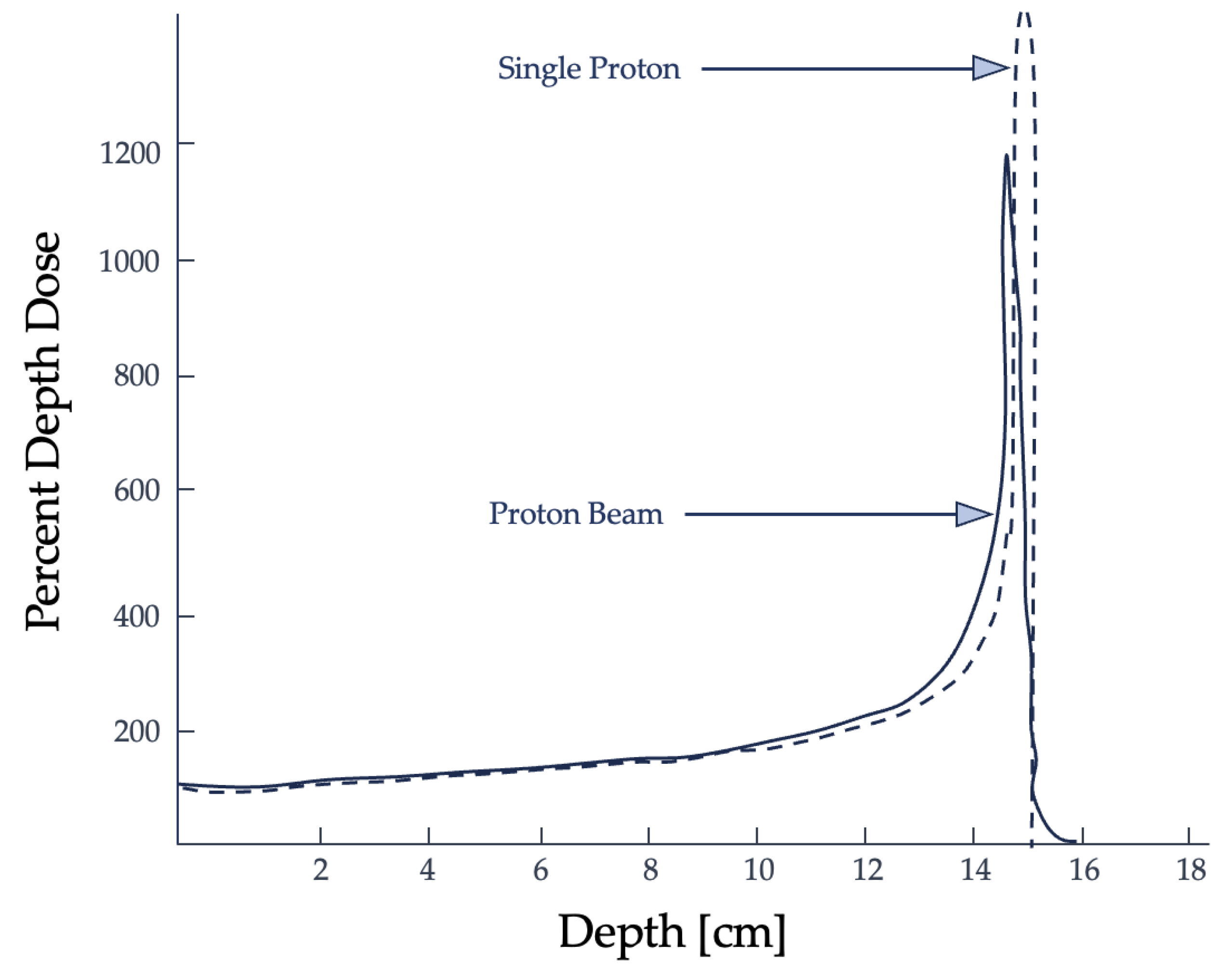
2.2. Ionizing Radiation Interactions
A radiation particle can interact with its environment to lose or deposit all or some of its energy into the medium. If the energy is great enough, the particle can cause an electron of a medium’s atom to be ejected from its orbital shell. The atom is then categorized as ionized and has a negative charge, which can continue interacting with the medium and cause damage. There are two ways for radiation-induced damage to occur: directly and indirectly. Direct action occurs when the projectile directly hits the target (e.g., DNA). Indirect action refers to a particle that hits near the target in the microenvironment, generating free radicals, or ionized atoms, that chemically react with the target [
15]. Free radicals are atoms or molecules with an unpaired orbital electron, making them highly chemically reactive.
Linear Energy Transfer (LET) describes the energy a particle transfers per unit length of a track as it traverses a medium [
2]. High-LET particles, such as heavy ions like alpha particles, cause damage and are more densely ionizing along their paths than that of low-LET particles, such as secondary electrons liberated by x- or gamma-rays [
12,
16]. This can result in more damage produced within the medium through high-density clusters of ionization and biological damage. Research suggests that direct-action damage is the dominant process responsible for space radiation exposure’s more concerning biological effects related to DNA damage and the probability of cell death or misrepair of the strand breaks.
Cellular response following irradiation depends on how the average LET is specified. There are two ways: track-average and energy-average LET. For track-averaged LET, one divides the particle’s path into equal-length segments, then reports the average energy transferred within a segment. For energy-averaged LET, one partitions the path length into equal energy-loss increments and then reports the mean of the iso-energy-loss path lengths [
2,
17]. The choice of the average LET used can sometimes make a big difference. While both averages yield similar results with x-rays and monoenergetic charged particles, neutrons are better described by the energy-average LET [
2]. Furthermore, as LET increases, the variability of an ionizing particle’s lethality across the cell’s cycle decreases so that radiosensitivity appears independent of the cell cycle at higher LET [
18]. Astronauts are exposed to continuous high-LET, at low fluence rates (i.e., low numbers of particles per area of interest) radiation environments for protracted periods. The radiobiological tools used to describe the long-term effects of protracted low-dose exposure are limited, especially when definitions, such as average LET, are inconsistent across different experimental and research analyses.
