1. Introduction
The external exposome plays a key role in the pathobiology of the inflammatory response to aeroallergens leading to subsequent clinical phenotypes of allergic disease in genetically predisposed individuals. Allergen immunotherapy (AIT) is the only available disease modifying-treatment for respiratory atopic individuals presenting with IgE-mediated allergic rhinitis and/or allergic asthma. Accurate identification of the causative underlying allergen source is mandatory to achieve a successful response to AIT in terms of symptomatic relief and even sustained clinical remission after stopping the treatment [
1]. The availability of single allergenic molecules has elicited a revolutionary age in precision allergy molecular diagnosis (P@MD), including the optimal planning of tailored AIT in polysensitized subjects [
2]. As the natural pathobiology of allergen sensitization across the lifespan has not been fully elucidated and given the prophylactic role of AIT in the so-called “atopic march”, the most appropriate timeframe to start therapy remains still uncertain. The aim of this post-hoc analysis is to determine the current eligibility for house dust mite (HDM) AIT of a selected cohort of youngsters afflicted with different atopic phenotypes [
3].
2. Materials and Methods
2.1. Subjects
The current work was restricted to consecutive children and young adults -from 5 to 20 years of age- with an allergist-confirmed diagnosis of active allergic rhinitis (AR), asthma (A), or atopic dermatitis (AD) according to current guidelines [
4,
5,
6] and enrolled from January 2021 to January 2023. This investigation was authorized by the local Ethical Committee and informed consent was properly collected from all participants, and/or parents/guardians for those under 18 years of age. Onset of allergic symptoms after 3 (or more) years of local residency was required to participate in the present investigation. Study workflow included retrieval of sociodemographic data, past and current medical conditions, and skin prick test (SPT) results to a battery of local inhalants.
2.2. Skin Prick Test, and IgE Assays
Percutaneous testing was carried out according to European standards [
7], enclosing a diagnostic panel (Inmunotek, Madrid, Spain) with standardized raw extracts (
Dermatophagoides pteronyssinus (
D. pteronyssinus),
Blomia tropicalis (
B. tropicalis),
Lepidoglyphus destructor (
L. destructor),
Glycyphagus domesticus (
G. domesticus)
Tyrophagus putrescentiae (
T. putrescentiae), cat and dog dander, grass mix (
Poa pratensis, Dactilis glomerata, Lolium perenne, Phleum pratense, and
Festuca pratensis), olive,
Parietaria judaica, Artemisa vulgaris,
Alternaria alternata,
Aspergillus fumigatus,
Cladosporium herbarum, and
Blatella. Histamine (10 mg/mL) and saline were used as positive and negative controls as usual. Antihistamines were withdrawn a week before the SPT and wheal diameters were immediately measured after 20 min, with diameters greater than 3 mm regarded as positive. Blood samples were obtained from all participating individuals, identified with a code label, stored at −40 °C, and thawed immediately prior to the in vitro assay. Total IgE levels and sIgE were measured (ALEX MacroArray Diagnostics, Vienna, Austria) according to the manufacturer’s instructions in all included subjects. In brief, ALEX is a multiplex array containing 282 reagents (157 whole allergens and 125 molecular components). The different allergens and components are coupled onto polystyrene nano-beads, and then the allergen beads are deposited onto a nitrocellulose membrane, as formerly published [
8]. Total IgE levels were expressed in international units per unit volume (IU/mL), and sIgE levels were expressed in kU
A/L. Values ≥ 0.35 kU
A/L were considered positive. A total of 17 mite molecular allergens were included: Der p 1, Der p 2, Der p 5, Der p 7, Der p 10, Der p 11, Der p 20, Der p 21, Der p23, Der f 1, Der f 2, Blo t 5, Blot 10, Blo t 21, Lep d 2, Gly d 2, and Tyr p 2. Only subjects with a positive SPT and/or a specific sIgE to at least one of the corresponding crude mite extracts were included in the study. Patients under treatment with past or current allergen immunotherapy or biologics were excluded.
