Submitted:
18 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Plant Materials and Growth Conditions
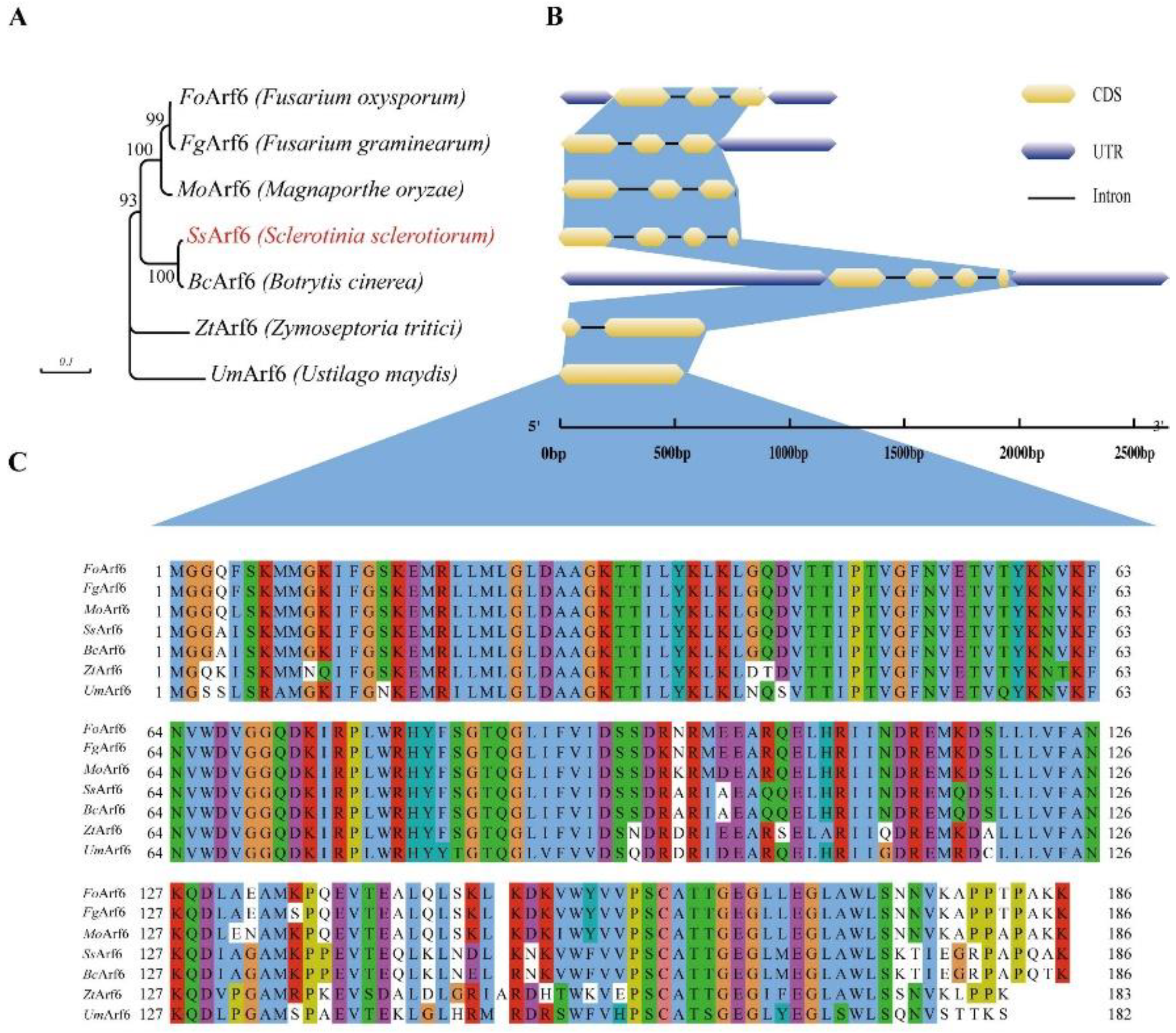
2.3. Phylogenetic Tree Construction and Sequence Analysis
2.4. Knockout and Complementation of SsArf6
2.5. DNA and RNA Manipulation
2.6. Colony Morphology Observation
2.7. Stress Treatment
2.8. Analysis of Compound Appressoria, OA and Virulence to Host Plants
3. Results
3.1. Identification of the Arf6 homolog in S. sclerotiorum
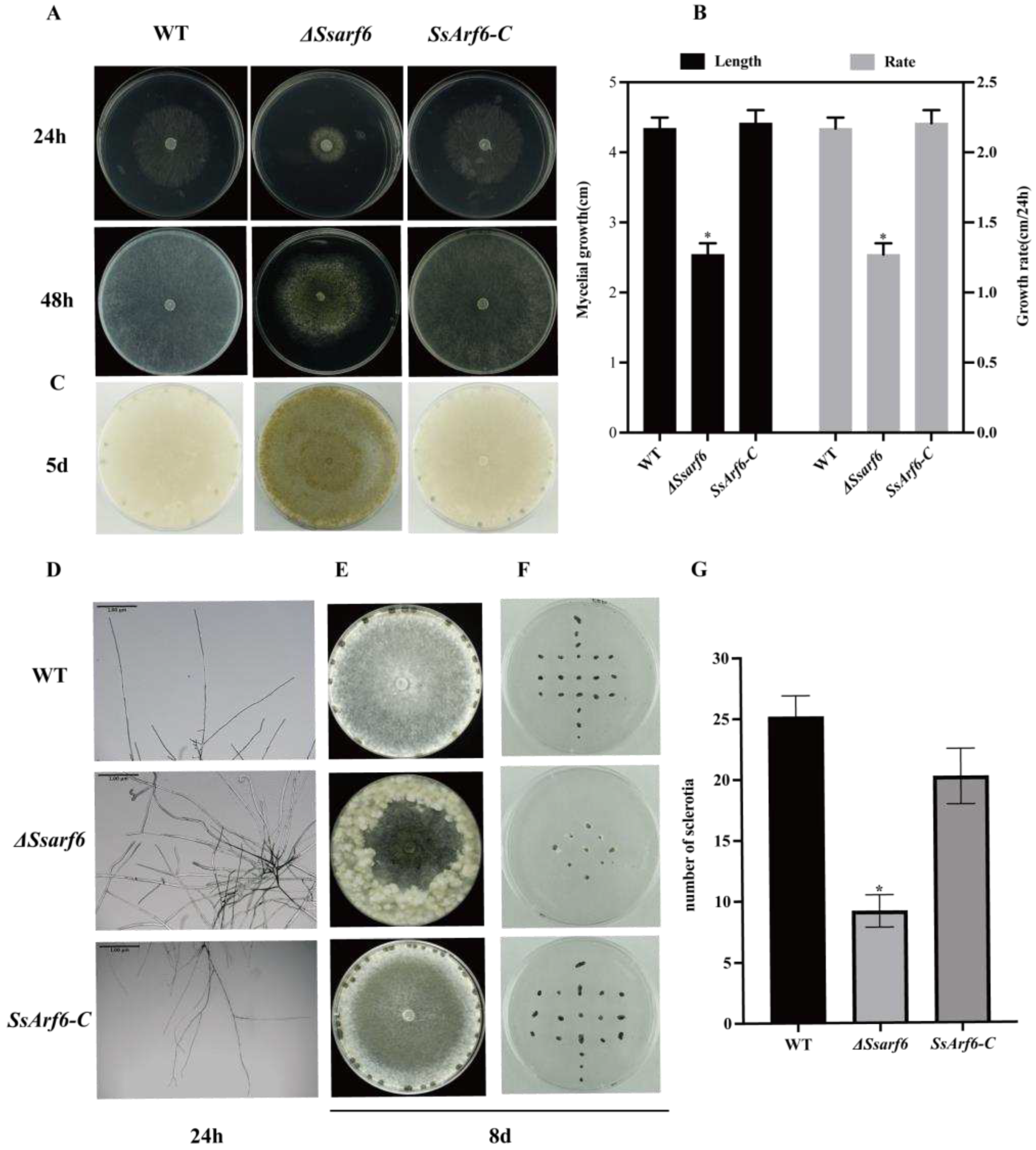
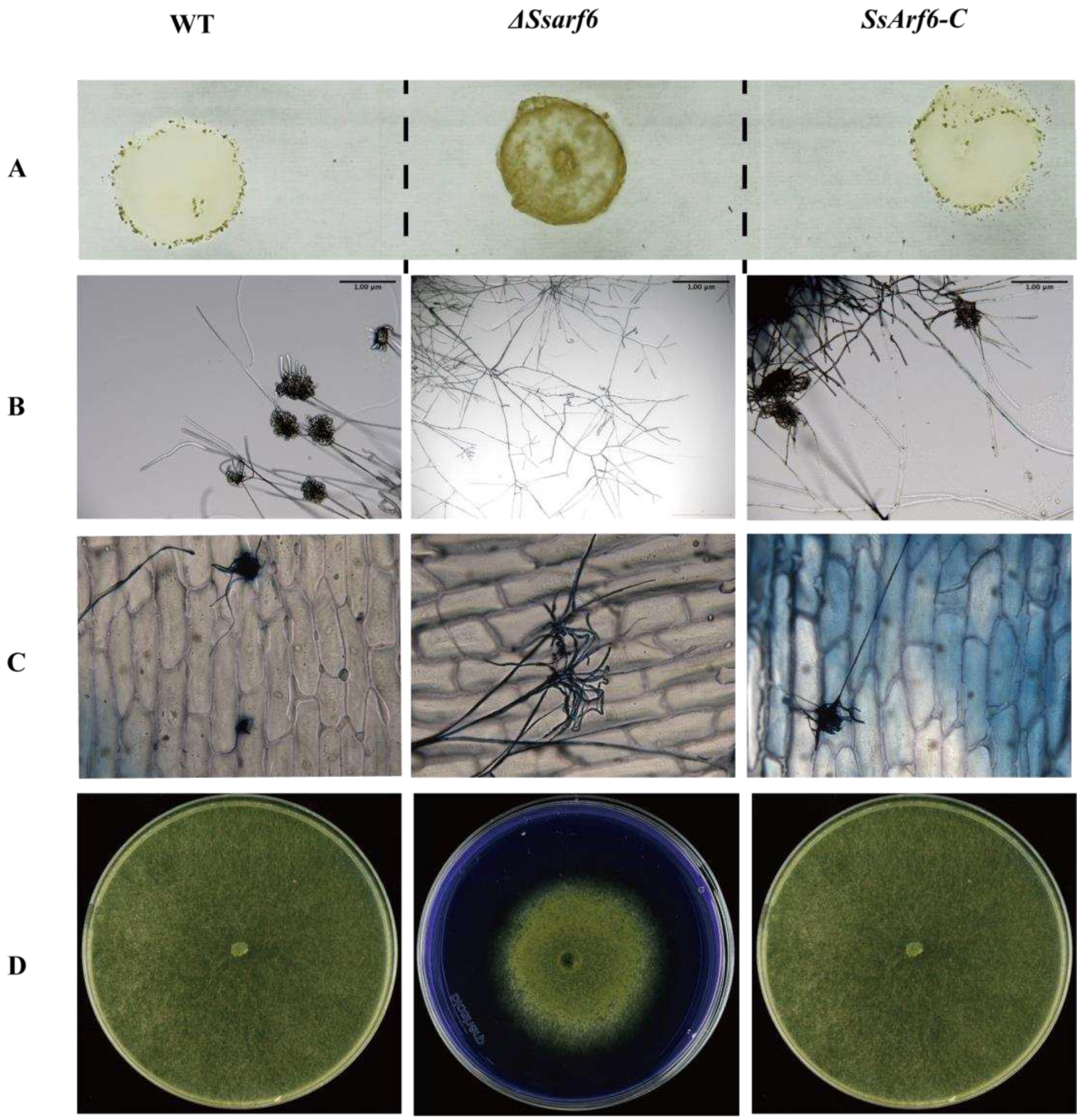
3.2. Knockout of SsArf6 leads to aberrant mycelium growth, increased melanin accumulation and decreased sclerotium production
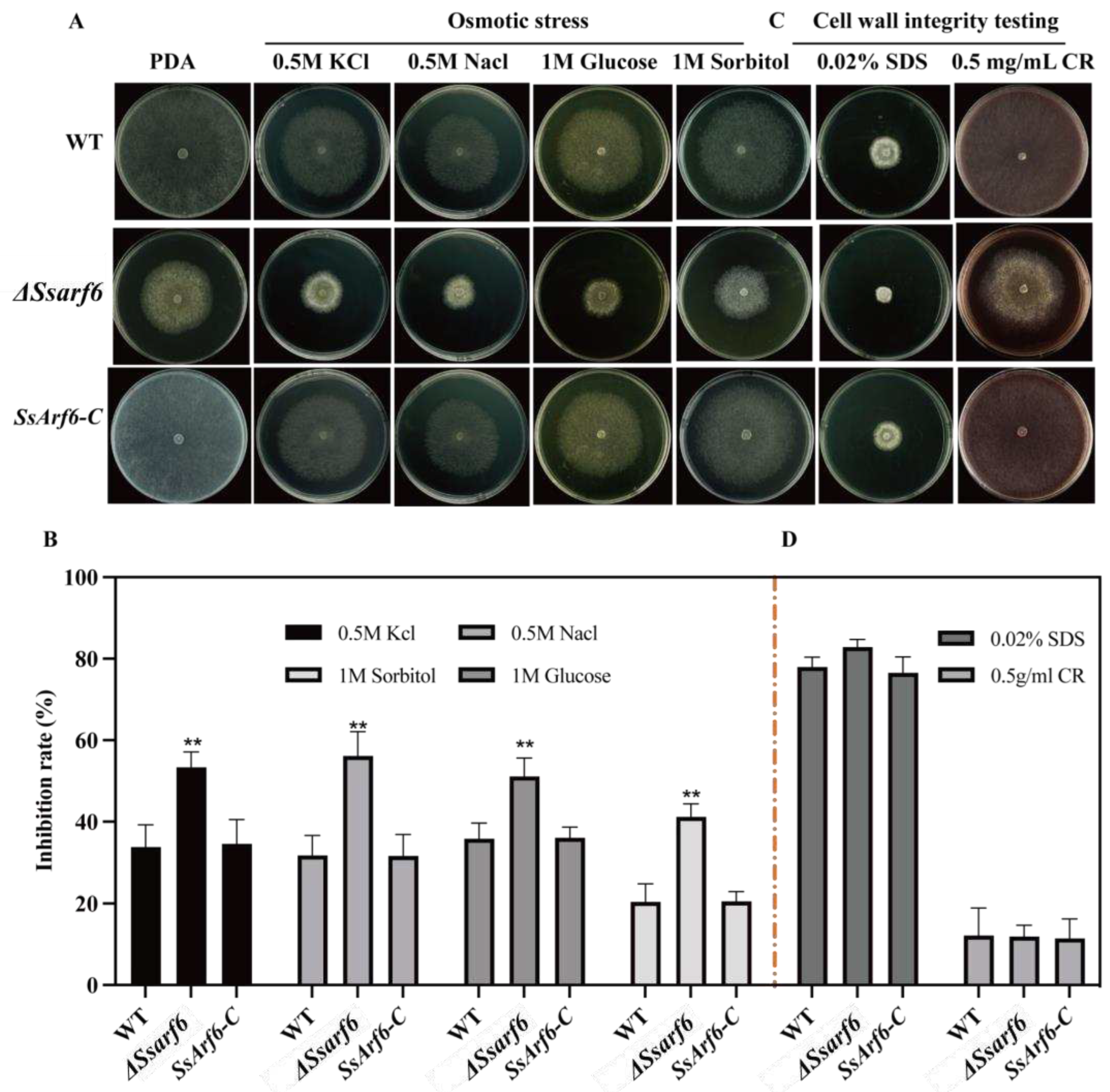
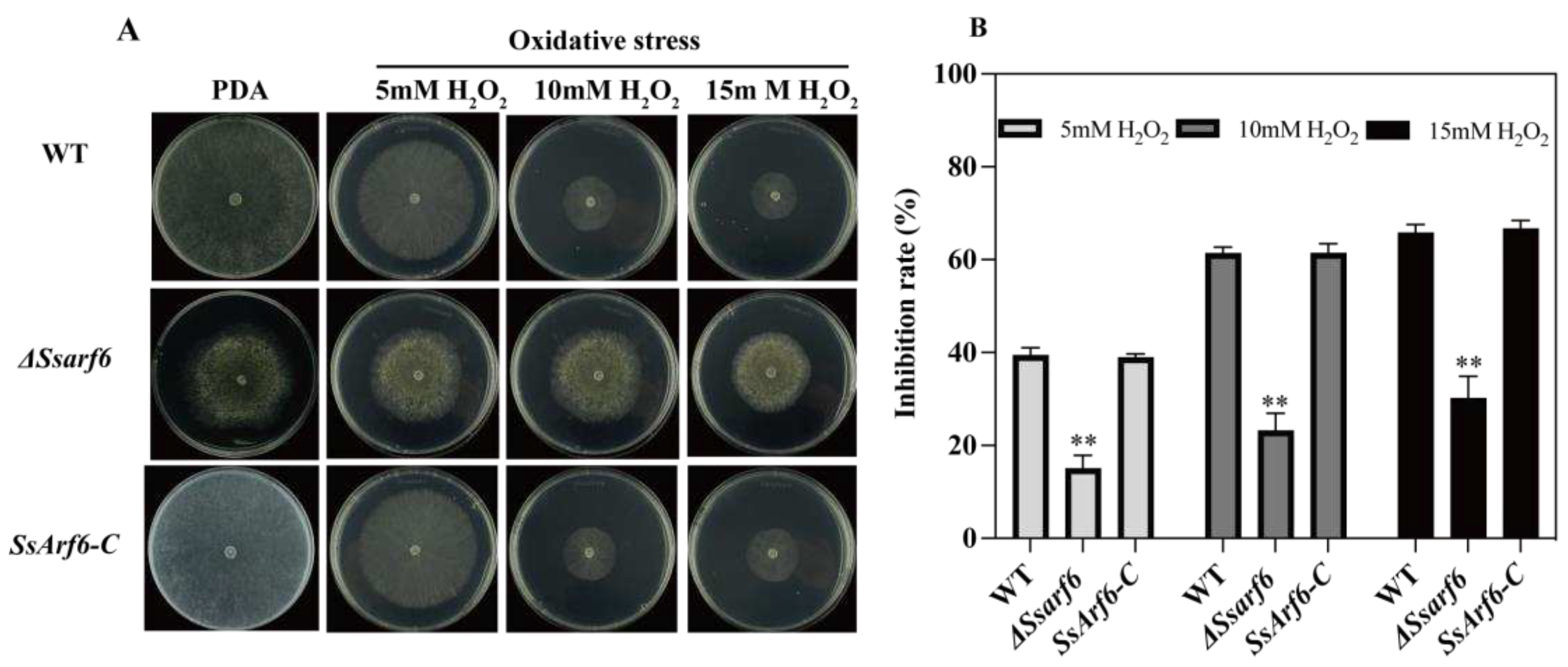
3.3. SsArf6 is implicated in abiotic stress response in S. sclerotiorum
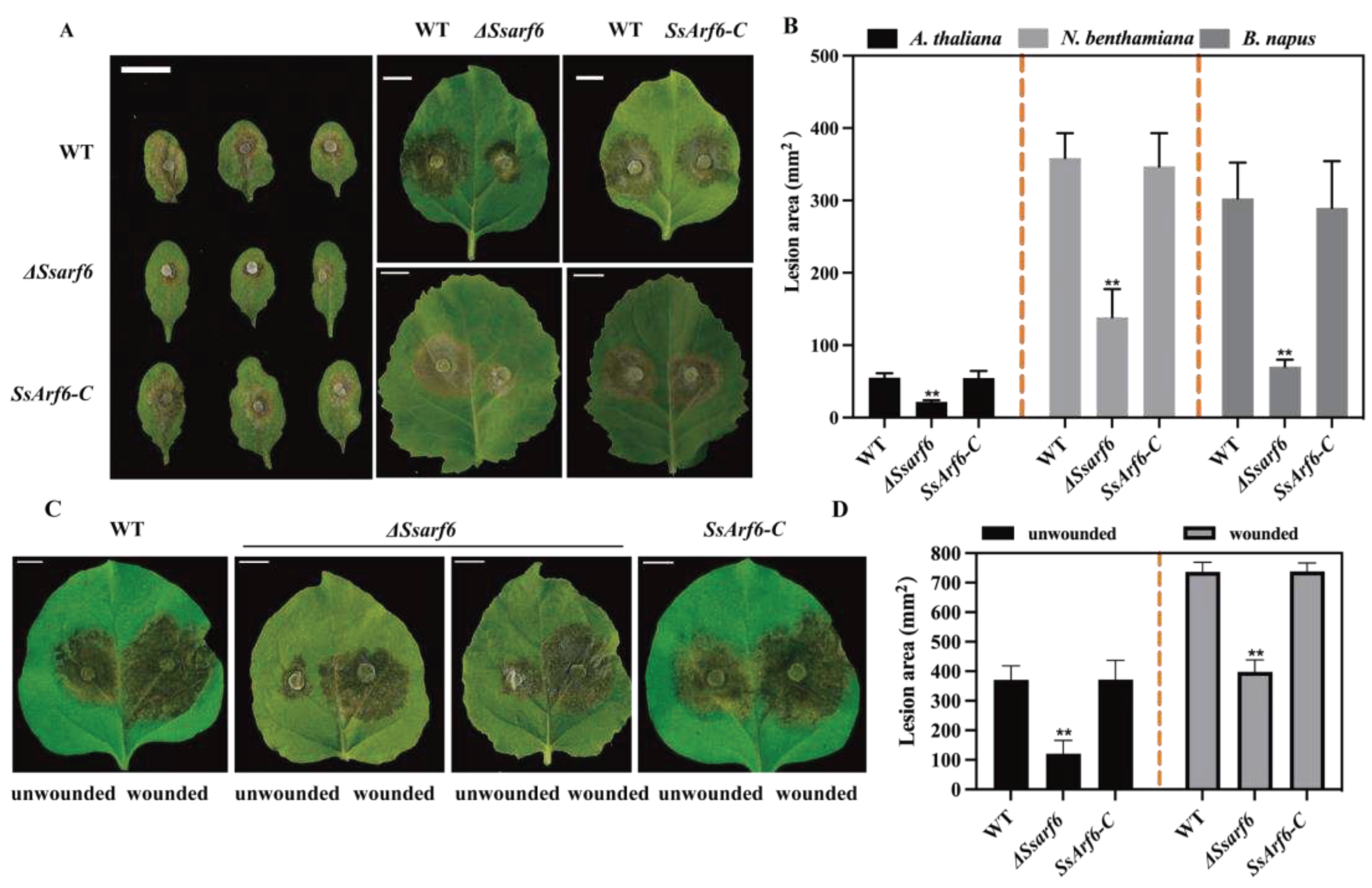
3.4. SsArf6 is involved in compound appressoria development
3.5. SsArf6 is essential for virulence to host plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, S.; Xu, Y.; Hoy, R.; Zhang, J.; Qin, L.; Li, X. The notorious soilborne pathogenic fungus Sclerotinia sclerotiorum: An update on genes studied with mutant analysis. Pathogens 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, T.; Guo, X.J.; Li, M.; Liu, X.Y.; Cao, J.; Tan, X.L. Sclerotinia stem rot resistance in rapeseed: Recent progress and future prospects. J. Agric. Food Chem. 2021, 69, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Fass, M.I.; Rivarola, M.; Ehrenbolger, G.F.; Maringolo, C.A.; Montecchia, J.F.; Quiroz, F.; García-García, F.; Blázquez, J.D.; Hopp, H.E.; Heinz, R.A.; et al. Exploring sunflower responses to Sclerotinia head rot at early stages of infection using RNA-seq analysis. Sci. Rep. 2020, 10, 13347. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.D.; Hartman, G.L.; Mueller, D.S.; Leitz, R.A.; Nickell, C.D.; Pedersen, W.L. Yield and seed quality of soybean cultivars infected with Sclerotinia sclerotiorum. Plant Dis. 1998, 82, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. CROP.J 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Lin, Y.; Fu, Y.; Xie, J.; Li, B.; Bian, X.; Feng, Y.; Liang, W.; Tang, Q.; et al. Editing homologous copies of an essential gene affords crop resistance against two cosmopolitan necrotrophic pathogens. Plant Biotechnol. J. 2021, 19, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tang, L.; Gong, Y.; Xie, J.; Fu, Y.; Jiang, D.; Li, G.; Collinge, D.B.; Chen, W.; Cheng, J. A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytol. 2018, 217, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.E.; Frampton, C.M.; Stewart, A. Factors influencing survival of sclerotia of Sclerotium cepivorum in New Zealand soils. N. Z. J. Crop Hortic. Sci 2002, 30, 29–35. [Google Scholar] [CrossRef]