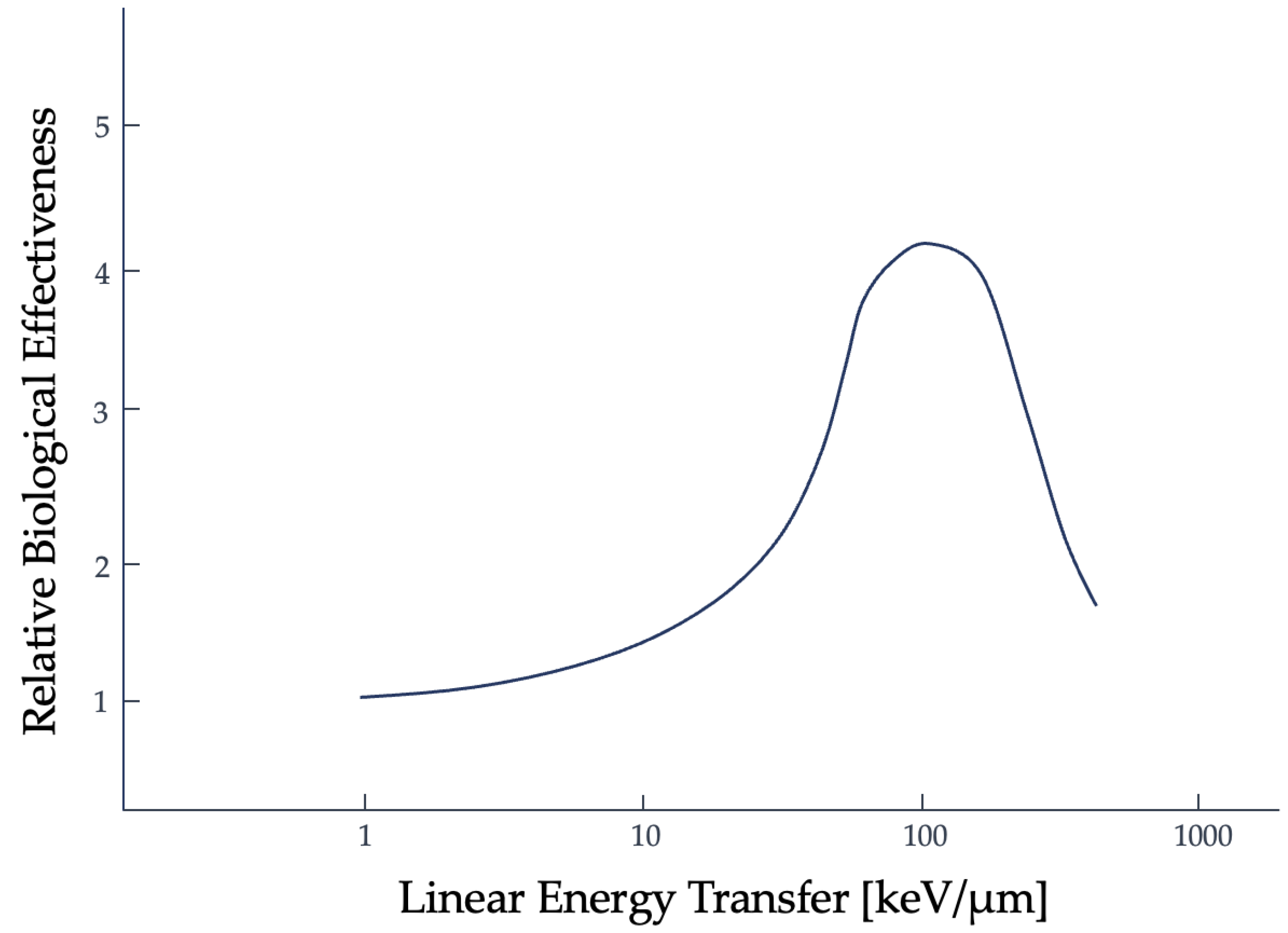
Another tool used to describe the efficacy of a radiation type is the relative biological effectiveness (RBE). RBE is the ratio of absorbed dose of one type of radiation to a specified, standard x-ray radiation (e.g., 250 kVp x-rays), for the same biological effect [
19,
20]. The trend for RBE against high-LET radiation, based on the clonogenic death of mammalian cells irradiated in culture, initially shows a positive correlation to about 100 keV/micron. Past this threshold, an inverse relationship is then seen, as shown in
Figure 2, and has been linked to double-strand DNA breaks [
21]. This is referred to as the overkill effect, or overkill phenomenon: while more LET is effective, the cell is already dead, so the additional energy is wasted [
22].
This simplistic definition of RBE, the "same biological effect” can refer to the likelihood of a particle’s lethality and the probability of producing nuclear DNA double-strand breaks [
22]. RBE can also be a measurement used to describe non-lethal radiation effects outside of DNA double-strand breaks, within the context of space radiation, but the uncertainties are large [
23,
24]. The uncertainties increase directly with increasing RBE [
25]. Instead of use as a definitive indication of whole-organ or whole-body non-malignant pathological outcomes, RBE is better utilized to describe the frequency and presence of lesions created by DNA strands from ionizing radiation that result in the cell culture’s inability to continue proliferating and eventual death [
23].
RBE and its uncertainties are critical aspects of clinical radiotherapy treatment planning [
25]. The error within RBE is due to the variability in stochastic processes (i.e., those involved in measuring cellular damage) and radiosenstivity (i.e., affected by cell type, radiation type, and microenvironment). Even with empirical evidence, RBE is roughly estimated because of these factors [
25]. Too large of an error in the RBE allocation can result in an underdosage or overdosage of the tumorous or normal tissues [
26]. The guidelines for RBE are partly set by the International Commission on Radiation Units and measurements (ICRU) and by a country’s legal limitations [
26]. ICRU report 78 requires that the contribution of error to the prescribed dose by RBE must fall within -5% to +7% and that an RBE of 1.1 is used for proton therapy at all dose levels, regardless of the factors present contributing to the variability [
27].
The conventional constant value of RBE for higher LET radiation and of protons (which are categorized as low LET radiation), is argued but there has yet to be a definitive reason for the deviations in dose per fraction seen experimentally [
26]. Between acute reaction (i.e., caused by cellular depopulation) and late reaction (i.e., from chronic inflammation) tissues, proton RBE data from in vitro and acute-reaction in vivo experiments are more likely to underetsimate RBE in late-reacting tissues [
26]. This is especially seen more heavily at lower doses per fraction.
Incorrect dosages given in the clinic could have significant biological consequences, especially in the probability of causing late-developing malignant (i.e., cancerous) or non-malignant (e.g., progressive fibrosis, vascular insufficiency, etc.) diseases in treated patients [
26]. This is also a concern in the realm of space radiation exposure. The reason for the variability in RBE per fraction seen experimentally is not definitively known. One possible reason could be that the technological advancements in treatment planning were not able to easily adjust for a variable RBE at the time the guidelines were developed [
26].
Another reason could be that there exists an incorrect labeling of the “sensitive volume” within the cell. In the following section, the concept of a “sensitive volume” and the assumptions made regarding radiation effects will be discussed in the context of radiobiological model development. Based on the simplified experiment that determined nuclear DNA as the primary target, or “sensitive volume”, of ionizing radiation, there is a variability in experimental results that has yet to be well-defined. The error in radiation effects and RBE seen clinically and experimentally could be from this lack of definitive evidence that there is only one target and it is nuclear DNA. This possibility that there could be multiple sensitive volumes that are responsible needs to be further investigated.
Figure 2.
Relationship between RBE, clonogenic cell death, and LET for mammalian cells with x-ray irradiation [
21]. Here, it can be seen that around 100 keV/micron, along the LET axis, there is a peak after which the RBE not only fails to increase but declines. Figure adapted from Okayasu [
21].
Figure 2.
Relationship between RBE, clonogenic cell death, and LET for mammalian cells with x-ray irradiation [
21]. Here, it can be seen that around 100 keV/micron, along the LET axis, there is a peak after which the RBE not only fails to increase but declines. Figure adapted from Okayasu [
21].
