Submitted:
20 November 2023
Posted:
21 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials Synthesis
2.2. Characterization and Electrochemical Measurement
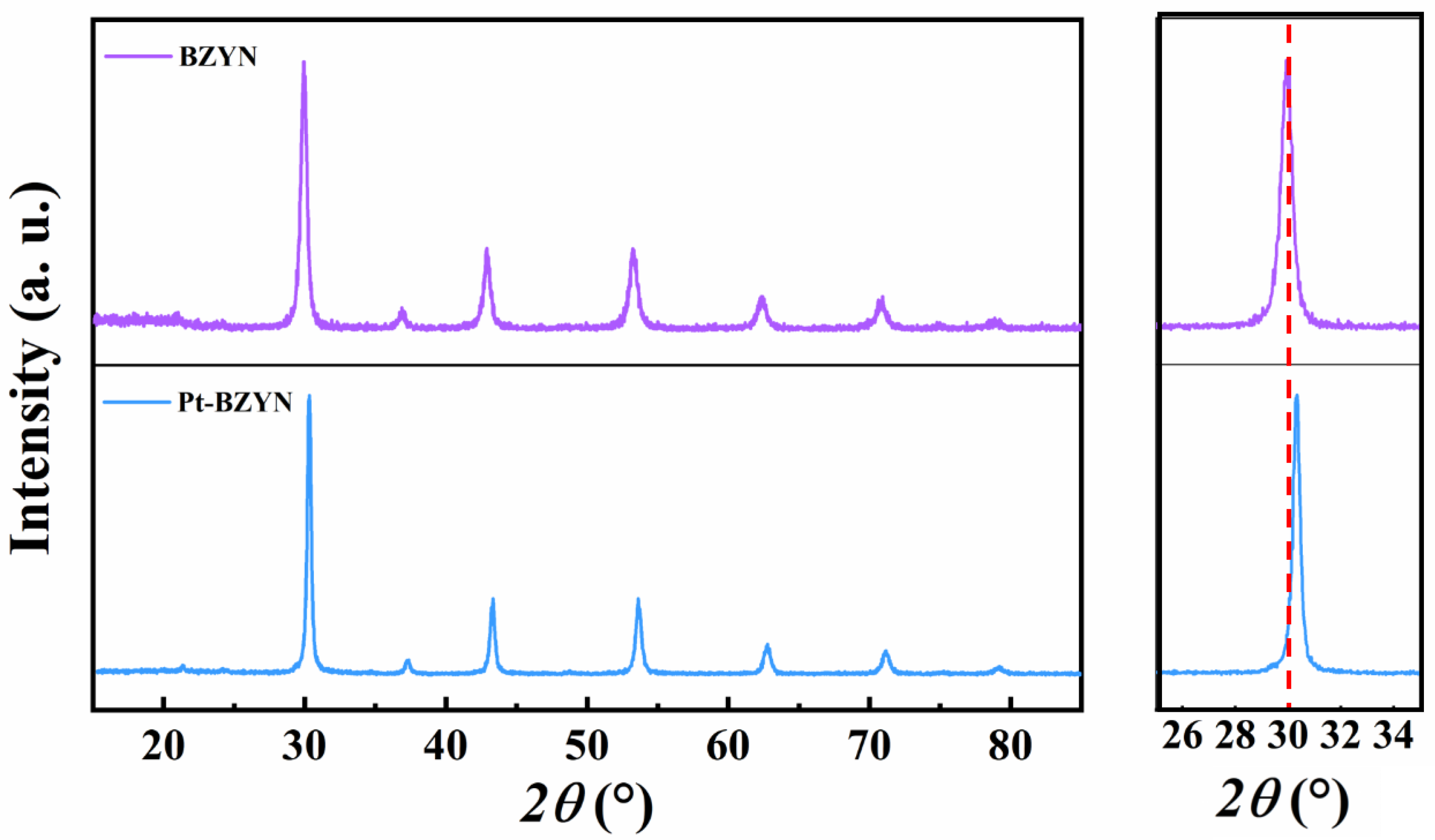
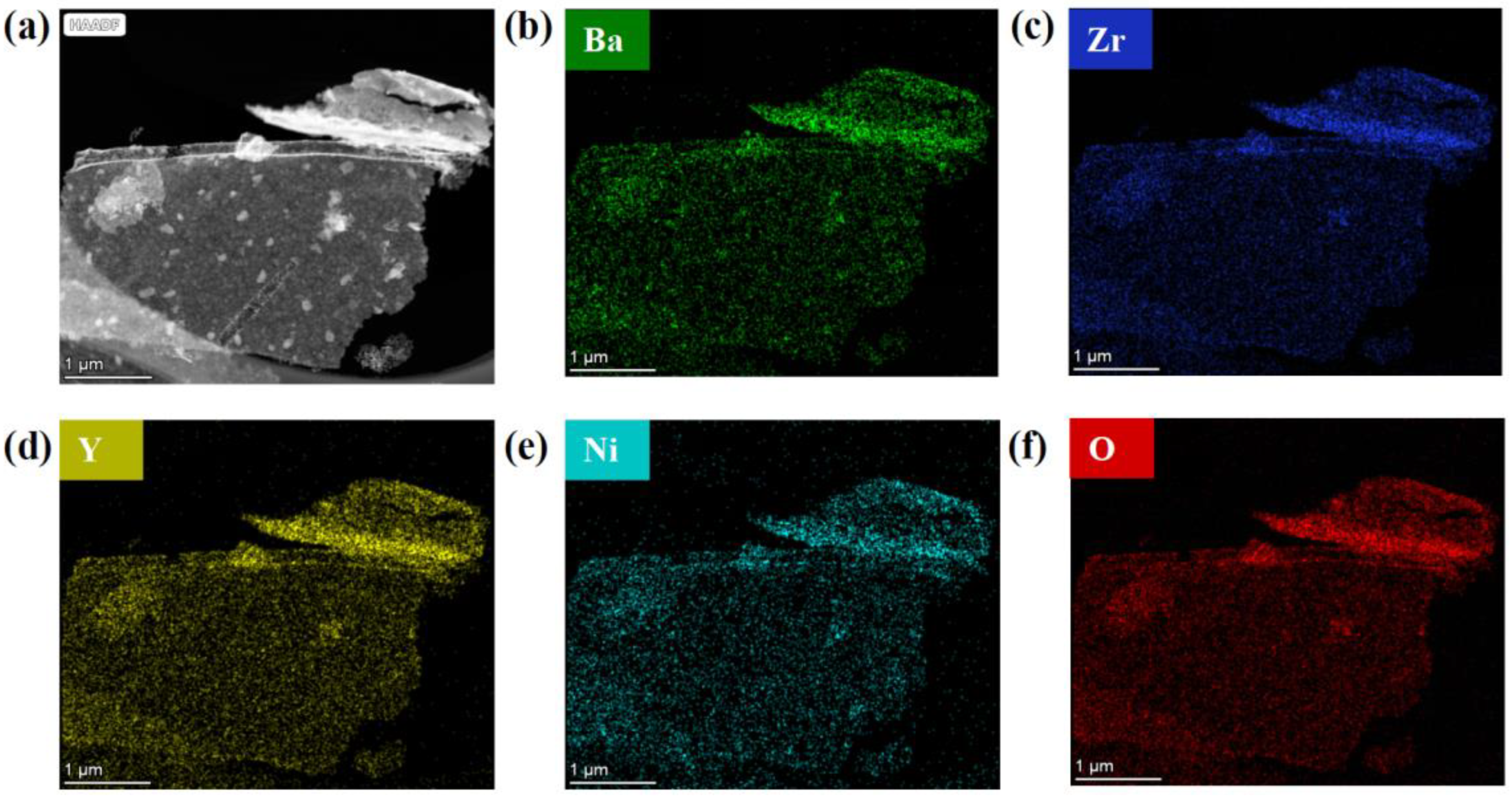
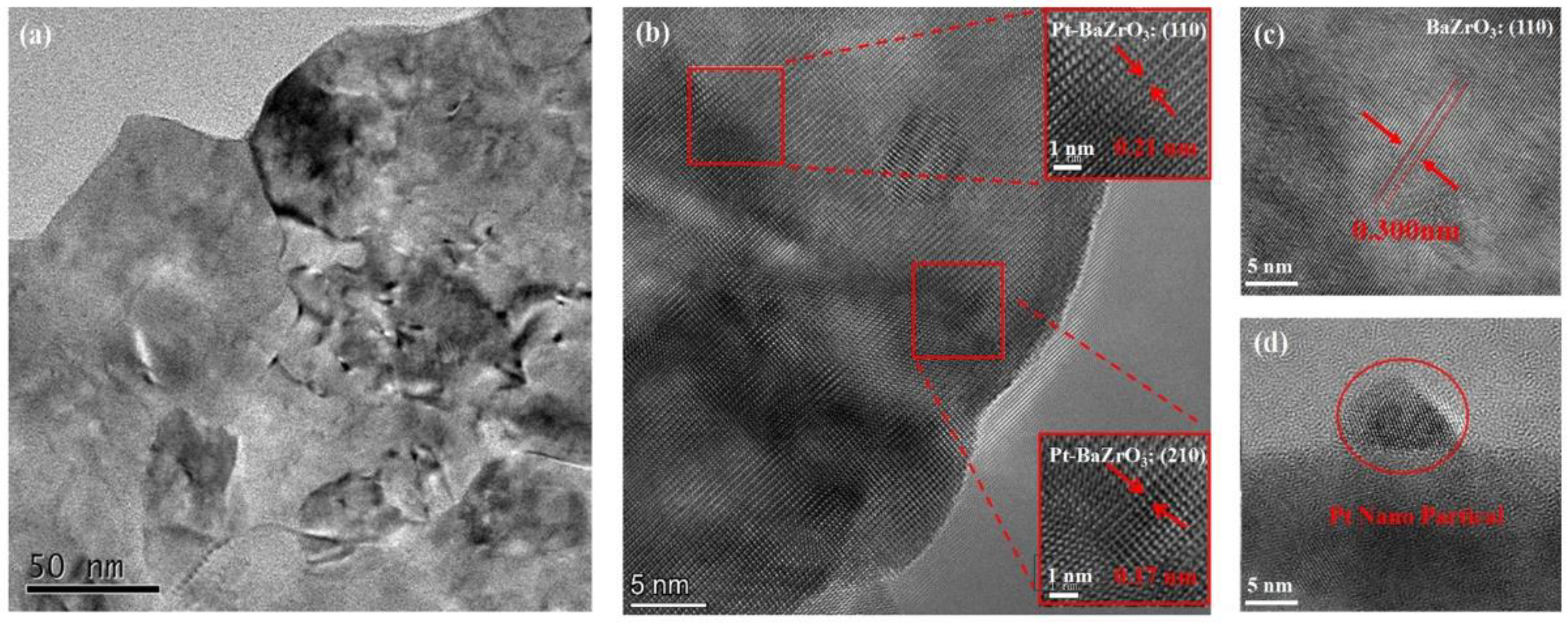
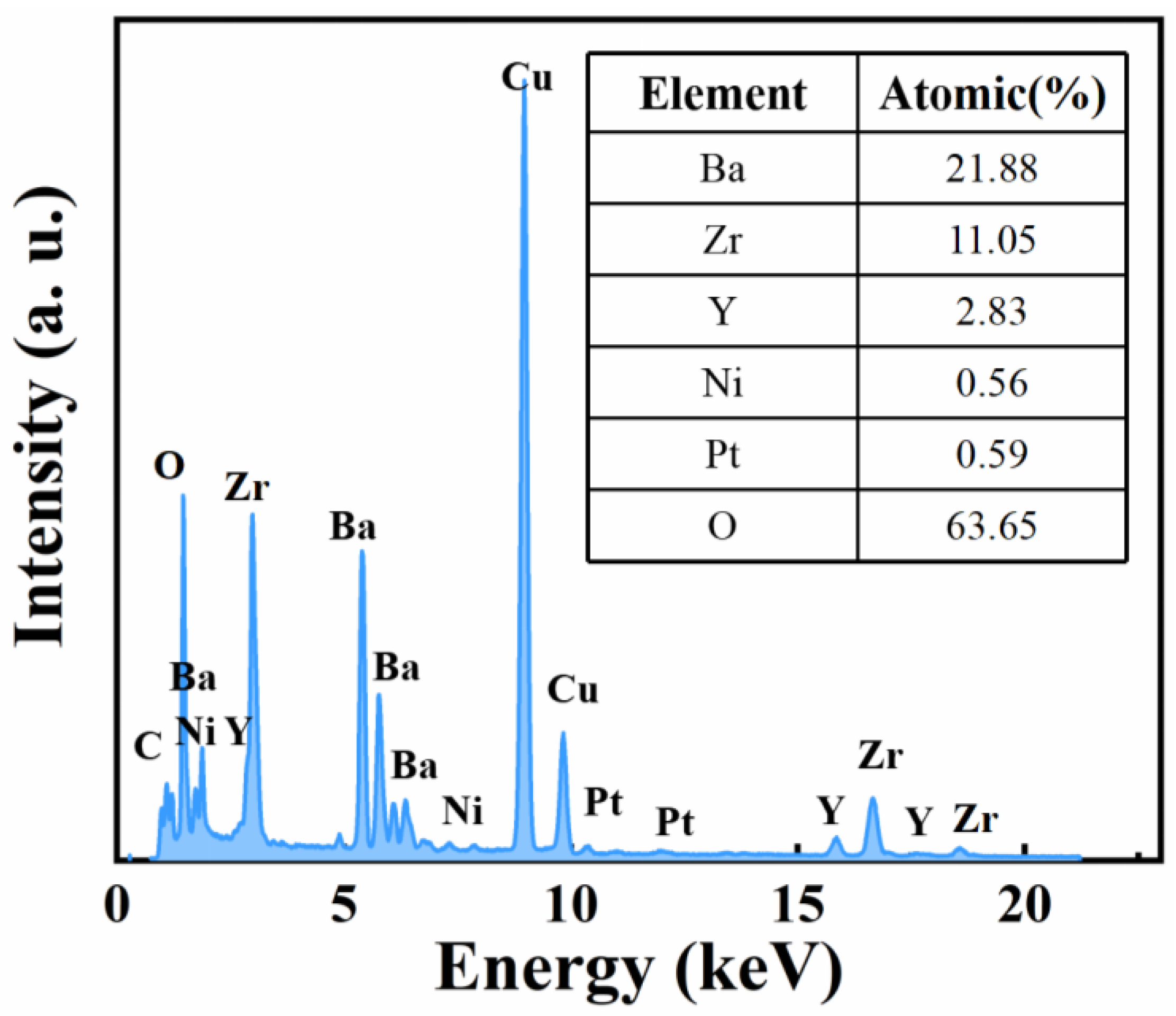
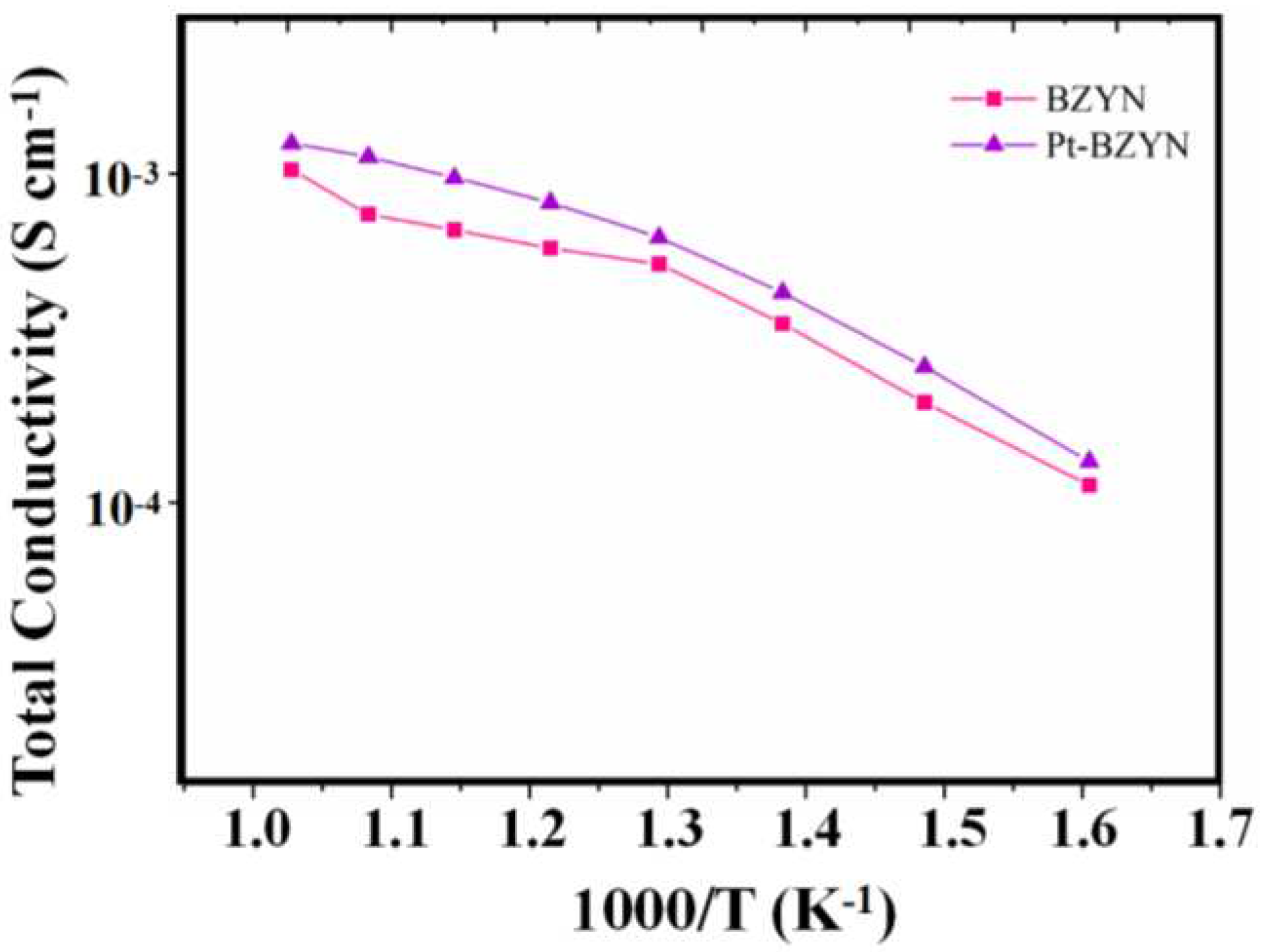
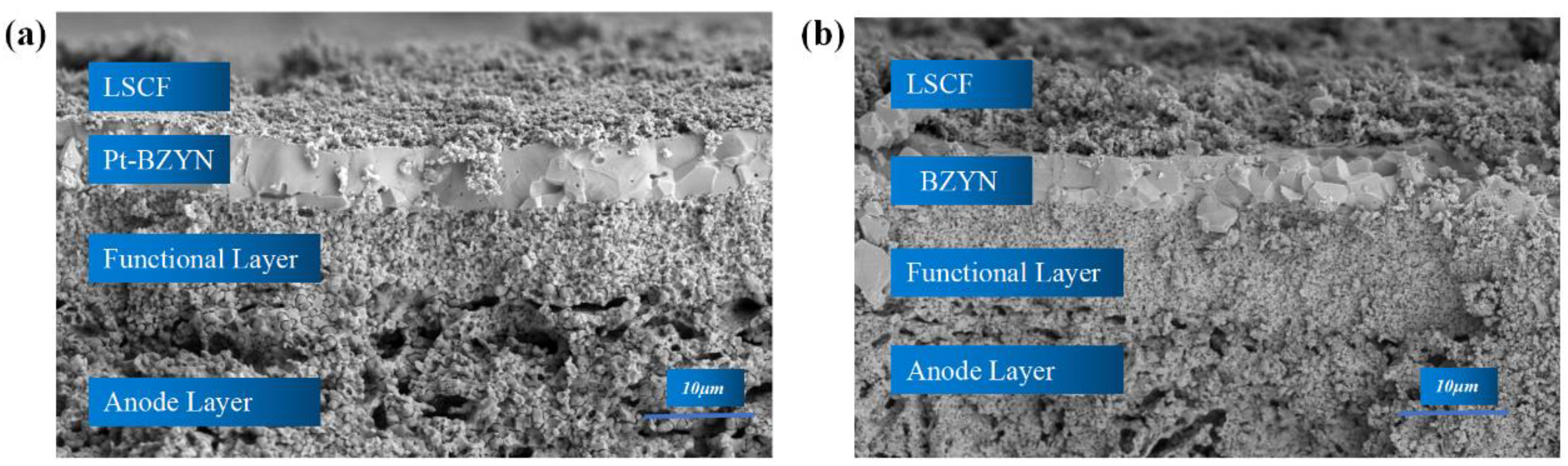
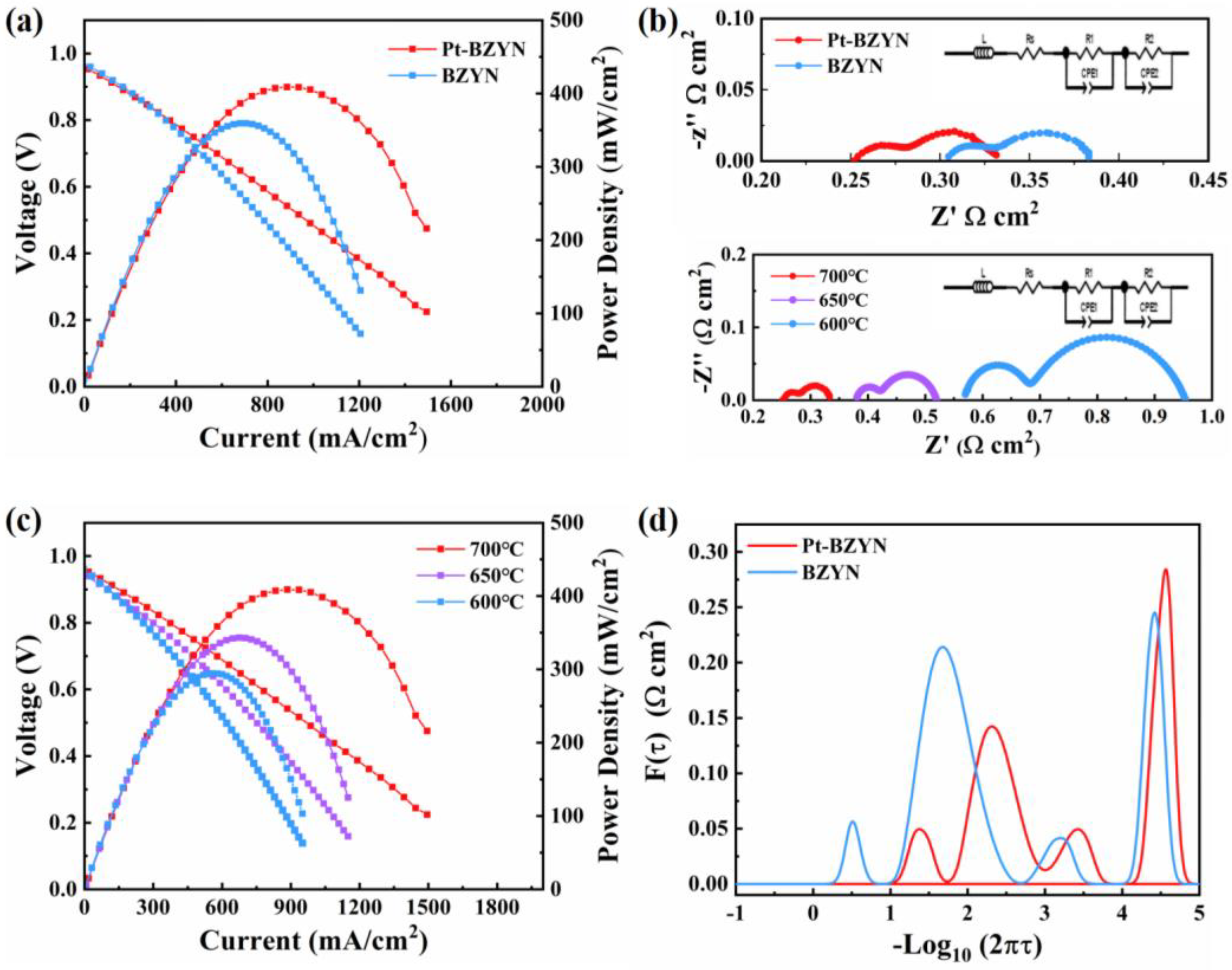
3. Results and Discussion
4. Conclusion
Acknowledgments
References
- Gao, Y.; Zhang, M.; Fu, M.; Hu, W.; Tong, H.; Tao, Z. A comprehensive review of recent progresses in cathode materials for Proton-conducting SOFCs. Energy Reviews 2023, 100038. [Google Scholar] [CrossRef]