- Willetts, H.J.; Wong, J.A.L. The biology of Sclerotinia sclerotiorum,S. trifoliorum, andS. minor with emphasis on specific nomenclature. Bot. Rev. 1980, 46, 101–165. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, K.; Li, M.; Hu, H.; Zhang, X.; Liu, J.; Pan, H.; Zhang, Y. SsAGM1-mediated uridine diphosphate-N-acetylglucosamine synthesis is essential for development, stress response, and pathogenicity of Sclerotinia sclerotiorum. Front.Microbiol. 2022, 13, 938784. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Buchenauer, H.; Han, Q.; Zhang, X.; Kang, Z. Ultrastructural and cytochemical studies on the infection process of Sclerotinia sclerotiorum in oilseed rape. J. Plant Dis. Prot. 2008, 115, 9–16. [Google Scholar] [CrossRef]
- Uloth, M.B.; Clode, P.L.; You, M.P.; Barbetti, M.J. Attack modes and defence reactions in pathosystems involving Sclerotinia sclerotiorum, Brassica carinata, B. juncea and B. napus. Ann. Bot. 2015, 117, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Rollins, J.A. Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology. 2018, 108, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, J.; Yu, B.; Liu, L.; Zhang, X.; Liu, J.; Pan, H.; Zhang, Y. Transcription factor SsSte12 was involved in mycelium growth and development in Sclerotinia sclerotiorum. Front.Microbiol. 2018, 9, 2476. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Xiao, K.; Jiao, W.; Zhang, C.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. The coupling between cell wall integrity mediated by MAPK kinases and SsFkh1 is involved in sclerotia formation and pathogenicity of Sclerotinia sclerotiorum. Front.Microbiol. 