2.3. Radiobiological Numerical Models
The cell is the fundamental building block of human tissues and organs. Development of the first models describing the biological effects seen following ionizing radiation exposure began before the “sensitive volume” within the cell was identified. Soon after the first radiographic image was taken, scientists attempted to model and explain the physiological, biological, and chemical phenomena at the subcellular level following irradiation to an organic medium [
6]. The first application of target theory was developed in 1924 by Crowther and improved upon by Lea. Target theory dominated the field of radiobiology until the 1980s and had two subcategories: the Single-Target Single-Hit (STSH) model developed by Crowther and the Multi-Target Single-Hit (MTSH) model from Lea [
28]. The term "hit" refers to the ionizing radiation particle interacting with the medium and depositing dose into the sensitive volume within the target cell. Crowther found an exponential loss in biological activity following exposure to ionizing radiation [
29,
30]. This biological activity, also referred to as cell survival, refers to the cell’s ability to continue proliferating after irradiation, and is given by
with
for the initial percentage of viable cells, V as the “sensitive volume”, and the ionization density, I, determining cell survival. An exponential relationship between radiation exposure and cell survival was expected. It was assumed that the administered radiation would enter the sensitive volume V and inactivate the cell. Crowther’s method used roentgens, a unit that better described the ionization of air particles and did not hold for condensed media [
29]. Eukaryotic cells are composed of organelles communicating with the cell’s internal and external environments. Exposure, or the ionization of air, was a reasonable quantity for the intent and purpose of Crowther’s experiments, but the quantities defining air effects are insufficient in describing complex biological damage.
Lea extended the sensitive volume concept within target theory with the (MTSH) model. He theorized the inactivation of the tested organic samples was related to the formation of lethal mutations and that there were multiple targets within V [
31,
32]. From irradiating bacteria and viruses, he made assumptions that cell killing was a multi-step process: there needed to be an absorption of energy within a sensitive volume, lesions in the cell are created by energy deposition, and in a subsequent step, these lesions resulted in the cell’s inability to proliferate [
5,
31]. Target theory models successfully described radiation effects in some microbiological systems but failed to describe radiation effects seen in higher plant types and mammalian samples [
5,
23].
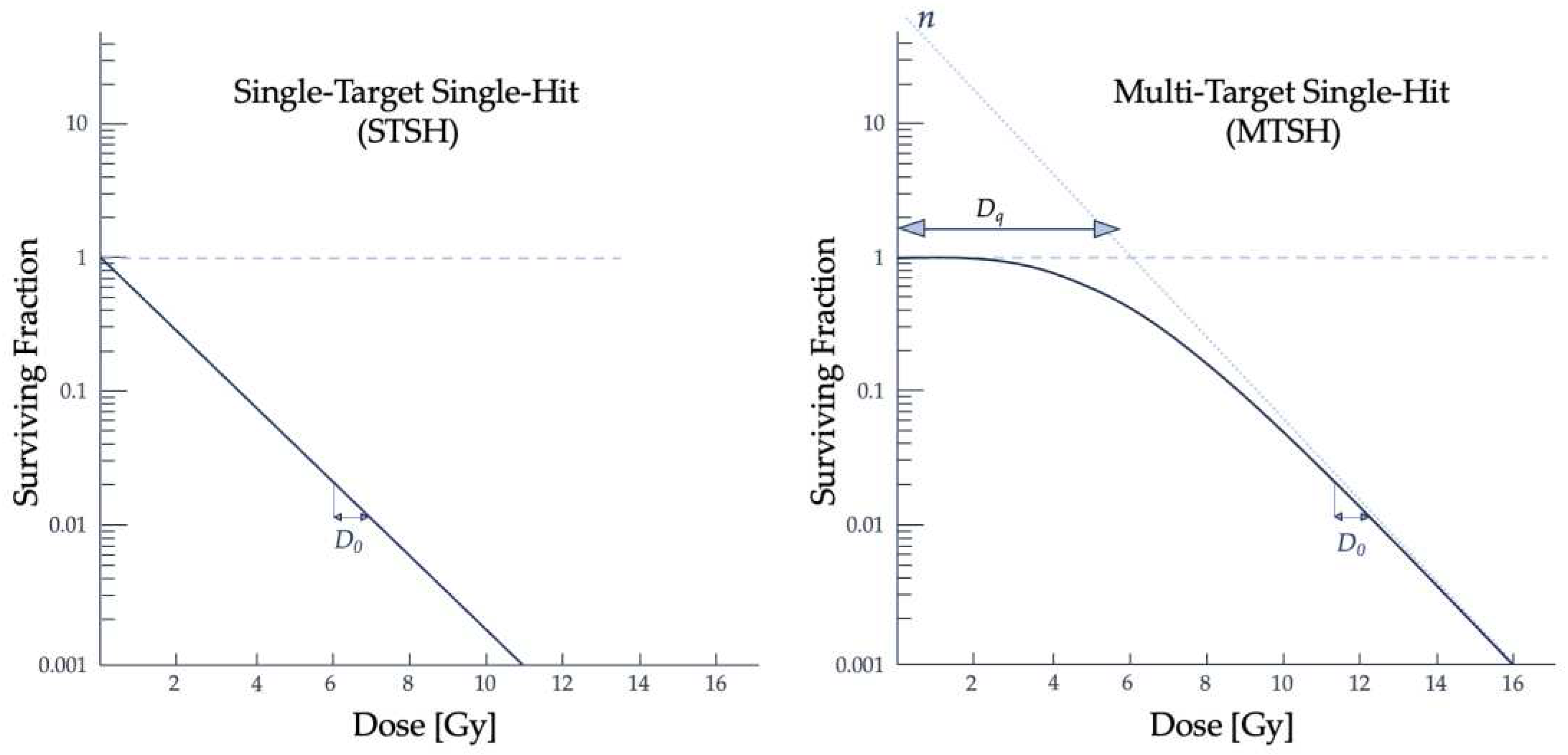
It was experimentally seen that more complex cells had a higher radiosensitivity than bacteria and viruses and that an initial dose to a sample does not always result in an exponential relationship with clonogenic cell survival. The range of dose where there is a delay in lethal damage to the cell is known as the quasi-threshold dose (D
q) and describes the "shoulder" of the cell survival curve, which can be seen in
Figure 3 [
12]. Building from the fundamental assumptions that each ionization causes damage to the molecular structure, Lea derived an equation that involved the molecular mass instead of a volume and D
q, which together describe the shoulder portion of the survival curve [
29]:
where D is dose, M is the molecular mass, and N is the number of hits within the target. This model assumed that no "hits" meant cell survival, that each target had an equal probability of being hit by ionizing radiation, and that a single hit was enough to inactivate the target [
28,
31]. The MTSH model appropriately follows experimental data in high dose ranges and is described by single- or multi-event killings, shown by the curved and linear portions of the curve, respectively [
31].
Radiosensitivity may vary depending on the period in the cell cycle when the radiation is received, the dose rate, and the microenvironment where the ROS are created [
2,
33]. Cell proliferation occurs via a cycle of mitosis, cell division, and DNA synthesis [
34]. Since radiosensitivity is independent of the cell cycle at higher LET, the models used to explain cell reactions to ionizing radiation are limited to abnormal tissue behaviors, such as the cancerous cells, which have a faster proliferation rate—again, cementing these models’ application in the clinical setting [
21]. The single- and multi-target models’ limitations are that they do not match experimental data in the lower dose range. It was expected that there would not be a shoulder to the curve in a lower dose range, whether for radiotherapy or space radiobiology purposes, but there is one present.