- Loureiro, F.J.; Nasani, N.; Reddy, G.S.; Munirathnam, N.; Fagg, D.P. A review on sintering technology of proton conducting BaCeO3-BaZrO3 perovskite oxide materials for Protonic Ceramic Fuel Cells. Journal of Power Sources 2019, 438, 226991. [Google Scholar] [CrossRef]
- Vignesh, D.; Sonu, B.K.; Rout, E. Factors constituting proton trapping in BaCeO3 and BaZrO3 perovskite proton conductors in fuel cell technology: a review. Energy & Fuels 2022, 36, 7219–7244. [Google Scholar] [CrossRef]
- Tong, H.; Fu, M.; Yang, Y.; Chen, F.; Tao, Z. A Novel Self-Assembled Cobalt-Free Perovskite Composite Cathode with Triple-Conduction for Intermediate Proton-Conducting Solid Oxide Fuel Cells. Advanced Functional Materials 2022, 32, 2209695. [Google Scholar] [CrossRef]
- Dudek, M.; Lis, B.; Lach, R.; Daugela, S.; Salkus, T.; Kezionis, A.; Mosialek, M.; Socha, R.P.; Morgiel, J.; Gajek, M.; Sitarz, M.; Ziabka, M. Ba0.95Ca0.05Ce0.9Y0.1O3 as an electrolyte for proton-conducting ceramic fuel cells. Electrochimica Acta 2019, 304, 70–79. [Google Scholar] [CrossRef]
- Xie, K.; Yan, R.Q.; Liu, X.Q. Stable BaCe0.7Ti0.1Y0.2O3-δ proton conductor for solid oxide fuel cells. Journal of Alloys and Compounds 2009, 479, L40–L42. [Google Scholar] [CrossRef]
- Hossain, M.K.; Iwasa, T.; Hashizume, K. Hydrogen isotope dissolution and release behavior in Y-doped BaCeO3. Journal of the American Ceramic Society 2021, 104, 6508–6520. [Google Scholar] [CrossRef]
- Han, D.; Uda, T. The best composition of an Y-doped BaZrO3 electrolyte: selection criteria from transport properties, microstructure, and phase behavior. Journal of Materials Chemistry A 2018, 6, 18571–18582. [Google Scholar] [CrossRef]
