1. Introduction
1.1. The Use of Fission Yeast to Study Eukaryotic Cytokinesis
Both mammalian cells and the fission yeast Schizosaccharomyces pombe use binary fission to divide medially. Fission yeast cells are encased in a cell-wall structure giving them their rod shape following growth by tip extension and divide equatorially. Therefore, the species is considered an excellent model organism for studying eukaryotic cytokinesis, where similar cellular processes occur.
Research using S. pombe has allowed the identification of many important conserved cell cycle regulators. As the mechanisms of assembly and constriction of the actin-myosin contractile ring (ACR) in S. pombe are very similar to those seen in mammalian cells, our current understanding of eukaryotic cytokinesis stems extensively from studies in fission yeast (Pollard and Wu, 2010).
During the 1970s, the work of Leland Hartwell and colleagues with the budding yeast Saccharomyces cerevisiae led to the discovery of a large number cell division cycle (CDC) mutants, and for the first-time eukaryotic genes required for cell division were characterized (Hartwell et al., 1970, Hartwell et al., 1973). Study of the cell division cycle continued in the distally related fission yeast S. pombe by Paul Nurse and colleagues, with the discovery of equivalent cdc gene mutants (Nurse, 1975, Nurse et al., 1976).
Later in the 1990s, two landmark reviews discussed aspects of the S. pombe cell cycle including the timing of events leading to cytokinesis, cell division, and mechanisms for determining the medial or equatorial division plane. At that time the cdc16 and cdc2 genes were thought to act as a molecular switch regulating S. pombe mitosis and cytokinesis (Chang and Nurse, 1993), and it was proposed that the division plane was determined by the position of the nucleus (Chang and Nurse, 1996). Subsequent research offered a deeper understanding of the S. pombe cell cycle regulation, including aspects of cytokinesis (reviewed in Nurse, 2020), and cell polarity (reviewed in Chang et al., 2019).
Establishment of cytokinesis is mediated by a cytokinetic ACR that leads to the final separation of two daughter cells. In this short review, we describe
S. pombe cytokinesis starting from the medial positioning of the division plane and the assembly of the cytokinetic ACR (
Section 2), to the forces that generate tension and lead to constriction of the ACR, and the final separation of the two daughter cells (
Section 3).
2. Actin-Myosin Contractile Ring (ACR) Assembly in Fission Yeast
2.1. Positioning of the Cell Division Plane
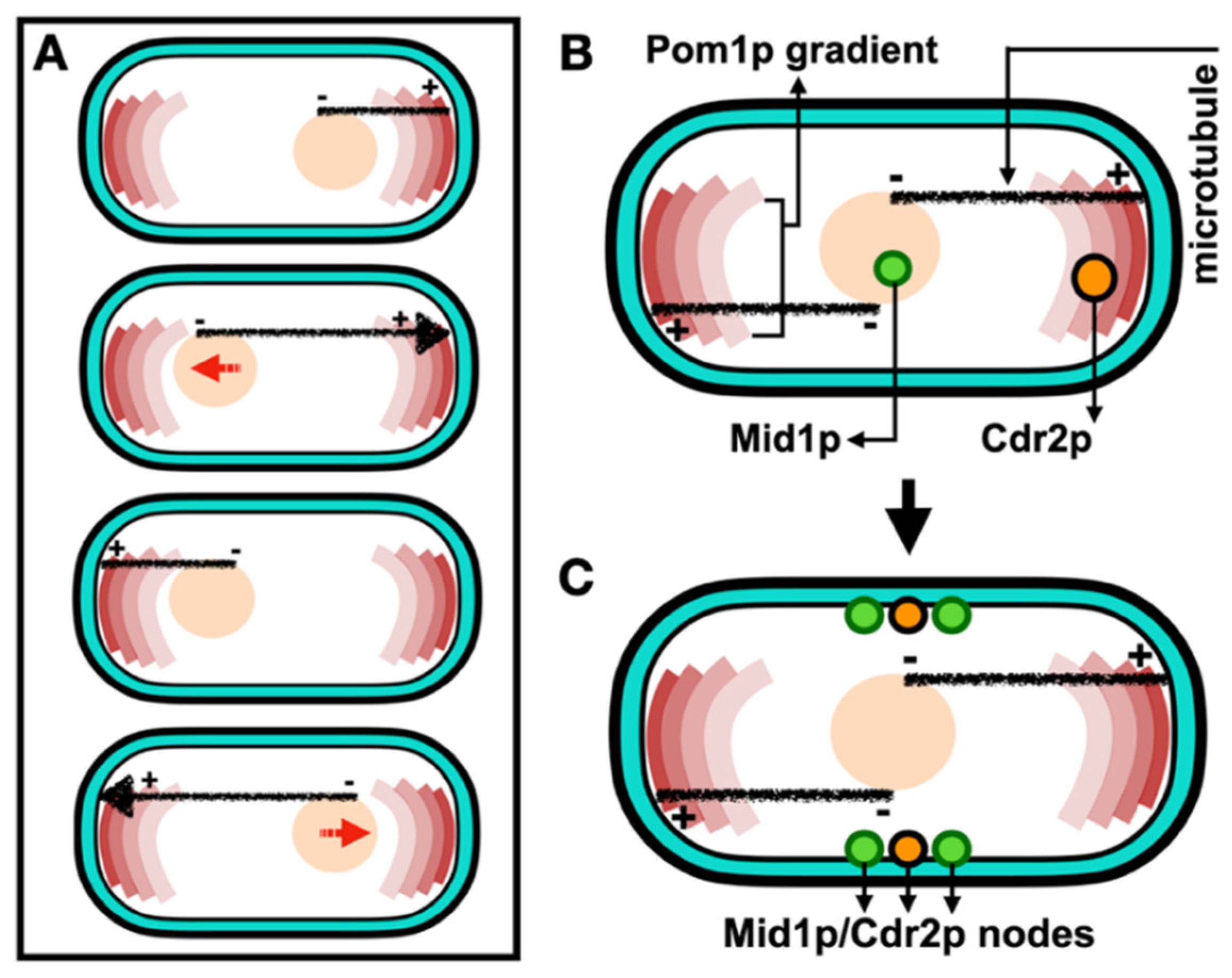
