Submitted:
17 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Sampling of environmental matrices
3. Sample preparation
3.1. Extraction
3.1.1. Extraction solvent
3.1.1.1. Dichloromethane
3.1.1.2. Water
3.1.1.3. Toluene
3.1.1.4. Ethyl acetate
3.1.1.5. Other solvents
3.1.2. Dissolved electrolyte effect
3.1.3. pH adjustment
4. Separation and analysis techniques
4.1. GC inlet
4.2. GC column
4.2.1. Stationary phase
4.2.2. Column dimensions
4.3. GC detectors
4.4. Internal standard
5. Conclusions
Acknowledgments
References
- Clark, E. Sulfolane and Sulfones. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; p. 1921120603120118.a01 ISBN 978-0-471-23896-6.
- Bak, A.; Kozik, V.; Dybal, P.; Kus, S.; Swietlicka, A.; Jampilek, J. Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential. Sustainability 2018, 10, 3677. [CrossRef]
- Travis, C.C.; Arms, A.D. Bioconcentration of Organics in Beef, Milk, and Vegetation. Environ. Sci. Technol. 1988, 22, 271–274. [CrossRef]
- The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; O’Neil, M.J., Ed.; 13th ed.; Merck: Whitehouse Station, N.J, 2001; ISBN 978-0-911910-13-1.
- Coetzee, J.F. Sulpholane: Purification, Tests for Purity and Properties. Pure Appl. Chem. 1977, 49, 211–216. [CrossRef]
- Willer, R. Sulfoxides. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley, 2000 ISBN 978-0-471-48494-3.
- Greene, E.; Gieg, L.; Coy, D.; Fedorak, P. Sulfolane Biodegradation Potential in Aquifer Sediments at Sour Natural Gas Plant Sites. Water Res. 1998, 32, 3680–3688. [CrossRef]
- Canadian Environmental Quality Guidelines for Sulfolane: Water and Soil : Scientific Supporting Document; Canadian Council of Ministers of the Environment: Winnipeg, Man., 2006; ISBN 978-1-896997-59-9.
- UOP SULFOLANE PROCESS. In Handbook of Petroleum Refining Processes; Meyers, R.A., Ed.; McGraw-Hill Education: New York, 2016 ISBN 978-0-07-185049-0.
- Kim, C.; Clarke, W.; Lockington, D. Competitive Adsorption of Sulfolane and Thiolane on Clay Materials. Korean J. Chem. Eng. 1999, 16, 215–220. [CrossRef]
- Stewart, O. Sulfolane Technical Assistance and Evaluation Report; 2010;
- Testing Status of Sulfolane 11054 Available online: https://ntp.niehs.nih.gov/go/ts-11054 (accessed on 17 September 2023).
- Mulder, D.; Yu, L.; Achari, G. A Laboratory and Field Investigation of Aerobic Biodegradation of Sulfolane in Groundwater. J. Chem. Technol. Biotechnol. 2021, 96, 2865–2871. [CrossRef]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Tenth edition.; Cengage: Boston, MA, 2022; ISBN 978-0-357-45043-7.
- Groundwater Sampling Operating Procedure Available online: https://www.epa.gov/sites/default/files/2015-06/documents/Groundwater-Sampling.pdf (accessed on 16 April 2023).
- Soil Sampling Operating Procedure Available online: https://www.epa.gov/sites/default/files/2015-06/documents/Soil-Sampling.pdf (accessed on 17 September 2023).
