1. Introduction
The solar power generation market has been growing rapidly worldwide over the past few years due to increased energy demand, expanded government support, increased interest in environmental pollution, etc., and solar module installations are increasing exponentially in line with this trend [
1,
2]. As of 2019, the cumulative solar cell installation worldwide exceeds 500 GW, and the total cumulative installation volume is expected to exceed TW by 2023 [
3,
4]. This market expansion has allowed it to produce electricity at a much lower price than other energy production methods in regions with favorable climatic conditions [
4]. However, the lifetime of solar modules is 20-30 years, and most of the modules that were initially installed have reached the end of their lifespan and are currently being partially scrapped and demolished [
5,
6]. The generation of waste solar modules is expected to exceed 1.7 million tons after 2030 [
6,
7,
8,
9]. The European Union (EU) revised the Waste Electrical and Electronic Equipment (WEEE) directive in 2012 in preparation for the renewable energy waste that will increase in the future, and classified waste solar modules as electrical and electronic waste [
10,
11]. In general, more than 95% of solar modules installed around the world are crystalline silicon-based modules and are composed of glass, aluminum frames, solar cells, PV ribbons, ethylene vinyl acetate (EVA) encapsulant, junction boxes, etc. [
12]. Of these, the high-purification process cost of polysilicon used in solar cell manufacturing accounts for more than 30% of the solar module manufacturing cost and expensive silver is used for the front part of the solar cell [
13,
14,
15]. In addition, it has been reported that when evaluating the environmental impact of the process of collecting the raw materials (2N grade silicon) required for solar cell manufacturing from quartz and sand through life cycle assessment (LCA), silicon per m
2 has the second highest global warming potential [
16]. Therefore, in this study, a study was conducted to effectively recover silicon and silver by utilizing acid leaching and substitution reactions from solar cells.
2. Materials and Methods
2.1. Experimental materials
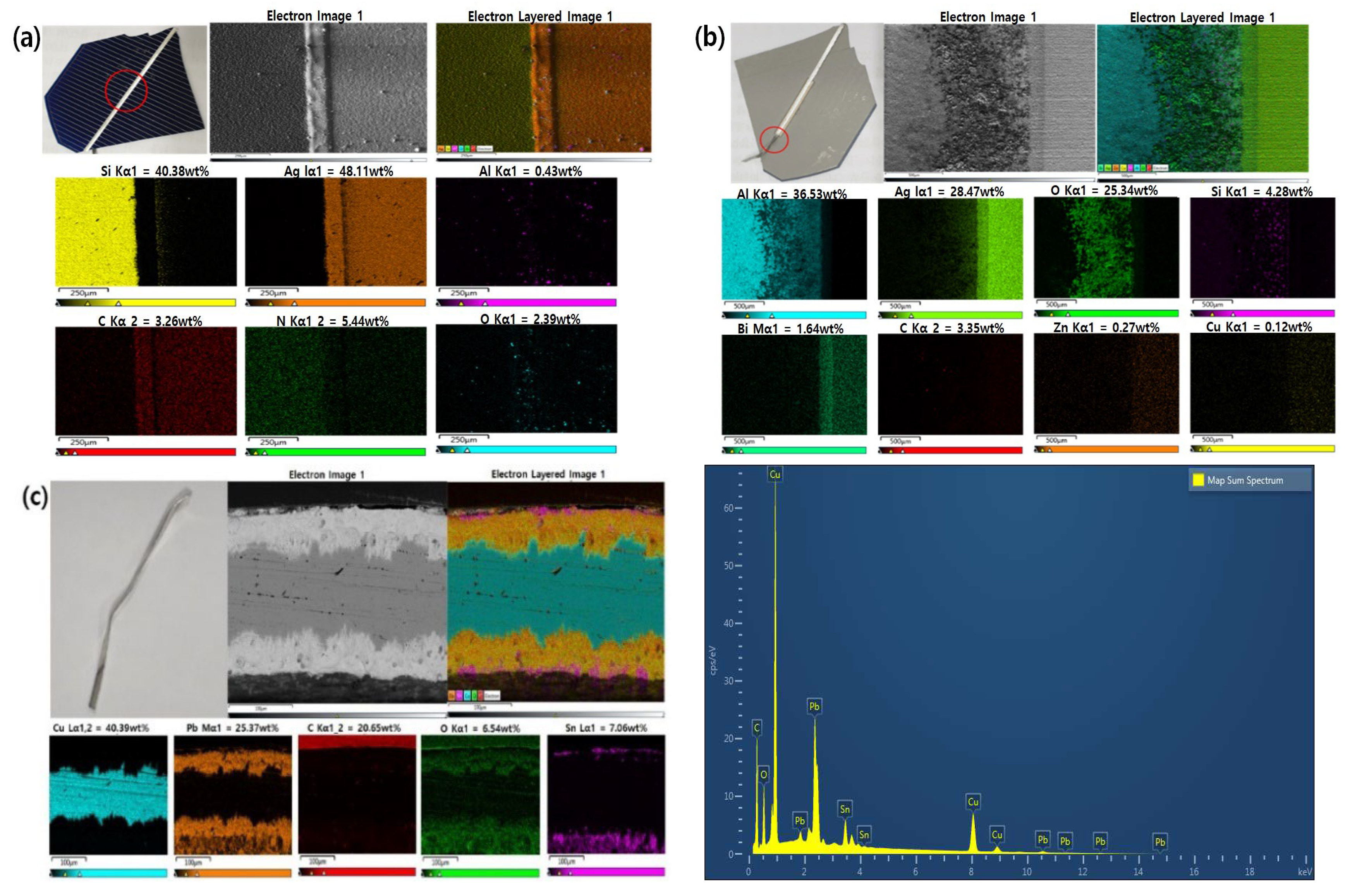
In this study, PV ribbon attached to solar cells and cells with glass, and aluminum frames were used and removed from EVA (ethylene vinyl acetate) encapsulation materials, junction boxes, etc. SEM-mapping analysis was performed on the front and back of solar cell that were mounted and polished as shown in
Figure 1a,b. The PV ribbon was removed from the solar cells and analyzed separately (