In S. pombe cellular growth occurs throughout a longer interphase period with this ceasing during the shorter mitosis and cytokinesis periods, after a certain cell length is achieved. During the cell cycle, “middle” and “end” or “tip” locations are specified by two spatial axes. The “middle” location is defined by the nucleus which is positioned at the cell centre by a microtubule-pushing mechanism where a force produced by the cytoplasmic microtubule bundles and is applied to the nucleus (Tran et al., 2001, Piel and Tran, 2009). Furthermore, this force is able to efficiently re-centre nuclei of cells exposed to nuclear displacement (Daga et al., 2005, Daga et al., 2006). The dynamic interplay between the nucleus and the microtubule cytoskeleton is illustrated and reviewed by Gallardo et al. (2019). Alternatively, the “end” or “tip” location is defined by formin-mediated actin assembly mechanism at cell tips (Martin et al., 2005), and polarity factors including the DYRK kinase Pom1p secreted in a gradient manner at cell poles. Additionally, Pom1p controls mitotic entry by phosphorylating the ACR scaffold protein Cdr2p at the cell “middle” through phosphorylating its membrane-binding C-terminal region (Gerganova et al., 2019).
Much evidence shows that the Anillin-like protein Mid1p localizes to the “middle” location and initiates ACR assembly (Paoletti and Chang, 2000, Almonacid
et al., 2011, Saha and Pollard, 2012, Rezig
et al., 2021). Mid1p has two membrane binding domains, the pleckstrin homology domain (PH) and the cryptic domain (C2) (Chatterjee
et al., 2019). However, it only binds the plasma membrane after it is activated and resealed from the nucleus (Bähler
et al., 1998, Almonacid
et al., 2011). The roles of Mid1p in positioning the ACR are now well understood in
S. pombe, and are reviewed in Rezig
et al. (2022), with medial positioning of the ACR described in
Figure 1.
In contrast, comprehension of the organization of proteins that assemble the complex cytokinetic machinery during cytokinesis is still relatively rudimentary. In S. pombe, cytokinesis proteins are recruited to the cell centre pre-determining the future division plane, these organized as cortical spots, called “nodes” (Akamatsu et al., 2014, Laplante et al., 2016). The next Section will discuss the nature of these nodes including their constituent proteins and spatiotemporal organization.
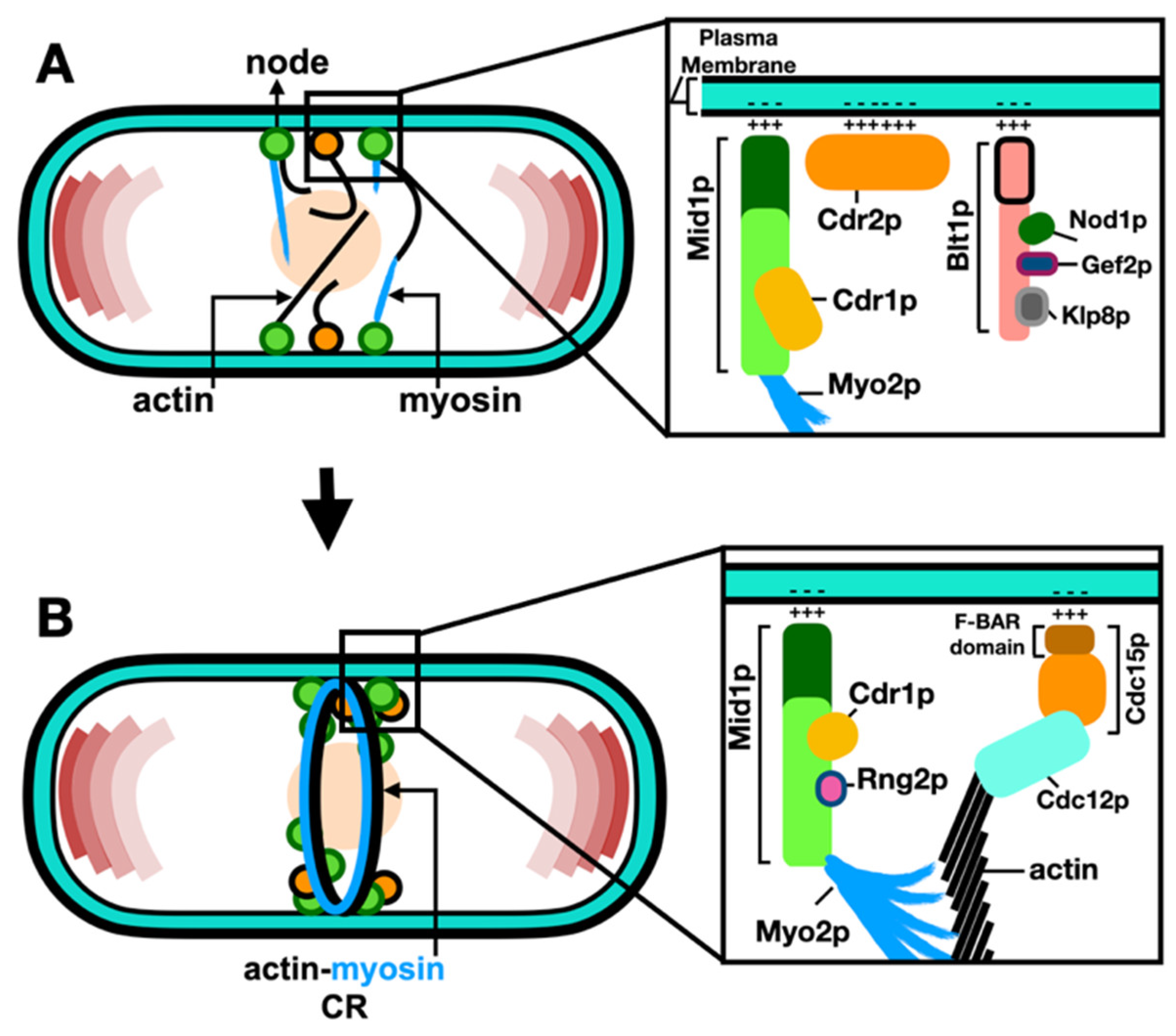
2.2. Molecular Organization of Nodes within the ACR
The current model for the ACR assembly includes two types of interphase nodes: type-1 “stationary” nodes containing Cdr1p, Cdr2p, Wee1p, Mid1p; and type-2 “anchoring” nodes containing Blt1p, Gef2p, Cdc15p, Rng2p, and Cdc12p (Zhu et al., 2013, Akamatsu et al., 2014). The “anchoring” nodes form the units that anchor the ends of myosin-II tails to the plasma membrane, with myosin-II heads extending into the cytoplasm. These nodes then merge into a ring-like structure, called the actin-myosin contractile ring (ACR) and, as its name implies, it is composed of actin filaments and myosin-II motors in addition to various classes of cytokinesis proteins (Malla et al., 2021).
