for a Juvenile Fish, Cottus Extensus. Oecologia 1994, 98, 247–256. [Google Scholar] [CrossRef]
- Abdel-Malek, S.A. Threespine Stickleback (Gasterosteus aculeatus L.) Juvenile Feeding in the Kandalaksha Bay of the White Sea. Vopr. Ikhtiologii 1968, 8, 294–302. [Google Scholar]
- Hangelin, C.; Vuorinen, I. Food Selection in Juvenile Three-Spined Sticklebacks Studied in Relation to Size, Abundance and Biomass of Prey. Hydrobiologia 1988, 157, 169–177. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Food of Fresh-Water Sticklebacks (Gasterosteus Aculeatus and Pygosteus Pungitius), with a Review of Methods Used in Studies of the Food of Fishes. J. Anim. Ecol. 1950, 19, 36. [Google Scholar] [CrossRef]
- Berezina, N.A.; Zhgareva, N.N.; Strelnikova, A.P. Feeding Features of the Nine-Spined Stickleback Pungitius Pungitius (Gasterosteidae) In Water Bodies of the North-West of Russia. J. Ichthyol. 2023, 63, 309–318. [Google Scholar] [CrossRef]
- Walkey, M. The Ecology of Neoechinorhynchus Rutili (Muller). J. Parasitol. 1967, 53, 795–804. [Google Scholar] [CrossRef]
- Ziuganov, V.V. Genetics of Osteal Plate Polymorphism and Microevolution of Threespine Stickleback (Gasterosteus Aculeatus L.). Theor. Appl. Genet. 1983, 65, 239–246. [Google Scholar] [CrossRef]
- Helenius, L.K.; Borg, J.P.G.; Nurminen, L.; Leskinen, E.; Lehtonen, H. The Effects of Turbidity on Prey Consumption and Selection of Zooplanktivorous Gasterosteus Aculeatus L. Aquat Ecol 2013, 47, 349–356. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Huntingford, F.A. Laboratory and Field Studies on Diet Choice in Three-Spined Sticklebacks, Gasterosteus Aculeatus L., in Relation to Profitability and Visual Features of Prey. J. Fish Biol. 1989, 34, 245–257. [Google Scholar] [CrossRef]
- Spilseth, S.A.; Simenstad, C.A. Seasonal, Diel, and Landscape Effects on Resource Partitioning between Juvenile Chinook Salmon (Oncorhynchus Tshawytscha) and Threespine Stickleback (Gasterosteus Aculeatus) in the Columbia River Estuary. Estuaries Coasts 2011, 34, 159–171. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hakala, T.; Saesmaa, S.; Viitasalo, M. Prey Selection by the Larvae of Three Species of Littoral Fishes on Natural Zooplankton Assemblages. Aquat Ecol 2007, 41, 85–94. [Google Scholar] [CrossRef]