- Saint-Fort, R. Sulfolane Attenuation by Surface and Subsurface Soil Matrices. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 1211–1231. [CrossRef]
- Doucette, W.; Chard, J.; Moore, B.; Staudt, W.; Headley, J. Uptake of Sulfolane and Diisopropanolamine (DIPA) by Cattails (Typha Latifolia). Michrochemical J. 2005, 81, 41–49. [CrossRef]
- Lee, J.K.; Lee, W.J.; Cho, Y.-J.; Park, D.H.; Lee, Y.-W.; Chung, J. Variation of Bacterial Community Immobilized in Polyethylene Glycol Carrier during Mineralization of Xenobiotics Analyzed by TGGE Technique. Korean J. Chem. Eng. 2010, 27, 1816–1821. [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C. Degradation of Sulfolane Using Activated Persulfate with UV and UV-Ozone. Water Res. 2017, 125, 325–331. [CrossRef]
- Dominic, J.A.; Somathilake, P.; Achari, G.; Langford, C.H.; Tay, J.-H. Sunlight Mediated Passive Wastewater Treatment Technology Using Photochemical Reduction of Ferric Iron for Decontamination of Various Aqueous Contaminants. Sol. Energy 2018, 173, 470–477. [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C. Mineralization of Sulfolane in Aqueous Solutions by Ozone/CaO2 and Ozone/CaO with Potential for Field Application. Chemosphere 2018, 197, 535–540. [CrossRef]
- Heydari, G.; Langford, C.; Achari, G. Passive Solar Photocatalytic Treatment of Emerging Contaminants in Water: A Field Study. Catalysts 2019, 9. [CrossRef]
- Khan, M.; Yu, L.; Achari, G.; Tay, J. Degradation of Sulfolane in Aqueous Media by Integrating Activated Sludge and Advanced Oxidation Process. Chemosphere 2019, 222, 1–8. [CrossRef]
- Khan, M.; Yu, L.; Tay, J.; Achari, G. Coaggregation of Bacterial Communities in Aerobic Granulation and Its Application on the Biodegradation of Sulfolane. J. Hazard. Mater. 2019, 377, 206–214. [CrossRef]
- Dinh, M.; Hakimabadi, S.G.; Pham, A.L.-T. Treatment of Sulfolane in Groundwater: A Critical Review. J. Environ. Manage. 2020, 263, 110385. [CrossRef]
- Khan, M.; Yu, L.; Achari, G. Field Evaluation of a Pressurized Ozone Treatment System to Degrade Sulfolane in Contaminated Groundwaters. J. Environ. Chem. Eng. 2020, 8. [CrossRef]
- Khan, M.; Yu, L.; Hollman, J.; Tay, J.; Achari, G. Integration of Aerobic Granulation and UV/H2O2 Processes in a Continuous Flow System for the Degradation of Sulfolane in Contaminated Water. Environ. Sci. Water Res. Technol. 2020, 6, 1711–1722. [CrossRef]
- Yang, Y.; Yu, L.; Gopal, A. Aerobic Biodegradation of Sulfolane Using Archaea and Pseudomonas Strains. J. Chem. Technol. Biotechnol. 2022, 97, 1763–1770. [CrossRef]
- Yu, L.; Iranmanesh, S.; Keir, I.; Achari, G. A Field Pilot Study on Treating Groundwater Contaminated with Sulfolane Using UV/H2O2. Water 2020, 12. [CrossRef]
- Dharwadkar, S.; Yu, L.; Achari, G. Enhancement of LED Based Photocatalytic Degradation of Sulfolane by Integration with Oxidants and Nanomaterials. Chemosphere 2021, 263. [CrossRef]
- Dharwadkar, S.; Yu, L.; Achari, G. Photocatalytic Degradation of Sulfolane Using a LED-Based Photocatalytic Treatment System. Catalysts 2021, 11. [CrossRef]
- Yu, L.; Mehrabani-Zeinabad, M.; Achari, G.; Langford, C. Application of UV Based Advanced Oxidation to Treat Sulfolane in an Aqueous Medium. Chemosphere 2016, 160, 155–161. [CrossRef]
- Kasanke, C.; Leigh, M. Factors Limiting Sulfolane Biodegradation in Contaminated Subarctic Aquifer Substrate. PLOS ONE 2017, 12. [CrossRef]
- Chang, S.; Wu, C.; Yang, C.; Lin, C. Evaluation Use of Bioaugmentation and Biostimulation to Improve Degradation of Sulfolane in Artificial Groundwater. Chemosphere 2021, 263. [CrossRef]
- Stevens, S.; Richardson, D. Analysis of Sulfolane in Biochar. Appita 2018, 71, 349–353.