Live cell imaging, high-speed fluorescence photo-activation localization microscopy (FPALM), and fluorescence resonance energy transfer (FRET) have shown to be excellent methods to dissect ACR nodes. They have revealed that nodes are discrete units with stoichiometric ratios and specific distribution of constituent proteins (Laplante
et al., 2016, Akamatsu
et al., 2017, McDonald
et al., 2017, Malla
et al., 2021). Furthermore, the localization of the ACR constituents is thought to be arranged in several layers relative to the plasma membrane, starting with plasma membrane-binding proteins and the tail of myosin-II, to intermediate cytokinesis proteins, and farthest from the plasma membrane lays myosin motor domains, F-actin and its cross-linkers (McDonald
et al., 2017). Advances in laser scanning microscopy, such as Airy-scanning using very low laser power to acquire high-quality images, increased the resolution and signal-to-noise ratio and enabled the detection and measurement of even feint individual cytokinesis nodes (Sayyad and Pollard, 2022). Coalescence of nodes leads to the ACR assembly through the search capture pull and release (SCPR) mechanism, whereby Cdc12p nucleates actin filaments as Myo2p pulls actin filaments, thus producing the force required to pull the individual nodes into the ACR (Vavylonis
et al., 2008, Laplante
et al., 2016, Zimmermann
et al., 2017). Such assembly of the ACR from node precursors is described in
Figure 2.
2.3. Anchorage of the ACR to the Plasma Membrane
After ACR assembly, Myo2p tails and Cdc15p localize to the plasma membrane, with the Myo2p heads, Myp2p and the bundle of actin filaments localizing 60 nm away from the plasma membrane (Swulius et al., 2018). It is suggested that this organization connects the bundle of actin filaments to the plasma membrane (Bellingham-Johnstun et al., 2021). Cdc15p next recruits Cdc12p to the ACR, and this interaction is thought to be essential for the ACR organization and stability (Snider et al., 2020).
The phospho-status of Cdc15p influences its ability to bind the plasma membrane, with phosphorylation of Cdc15p by Pom1p inhibiting its binding to the plasma membrane at cell tips (Bhattacharjee et al., 2020). Additionally, the p21-activated protein kinase (Pak1p), another polarity kinase, was found to regulate the function of Mid1p and Cdc15p (Magliozzi et al., 2020). Cdc15p has three regulatory components: an N-terminal Fre/Cip4 homology Bin/Maphiphysin/Rvs domain (F-BAR), a medial intrinsically disordered region (IDR), and a C-terminal Src homology 3 domain (SH3). While the F-BAR domain enables protein oligomerization and concentration on the plasma membrane to scaffold protein assemblies resulting in membrane deformation (Snider et al., 2021), it was recently found that phosphorylation of Cdc15p induces the separation of the Cdc15p IDR region resulting in an inhibition of Cdc15p phase separation, and the formation of condensate on the plasma membrane (Bhattacharjee et al., 2023).