2.3. Statistical Analysis
Demographic features were summarized by medians and standard deviations for continuous variables and percentages for categorical variables. Kruskal–Wallis, Mann–Whitney U, and Chi-square tests were required for parametric continuous, nonparametric continuous, and categorical variables, respectively. A p-value of less than 0.05 was considered statistically significant. All data were analyzed using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, La Jolla, CA, USA.
3. Results
3.1. Study population
A total of 73 patients were screened, with 60 of them finally confirming their eligibility for the study. The selected subset included 31 males and 29 females, with a mean age of 15.15 years (range 8–20). Most patients included in the current study were Caucasians (91.3%) living in urban dwellings (72.1%). The patients were divided into three different groups -AR, A, or AD- according to their current atopic disease. Globally, 12 out of 20 (60%) individuals suffered from severe AR, followed by 55% (11 out 20) with severe A, and 35% (7 out of 20) with severe AD. All subjects were on regular daily treatment, including environmental allergen avoidance measures and standard medical care according to their disease stage and severity. Confirmed food allergy (milk, egg, and/or tree-nuts) was associated in 13 subjects (23.66%), and 49 individuals (81.66%) alleged a documented family history of atopy. Thirteen patients—6 with AR, 5 with A, and 2 with AD—were excluded after a showing a negative skin prick test (SPT) to local aeroallergens (
n = 6) or current/former allergen immunotherapy (
n = 7). The quantitative analysis of total serum IgE showed a median (range) value of 380.5 (23.8–6,820) IU/mL in the investigated population. More specifically, a median value of total IgE of 73.15 (23.88–2160) IU/mL was found for AR, 551.5 (41–2242) IU/mL for A, and 965 (81.81–17,420) IU/mL for AD (
Table 1).
3.2. sIgE Reactivity, and Individual Molecular Profile
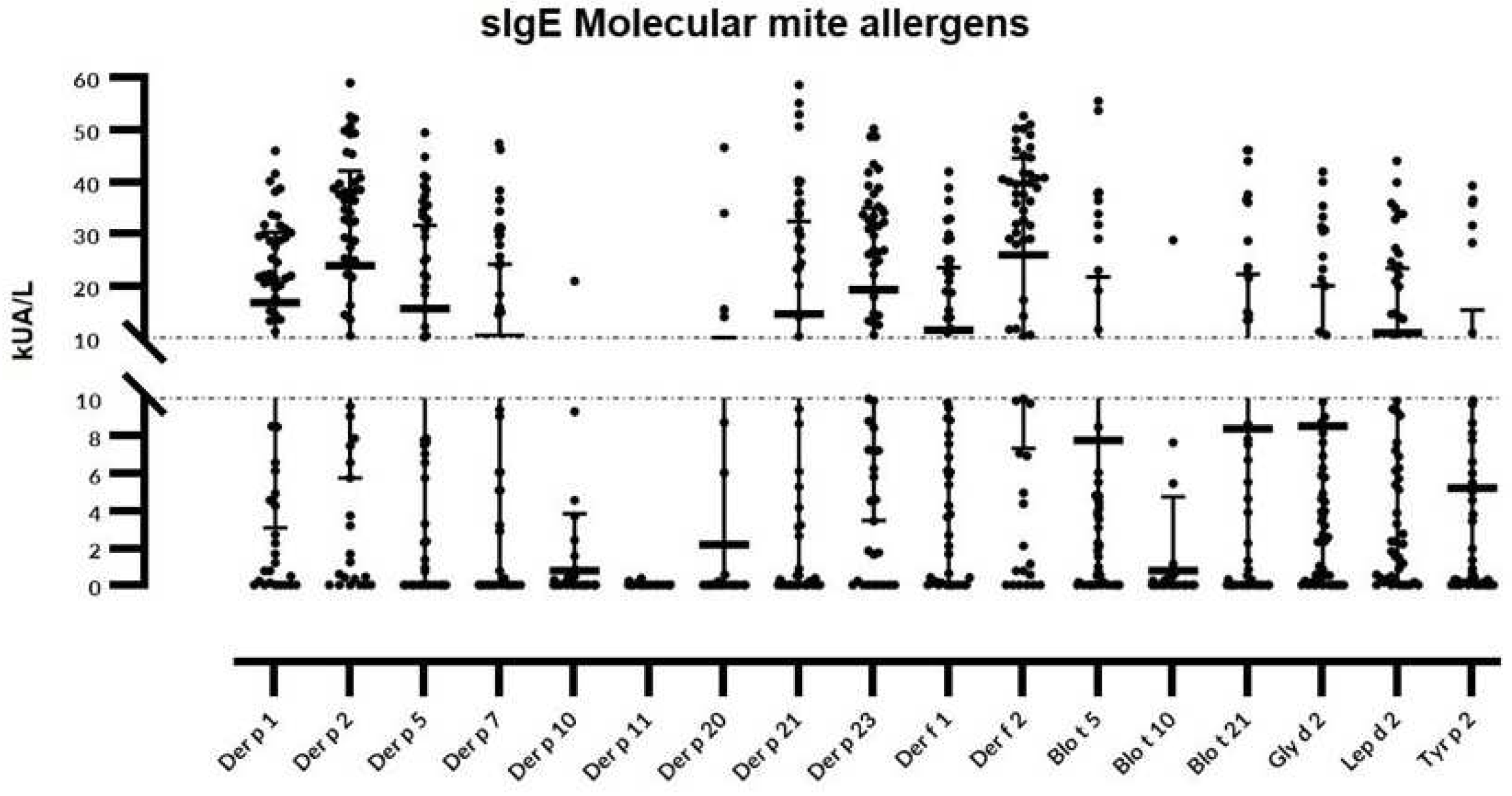
Considering individual molecular allergens exclusively, Der f 2 was most frequently identified with sIgE ≥0.35 kU
A/L in 54 out of 60 subjects (90%) and Der p 2 in 53 patients (88.3%), followed by Der p 23 (83.3%), Der p 1 and Lep d 2 (81.6%), Gly d 2 (78.3%), Der f 1 (75%), Der p 5 (70%), Der p 21 (66.6%), Blo t 5 (60%), and Der p 7 (50%) (
Figure 1).