2022, 13, 816091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Lai, W.; Huang, K.; Li, Y.; Wang, Z.; Chen, X.; Wang, A. SsATG8 and SsNBR1 mediated-autophagy is required for fungal development, proteasomal stress response and virulence in Sclerotinia sclerotiorum. Fungal Genet. Biol. 2021, 157, 103632. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Yu, H.; Cong, J.; Xiao, K.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. Transcription factor SsFoxE3 activating SsAtg8 is critical for sclerotia, compound appressoria formation, and pathogenicity in Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Yu, H.; Chen, X.; Xiao, K.; Jia, D.; Wang, F.; Zhang, Y.; Pan, H. The SsAtg1 activating autophagy is required for sclerotia formation and pathogenicity in Sclerotinia sclerotiorum. J. Fungi 2022, 8, 1314. [Google Scholar] [CrossRef]
- Jiao, W.; Ding, W.; Rollins, J.A.; Liu, J.; Zhang, Y.; Zhang, X.; Pan, H. Cross-talk and multiple control of Target of Rapamycin (TOR) in Sclerotinia sclerotiorum. Microbiol. Spectrum 2023, 11, e0001323. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, J.; Zhu, W.; Yang, Y.; Mei, J.; Bi, C.; Qian, W.; Qing, L.; Tan, W. Ss-Rhs1, a secretory Rhs repeat-containing protein, is required for the virulence of Sclerotinia sclerotiorum. Mol. Plant Pathol. 2017, 18, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Q.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. Ssams2, a gene encoding GATA transcription factor, is required for appressoria formation and chromosome segregation in Sclerotinia sclerotiorum. Front. Microbiol. 