The role of Cdc15p during S. pombe cytokinesis was examined using laser ablation. The ACR recoils after being severed, however, this recoil profile was greater and slower in the ablated ACR of Cdc15-depleted cells, suggesting that loss of Cdc15p decreases stiffness of the ACR material (Moshtohry et al., 2022). Furthermore, another F-BAR protein, Imp2p, was found to contribute to the stiffness of the ACR (Bellingham-Johnstun et al., 2021).
3. ACR Constriction and Septation in Fission Yeast
3.1. Constriction of the ACR
The endosomal sorting complex required for transport (ESCRT) machinery has a well characterized role in mammalian cytokinesis, where it induces membrane remodeling events to disassemble the constricted ACR (reviewed in Bhutta et al., 2014a). ESCRTs were initially characterized in S. pombe in the early 1990s for regulating vesicle-mediated protein sorting (Takegawa et al., 1995, 2003, Iwaki et al., 2007). ESCRTs were then found to be required for cytokinesis in S. pombe (Bhutta et al., 2014b); however, the precise role(s) of ESCRTs during cytokinesis in fission yeast remains unclear.
The ATPase ESCRT associated protein Vps4p plays a critical role in recycling ESCRT complexes: such an interaction is catalyzed during abscission and leads to the final “cutting” of the thin intracellular bridge connecting the two daughter cells. Vps4p has been suggested to interact directly or indirectly with the C-terminal domain of Mid1p, probably through the lipid-binding PH domain, and Mid1p localization to the nodes is disrupted in vps4Δ cells (Rezig et al., 2021). Interestingly, Mid1p leaves the ACR upon constriction through phosphorylation by Sid2p (Willet et al., 2019), and coordination between Vps4p and Sid2p might regulate Mid1p disassociation from the cell cortex; however, this hypothesis requires further investigation as Mid1p leaves the ACR before the onset of abscission, when Vps4p is activated (Rezig et al., 2021).
3.2. Septation Coordinates with ACR Constriction
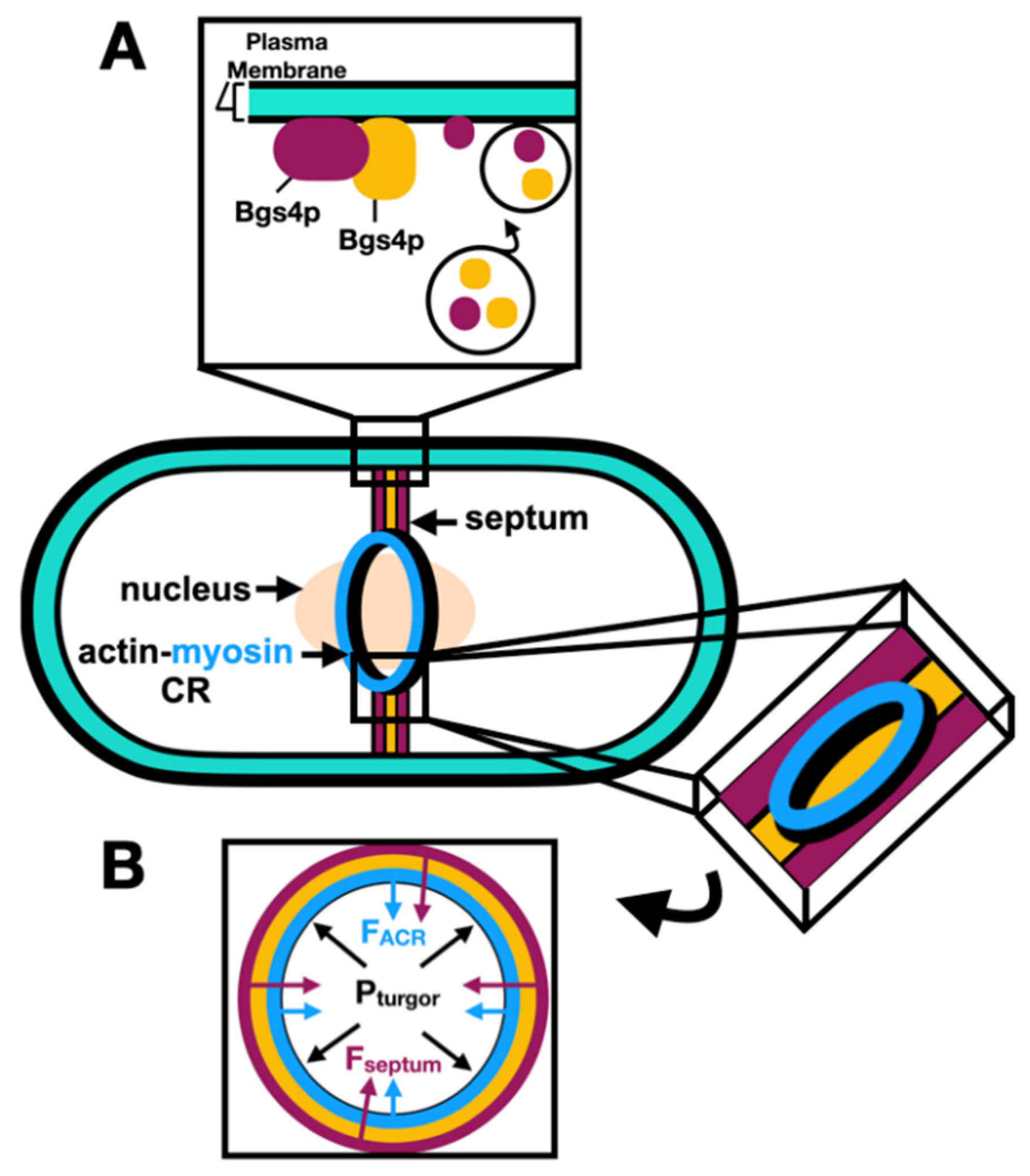
Unlike in mammalian cells where cytokinesis only implies the formation of a medial ACR, cytokinesis in S. pombe also requires the formation of a cell wall-like structure called the septum, physically separating the two daughter cells, at the division site. After ACR assembly and constriction, the septation initiation network (SIN) mediates the synapses of the amphid defective (SAD) kinase, Cdr2p, dispersal from the cell cortex into the cytoplasm, and septation is coordinated with ACR constriction (Rincon et al., 2017). The septum is composed of three layers: a middle primary septum layer that is later digested at the end of cytokinesis, and two flanking secondary septa which remain intact to form the new cell walls of separated daughter cells (reviewed by Pérez et al., 2018 and Hercyk et al., 2019).