The following molecules were found in <50% of the studied population: Tyr p 2 (46.6%), Blo t 21 (45%), Der p 10 (15%), Der p 20 (13.3%), Blo t 10 (11.6%), and Der p 11 (1.6%). Fifty-nine out of 60 (98.3%) individuals depicted sIgE responses one or more of the investigated molecules. Only 2 subjects (3.3%) were exclusively sensitized to group 1 or 2 HDM allergens, meanwhile single reactors were scarcely (5%) found, and limited to Der p 21 and Der p1 in 2 and 1 subjects respectively.
A higher frequency (
p < 0.05) of sIgE binding was found in patients with A (97.3%) or AD (86.6%) in contrast to those subjects with AR (49.5%). In addition, patients afflicted with A or AD showed quantitatively higher serum titers (
p < 0.05) to six mite molecules—Der p 2, Der p 5, Der p 7, Der p 21, Der f 1, and Der f 2,—than individuals with AR (
Table 2).
Aggregation of molecules beyond group 1 or 2 HDM allergens as Der p 5, Der p 7, Der p 21, Der p 23, Blo t 5, Lep d 2, and Gly d 2 confirmed a marked pleiomorphic molecular response in youngsters subjected to a high exposure to both HDM and SM, and influenced by perennial subtropical climate conditions. Both A and AD phenotypes showed a more complex aggregation pattern of molecules, including concurrent sensitization to ≥ 8 HDM mite allergens, in contrast to most of those subjects (>83%) with AR displaying a concomitant sIgE response to <8 HDM molecules (
Table 3).
4. Discussion
Our findings indicate that IgE reactivity profiles in children to multiple mite allergen components increase in concentration and complexity depending on their basal atopic disease. The commercial availability of further mite molecular allergens expands the diagnostic possibilities beyond the dichotomy of group 1 or 2 HDM allergen sensitization in the investigated population. In fact, 9, 11 and 13 mite molecules showed a corresponding sIgE binding frequency ≥50% in patients afflicted with AR, AD and A, respectively. Former longitudinal investigations -confirming the molecular spreading hypothesis- have elegantly showed that only children with concurrent sIgE responses to groups 1 and 2 mite allergens had the highest risk of asthma and significantly increased exhaled NO [
9,
10]. Interestingly, no significant (
p = 0.36) differences in the frequency of sensitization to both group 1 and 2 HDM allergens was found in our studied cohort regardless of their basal atopic condition.