- Bretzel, J.B.; Geist, J.; Gugele, S.M.; Baer, J.; Brinker, A. Feeding Ecology of Invasive Three-Spined Stickleback (Gasterosteus Aculeatus) in Relation to Native Juvenile Eurasian Perch (Perca Fluviatilis) in the Pelagic Zone of Upper Lake Constance. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Roch, S.; von Ammon, L.; Geist, J.; Brinker, A. Foraging Habits of Invasive Three-Spined Sticklebacks (Gasterosteus Aculeatus) – Impacts on Fisheries Yield in Upper Lake Constance. Fish. Res. 2018, 204, 172–180. [Google Scholar] [CrossRef]
- Campbell, C.E. Prey Selectivities of Threespine Sticklebacks (Gasterosteus Aculeatus) and Phantom Midge Larvae (Chaoborus Spp.) in Newfoundland Lakes. Freshw. Biol. 1991, 25, 155–167. [Google Scholar] [CrossRef]
- Delbeek, J.C.; Williams, D.D. Feeding Selectivity of Four Species of Sympatric Stickleback in Brackish-Water Habitats in Eastern Canada. J. Fish Biol. 1988, 32, 41–62. [Google Scholar] [CrossRef]
- Rick, I.P.; Mehlis, M.; Bakker, T.C.M. Male Red Ornamentation Is Associated with Female Red Sensitivity in Sticklebacks. PLoS ONE 2011, 6, e25554. [Google Scholar] [CrossRef]
- Paffenhofer, G.-A.; Mazzocchi, M.G. On Some Aspects of the Behaviour of Oithona Plumifera (Copepoda: Cyclopoida). J. Plankton Res. 2002, 24, 129–135. [Google Scholar] [CrossRef]
- Koski, M.; Kiørboe, T.; Takahashi, K. Benthic Life in the Pelagic: Aggregate Encounter and Degradation Rates by Pelagic Harpacticoid Copepods. Limnol. Oceanogr. 2005, 50, 1254–1263. [Google Scholar] [CrossRef]
- Van Duren, L.; Videler, J. Swimming Behaviour of Developmental Stages of the Calanoid Copepod Temora Longicornis at Different Food Concentrations. Mar. Ecol. Prog. Ser. 1995, 126, 153–161. [Google Scholar] [CrossRef]
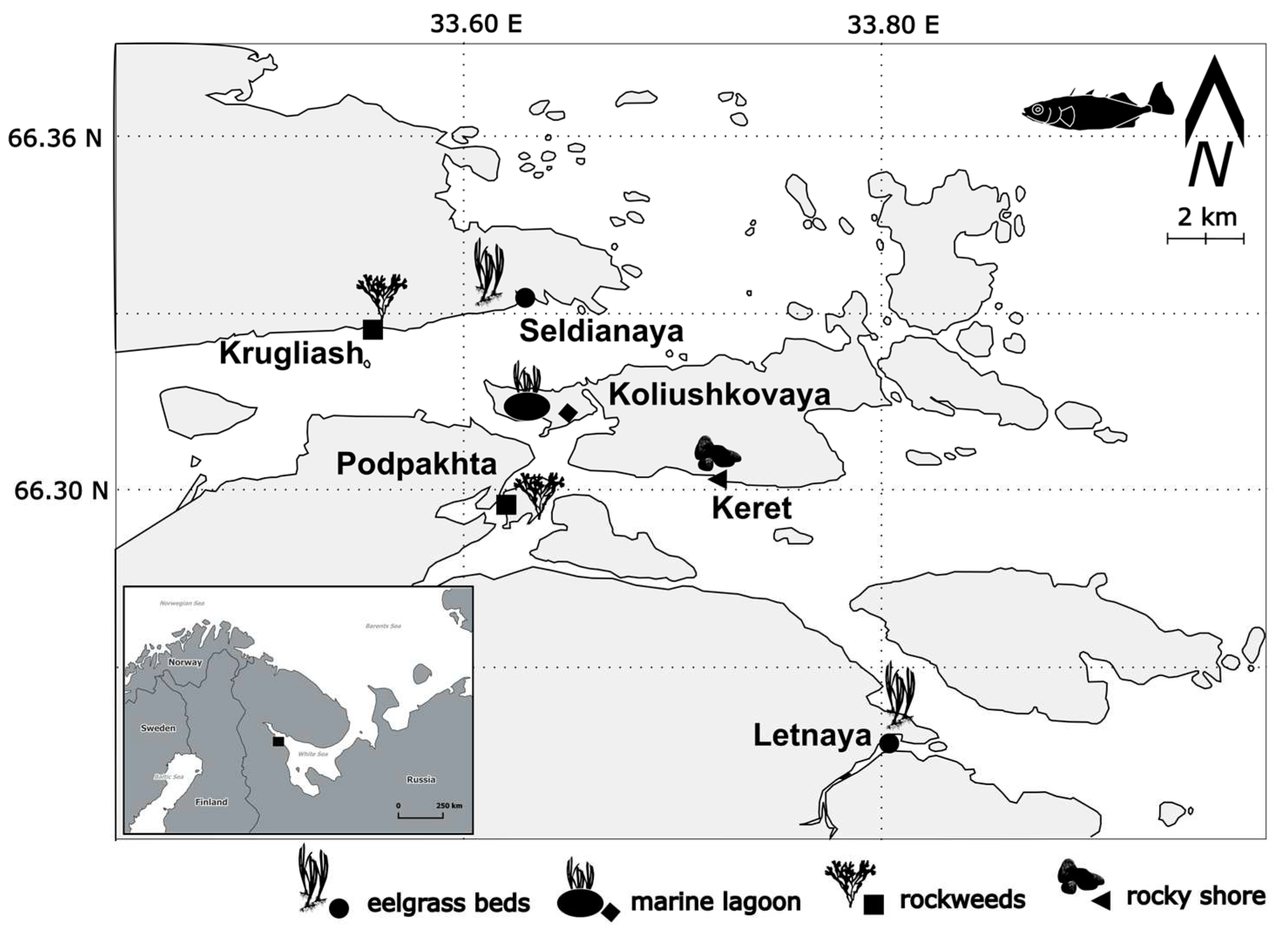