S. pombe septation occurs in three stages. First, deposition of the septum cell-wall structure material is carried out through membrane trafficking events where secretory vesicles deliver the septum beta-glucan synthase 1, Bgs1p (Onwubiko et al., 2021). Localization of cell-wall building enzymes including Bgs1p depends on both Cdc42p and Cdc15p (Wei et al., 2016, Campbell et al., 2022). Second, during anaphase B the ACR constricts at a slow rate as the septum ingression is initiated; however, after the delivery of the alpha-glucan synthase 1, Ags1p, and Bgs4p, the rate of constriction and septum ingression is increased (Cortés et al., 2018). Third, after the ACR constricts, exocytosis leads to the delivery of glucanases and digestion of the primary septum leaving the two daughter cells each with a new cell wall (secondary septa) (Pérez et al., 2015).
Septation is coupled to ACR constriction, and the interaction between Bgs1p and the ACR is mediated by the paxillin Pxl1p (Cortés et al., 2015). Furthermore, Pxl1p accumulation during septum formation is thought to be mediated by an interaction with both the N-terminal F-BAR (Snider et al., 2020) and C-terminal SH3 domains of Cdc15p (Bhattacharjee et al., 2020). The mechanism of engagement of Pxl1p to both distal domains of Cdc15p is not well understood. However, a recent study demonstrated this interaction and found that Pxl1p binds to the Cdc15p F-BAR cytosolic domain (Snider et al., 2022).
Proctor and colleagues (2012) found that beta-glucan synthesis in important for the ACR to overcome the high turgor pressure during cytokinesis, suggesting that other pathways might coordinate with ACR constriction. Recent research used a fission yeast mutant
cps1-191, defective in 1,3-beta-glucan-synthase septum synthesis, that arrests with a non-constricting ACR, to test if high turgor pressure restricted ACR constriction in such mutants (Chew
et al., 2020). This showed that a decreased turgor pressure inhibits the ACR constriction in
cps1-191 mutants, suggesting that the extracellular glycan network regulates the ACR constriction and septum ingression, and that subsequent remodeling of the extracellular components relieves this restriction and facilitates the ACR constriction. Septum synthesis and coordination with ACR constriction are described in
Figure 3.
3.3. Abscission and the Final Separation
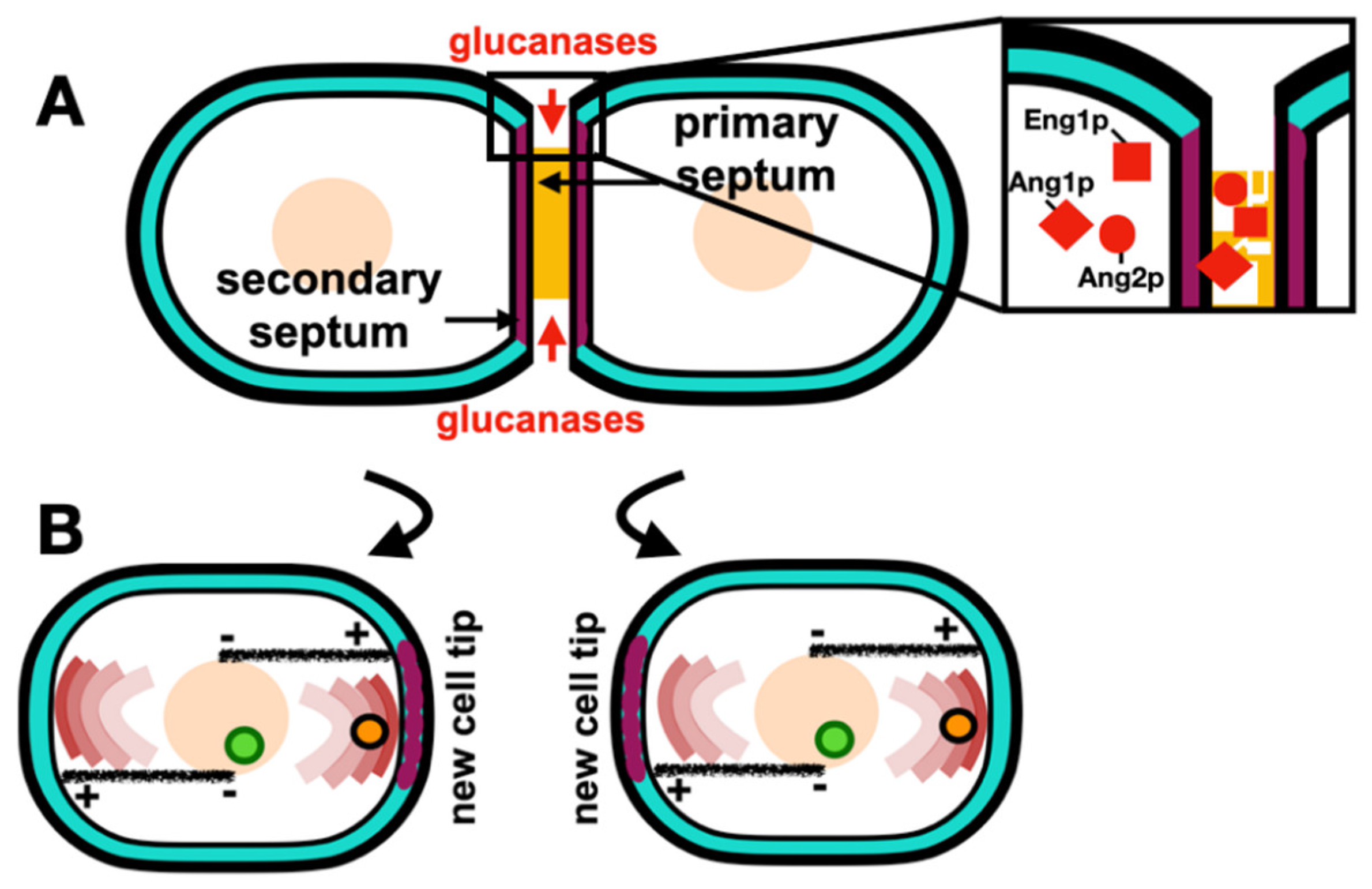
At the last stage of cytokinesis, abscission, completed primary septum digestion splits the two daughter cells apart, this thought to be driven by turgor pressure (Atilgan
et al., 2015). The primary septum is hydrolyzed by the digestive endo-(1,3)-beta-glucanase, Eng1p (Martin-Cuadrado
et al., 2003), and the endo-(1,3)-alpha-glucanase, Agn1p (Dekker
et al., 2004). A recent study found that Cdc42p is required to recruit Eng1p and Agn1p to the septum, thus directing their proper localization at the division site (Onwubiko
et al., 2020). The membrane trafficking events during abscission are tightly regulated to ensure complete abscission. Septins complexes are involved in the delivery of the digestive glucanases (Zheng
