1. Introduction
Hyperhomocysteinemia (HHcys) is a metabolic condition characterized by elevated blood homocysteine (Hcys) levels is implicated in various disorders, serving as a potential risk factor for serious complications [
1,
2,
3]. Despite its recognition, the assessment and management of HHcys remain contentious, marked by conflicting results in studies evaluating its impact on reducing cardiovascular and cerebrovascular disease risks.
Hcys, a non-essential amino acid derived from methionine (Met) metabolism, participates in a complex cycle involving Met and cysteine (Cys) through a transsulfuration mechanism [
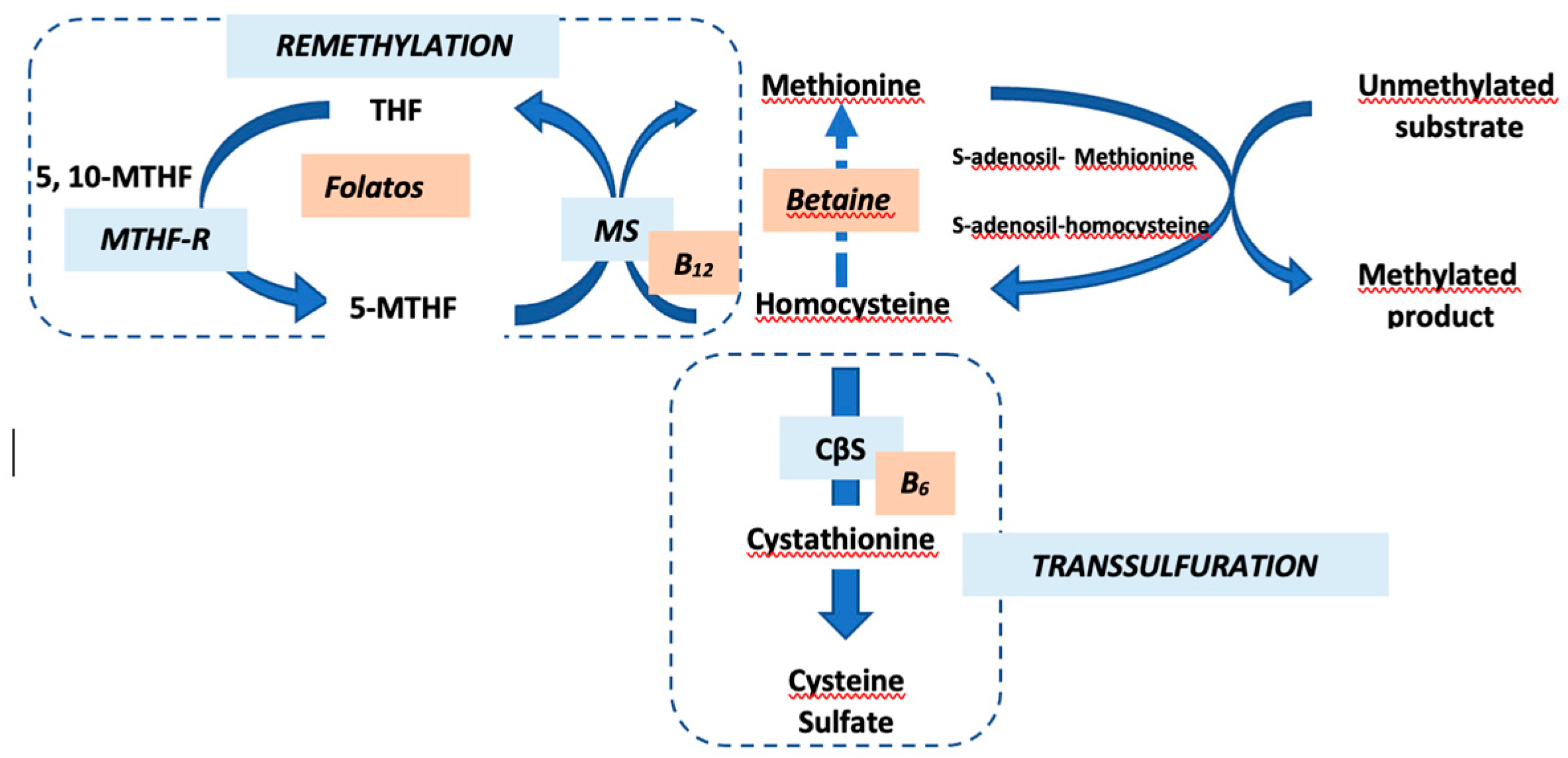
4]. Met, primarily obtained from dietary proteins, undergoes recycling into Hcys via a methyl group donation reaction. The remethylation of Hcys to Met, facilitated by methionine synthase (MS), requires folate derivatives and vitamin B12. The transsulfuration pathway, with vitamin B6 as a cofactor, converts Hcys into Cys and subsequently into sulfate (Figure1).
Figure 1.
Homocysteine (Hcy) metabolism. Hcy is remethylated into methionine (Met) by methionine synthase (MS) in the presence of vitamin B12 and folates; transsulfuration by cystathionine-β-synthase (CβS), whose cofactor is vitamin B6, allows Hcy to be transformed into cysteine (Cys) and then in sulfate. MTHF-R: methylenetetrahydrofolate reductase.
Figure 1.
Homocysteine (Hcy) metabolism. Hcy is remethylated into methionine (Met) by methionine synthase (MS) in the presence of vitamin B12 and folates; transsulfuration by cystathionine-β-synthase (CβS), whose cofactor is vitamin B6, allows Hcy to be transformed into cysteine (Cys) and then in sulfate. MTHF-R: methylenetetrahydrofolate reductase.
Enzymatic deficiencies in these pathways can lead to HHcys, with elevated Hcys levels indicating metabolic dysfunction and predisposing individuals to arterial and venous thromboembolism by damaging vascular endothelial cells [
5]. While some debate the direct link between HHcys and thrombosis risk [
6,
7], it is well-established that lowering Hcys levels reduces cardiovascular risk in classic homocystinuria (HCU) patients, afflicted by a severe deficiency of the CβS enzyme [
8,
9].
Classic HCU patients, marked by CβS enzyme deficiency, often encounter thrombotic or atherosclerotic diseases early in life, alongside neurological, psychiatric, and skeletal complications. Reduction of Hcys levels has demonstrated efficacy in slowing processes related to brain atrophy [
10]. However, a 2017 Cochrane review [
3] suggests that therapies targeting mild forms of HHcys may not significantly impact stroke prevention and minimally affect coronary heart disease prevention, focusing on mild elevations commonly encountered in clinical practice.