Figure 1c). The PV ribbon is the copper ribbon coated with tin and lead alloy. The solar cell separated from the PV ribbon was pulverized into a fine powder using a high alumina mortar and pestle. An analysis was conducted to identify the components of the powder by XRD and XRF analysis are shown in
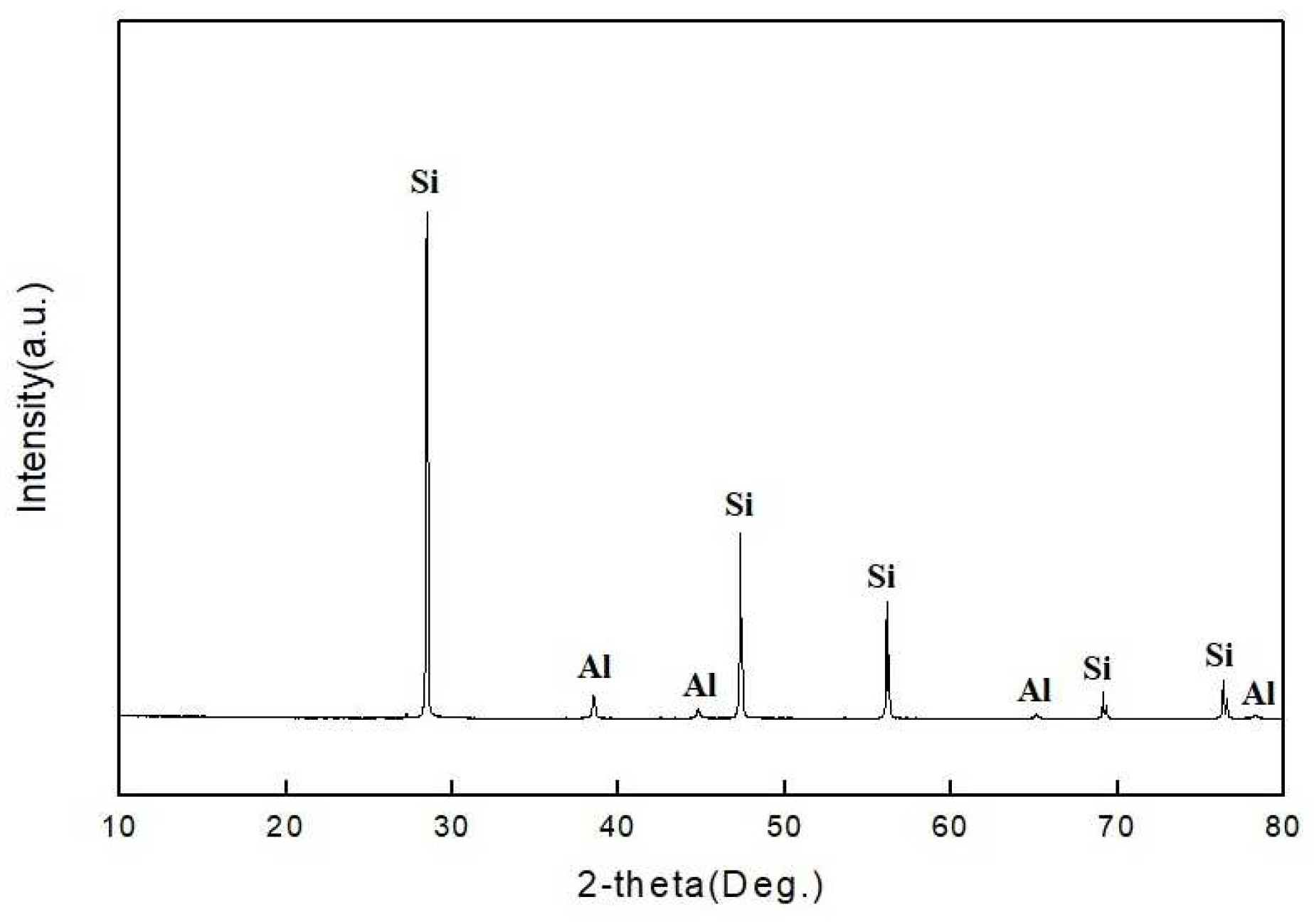
Figure 2 and
Table 1. It was detected that the powder of the solar cell consisted of silicon and aluminum according to the results of the XRD analysis. XRF analysis revealed that the powder contained 89.74 wt.% Si, 10.17 wt.% Al and a small amount of 0.074 wt.% Ag.
2.2. Selective leaching
Currently, various studies have been conducted to recover valuable metals from solar cells, and studies have been reported to identify the leaching behavior of solar cell components. Aluminum, which is the most abundant material contained in a solar cell, is reported to have a leaching effect with sulfuric acid, hydrochloric acid, and nitric acid. However, aluminum has the potential to form compounds with silicon in sulfuric acid. silver is expected to form AgCl precipitate in hydrochloric acid [
17]. In addition, the copper and lead contained in the PV ribbon are not oxidized in sulfuric acid [
18] and are reported to be soluble in nitric acid [
19,
20] and hydrochloric acid [
21]. In this study, nitric acid was used as a leaching agent based on the survey contents [
17,
18,
19,
20,
21], and a method of recovering high-purity silicon and silver in the recovered solution after leaching was derived.