- Eckardt, M.; Greb, A.; Simat, T.J. Polyphenylsulfone (PPSU) for Baby Bottles: A Comprehensive Assessment on Polymer-Related Non-Intentionally Added Substances (NIAS). Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1421–1437. [CrossRef]
- Headley, J.V.; Peru, K.M.; Dickson, L.C. Gas Chromatographic–Mass Spectrometric Determination of Sulfolane in Wetland Vegetation Exposed to Sour Gas-Contaminated Groundwater. J. Chromatogr. A 1999, 859, 69–75. [CrossRef]
- Headley, J.; Dickson, L.; Peru, K. Comparison of Levels of Sulfolane and Diisopropanolamine in Natural Wetland Vegetation Exposed to Gas-Condensate Contaminated Ground Water. Commun. Soil Sci. Plant Anal. 2002, 33, 3531–3544. [CrossRef]
- Versace, F.; Uppugunduri, C.R.S.; Krajinovic, M.; Théorêt, Y.; Gumy-Pause, F.; Mangin, P.; Staub, C.; Ansari, M. A Novel Method for Quantification of Sulfolane (a Metabolite of Busulfan) in Plasma by Gas Chromatography–Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2012, 404, 1831–1838. [CrossRef]
- Silinski, M.A.R.; Uenoyama, T.; Cooper, S.D.; Fernando, R.A.; Robinson, V.G.; Waidyanatha, S. Development and Validation of an Analytical Method for Quantitation of Sulfolane in Rat and Mouse Plasma by GC-MS. J. Anal. Toxicol. 2019, 43, 477–481. [CrossRef]
- Shipkowski, K.; Cora, M.; Cesta, M.; Robinson, V.; Waidyanatha, S.; Witt, K.; Vallant, M.; Fallacara, D.; Hejtmancik, M.; Masten, S.; et al. Comparison of Sulfolane Effects in Sprague Dawley Rats, B6C3F1/N Mice, and Hartley Guinea Pigs after 28 Days of Exposure via Oral Gavage. Toxicol. Rep. 2021, 8, 581–591. [CrossRef]
- Greene, E.; Fedorak, P. Nutrient Stimulation of Sulfolane Biodegradation in a Contaminated Soil from a Sour Natural Gas Plant and in a Pristine Soil. Environ. Technol. 2001, 22, 619–629. [CrossRef]
- Brandao, M.; Yu, L.; Garcia, C.; Achari, G. Advanced Oxidation Based Treatment of Soil Wash Water Contaminated with Sulfolane. Water 2019, 11. [CrossRef]
- BC Lab Manual for Sulfolane Analysis 2017.
- Sulfolane Key Elements Document, Version 4 Available online: https://dec.alaska.gov/media/6165/key-element-all-media-2013-7-22.pdf.
- Fedorak, P.; Coy, D. Biodegradation of Sulfolane in Soil and Groundwater Samples from a Sour Gas Plant Site. Environ. Technol. 1996, 17, 1093–1102. [CrossRef]
- Luther, S.; Dudas, M.; Fedorak, P. Sorption of Sulfolane and Diisopropanolamine by Soils, Clays and Aquifer Materials. J. Contam. Hydrol. 1998, 32, 159–176. [CrossRef]
- Greene, E.A.; Coy, D.L.; Fedorak, P.M. Laboratory Evaluations of Factors Affecting Biodegradation of Sulfolane and Diisopropanolamine. Bioremediation J. 1999, 3, 299–313. [CrossRef]
- Kim, C.; Clarke, W.; Lockington, D. Determination of Retardation Coefficients of Sulfolane and Thiolane on Soils by K-Ow-K-Oc and Solubility Parameter, Batch and Column Experiments. Environ. Geol. 2000, 39, 741–749. [CrossRef]
- Kim, C.; Lockington, D.; Clarke, W.; Kim, C. Preliminary Determination of Pollutants Plume in Groundwater at Hazardous Solid Waste Disposal Site by Employing CPT and Rig. Environ. Technol. 2000, 21, 17–30. [CrossRef]
- Kim, C.; Chon, B. Oxygen Uptake Characteristics of Soil Inoculum Amended with Thiophene Derivatives. Korean J. Chem. Eng. 2002, 19, 773–779. [CrossRef]
- Yang, C.; Liu, S.; Su, Y.; Chen, Y.; Lin, C.; Lin, K. Bioremediation Capability Evaluation of Benzene and Sulfolane Contaminated Groundwater: Determination of Bioremediation Parameters. Sci. Total Environ. 2019, 648, 811–818. [CrossRef]
- Lin, C.-W.; Liu, S.-H.; Wu, C.-F.; Chang, S.-H. Critical Factors for Enhancing the Bioremediation of a Toxic Pollutant at High Concentrations in Groundwater: Toxicity Evaluation, Degrader Tolerance, and Microbial Community. J. Environ. Manage. 2021, 277, 111487. [CrossRef]
- Li, Z.; Song, J.; Zhang, S.; Wang, J.; Jian, X. Measurement and Correlation of Liquid-Liquid Equilibrium for the Ternary System (Water+1,2-Dichloroethane plus Sulfolane) at 288.15, 298.15, and 308.15 K. Chin. J. Chem. Eng. 2022, 51, 109–114. [CrossRef]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental Risk-Based Ranking of Solvents Using the Combination of a Multimedia Model and Multi-Criteria Decision Analysis. Green Chem. 2017, 19, 1034–1042. [CrossRef]
- Hossaini, R.; Chipperfield, M.P.; Montzka, S.A.; Leeson, A.A.; Dhomse, S.S.; Pyle, J.A. The Increasing Threat to Stratospheric Ozone from Dichloromethane. Nat. Commun. 2017, 8, 15962. [CrossRef]
- Handbook of Sample Preparation; Pawliszyn, J., Lord, H.L., Eds.; Wiley: Hoboken, N.J, 2010; ISBN 978-0-470-09934-6.