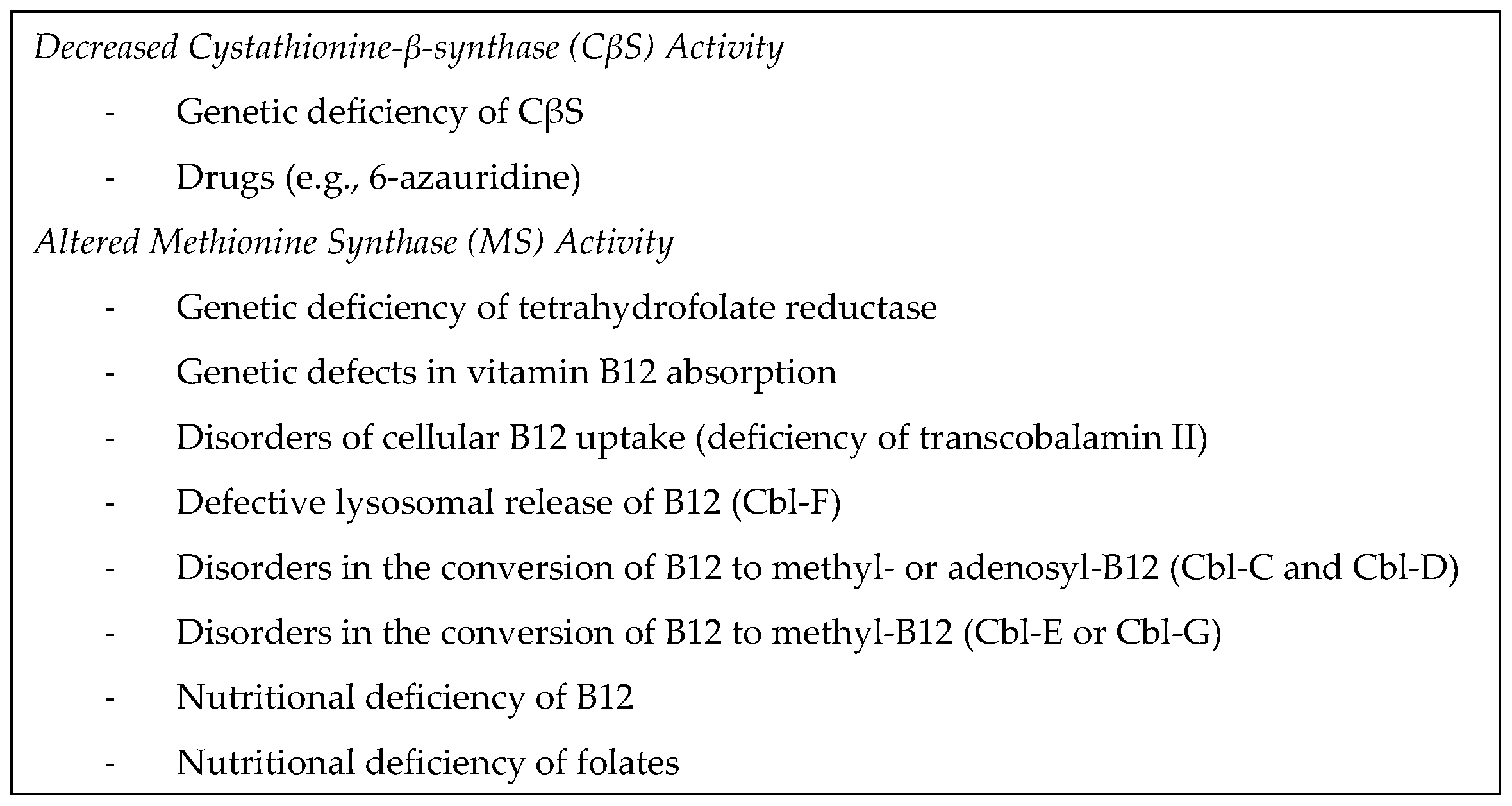
Despite analytical challenges associated with Hcys measurement, disorders in Hcys metabolism manifest through simple assessments of plasma Hcys concentrations. Marked elevations are observed in homozygous CβS deficiency, while more moderate increases occur in heterozygous CβS deficiency and folic acid metabolism disorders like methylenetetrahydrofolate reductase (MTHFR) deficiency. Additional metabolic studies, encompassing amino acids, plasma vitamins, and organic acids in urine, may be necessary for a comprehensive evaluation [
5].
The substantial delay in detecting HHcys, coupled with the absence of standardized therapeutic protocols and proper etiological diagnosis, complicates the management of this condition. This article underscores the need to investigate HHcys in various clinical contexts and advocates for fundamental metabolic studies to identify mechanisms involved in moderate or severe forms, guiding appropriate treatment.
2. Types of Hyperhomocysteinemias (HHcys)
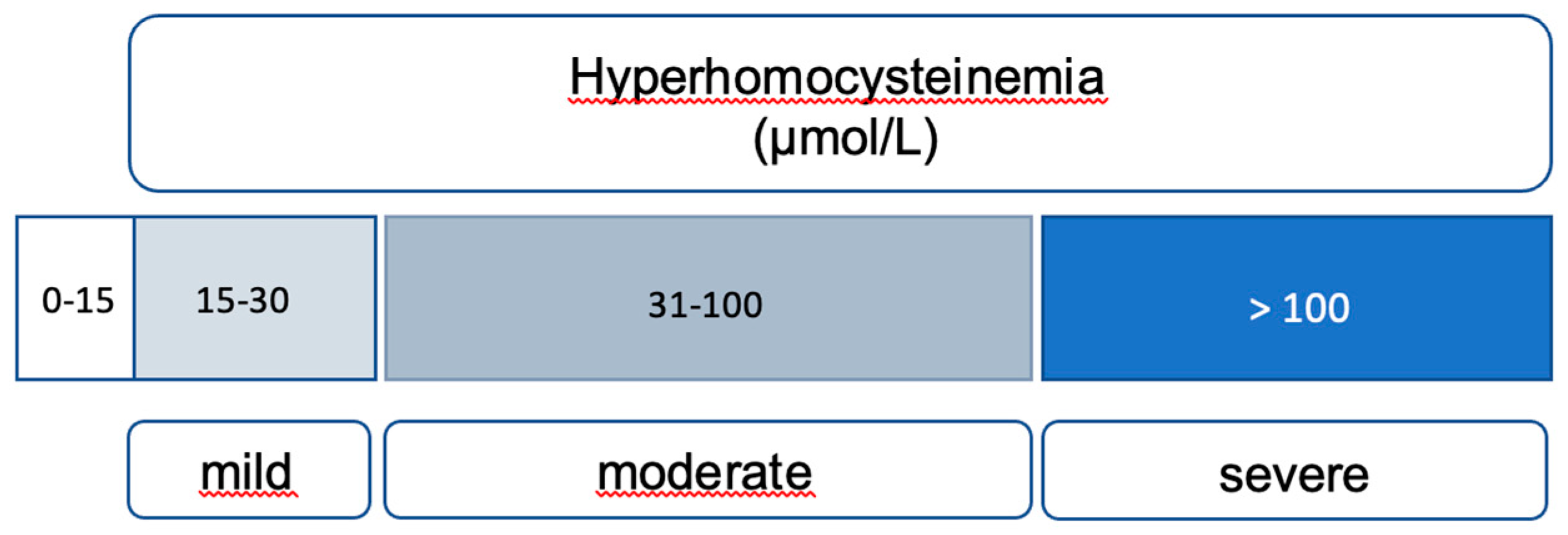
HHcys, characterized by elevated blood Hcys levels exceeding 15 μmol/L, is influenced by a series of reactions involving essential vitamins (B6, B12, and folates) and enzymes, notably MTHFR. Nutritional deficiencies, including alcohol consumption, untreated celiac disease, and prolonged use of proton pump inhibitors, can lead to elevated Hcys levels due to their crucial role in Hcys metabolism [
11,
12]. Elevated Hcys is also associated with various conditions such as cognitive impairment, chronic kidney disease, hypothyroidism, psychiatric disorders, and bone mineralization disorders [
13,
14,
15,
16]. Inborn errors of Hcys metabolism (IEM) result in severe HHcys, categorized based on clinical impact into mild (16 to 30 μmol/L), moderate (31 to 100 μmol/L), and severe (more than 100 μmol/L) forms [
17] (
Figure 2).