2.3. Recovering high-purity silicon from solar cells
When 13 g of solar cell was sampled, it was confirmed that the weight of the PV ribbon attached to the cell surface per unit surface area was 2.5 g, and this was conformed as the standard weight of the raw material. A leaching experiment was performed using an ultrasonic cleaner (Sae Han Ultrasonic Co., SH-2340D/40kHz/10L) after adding a raw material into a 100 ml nitric acid solution prepared by a molar solution. The concentration and time of leaching were set as variables. After the experiment, the powder solar cell was washed with distilled water and dried at 100 °C for 24 hours. XRD and XRF analysis were used to determine the chemical composition and phases of samples.
2.4. Recovery of silver from leachate reacted with solar cells
For the selective recovery of silver, a substitution reaction considering the ionization tendency of the metal (Cu>Hg>Ag) was used. Copper has a stronger oxidation tendency than silver, therefore powder copper was used as a variable amount according to the calculation. After the addition of copper powder to the solution, the precipitate was filtered under reduced pressure and dried at 100 °C for 24 hours, and analyzed by XRD and XRF. ICP analysis was used on the solution that reacted with solar cells.
3. Results and Discussion
3.1. Silicon recovery using nitric acid
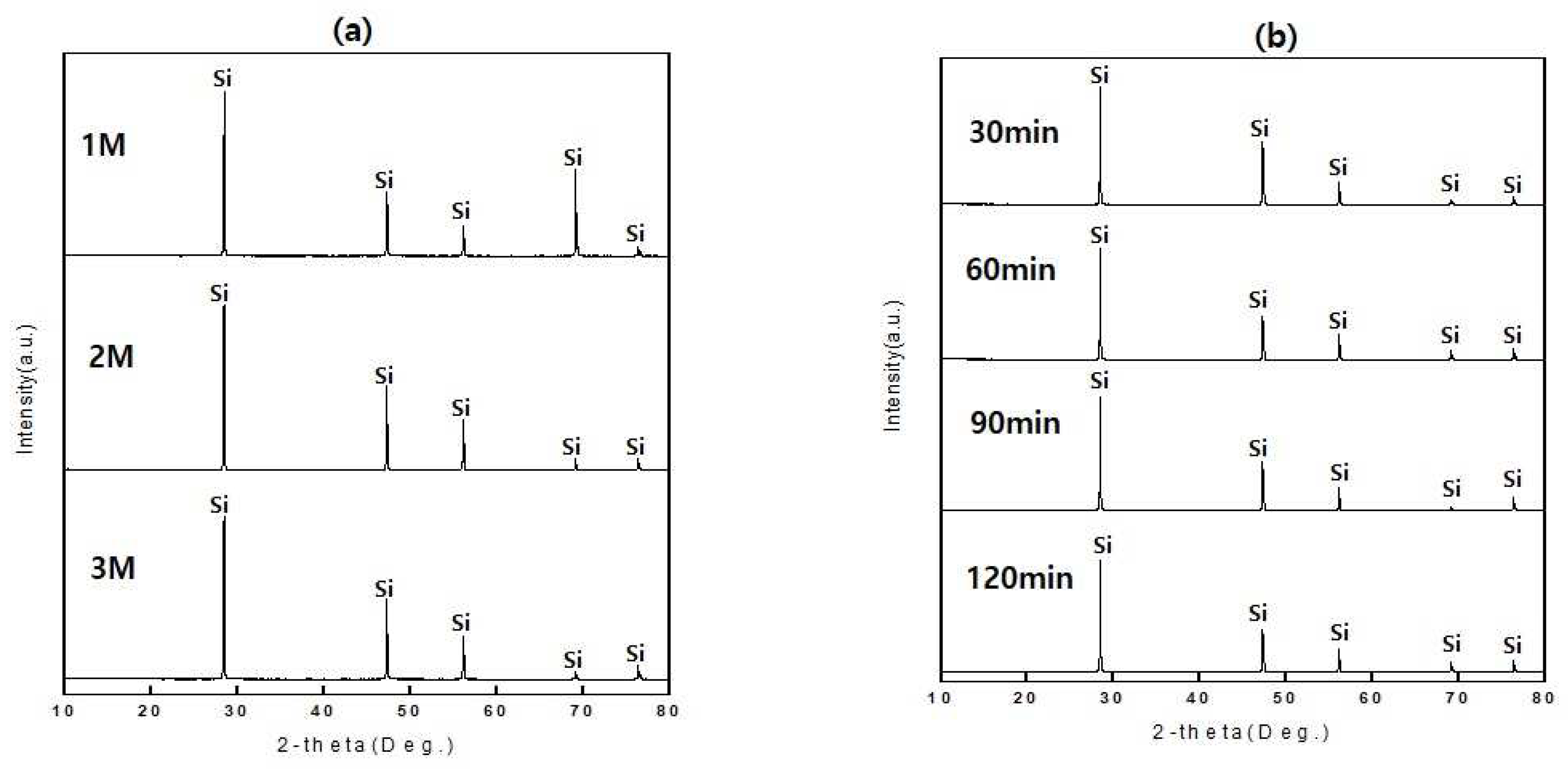