Chadwick et al. 1973, was one of the first to incorporate subcellular components into the numerical modeling. This approach was termed the molecular theory and encompassed a broad class of radiobiological models considering subcellular processes in a cell’s reaction to irradiation. This model allowed insight into the radiobiological variability seen in irradiation experiments and assumed that damage to the critical structures within the cell affecting reproduction was to the double-strand nuclear DNA [
18,
35]. It was hypothesized that what was seen experimentally was due to the cell’s ability to repair DNA damage from irradiation and combat the lethality of the administered dose. Single-strand breaks would be appropriately repaired, while double-stranded DNA damage would likely lead to permanent cellular damage [
18]. The purpose of the molecular theory model, shown in Equation 2, was to connect the physical and biochemical experimental observations; however, it bypassed several intracellular molecular functions and focused on repair mechanisms specific to nuclear DNA [
35].
Here, p is the proportionality factor connecting DNA double-strand breaks to cell death, fx is the proportion of DNA double-strand breaks not repaired, nx is the number of sites, kx is the dose per site needed to result in double-strand breaks, Δ represents the probability of a single event double-strand break, ε is the proportion of bonds broken to be DNA double-strand breaks, and D is the dose administered.
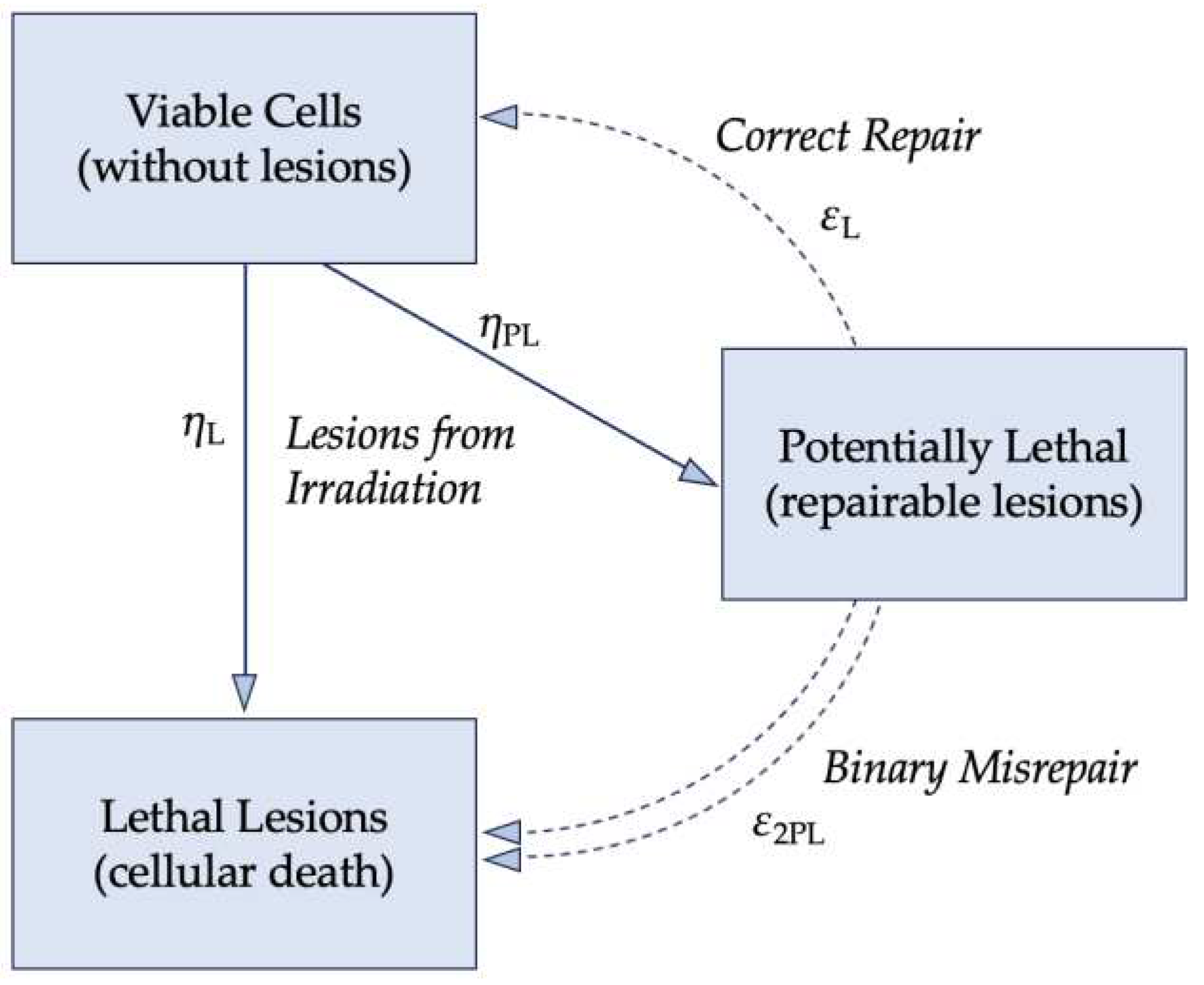
The lethal-potentially lethal (LPL) model, formulated by Curtis, further built upon the foundation that nuclear DNA is the primary target of ionizing radiation.
Figure 4 visualizes the LPL model with η as the implicit dose rate and ε as the repair rate, which is assumed constant; the repair mechanisms work at a fixed rate regardless of the concentration of damaged DNA. Potentially-lethal (PL) lesions may be repaired and return the cell to the viable, pre-damaged state or are lethal (L) lesions that result in cell death. The numerical description of the LPL model is shown in Equation 3. While ε and η are implicitly the repair and dose rates, respectively, t
r is the total repair time available after exposure to ionizing radiation.
linear quadratic (LQ) model was subsequently derived. This model has several limitations that have continued into the development of modern numerical models. Although these models hold for the irradiation of individual cells in vitro and in vivo, there are limitations in the validity of these models at low dose rates [
12]. Furthermore, the time dependence is implicit and may only describe the presence or absence of cell inactivation [
12,
37]. The experimental data described by this model was obtained from yeast, bacterial, and viral samples irradiated in vitro and is defined by the following equation:
with S as the percentage of irradiated cells that are able to continue proliferating and D for the total radiation dose administered [
28,
32]. The αD component describes the single hits on the DNA strands, while the βD
2 term describes multiple hits [
28,
32]. The behavior of the LQ model equation should result in a continuously curving relationship, which does not match experimental data for prolonged radiation dosage since there is a linear portion of the curve present [
32]. The curve in
Figure 5 shows the relationship between the dose given and the resulting proportion of surviving cells and the difference in radiation effectiveness for clonogenic death at varying dose levels and for particle types.