Severe forms, including classic HCU, stem from CβS enzyme deficiency, crucial for Hcys transsulfuration, accompanied by hypomethioninemia. Other IEMs leading to severe HHcys involve remethylation disorders, often due to MTHFR enzyme deficiency or defects in enzymes related to vitamin B12. Mutations in the MTHFR gene disrupt folate acid metabolism, resulting in methyltetrahydrofolate deficiency and HHcys with hypomethioninemia. Disorders related to cobalamin (cbl-) metabolism, particularly cblC, combine methylmalonic acidemia (MMA) with HCU [
4,
18]. These disorders manifest with significantly elevated Hcys levels (> 50 μmol/L).
Acquired HHcys may result from lifestyle factors, clinical conditions, and medications. Renal insufficiency and certain medications, including folate antagonists, carbamazepine, nitric oxide, methotrexate, cholestyramine, and niacin, contribute to acquired HHcys [
4,
10,
19]. Acquired forms may remain asymptomatic until the third or fourth decade of life, manifesting as thrombotic events. The recognition of these diverse types of HHcys highlights the need for comprehensive investigation, considering both congenital and acquired factors, to guide appropriate therapeutic interventions [
20].
Table 1.
3. Clinical Manifestations of HHcyss
Disorders affecting Hcys metabolism present a diverse clinical spectrum contingent upon the enzymatic impairment's nature and extent. Moderate to severe HHcys leads to significant morbidity and mortality, affecting multiple organ systems. In IEM of Hcys, symptoms often emerge in early childhood, resulting in neurodevelopmental, skeletal, ocular, and vascular complications.
Classic HCU, a severe form of HHcys due to CβS deficiency, manifests with skeletal anomalies, ocular issues, neuro-psychiatric concerns, and vascular problems. Without timely intervention, it can lead to psychiatric disorders, behavioral issues, and preventable intellectual disability. Recurrent severe vascular occlusions are a principal complication of HHcys, with even moderate elevation in blood Hcys contributing independently to vascular diseases. Hcys serves as an atherogenic risk predictor and potent prognostic indicator for adverse cardiovascular events [
19,
20,
21,
22], emphasizing the clinical significance of HHcys and the importance of interventions to mitigate risks and improve outcomes [
18]. Clinical suspicion arises with moderately elevated (31-100 μmol/L) or severe (>100 μmol/L) Hcys levels, and definitive diagnosis involves identifying mutations in the CβS gene [
23].
Other causes of HHcys include disorders affecting Hcys remethylation and sulfur amino acid metabolism. Vitamin B12 metabolism abnormalities, folate metabolism issues (MTHFR gene mutations), and abnormalities in MS metabolism contribute to HHcys. CblC deficiency exhibits neurological, ocular, and renal symptoms. MTHFR deficiency manifests with progressive encephalopathy, epilepsy, and psychiatric disorders. MS deficiency leads to megaloblastic anemia, intellectual disability, and psychiatric disorders. Understanding the varied clinical manifestations of HHcys and its underlying causes is crucial for effective diagnosis and intervention.
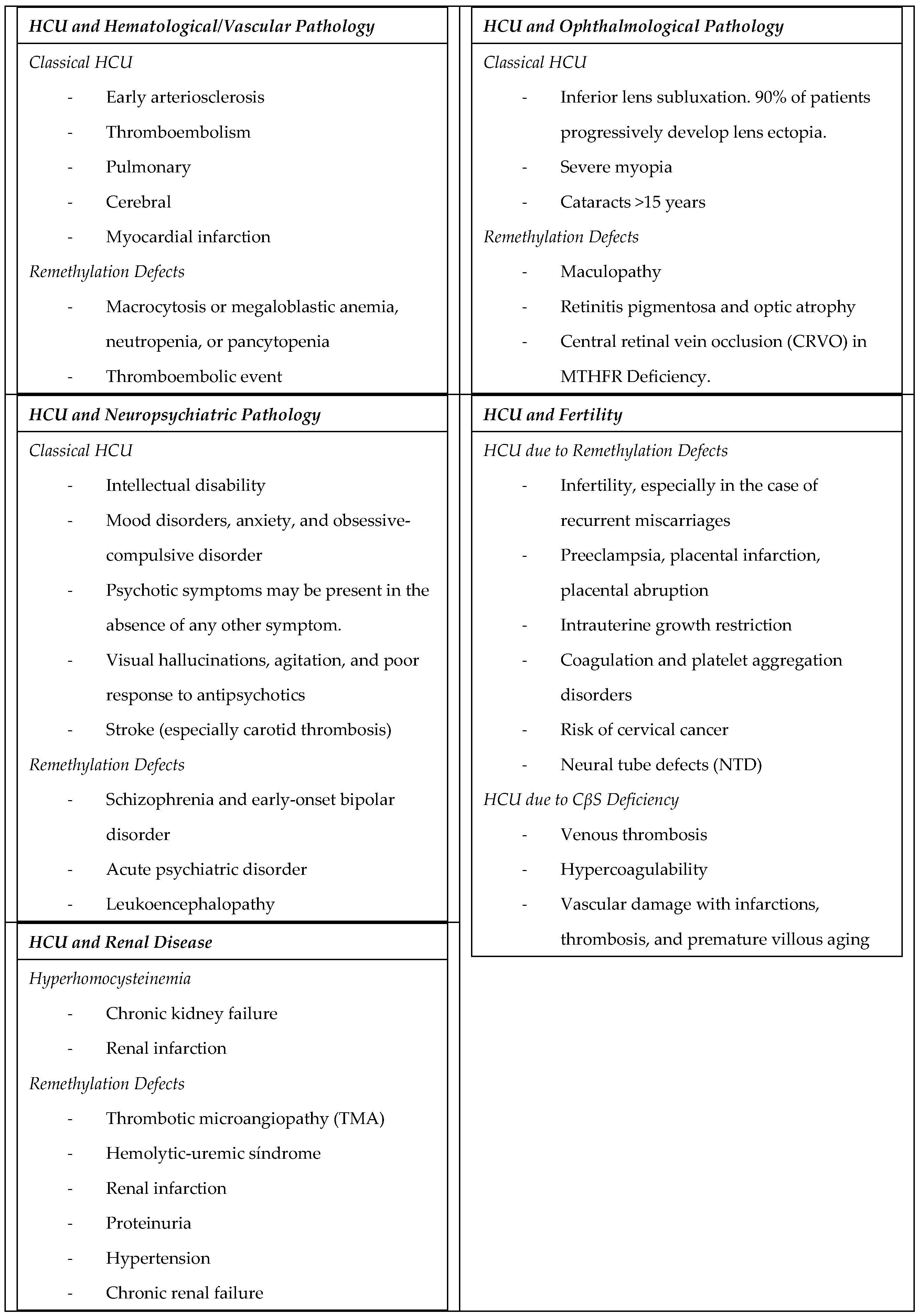
4. Specific Organ or System Manifestations (Table 2)
Table 2.