In line with previous research, Der p 5 and der p 21, two allergens with no relevant IgE cross-reactivity despite showing a similar three-dimensional structure were highly (>65%) identified in the present population. Although both allergens have been described as clinically relevant molecules -specially in asthmatic subjects- significant IgE-binding frequency was found also found for AD in contrast to those patients afflicted with AR [
11,
12].
Recently, Der p 23 -a peritrophin-like protein of 8 kD synthesized the intestinal tract of mites- has been also strongly associated with asthma in different European cohorts [
13,
14]. Despite Der p 23 has revealed a high allergenic activity -binding IgE in around 70% of mite allergic subjects [
15]- and Der p 23 monomolecularly sensitized subjects, without sIgE to other HDM allergens have been reported, no single reactors (0%) to Der p 23 were identified in the present cohort. In fact, sensitization to Der p 23 was found equally relevant in terms of prevalence and sIgE quantification across the three investigated atopic phenotypes. In addition, our study highlighted an increased sensitization rate (50%) for patients with AD and A to Der p 7 which has been biologically described as a potent allergenic molecule activity -like Der p 5, Der p 21 and/or Der p 23- in specific cohorts [
16,
17].
Although most of subjects included in the present investigation (95%) were polysensitized, no management strategies to AIT have been standardized for such patients yet [
18]. According to previous reports, clinical efficacy of AIT inducing protective-IgG was only achieved in those patients sensitized to Der p 1 and/or Der p 2 -and to a less extent to Der p 23- but not to other relevant allergens namely Der p 5, Der p 7 and or Der p 21 [
19,
20]. Conforming to this evidence most of the studied subjects in our population may not benefit from AIT as following these criteria only 8.3% of the selected individuals would be eligible for P@MD-driven AIT, regardless of their basal atopic phenotype. Conversely, as recent research state that sIgG4 to HDM components do not qualify as a biomarker to evaluate the efficacy of AIT [
21], additional research including the assessment of the allergenic activity and clinical impact of individual allergens is warranted to identify further prognostic markers for AIT efficacy in certain populations [
22].
Another intriguing issue -and specially in pediatric patients- is when should AIT be started. Current guidelines recommend that AIT can be initiated at age 5 years [
23] meanwhile allergen sensitization can start as early as 12 months of age [
24]. In this regard, a comprehensive individual patient selection -including the sensitization profile related to the current allergen exposome, the active underlying atopic disease and associated comorbidities- increase the adherence to prescribed medication and the success of AIT [
25,
26]. Further investigations are warranted to investigate the prophylactic role of AIT in those patients with a clinically relevant sensitization under the age of 5 years old before the development of complete longitudinal mite sensitization trajectories.
A few limitations should be mentioned, as the study has a relatively restricted sample size, and the reported data came from a single center. Moreover, 2 out of 60 individuals (3.33%) with a positive SPT to local aeroallergens—1 asthmatic and 1 subject with AD—could not be identified by the implemented multiplex array.
As planned AIT follows the general assumption that the patient is mainly sensitized to major allergens of the allergenic source and not to minor allergens [
27], two considerations may be addressed in this regard: first, recent research suggests abandoning the practice of classifying allergens based on their IgE-binding frequency to evaluate their clinical relevance in different geographical scenarios [
28,
29]. Secondly, rather than restricting the use of AIT for patients with a sensitization to the so-called “minor allergens”, these molecules should be ideally identified, and their presence ensured in commercially available AIT preparations [
30] to benefit a rising number of polysensitized -and potentially clinically polyallergic- subjects.