et al., 2018). Interestingly, a recently characterized different Anillin homologue, Mid2p is required for this process (Tasto
et al., 2003). It will be interesting to discover how Mid2p regulates the later stages of cytokinesis. The abscission mechanism is described in
Figure 4.
4. Concluding Remarks
Studying the function of gene products and their localization through the cell cycle in fission yeast has been a powerful way to understand eukaryotic cell division, especially with advanced live cell imaging, fluorescent macromolecules, and the integration of different research fields including cell molecular biology, cell mechanics and mathematical modelling. This enabled the generation of a model of S. pombe cytokinesis as described in this review. In summary, a ring first forms at the equator of the cell, and while the new cell-wall material is deposited through membrane trafficking, the ring constricts through a complex network of forces balanced by coordination between ring constriction and septum synthesis. Digestive enzymes then cut the remaining septum material connecting the two daughter cells leading to their final separation. This review addresses the dynamic nature of these processes and presents a visualized picture of current understanding of the proteins and processes that control this important event. It is anticipated that these mechanisms will be conserved across all eukaryotes and so will be informative about human cells and disease conditions, where they are defective.
Conflicts of interest
No potential conflict of interest was reported by the author(s).
References
- Akamatsu, M.; Berro, J.; Pu, K.M.; et al. Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase. J. Cell Biol. 2014, 204, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu M, Lin Y, Bewersdorf J; et al. Analysis of interphase node proteins in fission yeast by quantitative and superresolution fluorescence microscopy. Mol. Biol. Cell. 2017, 28, 3203–3214.
- Almonacid, M, Celton-Morizur, S, Jakubowski, JL; et al. Temporal control of contractile ring assembly by Plo1 regulation of Myosin II recruitment by Mid1/Anillin. Curr. Biol. 2011, 21, 473–479.
- Atilgan E, Magidson V, Khodjakov A; et al. Morphogenesis of the fission yeast cell through cell wall expansion. Curr. Biol. 2015, 25, 2150–2157.
- Bähler J, Steever AB, Wheatley S; et al. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol. 1998, 143, 1603–1616.
- Bellingham-Johnstun K, Anders EC, Ravi J; et al. Molecular organization of cytokinesis node predicts the constriction rate of the contractile ring. J. Cell Biol. 2021, 220, e202008032.
- Bhattacharjee R, Hall AR, Mangione MC; et al. Multiple polarity kinases inhibit phase separation of F-BAR protein Cdc15 and antagonize cytokinetic ring assembly in fission yeast. Elife 2023, 7, e83062.
- Bhattacharjee R, Mangione MC, Wos M; et al. DYRK kinase Pom1 drives F-BAR protein Cdc15 from the membrane to promote medial division. Mol. Biol. Cell. 2020, 31, 917–929.
- Bhutta MS, McInerny CJ, Gould GW. ESCRT function in cytokinesis: Location, dynamics and regulation by mitotic kinases. Int. J. Mol. Sci. 2014, 15, 21723–21739.