Specific organ o system manifestations of HHCys.
Table 2.
Specific organ o system manifestations of HHCys.
4.1. Vascular
Elevated blood Hcys levels are associated with atherogenic and prothrombotic properties, leading to specific histopathological features in vascular lesions, including intimal thickening, elastic lamina rupture, smooth muscle hypertrophy, platelet accumulation, and occlusive thrombi formation.
Studies report increased odds ratios (2.5 to 3.0) for venous thromboembolic disease (VTE) in individuals with Hcys levels exceeding 2 standard deviations above the normal range. Mild HHcys (16 to 30 μmol/L) may serve as a risk marker for recurrent VTE, although with some reservations.
At the arterial level, premature atherosclerosis and embolic complications affecting large and small vessels can manifest at any age. Arterial or venous thrombotic events, especially cerebral, occur when total Hcys concentration exceeds 100 μmol/L. Without treatment, around 50% of patients with severe genetically induced HHcys experience vascular complications before age 30. In hereditary HCU, untreated individuals face early arteriosclerosis and a heightened risk of vascular complications. While these complications may arise in childhood, they more commonly present as initial signs during adulthood in approximately 10% of cases [
24]. Up to 30% of HCU patients experience vascular events before turning 20, with half involving peripheral venous system thromboembolisms, 25% pulmonary embolisms, up to 33% cerebrovascular accidents, 11% peripheral arterial thromboembolisms, and 4% myocardial infarctions [
25]. Appropriate treatment significantly reduces the risk of these events [
26].
Consideration of HCU diagnosis is warranted in individuals under age 55 experiencing venous or arterial thromboembolic events, particularly if recurrent. A strong suspicion arises when such events are accompanied by psychiatric symptoms, mild cognitive impairment, a Marfanoid appearance, or early lens dislocation history. Triggering situations, such as physical stress, surgery, postpartum period, or prolonged fasting, should be considered. The absence or minimal relevance of other clinical manifestations may suggest milder phenotypes or responsiveness to vitamin B6 treatment [
27].
4.2. Hematological
For HHcyss resulting from remethylation disorders, hematological complications (megaloblastic anemia, neutropenia, and/or pancytopenia) are more prevalent than vascular complications. This association between hematological abnormalities and thromboembolic processes is more frequently observed in adult-onset presentations. These hematological abnormalities should be scrutinized, particularly in patients who also display visual deficits, retinopathy, peripheral neuropathy, ataxia, spinal degeneration, mild to moderate cognitive impairment, or psychiatric or behavioral disorders.
4.3. Marfanoid Habit
Skeletal involvement is marked by a Marfanoid phenotype and early osteoporosis. Elevated Hcys levels have been documented to disrupt collagen and elastin synthesis in connective tissue, giving rise to abnormalities in the skin, joints, and skeleton. It has been observed in cell cultures that fibrillin-1, the protein altered in Marfan syndrome, experiences reduced levels in cases of Cys deficiency, contributing to the characteristic phenotype exhibited by HCU patients. The Marfanoid syndrome, characterized by increased height due to elongated limbs with metaphyseal and epiphyseal overgrowth and a reduced upper-to-lower segment ratio, is common. Arachnodactyly, dry and thin skin, and brittle hair may also be present. Nearly constant osteoporosis predisposes individuals to conditions such as scoliosis, pathological fractures, and vertebral collapse. Other common deformities encompass genu valgum, pectus excavatum or carinatum, and cavus feet. Joint mobility limitation, especially in the limbs, often contrasts with the joint laxity seen in Marfan syndrome.
A certain degree of intellectual disability is prevalent, likely due to the competitive inhibition of amino acid transport through the blood-brain barrier and the challenges in neurotransmitter synthesis arising from high concentrations of Met and Hcys. Behavioral and personality disorders are also frequently observed [
9,
20,
28].
4.4. Neuropsychiatric Abnormalities
Neurological manifestations include psychomotor delays, psychiatric disorders, and seizures. Psychiatric symptoms affect up to 50% of HCU patients, predominantly manifesting as mood disorders, anxiety, and obsessive-compulsive disorder [
29]. Instances of psychotic symptoms have also been reported, and these can occur even in the absence of other clinical symptoms. Some HCU patients with no history of psychiatric issues or risk factors have exhibited visual hallucinations, agitation, and a poor response to antipsychotic medications [
30,
31]. Mental disability is prevalent and can be linked with other symptoms such as thromboembolic complications, lens dislocation, or a Marfanoid appearance [
32].
4.5. Kidney Disease
HHcys has notable implications for renal health, necessitating consideration in cases of atypical hemolytic uremic syndrome (aHUS) and unexplained renal function decline with associated symptoms. In adults, cblC deficiency may present with proteinuria, hypertension, chronic kidney disease (CKD), and aHUS. Routine genetic panels for aHUS and chronic renal disorders should include genes from the intracellular cobalamin pathway [
33].
Chronic Kidney Disease (CKD) emerges as a common acquired cause of HHcys across age groups. Hcys levels in CKD patients are significantly elevated, with a prevalence ranging from 85% to 100%. There is a positive correlation between creatinine levels and Hcys concentrations, emphasizing the link between Hcys and the degree of kidney disease. The pathogenic mechanism involves altered Hcys metabolism rather than reduced excretion. In cblC deficiency, early treatment with hydroxocobalamin and folates may potentially reverse renal insufficiency [
34,
35].