- EPA Method 3550C Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3550c.pdf (accessed on 17 April 2023).
- EPA Method 3540C Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-3540c-soxhlet-extraction.
- Neely, B.J.; Wagner, J.; Robinson, R.L.Jr.; Gasem, K.A.M. Mutual Solubility Measurements of Hydrocarbon–Water Systems Containing Benzene, Toluene, and 3-Methylpentane. J. Chem. Eng. Data 2008, 53, 165–174. [CrossRef]
- Khan, M.; Yu, L.; Achari, G. Sulfolane in Contaminated Sites: Environmental Toxicity and Bioremediation Technologies. Environ. Rev. 2022, 30, 217–227. [CrossRef]
- Altshuller, A.P.; Everson, H.E. The Solubility of Ethyl Acetate in Aqueous Electrolyte Solutions. J. Am. Chem. Soc. 1953, 75, 4823–4827. [CrossRef]
- Yang, Y.; Yu, L.; Iranmanesh, S.; Keir, I.; Achari, G. Laboratory and Field Investigation of Sulfolane Removal from Water Using Activated Carbon. J. Environ. Eng. 2020, 146. [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Seventh edition.; Cengage Learning: Australia, 2018; ISBN 978-1-305-57721-3.
- Kovacevik, B.; Zdravkovski, Z.; Mitrev, S. PESTICIDE ANALYSIS IN WATER SAMPLES USING GC-MS PULSED SPLITLESS INJECTION. 2016.
- Thiophene, Tetrahydro-, 1,1-Dioxide Available online: https://webbook.nist.gov/cgi/inchi?ID=C126330&Mask=200 (accessed on 22 September 2023).
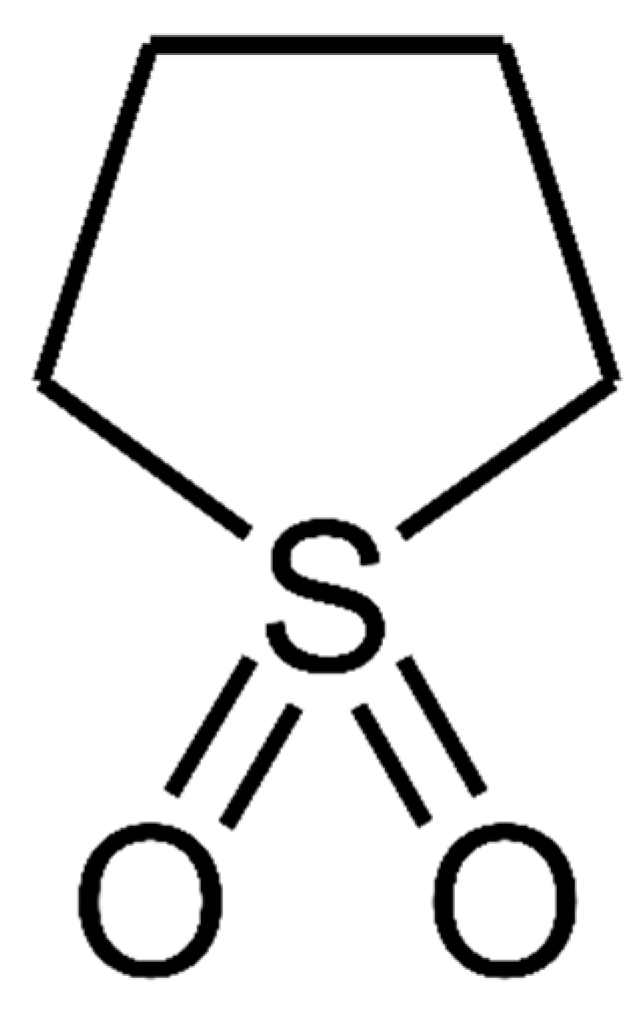
| property | Value | Ref |
| Molecular formula | C4H8SO2 | [1] |
| Molecular weight | 120.17 g/mol | [1] |
| Melting point | 28.5 °C | [1] |
| Boiling point | 287.3 °C | [1] |
| Log P | -0.4 | [3] |
| Solubility in water | Miscible at 30 °C | [4] |
| pKa | 12.9 * | [5] |
| Sample type | Method of extraction | Extraction phase | NO. of extraction aliquotes | Solvent to sample ratio (mL:mL or mL:g) | Centrifugation | Filtration | Salting out agent (saturation %) | Water removal of extract | Solvent evaporation (concentration factor) | pH adjustment | Internal standard | Recovery (%) | Ref. |
| Aqueous solution | AQ1 direct injection | N/A | N/A | N/A | Yes | Filter paper | No | No | No | No | No | N/A | [17] |
| Aqueous solution | LLE2 | DCM4 | 3 | 2:5 | No | No | No | No | No | No | No | 60 to 70 | [18] |
| Aqueous solution | LLE2 | DCM4 | 1 | 2:5 | No | Yes | NaCl (80%) | No | No | No | Ethylene glycol butyl ether | N/A | [19] |
| Aqueous solution | LLE2 | DCM4 | 1 | 1:1 | No | No | No | No | No | No | No | 80 | [20] |
| Aqueous solution | LLE2 | DCM4 | 1 | 2:5 | No | N/A | No | No | No | No | No | N/A | [21] |
| Aqueous solution | LLE2 | DCM4 | 1 | 2:1 | No | No | No | No | No | No | No | 80 | [22] |
| Aqueous solution | LLE2 | Ethyl acetate | 1 | 2:1 | No | No | No | No | No | No | No | N/A | [23] |
| Aqueous solution | LLE2 | DCM4 | 1 | 3:5 | No | Yes | No | No | No | No | No | 80±5 | [24] |
| Aqueous solution | LLE2 | DCM4 | 1 | 3:5 | No | Yes | No | No | No | No | No | 80±5 | [25] |