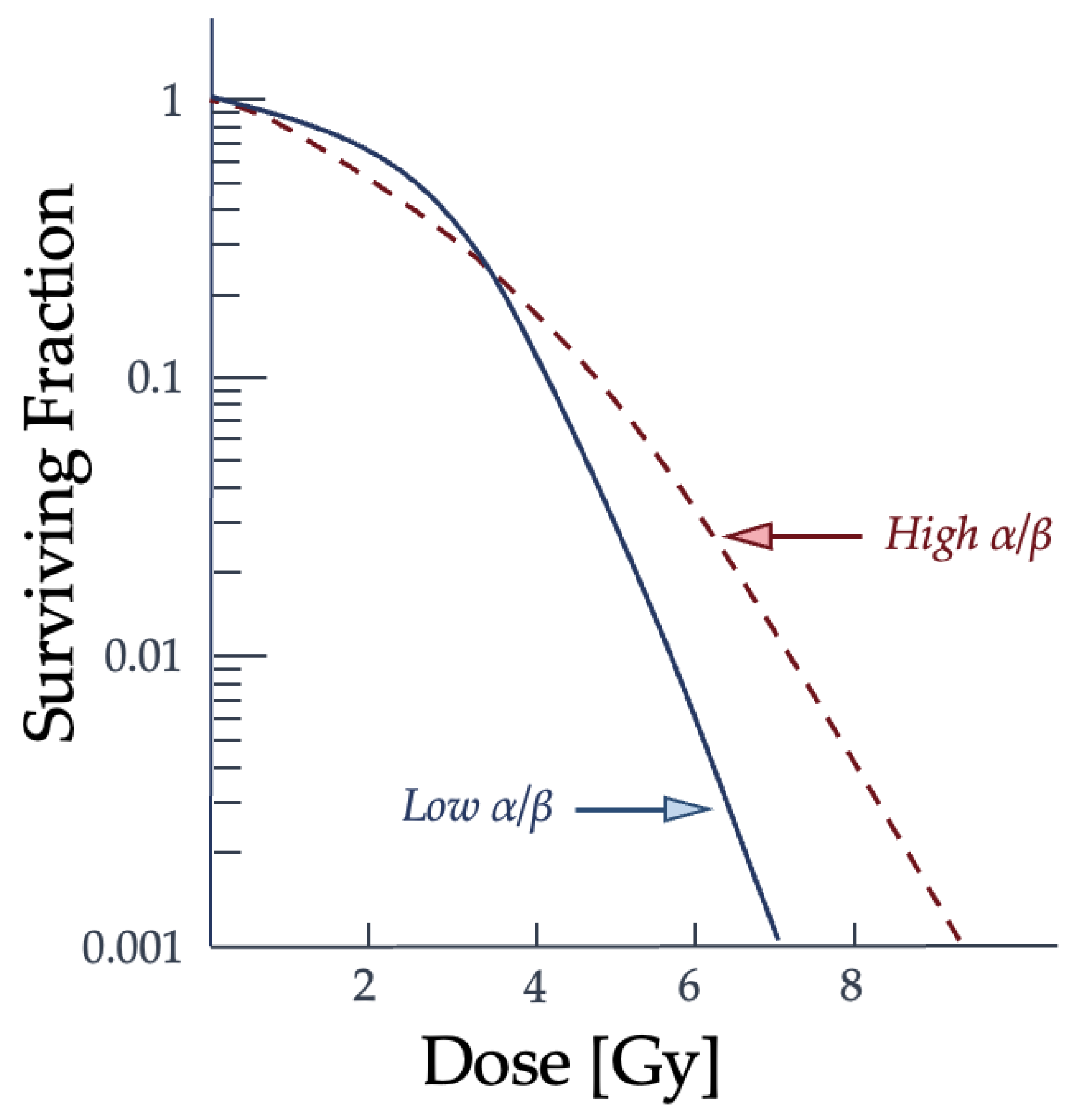
The α term is also used to refer to lethal damage from a single particle and the β term is reserved for the accumulating lethal damage caused by more than a single particle track [
32]. The α/β ratio corresponds to the dose at which each type of damage is equal and is often used to characterize the radiosensitivity of cell lines. Low α/β ratio cell lines have a more pronounced curvature to the cell survival graph, while higher α/β ratio cell lines show a more constant rate of cell-killing as the dose increases [
32]. How a cell survival curve, or response curve, is produced and whether the cell line is "high α/β" to "low α/β" is contingent upon the radiation conditions and potentially the microenvironment and type of cell. Changes to the cell cycle and target environment for low-LET radiation have been shown to cause shifts in the cell cycle and change a cell population from "high α/β" to "low α/β" [
32]. This comparison of how different cell line types may result in a difference in the resulting cell survival curve can be seen in
Figure 6.
As the dose increases, the surviving fraction decreases, but the severity and concentration of double-strand breaks vary between types of radiation and cell lines. The α/β ratio describes the type of damage the ionizing radiation is capable of at each dose in relationship to the cell line type and is reflected in the curvature of the graph [
32]. The α term is defined as the probability of cell death from a single incident particle causing lethal damage, while β reflects the probability of lethality from multiple hits [
32]. It should be noted that cell survival curves for individual cells, or asynchronous cell populations, differ from the irradiation behavior of synchronous cells, such as tissue. As a result, the LQ model has very low accuracy when describing impacts on cellular systems. Further evidence is needed to support how damage to subcellular structures beyond nuclear DNA, the cell’s type, or cellular microenvironment can alter the α/β ratio of a cell population. More models emerged from the LQ model to explain the biological phenomena of cell survival and to try and improve the accuracy, such as the repair-misrepair (RMR) model, the saturable repair model, two-lesion kinetic model, and repair-misrepair-fixation model [
28,
31]. These models failed to directly link radiation damage to double-strand breaks for all radiation and cell line types [
38].
Figure 6.
As the dose increases, the surviving fraction decreases, but the severity and concentration of double-strand breaks are variable between radiation types and cell lines. The lower an α/β ratio is, or higher the particle’s LET, the more likely double-strand breaks from a single particle interaction will occur when it traverses the biological medium [
32]. Graph adapted from McMahon [
32].
Figure 6.
As the dose increases, the surviving fraction decreases, but the severity and concentration of double-strand breaks are variable between radiation types and cell lines. The lower an α/β ratio is, or higher the particle’s LET, the more likely double-strand breaks from a single particle interaction will occur when it traverses the biological medium [
32]. Graph adapted from McMahon [
32].
The two-lesion kinetic (TLK) model aimed to connect the biochemical processes of double-strand break repairs with an ionizing radiation’s lethality [
38]. This model considers the variability in cellular DNA repair mechanisms and that these repair systems saturate the microenvironment at higher dosages. The TLK model also differentiated between the two types of DSBs and, while it is similar to the LPL and RMR models, it accounts for the local complexity of the damaged site [
38]. It can incorporate more parameters into its formalism, allowing for better agreement with experimental data and introducing additional complications.
The repair-misrepair-fixation (RMF) model combines the LPL and RMR models with microdosimetry concepts into their predictive model [
39]. By this combination, the RMF model considers the intra- and inter-track binary misrepairs of DSBs and relates this damage to RBE [
40]. However, as previously discussed, RBE is inadequate for describing radiation effects beyond the particle’s lethality. Furthermore, the RMF model assumes that the interactions of ionizing radiation with nuclear DNA resulting in double-strand breaks affect the nucleus as a whole [
39].