HHcys contributes to thrombotic microangiopathy (TMA), leading to HUS. Although rare, the association with HUS has been observed in newborns with cblC deficiency. Elevated plasma levels of Hcys and MMA play a role in TMA pathogenesis, disrupting antithrombotic properties of vascular endothelium and promoting vascular thrombosis. Hcys-thiolactone and MMA induce cellular damage, impacting renal cells. Tubulointerstitial nephritis and proximal renal tubular acidosis are reported in cblC remethylation defects [
36].
Renal infarction is an infrequent but serious consequence of HHcys, contributing to multiple thromboembolic events affecting various circulations. Patients with HCU may experience arterial hypertension due to renal artery thrombosis. Elevated Hcys levels contribute to atherosclerosis and thrombosis through various mechanisms, including LDL oxidation, endothelial growth inhibition, smooth cell proliferation stimulation, and interference with coagulation and fibrinolysis. Mild to moderate increases in Hcys may also serve as markers of tissue damage or repair, with the association between HHcys and vascular disease becoming more pronounced after a vascular event [
36].
Understanding the multifaceted impact of HHcys on renal health is crucial for comprehensive patient care. Routine inclusion of Hcys assessment in relevant genetic panels can aid in early detection and intervention, potentially mitigating severe complications associated with HHcys.
4.6. Ocular Abnormalities
Metabolic disorders affecting Hcys metabolism can have significant implications for various components of the eye, presenting challenges and opportunities in the field of ophthalmology. Ophthalmologists encounter two primary clinical scenarios: patients with elevated Hcys as part of a known metabolic disorder, where ocular abnormalities manifest because of the underlying condition, and those presenting with ocular issues, prompting suspicions of an underlying metabolic disorder. Ophthalmologists must be well-versed in the ocular manifestations associated with HHcys for diagnostic purposes and ongoing disease monitoring [
37].
In classic HCU, a hallmark feature is inferior lens subluxation, with ectopia lentis being a consistent clinical manifestation. Approximately 85% of non-responders to vitamin B6 experience lens dislocation before the age of 12, often bilaterally and located in the lower or nasal regions. This condition can lead to complications like retinal detachment, strabismus, severe myopic astigmatism, and cataracts, typically emerging around the age of 15 [
17].
MTHFR deficiency, among other remethylation disorders, is associated with thrombotic events like central retinal vein occlusion (CRVO). Evaluation of Hcys levels is recommended in CRVO patients, particularly in the absence of typical vascular risk factors, those under 55 years of age, or instances of bilateral involvement [
38,
39].
CblC deficiency homocystinuria presents a diverse ocular phenotype and is notable for its association with childhood maculopathy. Early-onset cases exhibit progressive retinal conditions, ranging from subtle retinal nerve fiber layer loss to advanced optic and macular atrophy with characteristic "bone spicule" pigmentation, accompanied by nystagmus, abnormal vision, and strabismus. Late-onset cases do not consistently show evidence of retinal degeneration or optic atrophy [
40,
41,
42]. Understanding these ocular manifestations is pivotal for ophthalmologists in providing comprehensive care and improving patient outcomes in the context of metabolic disorders affecting Hcys metabolism.
4.7. Reproductive Medicine and Pregnancy
Elevated Hcys levels during pregnancy can adversely affect implantation, embryonic development, fetal growth, and maternal health, increasing the risk of conditions such as preeclampsia [
43]. This is particularly significant in women with a history of pregnancies complicated by elevated Hcys, as it serves as a crucial risk indicator, impacting vascular function during pregnancy and heightening the risk of adverse effects on embryonic development [
43]. Pregnant women predisposed to thrombosis face an additional risk, with elevated Hcys potentially disrupting proper embryonic development. Folate deficiency is recognized as a contributor to an increased risk of neural tube defects in the developing embryo [
44].
Measurement of plasma homocysteine levels provides an indirect means of assessing folate deficiency, emphasizing the importance of monitoring Hcys as a potential marker for folate status during pregnancy. Moreover, moderately elevated Hcys levels before conception are associated with lower performance on neurodevelopmental tests at four months and cognitive assessments at six years [
45]. Understanding and addressing these relationships are crucial for maternal and fetal health during pregnancy.
5. Management of patients with suspected HHcys
5.1. Evaluation
When encountering arterial or venous thrombotic vascular events, the possibility of moderate to severe HHcys should be considered. The evaluation process begins with a thorough medical history and physical examination to identify signs consistent with HCU. Severe HHcys-related disorders may manifest as developmental delays or behavioral issues in children, while adults may present with vascular disease along with hematological, neuropsychiatric, ocular, or renal disorders. In women with high-risk pregnancies or infertility, HHcys should be considered. The presence of classic HCU is considered when certain features, such as a marfanoid morphotype, lens ectopia, severe non-familial myopia, skeletal deformities, arterial or venous thrombotic vascular events, intellectual disability, and psychiatric symptoms, are identified. Kidney disease and infertility are also associated conditions.
For pediatric and young adult patients, HHcys exclusion is crucial in those displaying a Marfanoid habitus or compatible dysmorphic features, particularly if accompanied by intellectual disabilities. In young adults, ruling out HHcys is recommended in cases of thromboembolic disease (especially under 55 years without apparent causes), recurrent or unusual thromboses, and patients with peripheral embolisms, early coronary disease, or pulmonary hypertension linked to chronic venous thromboembolic disease.