- Bhutta MS, Roy B, Gould GW; et al. A complex network of interactions between mitotic kinases, phosphatases and ESCRT proteins regulates septation and membrane trafficking in S. pombe. PLoS ONE 2014, 9, e111789.
- Campbell BF, Hercyk BS, Williams AR; et al. Cdc42 GTPase activating proteins Rga4 and Rga6 coordinate septum synthesis and membrane trafficking at the division plane during cytokinesis. Traffic 2022, 23, 478–495.
- Chang, F. Forces that shape fission yeast cells. Mol. Biol. Cell. 2017, 28, 1819–1824.
- Chang, F.; Nurse, P. Finishing the cell cycle: Control of mitosis and cytokinesis in fission yeast. Trends Genet. 1993, 9, 333–335.
- Chang, F.; Nurse, P. How fission yeast fission in the middle. Cell. 1996, 84, 191–194.
- Chatterjee, M.; Pollard, T.D. The functionally important N-terminal half of fission yeast Mid1p Anillin Is intrinsically disordered and undergoes phase separation. Biochemistry. 2019, 58, 3031–3041.
- Chew TG, Lim TC, Osaki Y; et al. Inhibition of cell membrane ingression at the division site by cell walls in fission yeast. Mol. Biol. Cell. 2020, 31, 2306–2314.
- Cortés JC, Pujol N, Sato M, Pet al. Cooperation between Paxillin-like protein Pxl1 and glucan synthase Bgs1 is essential for actomyosin ring stability and septum formation in fission yeast. PLoS Genet. 2015, 11, e1005358.
- Cortés JC, Ramos M, Konomi M; et al. Specific detection of fission yeast primary septum reveals septum and cleavage furrow ingression during early anaphase independent of mitosis completion. PLoS Genet. 2018, 14, e1007388.
- Daga, R.R.; Chang, F. Dynamic positioning of the fission yeast cell division plane. Proc. Natl. Acad. Sci. USA 2005, 8228–8232.
- Daga, R.R.; Yonetani, A.; Chang, F. Asymmetric microtubule pushing forces in nuclear centering. Curr. Biol. 2006, 16, 1544–1550.
- Dekker N, Speijer D, Grun CH; et al. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell. 2004, 15, 3903–3914.
- Gallardo P, Barrales RR, Daga RR; et al. Nuclear mechanics in the fission yeast. Cells 2019, 8, 1285.
- Gerganova V, Floderer C, Archetti A; et al. Multi-phosphorylation reaction and clustering tune Pom1 gradient mid-cell levels according to cell size. Elife 2019, 3, e45983.
- Hartwell, L.H.; Culotti, J.; Reid, B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl. Acad. Sci. USA 1970, 66, 352–359.
- Hartwell, L.H.; Mortimer, R.K.; Culotti, J.; et al Genetic control of the cell division cycle in yeast:, V. Genetic analysis of cdc mutants. Genetics 1973, 74, 267–286.
- Hercyk, B.S.; Onwubiko, U.N.; Das, M.E. Coordinating septum formation and the actomyosin ring during cytokinesis in Schizosaccharomyces pombe. Mol. Microbiol. 2019, 112, 1645–1657.
- Iwaki T, Onishi M, Ikeuchi M; et al. Essential roles of class E Vps proteins for sorting into multivesicular bodies in Schizosaccharomyces pombe. Microbiology 2007, 153, 2753–2764.
- Laplante, C, Huang, F, Tebbs, IR; et al. Molecular organization of cytokinesis nodes and contractile rings by super- resolution fluorescence microscopy of live fission yeast. Proc. Natl. Acad. Sci. USA 2016, 113, E5876–E5885.
- Magliozzi JO, Sears J, Cressey L; et al. Fission yeast Pak1 phosphorylates anillin-like Mid1 for spatial control of cytokinesis. J. Cell Biol. 2020, 219, e201908017.
- Malla, M.; Pollard, T.D.; Chen, Q. Counting actin in contractile rings reveals novel contributions of cofilin and type II myosins to fission yeast cytokinesis. Mol. Biol. Cell. 2022, 33, ar51.
- Martin SG, McDonald WH, Yates JR 3rd; et al. Tea4p links microtubule plus ends with the formin for3p in the establishment of cell polarity. Dev. Cell. 2005, 8, 479–491.
- Martin-Cuadrado AB, Duenas E, Sipiczki M; et al. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 2003, 116, 1689–1698.
- McDonald NA, Lind AL, Smith SE; et al. Nanoscale architecture of the Schizosaccharomyces pombe contractile ring. Elife 2017, 15, e28865.
- Moshtohry M, Bellingham-Johnstun K, Elting MW; et al. Laser ablation reveals the impact of Cdc15p on the stiffness of the contractile ring. Mol. Biol. Cell. 2022, 33, br9.
- Nurse, P. Genetic control of cell size at cell division in yeast. Nature. 1975, 256, 547–551.
- Nurse, P. Fission yeast cell cycle mutants and the logic of eukaryotic cell cycle control. Mol. Biol. Cell. 2020, 15, 2871–2873.
- Nurse, P.; Thuriaux, P.; Nasmyth, K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 1976, 146, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Onwubiko UN, Mlynarczyk PJ, Wei B; et al. A Cdc42 GEF, Gef1, through endocytosis organizes F-BAR Cdc15 along the actomyosin ring and promotes concentric furrowing. J. Cell Sci. 2020, 132, 223776.