Leaching experiments of solar cells were conducted according to nitric acid concentration as the 64.1 ml of nitric acid in 1000 ml of distilled water. After preparing nitric acid solutions by 1M, 2M, and 3M, leaching experiments were conducted using an ultrasonic cleaner at a reaction temperature of 60 °C for the leaching times of 30 to 120 minutes. The results of XRD and XRF analysis after leaching are shown in
Figure 3 and
Table 2. As the XRD analysis shown in
Figure 3, Si phase was detected at all experimental conditions of the nitric acid concentrations
Figure 3a and leaching times
Figure 3b. It is confirmed that the nitric acid concentration increased, and the removal of aluminum, copper, and lead was effective. When the nitric acid concentration was 3M, the purity of silicon reached above 99% as shown in
Table 2. The leaching process is considered according to the below reaction formulas.
According to the reaction time, Si phases were detected at all leaching reaction times, it was confirmed that the aluminum was removed effectively if the nitric acid leaching time was more than 30 minutes, and silicon with a purity above 99% could be recovered as shown in
Table 3.
3.2. Silver recovery using substitution reaction from leachate
After the leaching experiment, an experiment was conducted to precipitate metals by a substitution reaction from the leachate filtered through vacuum filtration. Four samples of solution were prepared for the experiment condition as the nitric acid concentration of 3M at the reaction temperature of 60 °C for a reaction time of 30 minutes. The four samples were analyzed by ICP-OES for determining compounds as shown in