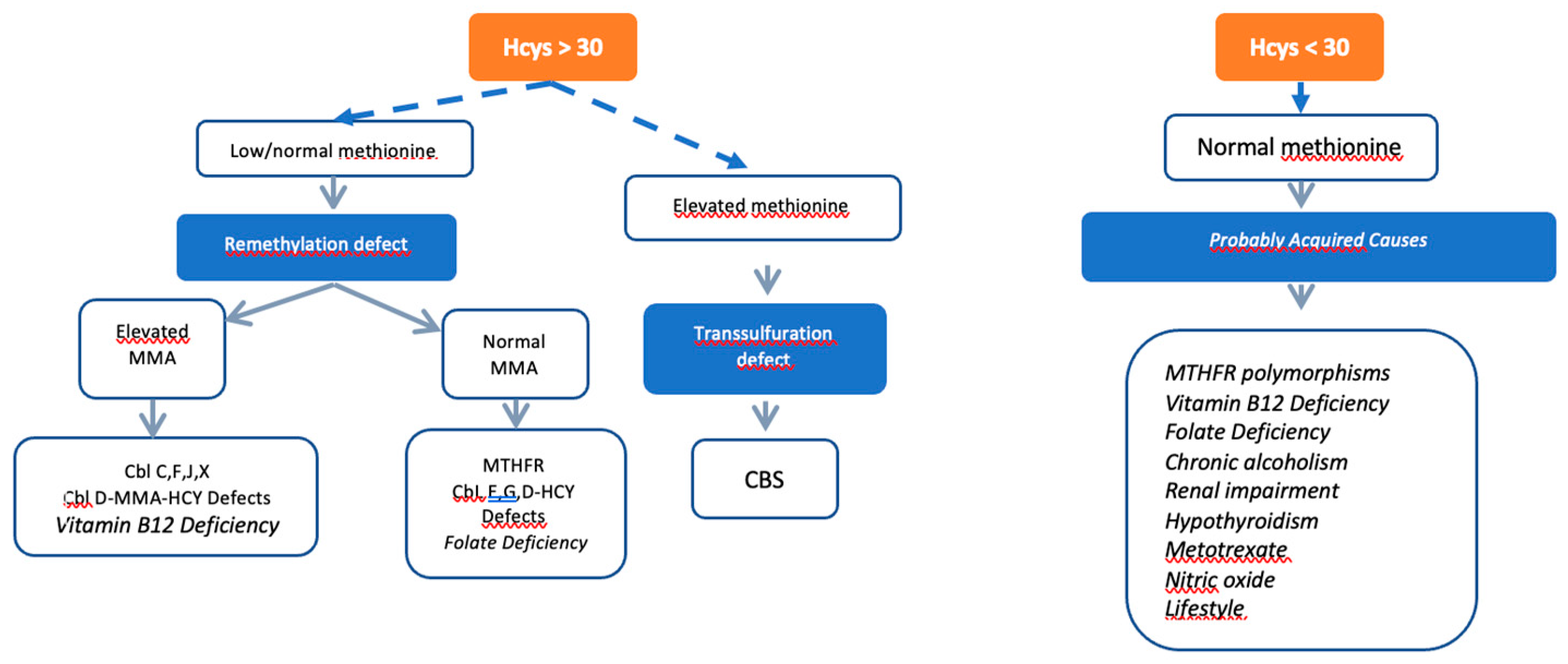
When moderate or severe HHcys is suspected (Hcys levels >31 μmol/L), comprehensive testing is recommended, including measurements of plasma Hcys, blood amino acids, vitamin B12, and folic acid levels. A study of organic acids in urine to determine methylmalonic acid (MMA) levels is also advised. The diagnostic possibilities are outlined in an algorithm (
Figure 3) and summarized in
Table 3, facilitating a systematic approach to confirm or rule out HHcys.
5.2. Treatment
The treatment approaches for different forms of HHcys vary, reflecting the diverse underlying causes and associated complications. In general, management options include vitamin B6 for susceptible forms or vitamin supplementations and a low-protein diet with betaine administration.
For mild HHcys, studies examining the impact of folic acid supplementation on cardiovascular and thromboembolic risk have yielded mixed results. The American Heart Association suggests that folic acid supplementation may reduce Hcys levels, but the reduction in cardiovascular risk is uncertain. However, treatment aiming to lower Hcys levels has shown promise in slowing carotid atherosclerosis progression, aiding primary stroke prevention, and delaying brain atrophy in mild cognitive impairment. The supplementation of vitamin B6, B12, or folic acid, considering the balance of risks and benefits, appears generally advantageous.
Classic Homocystinuria (HCU) necessitates a multifaceted approach. Initial treatment involves a therapeutic trial to determine vitamin B6 sensitivity, with one-third of patients responding positively. A low-protein diet, essential amino acid supplementation devoid of Met, and folate and vitamin B12 supplementation support Hcys remethylation via MS. Betaine supplementation, promoting remethylation through alternative pathways, is considered. The optimal dosage of betaine can vary, but general recommendations for betaine supplementation often range from 500 mg to 3 grams per day.
In cases resistant to vitamin B6, strict low-protein diets, Met-free essential amino acid formulas, and betaine administration are options. The recommended dose of betaine in children and adults is 100mg/kg/day divided in 2 doses per day, whereas in some patients doses above 200 mg/kg/day are needed to reach therapeutic goals. The primary treatment goal is to maintain total Hcys concentrations below 50 μmol/L, crucial for preventing vascular events or stabilizing neurological and bone involvement. However, the effectiveness in managing ocular involvement remains limited.
HCU due to remethylation disorders, exemplified by cblC deficiency, involves a comprehensive treatment approach. Hydroxocobalamin (OHCbl) is preferred over cyanocobalamin, administered parenterally to reach optimal vitamin B12 blood levels. Betaine doses are adjusted to optimize Hcys and Met levels. Unlike HCU, remethylation disorders don't necessitate protein restriction, emphasizing the importance of maintaining normal Met levels. Folic acid and carnitine supplementation have shown limited utility in treating remethylation disorders.
The varied treatment strategies underscore the complexity of HHcys management, requiring tailored approaches based on the specific form and its associated complications. Individualized treatment plans, incorporating dietary modifications and targeted supplementation, are essential for optimizing outcomes in patients with HHcys.
6. Hypoprotein Diet
Developing a hypoprotein diet involves meticulous consideration of food types, protein content, and overall nutritional value. The goal is to ensure adequate energy intake while controlling protein levels. The diet includes hypoprotein foods, amino acid mixtures without methionine, and supplements of minerals, vitamins, and trace elements. The principles of developing and implementing such a diet are crucial for patients managing metabolic disorders.
The hypoprotein diet categorizes foods based on Met or protein content into prohibited, controlled, and freely allowed groups. Prohibited foods, high in protein, include animal-origin foods like meat, fish, eggs, and certain plant-based foods such as legumes and nuts. Controlled foods, providing essential amino acids, are consumed in limited quantities based on individual tolerance. Freely allowed foods are either protein-free or have negligible protein content and include fats, sugary products, certain flours, seasonings, and specialized hypoprotein foods.
The System of Weighted Rations aids in recipe formulation, preventing the exceeding of daily protein rations. It involves selecting an arbitrary Met unit, simplifying the creation of varied menus without risking errors. Patient education focuses on understanding authorized foods, weight equivalences, and the system of weighted rations, ensuring proper meal planning.
Hypoprotein products, including bread and biscuits, are crucial for energy intake. These artificial foods, with reduced protein content, substitute traditional starchy items. Amino acid mixtures, devoid of methionine, are essential to prevent amino acid deficiencies in strict diets. Administered at least twice daily, they contribute to metabolic balance, albeit with potential taste challenges.
In addition to vitamins like pyridoxine (B6) and folic acid, minerals, vitamins, and trace elements are necessary, meeting recommended intake levels. Commercially prepared products can supplement these requirements when amino acid supplements are included, streamlining daily administration.