| site | coordinates | intertidal habitat | G.aculeatus sample size, ind. | ||
|---|---|---|---|---|---|
| date 1 | date 2 | ||||
| Keret | N 66.30261 | E 33.69054 | rocky shore | 30 | 12 |
| Krugliash | N 66.33070 | E 33.54565 | rockweeds | 30 | 11 |
| Koliushkovaya | N 66.31335 | E 33.64348 | lagoon, eelgrass bed | 30 | 30 |
| Letnaya | N 66.24456 | E 33.79524 | eelgrass bed | 30 | 30 |
| Podpakhta | N 66.29600 | E 33.61527 | rockweeds | 30 | 30 |
| Seldianaya | N 66.33765 | E 33.62485 | eelgrass bed | 30 | 30 |
| Factor | R-value | p-value | ||
|---|---|---|---|---|
| Date | 0.33 | 0.0001 | ||
| Site | 0.27 | 0.0001 | ||
| Date 1 | 0.38 | 0.0001 | ||
| Date 2 | 0.37 | 0.0001 | ||
| Pairwise, Date 1 | Pairwise, Date 2 | |||
| R-value | p-value | R-value | p-value | |
| Keret-Krugliash | 0.39 | 0.0001 | 0.44 | 0.0001 |
| Keret-Koliushkovaya | 0.73 | 0.0001 | 0.81 | 0.0001 |
| Keret-Letnaya | 0.44 | 0.0001 | 0.1 | 0.0851 |
| Keret-Podpakhta | 0.25 | 0.0001 | 0.13 | 0.0728 |
| Keret-Seldianaya | 0.5 | 0.0001 | 0.51 | 0.0001 |
| Krugliash-Koliushkovaya | 0.53 | 0.0001 | 0.55 | 0.0001 |
| Krugliash-Letnaya | 0.15 | 0.0002 | 0.41 | 0.0001 |
| Krugliash-Podpakhta | 0.12 | 0.0014 | 0.49 | 0.0002 |
| Krugliash-Seldianaya | 0.12 | 0.0005 | 0.5 | 0.0001 |
| Koliushkovaya-Letnaya | 0.75 | 0.0001 | 0.52 | 0.0001 |
| Koliushkovaya-Podpakhta | 0.57 | 0.0001 | 0.61 | 0.0001 |
| Koliushkovaya-Seldianaya | 0.55 | 0.0001 | 0.13 | 0.0001 |
| Letnaya-Podpakhta | 0.24 | 0.0001 | 0.15 | 0.0001 |
| Letnaya-Seldianaya | 0.16 | 0.0001 | 0.38 | 0.0001 |
| Podpakhta-Seldianaya | 0.18 | 0.0001 | 0.33 | 0.0001 |
| nursery ground type | Date 1 | Date 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | SE N | B | SE B | N | SE N | B | SE B | |
| lagoon | 126 | - | 5040 | - | 30 | - | 1500 | - |
| eelgrass beds | 35.8 | 15.05 | 327.7 | 222.64 | 13.2 | 11.22 | 282.2 | 239.39 |
| rocky shore | 17.6 | - | 142.7 | - | 12.5 | - | 465.9 | - |
| rockweeds | 7.9 | 3.51 | 50.8 | 12.29 | 16.4 | 11 | 440.2 | 329.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