- Fluri, A.; Marcolongo, A.; Roddatis, V.; Wokaun, A.; Pergolesi, D.; Marzari, N.; Lippert, T. Enhanced proton conductivity in Y-doped BaZrO3 via strain engineering. Advanced Science 2017, 4, 1700467. [Google Scholar] [CrossRef]
- Hu, H.J.; Zou, J.; Shan, L.; Jiang, X.Q.; Ni, Y.J.; Li, X.B.; Qian, X.W.; Chen, W.W.; Zhou, Y.C.; Zhang, W.F.; Wei, S.H.; Jian, J.W. Conductivities in Yttrium-Doped Barium Zirconate: A First-Principles Study. Crystals 2023, 13. [Google Scholar] [CrossRef]
- Bondevik, T.; Bjørheim, T.S.; Norby, T. Assessing common approximations in space charge modelling to estimate the proton resistance across grain boundaries in Y-doped BaZrO3. Physical Chemistry Chemical Physics 2020, 22, 11891–11902. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Z.; Shi, Z.; Liu, W. Chemically stable and easily sintered high-temperature proton conductor BaZr0.8In0.2O3−δ for solid oxide fuel cells. Journal of power sources 2013, 229, 95–101. [Google Scholar] [CrossRef]
- Sun, W.; Shi, Z.; Liu, M.; Bi, L.; Liu, W. An easily sintered, chemically stable, barium zirconate-based proton conductor for high-performance proton-conducting solid oxide fuel cells. Advanced Functional Materials 2014, 24, 5695–5702. [Google Scholar] [CrossRef]
- Ji, S.; Tanveer, W.H. Thickness determination of porous Pt cathode thin film capped by atomic layer-deposited alumina for low-temperature solid oxide fuel cells. Applied Surface Science 2020, 514. [Google Scholar] [CrossRef]