| Aqueous solution | LLE2 | Toluene | 1 | 2:1 | No | No | No | No | No | No | Sulfolane-d8 | N/A | [26] |
| Aqueous solution | LLE2 | DCM4 | 1 | 3:5 | No | Yes | No | No | No | No | No | 80±5 | [27] |
| Aqueous solution | LLE2 | DCM4 | 1 | 3:5 | No | Yes | No | No | No | No | No | 80±5 | [28] |
| Aqueous solution | LLE2 | DCM4 | 1 | 3:5 | No | Yes | No | No | No | No | No | N/A | [29] |
| Aqueous solution | LLE2 | DCM4 | 1 | 3:5 | No | Yes | No | No | No | No | No | 80±5 | [30] |
| Aqueous solution | LLE2 | DCM4 | 1 | 1:1 | No | 0.2 µm PTFE filter | No | No | No | No | No | N/A | [31] |
| Aqueous solution | LLE2 | DCM4 | 1 | 1:1 | No | 0.2 µm PTFE filter | No | No | No | No | No | N/A | [32] |
| Aqueous suspension | AQ1 direct injection | N/A | N/A | N/A | Yes | 0.22 µm nylon filter | No | No | No | No | No | N/A | [33] |
| Aqueous suspension | LLE2 | DCM4 | 3 | N/A | N/A | N/A | No | No | No | No | Sulfolane-d8 and nitrobenzene-d8 | N/A | [34] |
| Aqueous suspension | LLE2 | DCM4 | 1 | 1:10 | No | 0.22 µm Teflon filter | NaCl (100%) | No | No | No | No | N/A | [35] |
| Aqueous suspension | LLE2 | DCM4 | 1 | 3:5 | No | 0.45 µm syringe filter | No | No | No | No | No | N/A | [29] |
| Biochar | Soxhlet extraction | Ethyl acetate | 6 | N/A | No | No | No | No | N/A | No | N-butanol | 43 to 50 | [36] |
| Ethanol/water (1:1) mix | LLE2 | Chloroform | 1 | 1:5 | No | No | No | By Na2SO4 | No | No | Dicyclohexylmethanol | 45.5 | [37] |
| Homogenized water plant tissue mixture | LLE2 | Toluene | 3 | 5:1 | Yes | 0.2 µm cellulose acetate membrane filter | No | No | Yes (15) | No | No | 80±12 | [38] |
| Homogenized water plant tissue mixture | LLE2 | Toluene | 3 | 5:1 | Yes | 0.2 µm cellulose acetate membrane filter | No | No | Yes (15) | No | No | 80±12 | [39] |
| Homogenized water plant tissue mixture | LLE2 | DCM4 | 3 | 2:5 | No | 0.2 µm membrane filter | No | No | No | No | No | 50 to 60 | [18] |
| Plasma | LLE2 | Ethyl acetate | 1 | 5:1 | Yes | No | Yes | No | Yes (8) | by NaOH | Sulfolane-d8 | 93 | [40] |
| Plasma | LLE2 | Ethyl acetate | 1 | 5:1 | Yes | No | Yes | No | Yes (8) | by NaOH | Sulfolane-d8 | 74.4 to 88.7 | [41] |
| Plasma | LLE2 | Ethyl acetate | 1 | 5:1 | Yes | No | Yes | No | No | by NaOH | Sulfolane-d8 | >85 | [42] |
| PPSF polymer | SLE3 | Acetonitrile | 1 | 20:1 | No | No | No | No | No | No | Dicyclohexylmethanol | N/A | [37] |
| Soil | SLE3 | Water | 2 | 5:1 | Yes | No | No | No | No | No | No | 65 to 102 | [43] |
| Soil | SLE3 | Water | 1 to 3 | 1:1 to 3:1 | Yes | 0.45 µm filter | No | No | No | No | No | 82 to 99 | [44] |
| Soil | SLE3 | DCM4 | 1 | 1:1 | Yes | No | No | By Na2SO4 and NaCl | No | No | Sulfolane-d8 | 80-120 | [45] |
| Soil | SLE3 | DCM4 | 3 | 5:2 | Yes | Yes | No | By Na2SO4 | If required | No | Sulfolane-d8 | 70-120 | [46] |