There is a continued attempt to incorporate more modern tools into the radiobiological models. The Monte Carlo damage simulation (MCDS) has been combined with the RMF model, dosimetric data, and a Monte Carlo radiation transport model to improve the formalism for cell survival prediction [
41,
42]. This method can predict some double-strand and single-strand break and repair behaviors and can be applied to hypoxic microenvironments with differing types of ionizing radiation. Additionally, the local effect model (LEM) and the microdosimetric kinetic model (MKM) are two that aim to directly correlate energy deposition and subsequent cellular effects [
32]. These models were developed to connect the deposited energy with the radiation-induced biological effect, though their usage remains primarily limited to radiotherapy. The field of space radiobiology currently relies on the LQ model, and the potential for applying these newer models has yet to be seen. Importantly, radiobiological models are built on the hypotheses of Crowther and Lea and the common assumption that nuclear DNA is the only target of concern when studying radiation effects. However, if this were the case, it can be argued that the survival curves of different cells should be very similar in shape and slope.
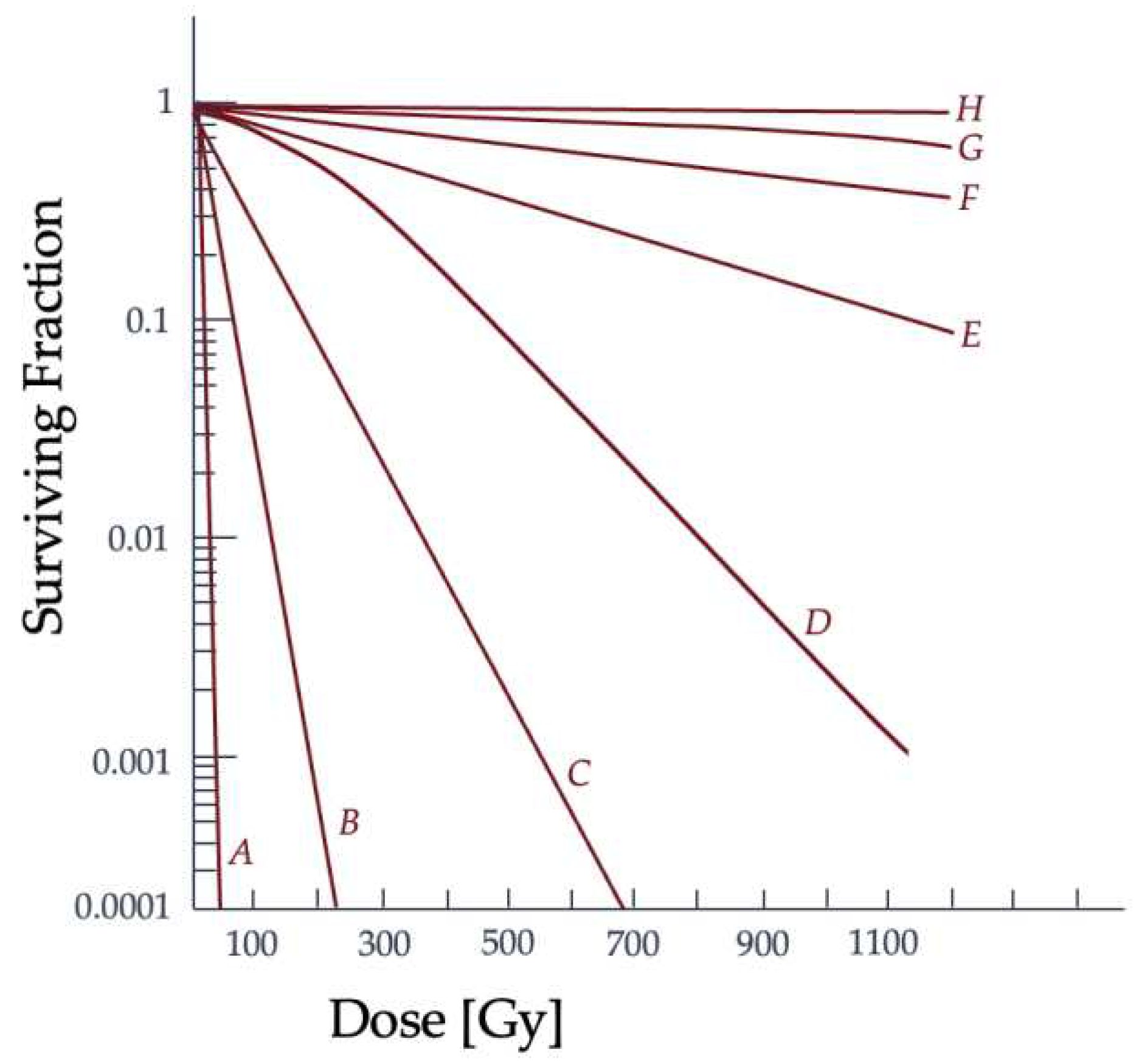
In vitro radiation studies of irradiating cancerous and noncancerous mammalian cells have confirmed higher radiosensitivity than bacteria and viruses, shown with an increased slope in their survival curves [
43]. The resulting survival curve comparison between cell types can be seen in
Figure 7, where there are distinct slope and shoulder width differences. While prokaryotic cells lack distinct nuclei and organelles, eukaryotic cell structure is much more complex [
44]. Mammalian cells have a nucleus and most contain mitochondria, with mitochondrial DNA, as well as other large subcellular structures. Since the radiosensitivity of mammalian cells is greater, there could potentially be additional targets within the cell outside of nuclear DNA, a theory in need of further investigation [
43].
Leaders of the early models stated that more survival curve analyses were necessary to prove conclusively that nuclear DNA is the primary target of radiation. The need remains. Recently, space radiation protection and guidelines noted a need for more data collection on subcellular targets radiation effects, and for the validation of computational transport models [
7,
28,
32].
2.4. DNA Repair Mechanisms
DNA comprises two strands of a sugar-phosphate backbone and is connected by four nitrogen base pairs (bp) [
2]. The order and pairings of the bases and these chains of molecules determine the task for each cell and the overall genetic blueprint of the organism [
46]. AP, or apurinic and apyrimidinic, sites are where lesions are present and can hinder DNA replication and transcription processes [
47,
48]. Ionizing radiation interacting with an organism’s cellular structures commonly triggers a stress response in which the free radical production increases, altering the redox state, and/or cellular homeostasis [
49]. The presence and elimination of free radicals are part of an organism’s normal biological function, even in the absence of irradiation. The chain reaction of free radical mechanisms converts nutrients into chemical energy. It maintains redox homeostasis, the cellular function of response and feedback, and is part of maintaining a physiologic steady state [
49]. However, when radiation exposure triggers a stress response, tissue inflammation can occur to remove diseased and damaged cells and prompt tissue repair mechanisms. During prolonged, continuous exposure to ionizing radiation, the biochemical processes that maintain homeostasis can malfunction, depending on the dose rate. This altered cellular environment affects subcellular response, leading to more than an acceptable amount of DNA misrepair, secondary oxidative stress responses, deficiencies in DNA repair enzymes, mutations, and can ultimately result in cell death [
49,
50].