- Gandavarapu, S.R.; Sabolsky, K.; Gerdes, K.; Sabolsky, E.M. Direct foamed and nano-catalyst impregnated solid-oxide fuel cell (SOFC) cathodes. Materials Letters 2013, 95, 131–134. [Google Scholar] [CrossRef]
- Xu, Y.S.; Kou, H.N.; Fang, S.H.; Wang, X.F.; Bi, L. Evaluation of potential reaction between BaZr0.8Y0.2O3-δ ceramics and Pt at high temperatures. Ceramics International 2019, 45, 22383–22387. [Google Scholar] [CrossRef]
- Lussier, J.A.; Shafi, S.P.; Donaberger, R.L.; Bieringer, M. Platinum Uptake and Ba2CePtO6 Formation During in Situ BaCe1-xMxO3-δ (M=La, In) Formation. Inorganic Chemistry 2014, 53, 8809–8815. [Google Scholar] [CrossRef]
- Xu, Y.; Kou, H.; Fang, S.; Wang, X.; Bi, L. Evaluation of potential reaction between BaZr0.8Y0.2O3-δ ceramics and Pt at high temperatures. Ceramics International 2019, 45, 22383–22387. [Google Scholar] [CrossRef]
- Fu, M.; Lin, X.; Li, X.Y.; Tao, Z.T. Applications of microwave technology in the field of solid oxide fuel cell - a review. Russian Chemical Reviews 2023, 92. [Google Scholar] [CrossRef]
- Xu, Y.; Kou, H.; Fang, S.; Wang, X.; Bi, L. Evaluation of potential reaction between BaZr0.8Y0.2O3-δ ceramics and Pt at high temperatures. Ceramics International 2019, 45, 22383–22387. [Google Scholar] [CrossRef]
- Shi, N.; Xie, Y.; Huan, D.; Yang, Y.; Xue, S.; Qi, Z.; Pan, Y.; Peng, R.; Xia, C.; Lu, Y. Controllable CO2 conversion in high performance proton conducting solid oxide electrolysis cells and the possible mechanisms. Journal of Materials Chemistry A 2019, 7, 4855–4864. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A.; Kadi, M.W.; Alresheedi, A.S.; Mkhalid, I.A.J.A. o. Fabrication of mesoporous PtO–ZnO nanocomposites with promoted photocatalytic performance for degradation of tetracycline. 2021, 6, 6438–6447. [CrossRef]
- Zheng, L.; Xie, J.; Liu, X.; Yang, C.; Zheng, W.; Zhang, J.J.A.A.M. Interfaces, Unveiling the electronic interaction in ZnO/PtO/Pt nanoarrays for catalytic detection of triethylamine with ultrahigh sensitivity. 2020, 12, 46267–46276. [CrossRef]
- Wang, X.; Ma, Z.; Zhang, T.; Kang, J.; Ou, X.; Feng, P.; Wang, S.; Zhou, F.; Ling, Y. Charge-transfer modeling and polarization DRT analysis of proton ceramics fuel cells based on mixed conductive electrolyte with the modified anode–electrolyte interface. ACS applied materials & interfaces 2018, 10, 35047–35059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