| Soil | Soxhlet extraction | DCM4 | 1 | 30:1 | No | No | No | By Na2SO4 | Yes (3) | No | Sulfolane-d8 | 70-120 | [46] |
| Soil Slurry | AQ1 direct injection | Water | N/A | N/A | Yes | No | No | No | No | No | No | N/A | [7] |
| Soil Slurry/soil water mix | LLE2 | DCM4 | 3 | 1:2 | Yes | No | NaCl (80%) | By Na2SO4 | Yes (N/A) | No | Dibenzothiophene | N/A | [47] |
| Soil water mix | LLE2 | Toluene | 3 | 5:1 | Yes | 0.2 µm cellulose acetate membrane filter | No | No | Yes (15) | No | No | 126 | [39] |
| Water | AQ1 direct injection | N/A | 0 | N/A | Yes | No | No | No | No | No | No | N/A | [48] |
| Water | AQ1 direct injection | Water | N/A | N/A | Yes | No | No | No | No | No | No | N/A | [49] |
| Water | LLE2 | DCM4 | 1 | 1:1 | Yes | 0.2 µm membrane filter | No | By Na2SO4 | No | No | No | N/A | [10] |
| Water | LLE2 | DCM4 | 1 | 1:1 | Yes | 0.2 µm embrane filter | No | No | No | No | No | N/A | [50] |
| Water | LLE2 | DCM4 | 3 | 1:20 | No | Glass wool | No | By Na2SO4 | Yes (15) | By NaOH to pH 10 | No | N/A | [51] |
| Water | LLE2 | Toluene | 3 | 5:1 | Yes | 0.2 µm cellulose acetate membrane filter | No | No | Yes (15) | No | No | 127 | [39] |
| Water | LLE2 | DCM4 | 1 | 1:1 | No | 0.2 µm membrane filter | No | By Na2SO4 | No | No | No | N/A | [52] |
| Water | LLE2 | DCM4 | 1 | 1:10 | No | No | NaCl (100%) | No | No | By Na2CO3 to pH 5-8 | No | N/A | [53] |
| Water | LLE2 | DCM4 | 1 | 1:10 | No | No | NaCl (100%) | No | No | By Na2CO3 to pH 5-8 | No | N/A | [54] |
| Water | LLE2 | DCM4 | 1 | 5:2 | No | 0.45 µm PTFE | No | No | No | No | No | N/A | [13] |
| Water | LLE1 | 1,2-dichloroethane | 1 | 1:1 | No | No | No | No | No | No | No | N/A | [55] |
| Water | LLE2 | DCM4 | 2 | 1:1 | No | No | Yes | By Na2SO4 | Yes (200) | Yes (pH<2) | Sulfolane-d8 | 80-120 | [45] |
| Sample type | Sample solvent | Carrier gas | Carrier gas flow rate/Pressure | Mode of injection | Injection volume | Inlet temperature (°C) | Type of column | Column stationary phase | Column dimensions | Detector | LLOD4 | LLOQ5 | Detector temperature (°C) | Ref. |
| Aqueous solution | DCM1 | N2 | 10 mL/min | N/A | N/A | N/A | WCOT2 | DB-5 | 30 m x 0.45 mm x 0.25 μm | FID | N/A | N/A | N/A | [18] |
| Aqueous solution | Water | N2 | 360 mL/min | Splitless | N/A | 280 | SCOT3 | OS-138 | 7.5 m x 0.51 mm | FID | 6 mg/L | N/A | N/A | [17] |
| Aqueous solution | DCM1 | He | 1 mL/min | Splitless | 1 | 250 | WCOT2 | DB-WAXETR | 30 m x 0.32 mm x 1 μm | MS | N/A | N/A | N/A | [19] |
| Aqueous solution | DCM1 | He | 250 KPa | Splitless | 2 | 165 | WCOT2 | ZB-5 | N/A | FID | 1 mg/L | N/A | 250 | [20] |
| Aqueous solution | DCM1 | He | 10 mL/min | Splitless | 2 | 250 | WCOT2 | ZB-5 | N/A | FID | N/A | N/A | 320 | [21] |
| Aqueous solution | DCM1 | He | 250 kPa | Splitless | 2 | 165 | WCOT2 | ZB-5 | N/A | FID | 1 mg/L | N/A | 250 | [22] |