2018, 9, 3031. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Li, L.; Liu, J.; Zhang, Y.; Pan, H. Proteomics analysis of SsNsd1-mediated compound appressoria formation in Sclerotinia sclerotiorum. Int. J. Mol. Sci. 2018, 19, 2946. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Yan, B.; Liao, H.; Dong, M.; Meng, X.; Wan, H.; Qian, W. Host-induced gene silencing of a multifunction gene Sscnd1 enhances plant resistance against Sclerotinia sclerotiorum. Front.Microbiol. 2021, 12, 693334. [Google Scholar] [CrossRef] [PubMed]
- Kabbage, M.; Yarden, O.; Dickman, M.B. Pathogenic attributes of Sclerotinia sclerotiorum: switching from a biotrophic to necrotrophic lifestyle. Plant Sci. 2015, 233, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Monazzah, M.; Rabiei, Z.; Enferadi, S.T. The effect of oxalic acid, the pathogenicity factor of Sclerotinia Sclerotiorum on the two susceptible and moderately resistant lines of sunflower. Iran. J. Biotechnol. 2018, 16, e1832. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Kabbage, M.; Kim, H.J.; Britt, R.; Dickman, M.B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011, 7, e1002107. [Google Scholar] [CrossRef]
- Wei, W.; Pierre-Pierre, N.; Peng, H.; Ellur, V.; Vandemark, G.J.; Chen, W. The D-galacturonic acid catabolic pathway genes differentially regulate virulence and salinity response in Sclerotinia sclerotiorum. Fungal Genet. Biol. 2020, 145, 103482. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yajima, W.; Das, D.; Suresh, M.R.; Kav, N.N. Isolation, expression and characterization of two single-chain variable fragment antibodies against an endo-polygalacturonase secreted by Sclerotinia sclerotiorum. Protein Expr Purif. 2009, 64, 237–243. [Google Scholar] [CrossRef]
- Fan, H.; Yang, W.; Nie, J.; Zhang, W.; Wu, J.; Wu, D.; Wang, Y. A novel effector protein SsERP1 inhibits plant ethylene signaling to promote Sclerotinia sclerotiorum infection. J. Fungi 2021, 7. [Google Scholar] [CrossRef]
- Seifbarghi, S.; Borhan, M.H.; Wei, Y.; Ma, L.; Coutu, C.; Bekkaoui, D.; Hegedus, D.D. Receptor-like kinases BAK1 and SOBIR1 are required for necrotizing activity of a novel group of Sclerotinia sclerotiorum necrosis-inducing effectors. Front. Plant Sci. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the pathogenomic features of a global pathogen. Cells 2023, 12. [Google Scholar] [CrossRef]
- Seifbarghi, S.; Borhan, M.H.; Wei, Y.; Coutu, C.; Robinson, S.J.; Hegedus, D.D. Changes in the Sclerotinia sclerotiorum transcriptome during infection of Brassica napus. BMC Genomics 2017, 18, 266. [Google Scholar] [CrossRef]
- Yang, G.; Tang, L.; Gong, Y.; Xie, J.; Fu, Y.; Jiang, D.; Li, G.; Collinge, D.B.; Chen, W.; Cheng, J. A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytol. 2018, 217, 739–755. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.L.; Li, J.L.; Zhu, F.X. Dimethachlon Resistance in Sclerotinia sclerotiorum in China. Plant Dis. 2014, 98, 1221–1226. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.-P.; Chen, C.-J.; Zhou, M.-G. Detection of resistance in Sclerotinia sclerotiorum to carbendazim and dimethachlon in Jiangsu Province of China. Australas. Plant Pathol. 2014, 43, 307–312. [Google Scholar] [CrossRef]
- Donaldson, J.G. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003, 278, 41573–41576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, T.; Chen, L.; Zheng, S.; Chen, S.; Zhang, D.; Li, G.; Wang, Z. Arf6 controls endocytosis and polarity during asexual development of Magnaporthe oryzae. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Gamara, J.; Davis, L.; Leong, A.Z.; Page, N.; Rollet-Labelle, E.; Zhao, C.; Hongu, T.; Funakoshi, Y.; Kanaho, Y.; Aoudji, F.; et al. Arf6 regulates energy metabolism in neutrophils. Free Radical Biol. Med. 2021, 172, 550–561. [Google Scholar] [CrossRef]
- Li, R.; Peng, C.; Zhang, X.; Wu, Y.; Pan, S.; Xiao, Y. Roles of Arf6 in cancer cell invasion, metastasis and proliferation. Life Sci 2017, 182, 80–84. [Google Scholar] [CrossRef]
- Schweitzer, J.K.; Sedgwick, A.E.; D'Souza-Schorey, C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin. Cell Dev. Biol. 2011, 22, 39–47. [Google Scholar] [CrossRef]