Table 4. The prepared solutions contained Cu, Al, Si, Ag, Pb, and Sn. The Ag content in the solutions was about 1.8 – 2.5 mg/l according to the sample number.
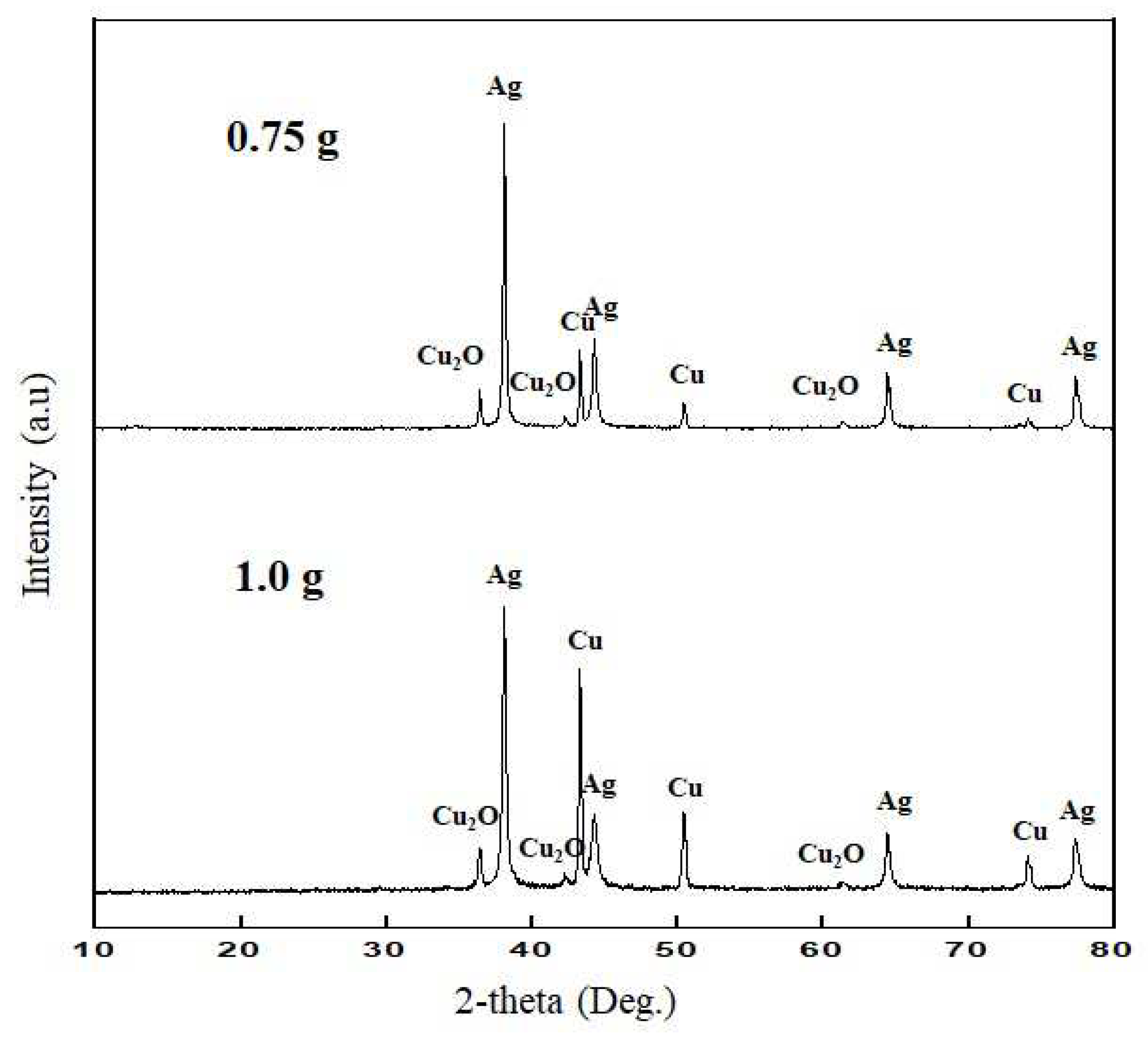
As a result of ICP analysis, the components of the leachate mostly contained copper, lead, aluminum, and silver. Taking advantage of the fact that among these metals, the silver was precipitated by adding copper, which has greater reducibility than Cu, Al, Si, Pb, and Sn. In the precipitation process, 0.25, 0.5, 0.75, and 1g of copper powder were added to solutions of 1, 2, 3, and 4, respectively, and stirred at room temperature for 30 minutes. As a result of the addition of copper powder when 0.25 and 0.5g were added, the precipitate was not observed during the experiment. When the addition of copper powder was 0.75 and 1.0 g, precipitate was produced 0.23 and 0.55 g, respectively. The produced powder was filtered under reduced pressure and dried, and then XRD, XRF, and ICP-OES analyses were performed. The analysis results are presented in
Figure 4 and
Table 5 and
Table 6, respectively.
As the result of XRF analysis (
Table 5), the solid powder contained when the addition of copper powder was 075 g, compounds were 49.9 wt.% Cu, 48.65 wt.% Ag, 1.23 wt.% Al and 0.23 wt.% Si, but, when copper addition was 1 g, compounds were 73.88 wt.% Cu, 25.24wt.% Ag, 0.71 wt.% Al and 0.17 wt.% Si. It was observed that copper addition was increased, copper content in the solid powder increased 73.88 wt.% which increased about two times, on the contrary, silver content in the solid powder decreased to 25.24 wt.%.