| Aqueous solution | Ethyl acetate | N/A | N/A | N/A | N/A | N/A | WCOT2 | ZB-5 | N/A | FID | N/A | N/A | N/A | [23] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 250 | WCOT2 | ZB-5 | N/A | MS | 10 μg/L | N/A | N/A | [24] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 250 | WCOT2 | ZB-5 | N/A | MS | 10 μg/L | N/A | N/A | [25] |
| Aqueous solution | Toluene | He | 1 mL/min | Splitless | 0.5 | 285 | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | MS | 20 μg/L | 70 μg/L | Ion source: 285 | [26] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 250 | WCOT2 | ZB-5 | N/A | MS | 10 μg/L | N/A | N/A | [27] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 250 | WCOT2 | ZB-5 | N/A | MS | 10 μg/L | N/A | N/A | [28] |
| Aqueous solution | DCM1 | He | N/A | Splitless | 1 | 165 | WCOT2 | ZB-5 | N/A | FID | 0.3 mg/L | 1 mg/L | 330 | [64] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 250 | WCOT2 | ZB-5 | N/A | MS | 10 μg/L | N/A | N/A | [30] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 165 | WCOT2 | ZB-5 | N/A | FID | N/A | N/A | 330 | [31] |
| Aqueous solution | DCM1 | He | 1.07 mL/min | Splitless | 1 | 165 | WCOT2 | ZB-5 | N/A | FID | N/A | N/A | 330 | [32] |
| Aqueous suspension | Water | He | N/A | Splitless | 1 | 165 | WCOT2 | ZB-5 | N/A | FID | 1 mg/L | N/A | 250 | [33] |
| Aqueous suspension | DCM1 | N/A | N/A | Pulsed-splitless | N/A | N/A | WCOT2 | RTX-200 | 30 m | MS | N/A | 40 μg/L | N/A | [34] |
| Aqueous suspension | DCM1 | N2 | N/A | Splitless | 1 | 200 | WCOT2 | Stabliwax | 30 m x 0.53 x 1 µm | FID | N/A | N/A | 250 | [35] |
| Aqueous suspension | DCM1 | He | N/A | Splitless | 1 | N/A | WCOT2 | 5-MSI | N/A | FID | N/A | N/A | 330 | [29] |
| Biochar | Ethyl acetate | He | N/A | Split (10:1) | 1 | N/A | WCOT2 | ZB-Wax-Plus | 30 m x 0.32 mm x 0.5 μm | FID | 10 mg/L | N/A | N/A | [36] |
| Ethanol/water (1:1) solution | Chloroform | He | 1 mL/min | Splitless | 1 | N/A | WCOT2 | HP-5 | 30 m x 0.25 mm x 0.25 µm | MS | 10 μg/kg of ethanol/water (1:1) | N/A | N/A | [37] |
| Homogenized water plant tissue mixture | Toluene | He | 25 cm/s | Splitless | N/A | 250 | WCOT2 | DB-5 | 25 m x 0.25 mm x 0.25 µm | MS | 90 ng per gram of wet plant tissue | 300 ng per g of wet plant tissue | Ion source: 280 | [38] |
| Homogenized water plant tissue mixture | Toluene | He | 25 cm/s | Splitless | N/A | 250 | WCOT2 | DB-5 | 25 m x 0.25 mm x 0.25 µm | MS | 90 ng per gram of wet plant tissue | 300 ng per g of wet plant tissue | Ion source: 280 | [39] |
| Homogenized water plant tissue mixture | DCM1 | N2 | 10 mL/min | N/A | N/A | N/A | WCOT2 | DB-5 | 30 m x 0.45 mm x 0.25 µm | FID | N/A | N/A | N/A | [18] |
| Plasma | Isopropanol | He | 1 mL/min | Splitless | 1 | 250 | WCOT2 | ZB-5 | 15 m x 0.25 mm x 0.25 µm | MS/MS | N/A | 20 μg/L | Ion source: 200 | [40] |