Changes to the cellular microenvironment from ionizing radiation interactions can damage subcellular structures like nuclear DNA by creating reactive oxygen species (ROS) or free radicals. ROS can be in the form of superoxide anion (O
2-) and the hydroxyl radical (OH
-) and subsequently form the hydrogen peroxide molecule (H
2O
2) [
51]. ROS are involved in a cell’s normal function. Approximately 104 lesions per cell from endogenous ROS formed in normal cellular processes are expected to occur daily [
52]. Ionizing radiation adds to the number of lesions present, or damage load, and at large doses may overwhelm the cell’s antioxidative defenses [
53]. These free radicals can be created directly from ionizing radiation interactions and come from the cell’s response to repair the damage from those interactions. As the organism ages, the lesions present on the DNA strands may also accumulate, and if misrepaired, these damaged sites can lead to DNA mutations and dysregulated cellular function [
54]. Cell survival experiments suggest that the cell’s repair mechanism’s effectiveness decreases with increasing LET [
42]. At the same dose rates, high-LET particles cause more oxidative clustered DNA lesions than low-LET radiation sources, since there is a higher percentage of non-repairable and more complex double-strand breaks present [
55,
56]. There are observed repair mechanisms supporting some of the postulated radiobiological models. Once a single-strand break (SSB) or double-strand break (DSB) has been created in the target, processes are simultaneously triggered to repair the breaks.
Between low- and high-LET damage to DNA, the resultant type of lesion will cause the cell to favor one repair pathway over the other [
50]. Depending on the distance between lesions along the DNA strands, the resulting breaks are categorized as DSB or SSB. For example, the term "clustered DNA lesions" is ascribed to multiple damaged sites within 20bp of each other; these can be caused by endogenous or exogenous sources, such as ionizing radiation, normal cell function, or chemical toxicity [
54]. When such multiple damaged sites are bunched within a short length, they may be more difficult to fully and faithfully repair through homologous recombination repair (HRR) since more of the DNA sequence will likely be lost, making clustered DNA lesions the most lethal form of all DNA damage caused by ionizing radiation [
57]. Nonhomologous end-joining (NHEJ) is suggested to be the primary repair mechanism for high-LET DNA damage [
50]. DSBs induced by ionizing radiation have blunt double-strand ends or short single-strand ends, which can be repaired by NHEJ [
58].
Compared to DSBs, SSBs with ample space between events have a higher chance of repair. The most common repair method for SSBs is base excision repair (BER), which is an epigenetic regulation of gene expression [
59,
60]. BER restores the complementary nature of the bases in opposite DNA strands and is the most versatile [
47,
48,
57,
61]. For a BER process to be successful, it needs to result in no significant change to the nuclear DNA strand’s radiosensitivity [
52]. Low-LET is more likely to cause such sparsely clustered lesions, and this type of damage can utilize either NHEJ or HRR, whereas high-LET interactions and damage tend to be more densely clustered and, therefore, more complex in their repair [
50]. Notably, the impact of clustered damage and DSBs on the cell’s ability to proliferate depends on the efficacy of the cell’s repair mechanisms, the dose rate, and the type of radiation [
42].
2.5. Mitochondrial DNA
Recent research suggests a link between mitochondrial DNA (mtDNA) and radiation effects, but the more commonly used radiobiological models, like the LQ model, do not take mtDNA into account [
62]. mtDNA plays a significant role in the function of a mammalian cell and is the primary oxygen-consuming source of cellular energy [
63]. Its importance disputes the assumption that nuclear DNA is the only subcellular structure of interest in radiobiological models [
62]. The primary role of mtDNA is to prepare for oxidative phosphorylation, a more efficient metabolic state for generating cellular energy [
63]. In cases of repair, the microenvironment of the mitochondrion differs from that of nuclear DNA [
52,
64]. It should also be noted that the metabolism of mitochondria has been implicated in bystander radiation effects, but more research is needed to confirm their direct link [
62].
Different cell types can be more susceptible to oxidative damage. mtDNA is more vulnerable to oxidative damage and will mutate at a greater rate than nuclear DNA when damaged. This is because their proximity to the electron transport chain increases the chance of accumulating toxic ROS [
52]. When the cellular environment’s redox stasis is imbalanced with an increased ROS level, this can lead to mitochondrial dysfunction and trigger intra- and extracellular distress signaling [
63]. Oxidative stress, cellular respiration levels, and the mitochondrion’s metabolism responds to the environment by undergoing a morphological change to regulate its repair. The mitochondrial double-membrane can go through fission and fusion actions to restore its function. The fission process allows the isolation and separation of the damaged proteins within the organelle.
In contrast, the fusion process mixes partially and fully functional mitochondria to create more fully functional ones [
63]. Because of the high consumption and production of oxygen species present when compared to other cell types, neuronal and muscular cells are more susceptible to oxidative stress effects [
53]. Tissues with a higher concentration of mtDNA are expected to have a higher sensitivity to oxidative damage. They are more likely to result in mutations and deletions involved in ATP production. Adenosine triphosphate, or ATP, is the molecule involved in cellular energy generation and in the production of RNA, or ribonucleic acid, which aids in carrying out instruction from nuclear DNA [
53]. Accumulation of mtDNA mutations may be linked to neurodegenerative diseases, such as amyotrophic lateral sclerosis [
53].
Since each cell has multiple copies of mtDNA, it was suggested that strand breaks might not affect overall mitochondrial function [
65,
66]. While not as thoroughly researched as nuclear DNA repair, studies of mtDNA show similar repair mechanisms. The typical nuclear DNA DSB repair pathway NHEJ was undetectable in mammalian mitochondria, but microhomology-mediated end joining, or MMEJ, was active [
67]. MMEJ is a DSB repair mechanism that employs microhomologous, or similar short sequence base regions [
68]. Ionizing radiation causes oxidative stress, leading to mtDNA mutations and deletions. It is suggested that the damaged molecules undergo degradation after a DSB in mtDNA. This will only happen if a small amount of mtDNA is damaged, because certain cell types can have up to thousands of mtDNA molecules and the loss of a few would not compromise function [
69]. So, the outcome of compromised cellular operation due to mtDNA damage, as suggested, is unlikely but still possible.
Note that the ISS is within the LEO and those aboard the station benefit from the added radiation protection that the Earth’s magnetosphere provides [
70,
71]. This study of the twins did not conclude that genes altered in-flight, compared to pre-flight and post-flight samples, and had increased levels of DNA damage response (DDR), which describes the cell’s process to repair and replicate DNA and continue through cell-cycle checkpoints, pathways present [
71]. The study instead saw changes to mitochondria within the subjects. The levels of mtDNA present within the subject aboard the ISS were higher than the pre-flight and post-flight sampling [
71]. There is a positive correlation between time spent on the ISS and the concentration of mtDNA in the subject’s blood sample. The presence of mtDNA within the blood is possibly linked to inflammation, a typical result of radiation exposure [
72]. With a limited testing pool and bias towards Caucasian middle-aged men, the result of this 2019 study implies the effects of prolonged exposure to ionizing radiation. Still, additional research is needed to conclude the causation of these physiological changes in post-flight samples.