- Onwubiko UN, Rich-Robinson J, Mustaf RA; et al. Cdc42 promotes Bgs1 recruitment for septum synthesis and glucanase localization for cell separation during cytokinesis in fission yeast. Small GTPases 2021, 12, 257–264.
- Paoletti, A.; Chang, F. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. biol cell. 2000, 11, 2757–2773.
- Pérez P, Cortés JCG, Cansado J; et al. Fission yeast cell wall biosynthesis and cell integrity signalling. Cell Surf. 2018, 4, 1–9.
- Pérez, P.; Portales, E.; Santos, B. Rho4 interaction with exocyst and septins regulates cell separation in fission yeast. Microbiology 2015, 161, 948–959.
- Piel, M.; Tran, P.T. Cell shape and cell division in fission yeast. Curr. Biol. 2009, 19, R823–R827.
- Proctor SA, Minc N, Boudaoud A et al. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr. Biol. 2012, 22, 1601–1608.
- Pollard, T.D.; Wu, J.Q. Understanding cytokinesis: Lessons from fission yeast. Nat. Rev. Mol. Cell Biol. 2010, 11, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Rezig IM, Yaduma WG, Gould GW; et al. Anillin/Mid1p interacts with the ESCRT-associated protein Vps4p and mitotic kinases to regulate cytokinesis in fission yeast. Cell Cycle. 2021, 20, 1845–1860.
- Rezig IM, Yaduma WG, Gould GW; et al. The role of anillin/Mid1p during medial division and cytokinesis: From fission yeast to cancer cells. Cell Cycle 2022, 22, 633–644.
- Rincon SA, Estravis M, Dingli F; et al. SIN-dependent dissociation of the SAD kinase Cdr2 from the cell cortex resets the division plane. Curr. Biol. 2017, 27, 534–542.
- Saha, S.; Pollard, T.D. Anillin-related protein Mid1p coordinates the assembly of the cytokinetic contractile ring in fission yeast. Mol. Biol. Cell. 2012, 23, 3982–3992.
- Sayyad, W.A.; Pollard, T.D. The number of cytokinesis nodes in mitotic fission yeast scales with cell size. Elife 2022, 12, e76249.
- Snider CE, Bhattacharjee R, Igarashi MG; et al. Fission yeast paxillin contains two Cdc15 binding motifs for robust recruitment to the cytokinetic ring. Mol. Biol. Cell. 2022, 33, br4.
- Snider CE, Chandra M, McDonald NA; et al. Opposite surfaces of the Cdc15 f-bar domain create a membrane platform that coordinates cytoskeletal and signaling components for cytokinesis. Cell Rep. 2020, 33, 108526.
- Snider CE, Wan Mohamad Noor WNI, Nguyen NTH; et al. The state of F-BAR domains as membrane-bound oligomeric platforms. Trends Cell Biol. 2021, 31, 644–655.
- Sohrmann, M, Fankhauser, C, Brodbeck, C et al. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes. Dev. 1996, 10, 2707–2719.
- Swulius MT, Nguyen LT, Ladinsky MS; et al. Structure of the fission yeast actomyosin ring during constriction. Proc. Natl. Acad. Sci. USA 2018, 115, e1455–E1464.
- Takegawa K, DeWald DB, Emr SD. Schizosaccharomyces pombe Vps34p, a phosphatidylinositol-specific PI 3-kinase essential for normal cell growth and vacuole morphology. J. Cell Sci. 1995, 108, 3745–3756.
- Takegawa K, Iwaki T, Fujita Y; et al. Vesicle-mediated protein transport pathways to the vacuole in Schizosaccharomyces pombe. Cell Struct. Funct. 2003, 28, 399–417.
- Tasto, J.J.; Morrell, J.L.; Gould, K.L. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 2003, 160, 1093–1103.
- Tran PT, Marsh L, Doye V; et al. A mechanism for nuclear positioning in fission yeast based upon microtubule pushing. J. Cell Biol. 2001, 153, 397–411.
- Vavylonis D, Wu JQ, Hao S; et al. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 2008, 319, 97–100.
- Wei B, Hercyk BS, Mattson N; et al. Unique spatiotemporal activation pattern of Cdc42 by Gef1 and Scd1 promotes different events during cytokinesis. Mol. Biol. Cell. 2016, 27, 1235–1245.
- Willet AH, DeWitt AK, Beckley JR; et al. NDR kinase Sid2 drives Anillin-like Mid1 from the membrane to promote cytokinesis and medial division site placement. Curr. Biol. 2019, 29, 1055–1063.
- Wu JQ, Sirotkin V, Kovar DR; et al. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 2006, 174, 391–402.
- Zimmermann D, Homa KE, Hocky GM; et al. Mechanoregulated inhibition of formin facilitates contractile actomyosin ring assembly. Nat. Commun. 2017, 8, 703.
- Zheng S, Dong F, Rasul F; et al. Septins regulate the equatorial dynamics of the separation initiation network kinase Sid2p and glucan synthases to ensure proper cytokinesis. FEBS J. 2018, 285, 2468–2480.
- Zhu YH, Ye Y, Wu Z; et al. Cooperation between Rho-GEF Gef2 and its binding partner Nod1 in the regulation of fission yeast cytokinesis. Mol. Biol. Cell. 2013, 24, 3187–3204.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).