- Lee, S.C.; Schmidtke, S.N.; Dangott, L.J.; Shaw, B.D. Aspergillus nidulans ArfB plays a role in endocytosis and polarized growth. Eukaryot Cell. 2008, 7, 1278–1288. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.S. Targeted gene replacement in fungi using a split-marker approach. Methods Mol. Biol. 2012, 835, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ao, K.; Tian, L.; Qiu, Y.; Huang, X.; Liu, X.; Hoy, R.; Zhang, Y.; Rashid, K.Y.; Xia, S.; et al. A forward genetic screen in Sclerotinia sclerotiorum revealed the transcriptional regulation of its sclerotial melanization pathway. Mol. Plant-Microbe Interact. 2022, 35, 244–256. [Google Scholar] [CrossRef]
- Yang, C.; Tang, L.; Qin, L.; Zhong, W.; Tang, X.; Gong, X.; Xie, W.; Li, Y.; Xia, S. mRNA turnover protein 4 is vital for fungal pathogenicity and response to oxidative stress in Sclerotinia sclerotiorum. Pathogens 2023, 12, 281. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Smith, D.F.Q.; Casadevall, A. The role of melanin in fungal pathogenesis for animal hosts. Curr. Top. Microbiol. Immunol. 2019, 422, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Mo, C.; Wei, X.; Ma, A. Melanin of fungi: from classification to application. World J. Microbiol. Biotechnol. 2022, 38, 228. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fu, Y.; Xie, J.; Li, B.; Chen, T.; Lin, Y.; Chen, W.; Jiang, D.; Cheng, J. Sclerotinia sclerotiorum SsCut1 modulates virulence and cutinase activity. J Fungi 2022, 8, 526. [Google Scholar] [CrossRef]
- Kim, K.S.; Min, J.Y.; Dickman, M.B. Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol. Plant-Microbe Interact. 2008, 21, 605–612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