| Plasma | Isopropanol | He | 1 mL/min | Split (50:1) | 1 | 250 | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | MS | 0.516 μg/L | 20 μg/L | Ion source: 230 | [41] |
| Plasma | Ethyl acetate | He | 1 mL/min | Split (50:1) | 1 | 250 | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | MS | 1.25 μg/L | N/A | Ion source: 230 | [42] |
| PPSF polymer | Acetonitrile | He | 1 mL/min | Splitless | 1 | N/A | WCOT2 | HP-5 | 30 m x 0.25 mm x 0.25 µm | MS | N/A | N/A | N/A | [37] |
| Soil | Water | He | 25 mL/min | Splitless | 2 | 250 | Packed | Tenax-GC coated with 5% polyphenyl ether | 1.2 m x 0.32 cm | FID | 1 mg/L | N/A | 250 | [43] |
| Soil | DCM1 | He | 250 KPa | Splitless | 1 | 250 | WCOT2 | ZB-5 | N/A | MS | <1 mg/L | <1 mg/L | 330 | [44] |
| Soil Slurry | Water | He | 24 mL/min | Splitless | 2 | 250 | Packed | Tenax-GC coated with 5% polyphenyl ether | 2 m x 0.3cm | FID | 0.5 mg/L | N/A | 250 | [7] |
| Soil Slurry or soil water mixture | DCM1 | N/A | N/A | Split (20:1) | N/A | 250 | WCOT2 | DB-5 | 25 m x 0.25 mm x 0.25 µm | FID | N/A | N/A | 250 | [47] |
| Soil water mixture | Toluene | He | 25 cm/s | Splitless | N/A | 250 | WCOT2 | DB-5 | 25 m x 0.25 mm x 0.25 µm | MS | 90 ng per gram of wet plant tissue | N/A | ion source: 280 | [39] |
| Water | Water | N2 | 30 mL/min | Splitless | 2 | 250 | Packed | Tenax-GC coated with 5% polyphenyl ether | 1.8 m x 0.32 cm | FID | 5 mg/L | N/A | 250 | [48] |
| Water | Water | He | 25 mL/min | Splitless | 2 | 250 | Packed | Tenax-GC coated with 5% polyphenyl ether | 1.2 m x 0.32 cm | FID | 1 mg/L | N/A | 250 | [49] |
| Water | DCM1 | He | 1.7 mL/min | N/A | N/A | 300 | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | FID | N/A | N/A | 350 | [10] |
| Water | DCM1 | He | N/A | N/A | N/A | N/A | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | FID | N/A | N/A | N/A | [50] |
| Water | DCM1 | He | 1.7 mL/min | Split (N/A) | 5 | 300 | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | FID | N/A | N/A | 350 | [51] |
| Water | Toluene | He | 25 cm/s | Splitless | N/A | 250 | WCOT2 | DB-5 | 25 m x 0.25 mm x 0.25 μm | MS | 1 ng/mL | N/A | ion source: 280 | [39] |
| Water | DCM1 | He | 1.7 mL/min | N/A | N/A | 300 | WCOT2 | DB-5 | 30 m x 0.25 mm x 0.25 µm | FID | N/A | N/A | 350 | [52] |
| Water | DCM1 | He | N/A | N/A | 0.2 | 200 | WCOT | Stabliwax | 30 m x 0.53 mm x 1 µm | FID | N/A | N/A | 250 | [53] |
| Water | DCM1 | He | N/A | N/A | 0.2 | 200 | WCOT2 | Stabliwax | 30 m x 0.53 mm x 1 µm | FID | N/A | 0.44 mg/L | 250 | [54] |
| Water | DCM1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | FID | 1 mg/L | N/A | N/A | [13] |
| Water | 1,2-dichloroethane | N2 | 4.5 mL/min | Split (8:1) | 0.2 | 250 | WCOT2 | DB-FFAP | 30 m x 0.53 mm x 1 µm | FID | N/A | N/A | 250 | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