Author Contributions
Conceptualization, R.G.-P., P.P.-G., and F.P.; methodology, R.G.-P., P.P.-G., and F.P.; software, F.P.; validation and formal analysis, I.S.-M. and F.P.; investigation, I.S.-M., R.G.-P., P.P.-G., and F.P.; resources, I.S.-M., and F.P.; data curation, R.G.-P., P.P.-G., and F.P.; writing—original draft preparation, R.G.-P. and P.P.-G.; writing—review and editing, R.G.-P., P.P.-G., and F.P.; project administration R.G.-P., P.P.-G., and I.S.-M.; funding acquisition R.G.-P. and P.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundación Canaria Instituto de Investigación Sanitaria de Canarias (FIISC), Servicio Canario de Salud, grant number OA17/042.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of CEIC Hospital Universitario de Canarias, Tenerife, Spain, with reference number P.I.-2017/72 on 30 October 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from Servicio Canario de Salud, but restrictions apply to the availability of these data, which were used under license for the current study and thus are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission from the Servicio Canario de Salud.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Durham, S.R.; Shamji, M.H. Allergen immunotherapy: past, present and future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Dramburg, S.; Hilger, C.; Santos, A.F.; Vecillas, L.d.L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User's Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, e13854. [Google Scholar] [CrossRef]
- González-Pérez, R.; Poza-Guedes, P.; Pineda, F.; Galán, T.; Mederos-Luis, E.; Abel-Fernández, E.; Martínez, M.J.; Sánchez-Machín, I. Molecular Mapping of Allergen Exposome among Different Atopic Phenotypes. Int. J. Mol. Sci. 2023, 24, 10467. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef]
- 2022 GINA Main Report. Available online: https://ginasthma.org/gina-reports/ (accessed on 23 September 2023).
- Eichenfield, L.F.; Ahluwalia, J.; Waldman, A.; Borok, J.; Udkoff, J.; Boguniewicz, M. Current guidelines for the evaluation and management of atopic dermatitis: A comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J. Allergy Clin. Immunol. 2017, 139, S49–S57. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Bojcukova, J.; Vlas, T.; Forstenlechner, P.; Panzner, P. Comparison of two multiplex arrays in the diagnostics of allergy. Clin. Transl. Allergy 2019, 9, 31. [Google Scholar] [CrossRef]
- Custovic, A.; Sonntag, H.-J.; Buchan, I.E.; Belgrave, D.; Simpson, A.; Prosperi, M.C.F. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J. Allergy Clin. Immunol. 2015, 136, 1645–1652.e8. [Google Scholar] [CrossRef] [PubMed]
- Hatzler, L.; Panetta, V.; Lau, S.; Wagner, P.; Bergmann, R.L.; Illi, S.; Bergmann, K.E.; Keil, T.; Hofmaier, S.; Rohrbach, A.; et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J. Allergy Clin. Immunol. 2012, 130, 894–901.e5. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Gosavi, R.A.; Krahn, J.M.; Edwards, L.L.; Cuneo, M.J.; Glesner, J.; Pomés, A.; Chapman, M.D.; London, R.E.; Pedersen, L.C. Der p 5 Crystal Structure Provides Insight into the Group 5 Dust Mite Allergens. J. Biol. Chem. 2010, 285, 25394–25401. [Google Scholar] [CrossRef] [PubMed]
- Resch, Y.; Michel, S.; Kabesch, M.; Lupinek, C.; Valenta, R.; Vrtala, S. Different IgE recognition of mite allergen components in asthmatic and nonasthmatic children. J. Allergy Clin. Immunol. 2015, 136, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Weghofer, M.; Grote, M.; Resch, Y.; Casset, A.; Kneidinger, M.; Kopec, J.; Thomas, W.R.; Fernández-Caldas, E.; Kabesch, M.; Ferrara, R.; et al. Identification of Der p 23, a Peritrophin-like Protein, as a New Major Dermatophagoides pteronyssinus Allergen Associated with the Peritrophic Matrix of Mite Fecal Pellets. J. Immunol. 