As a result of XRD analysis (
Figure 4) of the recovered solid powder by adding copper powder into the leachate, silver was determined as the main phase, Cu and CuO phases were detected as the impurities which were confirmed by XRF analysis.
Based on the results of the ICP-OES analysis, the recovery rate of silver was calculated below Equation 5 and presented in
Table 6. Although, the copper addition includes the increase of copper content in the precipitate, the recovery rate of silver increased to 93.3% when copper addition was 1 g which is the maximum amount of addition.
4. Conclusions
In this study, a study was conducted to recover silicon and silver, which are valuable metals, using acid solutions and substitution reactions from waste solar cells. In order to effectively recover silicon and silver, the leaching solutions was selected the acid solution (HNO3). According to the concentration of the nitric acid solution, when the concentration of the acid was 3M, copper, lead and others were completely removed from powder solar cell, and the residual silicon could be purified above 99%. As well, depending on the leaching time, it was found that 30 minutes of leaching time is enough to purify silicon with the purity of more than 99%. For recovery of silver using substitution reaction from leachate, there were no products when 0.25 g and 0.5 g of copper powder were added, but, the precipitate was produced when 0.75 g and 1 g of copper were added.
As a result of calculating the recovery rate of silver through the weight of silver of the recovered precipitate and the weight of silver in the leachate, more than 90% of silver could be recovered. The aluminum was deposited in the leachate due to it’s lower reducibility than silver and copper during precipitation reaction by copper, and it is considered that an appropriate amount of copper powder needs to be used to effectively use it in the manufacture of modules. In this study, it was achieved that recovery of silicon with more than 99% purity and the recovery rate of silver reached more than 90%, will contribute to economic and environmental benefits such as reducing the raw material of solar modules and environmental pollution from waste landfills.
Author Contributions
Hyun Jong Kim performed the experiments, organized the study plan, Urtnasan Erdenebold, wrote manuscript, and Jei-Pil Wang reviewed and checked the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This study was supported by the Korea Ministry of Environment (MOE) and the Korea Environment Industry & Technology Institute (KEITI).
Conflict of Interest
The authors declare that have no conflict of interest.
References
- Lineesh Punathil, K. Mohanasundaram, K.S.Tamilselavan, Ravishankar Sathyamyrthy, and Ali J.Chamkha, Recovery of Pure Silicon and other materilas from disposed solar cells. Int.J.Photoenergy, 2021, 1-4. [CrossRef]
- Dae-Sik Yoon,Young-Soo Ahn, Gi-Hawn Kang,Hyo Sik Chang, Jin-Seok Lee, Silicon Recovery from Solar Module Waste by a Physical Method. J.Korean Solar Energy, 2022, 42(3), 1-11. [CrossRef]
- Fatih Birol. Renewables 2018, Analysis and Forecast to 2023. International Energy Agency, OECD/IEA, 2018.
- Nancy, M. Haegel, Robert Margolis, Tonio Buonassisi, David Feldman, and Sarah Kurtz, Terawatt-scale photovoltaics: Trajectrories and challenges. Science, 2017, 6334(356), 141-143. [CrossRef]
- Ga Min Kim, Hyo Sik Chang. SiC Powder Manufacturing through Silicon Recovery from Waste Si Solar Cells. J.Korean Solar Energy, 2021, 42(3), (2021) 173-180.
- Kyounga Lee, Jongmun Cha, Lee Kyung-ah, Chajong Mun. Management of Solar Photovoltaic Panels under the Extended Producer Responsibility Legislation. J.Korean Soc.Miner.Energy.Resour, 2019, 56(4), 367-376. [CrossRef]
- Daniela Sica, Ornella Malandrino, Stefania Supino, Mario Testa, Maria Claudia Lucchetti. Management of end-of-life Photovoltaic panels as a step towards a circular economy. Renew.Sustain. Energy Rev, 2018, 82(3), 2934-2945. [CrossRef]
- Stephanie Weckend, Andreas Wade, Garvin Heath. End of Life Management: Solar Photovoltaic panels. IRENA-International Renewable Energy Agency. June 2016.