As previously stated, there are differences in the microenvironments surrounding mtDNA and nuclear DNA, as well as differences in their composition and damage repair. Nuclear DNA is linked with histones and chromatin-associated proteins that are involved in scavenging free radicals [
52,
73]. Although mitochondrial repair proteins are imported from the nucleus, mtDNA strands lack these scavengers. Furthermore, mtDNA has a higher density of coding sequences related to ATP production, which, if altered, affects overall cellular function. The danger arises if a damaged genome results in impaired oxidative phosphorylation and defective ATP production [
74]. With a decrease in ATP production comes an increase in ROS production, which can trigger and accelerate the progression of different mammalian diseases [
75].
This damage to mitochondria and mtDNA can be more extensive than seen in nuclear DNA [
73]. Once oxidative stress damages mtDNA, it lingers much longer than nuclear DNA damage and is more destructive. As a result of these differences in the presence of oxidative stress response, BER is the primary repair process available for mtDNA [
52]. Though this is a repair mechanism for SSBs, it is still able to remove and repair deaminated and oxidized DNA bases [
76]. It excises smaller DNA lesions caused by stressors, but most lesions induced by ionizing radiation are larger double-strand lesions that are irreparable or severely complicated to repair. Thus, the maintenance of mtDNA is vital because of the risks involved with untended mutations.
Furthermore, it has been shown that when the cytoplasm of a more complex mammalian cell is altered or damaged, it can cause changes in mitochondrial function [
77]. The component primarily involved in the process of mitochondrial fission is dynamin related protein 1 (DRP1). This protein activates the autophagy process of the cell, which is also oxyradical-dependent [
77]. This action, where the dysfunctional mitochondria are isolated and degraded by the autophagy process, is thought to protect surrounding structures from the subsequent effects of irradiated cytoplasm. Additional research is needed to confirm the roles each subcellular organelle and gene expression play in radiation effects, since even cell cytoplasm alterations can damage the organism [
77].
2.6. Epigenetics
Besides direct DNA damage, it is becoming increasingly recognized that radiation exposure can also affect DNA and histone modifications, i.e., methylation. These modifications, generally known as epigenetic, are the key regulators of the expression of genetic information. DNA methylation is the most studied epigenetic modification of DNA, where the methyl group is bonded to the 5th position of carbon in the process facilitated by the enzymes called DNA methyltransferases and methyl-binding proteins [
26].
Evidence accumulated through the last several decades convincingly demonstrates the potential ionizing radiation holds toward affecting DNA methylation patterns. In rodent models, whole-body exposure to either γ radiation or x-rays at doses of 1 Gy and above usually results in the loss of global DNA methylation in many organs and tissues within hours of irradiation [
78,
79,
80]. This effect may persist, typically in target organs for radiation-induced carcinogenesis, i.e., in the hematopoietic system (hematopoietic stem and progenitor cells, thymus) and mammary gland [
78,
81]. Loss of global DNA methylation in other organs (i.e., muscle or lung) has been shown to have largely transitory effects [
78,
82].
It must be emphasized that the loss of global DNA methylation is generally accepted as a hallmark of cancer [
83]. As persistent DNA hypomethylation after exposure to IR was observed mainly in target organs for radiation-induced carcinogenesis, this led to the hypothesis that IR besides exerting its genotoxic potential, may also cause cancer via an epigenetic mode of action [
78]. While this hypothesis has not been fully confirmed, several mechanisms tightly associated with carcinogenesis provide strong support. For instance, it is generally accepted that loss of DNA methylation usually occurs from otherwise heavily methylated repetitive elements that cover up to two-thirds of mammalian genomes [
84]. DNA methylation serves as a key mechanism of transcriptional silencing for repetitive elements [
85]. For instance, Long Interspersed Nucleotide Element 1 (LINE-1) – the most abundant repetitive element in mammalian genomes – is a retrotransposon whose 5’-UTR sequence is heavily methylated to prevent its aberrant transcriptional activity [
86]. As it covers ~17% and 22% of human and mouse genomes, respectively, loss of methyl groups from its promoter can result in its aberrant expression and retrotransposon activity. The latter is exhibited as a random introduction of its copy elsewhere in the genome. Such aberrant LINE-1 activity can lead not only to genome amplification but also significantly increase probability of mutations, as LINE-1’s copy can be introduced within the open reading frame (ORF) of a gene, thus affecting its transcription [
87,
88].
Besides global DNA hypomethylation, gene-specific DNA hypermethylation can occur due to exposure to ionizing radiation. Such events, if located within the gene promoters, are usually associated with transcriptional silencing, as the acquisition of methyl groups within the transcription start sites precludes the binding of transcription factors in the initiation of transcription. Similarly to global DNA hypomethylation, hypermethylation-induced silencing of tumor-suppressor genes is frequently observed in many cancers, including lung cancer of workers occupationally exposed to radiation [
89,
90].
Interestingly, exposure to high-LET radiation often shows differential patterns of DNA methylation alterations. For instance, several studies demonstrated loss of global DNA methylation in cell culture after exposure to low mean absorbed doses of protons or 56Fe ions [
91,
92]. However, the results of the in vivo studies appear contradictory as we and others observed global DNA hypermethylation that stemmed from both repetitive elements and genes [
93,
94,
95].
Another interesting outcome of high-LET radiation exposure is persistent changes in DNA methylation observed in organs that are considered targets for radiation-induced degenerative disease rather than carcinogenesis. For instance, persistently (i.e., 3-9 months after irradiation) altered DNA methylation was reported in the lungs and hearts of experimental mice after exposure to low mean absorbed doses of protons or heavy ions. These results were observed in several independently conducted experiments utilizing different sources and dose/rates of high-LET radiation [
93,
95,
96,
97,
98].
Opposite to expectations, high-LET-induced DNA hypermethylation of repetitive elements often resulted in paradoxical reactivation of LINE-1 elements [
95,
99]. It is plausible to hypothesize that the complex interplay between DNA methylation and histone modifications, where the latter may "overwrite" the silencing effects of the former, is responsible for this effect [
100]. There is a shortage of knowledge regarding the effects high-LET radiation exerts on histone modifications, and future research is warranted to explore this phenomenon.
Epigenetic effects of exposure to high-LET radiation are much more complex and less understood compared to the effects exerted by terrestrial ionizing radiation. Nevertheless, elucidating epigenetic reprogramming, its mechanisms, and its effects on gene expression offers multiple opportunities to better understand the long-term effects of such exposures. Another important implication of epigenetics in space biology is the potential to utilize the methylation status of selective LINE-1 elements as biomarkers for previous exposures. As evident from the discussion above, exposure to ionizing radiation (including high-LET radiation) leaves scars not only as mutations and irreparable damage on DNA itself but also permanently present alterations of DNA methylation within repetitive sequences (i.e., within the promoter regions of LINE-1 elements). Importantly, these altered patterns of DNA methylation can be detected not only in experimental systems, but also in humans previously exposed to ionizing radiation [
93,
95,
101].