Overall, adherence to the principles of a hypoprotein diet is vital for patients with IEM. Categorizing foods, understanding weighted rations, incorporating hypoprotein products, amino acid mixtures, and supplements contribute to effective management and improved patient outcomes. Education plays a key role in empowering patients to make informed dietary choices, ensuring the successful implementation of a hypoprotein diet tailored to their needs.
7. Conclusions
Based on clinical experience and a review of the literature, it is recommended to measure plasma Hcys levels in any situations where treatable HCU is a possibility. Adequate evaluation is advised for all patients in whom moderate or severe HHCys (Hcys > 30 μmol/L) is detected.
It is particularly advisable to determine Hcys levels for patients who have experienced arterial or venous thrombotic events, as well as those with mild to moderate cognitive impairment, neuropsychiatric disorders, or associations with ocular or skeletal abnormalities. When a patient exhibits a Marfanoid morphotype, lens ectopia, severe non-familial myopia, skeletal deformities, intellectual disability, and psychiatric symptoms, even if each of these alterations appears in isolation, HCU should be considered. Specific hematological abnormalities, kidney disease, and infertility are clinical situations that may be associated with elevated Hcys levels.
ay be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
AECOM The Spanish Society for the Study of Inborn Errors of Metabolism collaborates in the publication and promotion of the article. Recordati Rare disease collaborates with AECOM to promote the identification of HCU patients and supports meetings and actions in education of professionals.
References
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke. 2010 Jun;41(6):1205-12. [CrossRef]
- Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2016 Aug 15;5(8):e003768. [CrossRef]
- Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017 Aug 17;8(8):CD006612. [CrossRef]
- Huemer M, Diodato D, Schwahn B, Schiff M, Bandeira A, Benoist JF, Burlina A, Cerone R, Couce ML, Garcia-Cazorla A, la Marca G, Pasquini E, Vilarinho L, Weisfeld-Adams JD, Kožich V, Blom H, Baumgartner MR, Dionisi-Vici C. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017 Jan;40(1):21-48. [CrossRef]
- Veeranki S, Gandhapudi SK, Tyagi SC. Interactions of hyperhomocysteinemia and T cell immunity in causation of hypertension. Can J Physiol Pharmacol. 2017 Mar;95(3):239-246. [CrossRef]
- Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, Selhub J, Jacques PF, Cole E, Gravens-Mueller L, House AA, Kew C, McKenney JL, Pacheco-Silva A, Pesavento T, Pirsch J, Smith S, Solomon S, Weir M. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011 Apr 26;123(16):1763-70. [CrossRef]
- Park WC, Chang JH. Clinical Implications of Methylenetetrahydrofolate Reductase Mutations and Plasma Homocysteine Levels in Patients with Thromboembolic Occlusion. Vasc Specialist Int. 2014 Dec;30(4):113-9. [CrossRef]
- Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1999 Jan 5-12;99(1):178-82. [CrossRef]
- Wilcken DE, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis. 1997 Jun;20(2):295-300. [CrossRef]
- Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010 Sep 8;5(9):e12244. [CrossRef]
- Cylwik B, Chrostek L. Disturbances of folic acid and homocysteine metabolism in the context of alcohol abuse. Pol Merkur Lekarski. 2011 Apr;30(178):295-9. (Published in Polish) PMID: 21595178.
- Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2008 Jun 1;27(11):1110-21. [CrossRef]
- Cianciolo G, De Pascalis A, Di Lullo L, Ronco C, Zannini C, La Manna G. Folic Acid and Homocysteine in Chronic Kidney Disease and Cardiovascular Disease Progression: Which Comes First? Cardiorenal Med. 2017 Oct;7(4):255-266. [CrossRef]
- Smach MA, Jacob N, Golmard JL, Charfeddine B, Lammouchi T, Ben Othman L, Dridi H, Bennamou S, Limem K. Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer's disease or dementia: a case-control study. Eur Neurol. 2011;65(5):270-8. [CrossRef]
- Dietrich-Muszalska A, Malinowska J, Olas B, Głowacki R, Bald E, Wachowicz B, Rabe-Jabłońska J. Oxidative stress may be induced by elevated homocysteine in schizophrenic patients. Neurochem Res. 2012 May;37(5):1057-62. [CrossRef]
- Leboff MS, Narweker R, LaCroix A, Wu L, Jackson R, Lee J, Bauer DC, Cauley J, Kooperberg C, Lewis C, Thomas AM, Cummings S. Homocysteine levels and the risk of hip fracture in postmenopausal women. J Clin Endocrinol Metab. 2009 Apr;94(4):1207-13. [CrossRef]
- Morris AA, Kožich V, Santra S, Andria G, Ben-Omran TI, Chakrapani AB, Crushell E, Henderson MJ, Hochuli M, Huemer M, Janssen MC, Maillot F, Mayne PD, McNulty J, Morrison TM, Ogier H, O'Sullivan S, Pavlíková M, de Almeida IT, Terry A, Yap S, Blom HJ, Chapman KA. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2017 Jan;40(1):49-74. [CrossRef]
- Suárez García I, Gómez Cerezo JF, Ríos Blanco JJ, Barbado Hernández FJ, Vázquez Rodríguez JJ. Homocysteine: The cardiovascular risk factor of the next millennium? An Med Interna. 2001 Apr;18(4):211-7. (Published in Spanish) PMID: 11496543.
- Nuño-Ayala M, Carnicera R, Guzmán MA, Guillén N, Navarro MA, et al. Homocysteine: Current panorama and the mouse's contribution to its study. Clínica e Investigación en Arteriosclerosis. 22, 5, (200-219), 2010. [CrossRef]
- Couce ML, Balcells S, Sánchez-Pintos P, Aldámiz Echevarría L, del Toro M. Protocol for homocystinuria. In: Protocols for the Diagnosis and Treatment of Congenital Metabolic Errors (AECOM), 2nd edition. Ed. D. Ortega, Ergon 2018, p. 167-179.
- Mudd H, Levy HL, Skovby F. Disorders of transulfuration. In: The Metabolic and Molecular Basis of Inherited Diseases, 7th edition. Ed. CR Scriver, AL Beaudet, WS Sly, and D Valle. New York: McGraw-Hill 1995. p. 1279-1327.
- Sánchez O, Fabregate R, Sabán Ruiz J. Metabolic Factors I: Homocysteine, lipoproteins. In: Sabán Ruiz J, editor. Global Control of Cardiometabolic Risk. Endothelial Dysfunction as the Preferential Target. Vol. I. Physiopathological, Clinical, and Diagnostic Bases of Cardiovascular Risk Factors. Pathogenesis. Madrid: Ediciones Díaz de Santos, 2009. p. 609-622.