- Lu Sun, Minoru Fujii, Tomohiro Tasaki, Huijuan Dong, Satoshi Ohnishi. Improving waste to energy rate by promoting an integrated municipal solid-waste management system. Resour. Conserv. Recycl., 2018, 136 289–29. [CrossRef]
- S.E. Kabir, M.N.I. Mondal, M.K. Islam, I.A. Alnaser, M.R. Karim, M.A. Ibrahim, K. Sopian, M. Akhtaruzzaman. Adoption and implementation of extended producer responsibility for sustainable management of end-of-life solar photovoltaic panels. Global J. Environ. Sci. Manage., 2023, 9(2023) 251-270. [CrossRef]
- Carl L.Yaws, Ku-Yen Li, Thomas C.T. Chu, C.S. Fang, Ralph Lutwack, Anthony Briglio J. New technologies for solar energy silicon-Cost analysis of dichlorosilane process. Sol. Energy. 1981, 27, 539-546. [CrossRef]
- D. M. Powell, M. T. Winkler, H. J. Choi, C. B. Simmons, D. Berney Needleman, T. Buonassisi. Crystalline silicon photovoltaics: a cost analysis framework for determining technology pathways to reach baseload electricity costs. Energy Environ. Sci. 2012, 5, 5874-5883. [CrossRef]
- Abhishek Kumar, Monika Bieri, Thomas Reindl, Armin G. Aberle. Economic Viability Analysis of Silicon Solar Cell Manufacturing: Al-BSF versus PERC. Energy Procedia, 2017, 130, 43-49. [CrossRef]
- Anna Kuczyńska-Łażewskaa, Ewa Klugmann-Radziemskaa, Zuzanna Sobczakb, Tomasz Klimczukb. Recovery of silver metallization from damaged silicon cells. Sol. Energy Mater. Sol. Cells. 2018, 176, 190-195. [CrossRef]
- Jing Tao, Suiran Yu. Review on feasible recycling pathways and technologies of solar photovoltaic modules. Sol. Energy Mater. Sol. Cells. 2015, 141, 108-124. [CrossRef]
- Lizabeth Markert, Ilke Celik, Defne Apul. Private and Externality Costs and Benefits of Recycling Crystalline Silicon (c-Si) Photovoltaic Panels. Energies. 2020, 13(14), 3650. [CrossRef]
- P. M. Tembo,1 M. Heninger,2 and V. Subramanian. An Investigation of the Recovery of Silicon Photovoltaic Cells by Application of an Organic Solvent Method. ECS J. Solid State Sci. Technol. 2021, 10, 025001. [Google Scholar] [CrossRef]
- Qi Han, Yidi Gao, Ting Su, Jiabao Qin, Chi Wang, Zhan Qu, Xianze Wang. Hydrometallurgy recovery of copper, aluminum and silver from spent solar panels. J. Environ.Chem.Eng. 2023, 11, 109236. [CrossRef]
- Jeongeun Shina, Jongsung Parkb, Nochang Park. A method to recycle silicon wafer from end-of-life photovoltaic module and solar panels by using recycled silicon wafers. Sol.Energy. Mater Sol. Cells . 2017, 162, 1–6. [CrossRef]
- Dina Magdy Abdo 1, Ayat Nasr El-Shazly 1 and Franco Medici. Recovery of Valuable Materials from End-of-Life Photovoltaic Solar Panels. Materials. 2023, 16, 2840. [CrossRef]
- Youn Kyu Yi, Hyun Soo Kim, Tam Tran, Sung Kil Hong & Myong Jun Kim. Recovering valuable metals from recycled photovoltaic modules. J Air Waste Manag Assoc. 2014, 64(7), 797-807. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).