2013, 190, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Celi, G.; Brusca, I.; Scala, E.; Villalta, D.; Pastorello, E.; Farioli, L.; Cortellini, G.; Deleonardi, G.; Galati, P.; Losappio, L.; et al. House dust mite allergy in Italy—Diagnostic and clinical relevance of Der p 23 (and of minor allergens): A real-life, multicenter study. Allergy 2019, 74, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Randall, T.A.; Glesner, J.; Pedersen, L.C.; Perera, L.; Edwards, L.L.; DeRose, E.F.; Chapman, M.D.; London, R.E.; Pomés, A. Serological, genomic and structural analyses of the major mite allergen Der p 23. Clin. Exp. Allergy 2016, 46, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Curin, M.; Huang, H.-J.; Garmatiuk, T.; Gutfreund, S.; Resch-Marat, Y.; Chen, K.-W.; Fauland, K.; Keller, W.; Zieglmayer, P.; Zieglmayer, R.; et al. IgE Epitopes of the House Dust Mite Allergen Der p 7 Are Mainly Discontinuous and Conformational. Front. Immunol. 2021, 12, 687294. [Google Scholar] [CrossRef]
- Muddaluru, V.; Valenta, R.; Vrtala, S.; Schlederer, T.; Hindley, J.; Hickey, P.; Larché, M.; Tonti, E. Comparison of house dust mite sensitization profiles in allergic adults from Canada, Europe, South Africa and USA. Allergy 2021, 76, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Passalacqua, G.; Pfaar, O.; Sastre, J.; Wahn, U. Management of the polyallergic patient with allergy immunotherapy: a practice-based approach. Allergy, Asthma Clin. Immunol. 2016, 12, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, A.; Berings, M.; Rohrbach, A.; Huang, H.-J.; Curin, M.; Gevaert, P.; Matricardi, P.M.; Valenta, R.; Vrtala, S. Molecular profiling of allergen-specific antibody responses may enhance success of specific immunotherapy. J. Allergy Clin. Immunol. 2020, 146, 1097–1108. [Google Scholar] [CrossRef]
- Chen, K.-W.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P.; Horak, F.; Bunu, C.P.; Valenta, R.; Vrtala, S. Selection of house dust mite–allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J. Allergy Clin. Immunol. 2018, 143, 1248–1252.e12. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Xu, Q.; Zhang, W.; Jiang, Q.; Li, W.; Wang, Y.; Ma, D.; Lin, X.; Sun, B.; et al. Specific IgE and IgG4 Profiles of House Dust Mite Components in Allergen-Specific Immunotherapy. Front. Immunol. 2022, 12, 786738. [Google Scholar] [CrossRef]
- Caraballo, L.; Valenta, R.; Puerta, L.; Pomés, A.; Zakzuk, J.; Fernandez-Caldas, E.; Acevedo, N.; Sanchez-Borges, M.; Ansotegui, I.; Zhang, L.; et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ. J. 2020, 13, 100118. [Google Scholar] [CrossRef]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. Allergen Immunotherapy in Children User’s Guide. Pediatr. Allergy Immunol. 2020, 31, 1–101. [Google Scholar] [CrossRef]
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E.; et al. International consensus statement on allergy and rhinology: Allergic rhinitis – 2023. Int. Forum Allergy Rhinol. 2023, 13, 293–859. [Google Scholar] [CrossRef]
- Halken, S.; Larenas-Linnemann, D.; Roberts, G.; Calderón, M.A.; Angier, E.; Pfaar, O.; Ryan, D.; Agache, I.; Ansotegui, I.J.; Arasi, S.; et al. EAACI guidelines on allergen immunotherapy: Prevention of allergy. Pediatr. Allergy Immunol. 2017, 28, 728–745. [Google Scholar] [CrossRef]
- Ridolo, E.; Nicoletta, F.; Barone, A.; Ottoni, M.; Senna, G.; Canonica, G.W. Causes of Non-Adherence to Allergen-Specific Immunotherapy: A Foundation towards a Patient-Personalized Approach. J. Pers. Med. 2023, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Luengo, O.; Labrador-Horrillo, M. Molecular Allergy Diagnosis in Clinical Practice: Frequently Asked Questions. J. Investig. Allergol. Clin. Immunol. 2022, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Valenta, R.; Acevedo, N.; Zakzuk, J. Are the Terms Major and Minor Allergens Useful for Precision Allergology? Front. Immunol. 2021, 12, 651500. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, R.; Poza-Guedes, P.; Pineda, F. Comment on Platts-Mills et al. Pediatr. Allergy Immunol. 2023, 34, e13986. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Bonertz, A.; Vieths, S. Quality requirements for allergen extracts and allergoids for allergen immunotherapy. Allergol. et Immunopathol. 2017, 45, 4–11. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).