- Urreizti R, Asteggiano C, Bermudez M, Córdoba A, Szlago M, Grosso C, de Kremer RD, Vilarinho L, D'Almeida V, Martínez-Pardo M, Peña-Quintana L, Dalmau J, Bernal J, Briceño I, Couce ML, Rodés M, Vilaseca MA, Balcells S, Grinberg D. The p.T191M mutation of the CBS gene is highly prevalent among homocystinuric patients from Spain, Portugal, and South America. J Hum Genet. 2006;51(4):305-313.
- Magner M, Krupková L, Honzík T, Zeman J, Hyánek J, Kožich V. Vascular presentation of cystathionine beta-synthase deficiency in adulthood. J Inherit Metab Dis. 2011 Feb;34(1):33-7. [CrossRef]
- Linnebank M, Junker R, Nabavi DG, Linnebank A, Koch HG. Isolated thrombosis due to the cystathionine beta-synthase mutation c.833T>C (1278T). J Inherit Metab Dis. 2003;26(5):509-511. [CrossRef]
- Mudd SH. Vascular disease and homocysteine metabolism. N Engl J Med. 1985 Sep 19;313(12):751-753. [CrossRef]
- Yap S, Boers GH, Wilcken B, Wilcken DE, Brenton DP, Lee PJ, Walter JH, Howard PM, Naughten ER. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol. 2001 Dec;21(12):2080-2085. [CrossRef]
- Andria G, Fowler B, Sebastio G. Disorders of sulfur amino acid metabolism. In: Fernandes J, Saudubray JM, Van den Berghe G, Walter Jh (eds.). Inborn Metabolic Diseases, 4th edition. Berlin: Springer-Verlag; 2006. p. 273-282.
- Abbott MH, Folstein SE, Abbey H, Pyeritz RE. Psychiatric manifestations of homocystinuria due to cystathionine beta-synthase deficiency: prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am J Med Genet. 1987 Apr;26(4):959-969. [CrossRef]
- Colafrancesco G, Di Marzio GM, Abbracciavento G, Stoppioni V, Leuzzi V, Ferrara M. Acute psychosis in an adolescent with undiagnosed homocystinuria. Eur J Pediatr. 2015 Sep;174(9):1263-1266. [CrossRef]
- Li SC, Stewart PM. Homocystinuria and psychiatric disorder: a case report. Pathology. 1999 Aug;31(3):221-224. [CrossRef]
- Sedel F, Baumann N, Turpin JC, Lyon-Caen O, Saudubray JM, Cohen D. Psychiatric manifestations revealing inborn errors of metabolism in adolescents and adults. J Inherit Metab Dis. 2007 Oct;30(5):631-641. [CrossRef]
- Shankar A, Wang JJ, Chua B, Rochtchina E, Flood V, Mitchell P. Positive association between plasma homocysteine level and chronic kidney disease. Kidney Blood Press Res. 2008;31(1):55-62. [CrossRef]
- Long Y, Nie J. Homocysteine in Renal Injury. Kidney Dis (Basel). 2016;2(2):80-87. [CrossRef]
- Kalantari S, Brezzi B, Bracciamà V, Barreca A, Nozza P, Vaisitti T, Amoroso A, Deaglio S, Manganaro M, Porta F, Spada M. Adult-onset CblC deficiency: a challenging diagnosis involving different adult clinical specialists. Orphanet J Rare Dis. 2022 Feb 2;17(1):33. [CrossRef]
- Castelli E, Terrone C, Faraone N, Tizzani A. Renal infarction in a hyperhomocysteinemic patient. Nephron. 2002;92(3):749-750. [CrossRef]
- Guevara-Márquez, Y. C., Vela-Amieva, M., Juárez Echenique, J. C., Ordaz Favila, J. C., & Belmont-Martínez, L. (2013). Ophthalmic manifestations of inborn errors of metabolism. Acta Pediátrica de México, 34(4), 212-224.
- Huang X, Yang Y, Duan Y, Kuang YQ, Lin D. Homocysteine in retinal artery occlusive disease: A meta-analysis of cohort studies. Sci Rep. 2017 Nov 16;7(1):15708. [CrossRef]
- Management of Retinal Vein Occlusions. Clinical Practice Guidelines of the SERV (Spanish Society of Retina and Vitreous) March 2015. ISBN: 978-84-606-5721-7. www.serv.es.
- Baumgartner MR, Hörster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, Huemer M, Hochuli M, Assoun M, Ballhausen D, Burlina A, Fowler B, Grünert SC, Grünewald S, Honzik T, Merinero B, Pérez-Cerdá C, Scholl-Bürgi S, Skovby F, Wijburg F, MacDonald A, Martinelli D, Sass JO, Valayannopoulos V, Chakrapani A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014 Sep 2;9:130. [CrossRef]
- Weisfeld-Adams JD, McCourt EA, Diaz GA, Oliver SC. Ocular disease in cobalamin C defect: A review of the literature and a suggested framework for clinical surveillance. Mol Genet Metab. 2015 Apr;114(4):537-546. [CrossRef]
- Brooks BP, Thompson AH, Sloan JL, Manoli I, Carrillo-Carrasco N, Zein WM, Venditti CP. Ophthalmic Manifestations and Long-Term Visual Outcomes in Patients with Cobalamin C Deficiency. Ophthalmology. 2016 Mar;123(3):571-582. [CrossRef]
- Canto-Cetina T, Polanco-Reyes L, Ballote-Zapata M, Ordóñez-Luna M. Homocisteína y perfil de lípidos en embarazo normal y embarazo complicado con preeclampsia. Rev Esp Med Quir. 2014;19:423-430.
- van Gool JD, Hirche H, Lax H, De Schaepdrijver L. Folic acid and primary prevention of neural tube defects: A review. Reprod Toxicol. 2018 Sep;80:73-84. [CrossRef]
- Kožich V, Sokolová J, Morris AAM, Pavlíková M, Gleich F, Kölker S, Krijt J, Dionisi-Vici C, Baumgartner MR, Blom HJ, Huemer M; E-HOD consortium. Cystathionine β-synthase deficiency in the E-HOD registry-part I: pyridoxine responsiveness as a determinant of biochemical and clinical phenotype at diagnosis. J Inherit Metab Dis. 2021 May;44(3):677-692. [CrossRef]
- Valayannopoulos V, Schiff M, Guffon N, Nadjar Y, García-Cazorla A, Martinez-Pardo Casanova M, Cano A, Couce ML, Dalmau J, Peña-Quintana L, Rigalleau V, Touati G, Aldamiz-Echevarria L, Cathebras P, Eyer D, Brunet D, Damaj L, Dobbelaere D, Gay C, Hiéronimus S, Levrat V, Maillot F. Betaine anhydrous in homocystinuria: results from the RoCH registry. Orphanet J Rare Dis. 2019 Mar 14;14(1):66. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).