1. Introduction
Benign Paroxysmal Positional Vertigo (BPPV) is the most common cause of peripheral vestibular disorders [
1] with an overall prevalence of 2.4% in the adult general population [
2]; BPPV is characterized by paroxysms of vertigo triggered by head position changes in the direction of gravity. BPPV is explained by migration of degenerated otoconia into the semicircular canals, rendering them sensitive to head motion [
3]. Most cases of BPPV are idiopathic in origin and probably result from degeneration of the macula. Secondary causes of BPPV refer to identifiable causes of otoconial dislodgement including otologic and non-otologic surgery, head trauma and inner ear disorders which ultimately lead to the degradation of otoconia (vestibular neuritis, Meniere’s disease, and sudden sensorineural hearing loss) [
4,
5]. Although BPPV could usually be resolved without special treatment, the canalith repositioning procedure (CRP) can promote the recovery of BPPV [
1,
3,
6,
7]. CRP can help patients to get relief from BPPV by moving the otoconia utriculus where they are reabsorbed or dissolve. Successful CRP means that nystagmus (negative Dix–Hallpike test results) and vertigo symptoms disappear after CRP [
1,
3,
6]. However, after a seemingly successful physical treatment, up to two-third of patients are still dizzy and off-balance (residual dizziness, RD) [
8,
9,
10]. RD seems to be a syndrome caused by multiple factors (age, female gender, secondary BPPV, a longer duration of BPPV before treatment, higher Dizziness Handicap Index score before treatment, anxiety, osteopenia, [
10,
11] whose early recognition could contribute to the prevention and treatment of RD.
Among the several tests which can be applied to better understand the pathophysiology of a disease, metabolomics represents a promising possibility in describing metabolic modifications during an illness and after a therapeutic intervention, which can subsequently help to elucidate the mechanisms underlying some biological processes [
12]. Metabolomics focuses on measuring in biological specimens low-molecular-weight metabolites, which are either metabolic intermediates or metabolic end-products resulting from cellular metabolism [
13,
14]. Most biological specimens studied in metabolomics include blood samples (either serum or plasma), saliva and urine [
15]. To date, metabolomics is considered a research tool that can give novel insights in the diagnosis, treatment, and prognosis of ENT diseases, such as head and neck cancers, allergic rhinitis, sudden sensorineural hearing loss, noise-induced hearing loss and Menière’s Disease [
16,
17,
18]. Despite its high clinical incidence, to date, no metabolomic studies have been conducted on BPPV and on residual dizziness after successful treatment with CRM. Several medications have been proposed to approach RD, but the results reported are conflicting. Recently good therapeutic results were obtained using a supplementation with a polyphenol compound, which it has proven to be able to reduce subjective symptoms and improve instability earlier, decreasing the risk of potential complications [
19]. The present study aims to correlate the metabolic profiles of patients suffering from RD treated for 60 days with a supplementation with a polyphenol compound (Vertigoval
®), containing ViNitrox, Vitamin B6, Citicoline, Melissa officinalis, and Ginger having in mind three main objectives, to investigate a possible urine metabolic profile in patients suffering from residual dizziness after successful treatment of BPPV, to improve medical knowledge on pathophysiology of residual dizziness and to predict clinical outcomes as to avoid unnecessary treatment.
2. Materials and Methods
2.1. Participants
The enrolled population included 30 subjects (18 females and 12 males), mean age 56 years (range 25-65), consecutively admitted to the two tertiary referral centers involved in the study (17 patients from the Department of Otolaryngology of the University Hospital of Torino and 13 patients from the Department of Otolaryngology of the University Hospital of Pisa). Each patient suffered from BPPV due to the involvement of the posterior semicircular canal (PSC) and successfully treated with CRP [
6]. The affected side was the right in 17 cases (57%) and the left in 13 cases (43%). The positive result of CRP was evaluated 2/3 days after the treatment (at the next follow-up in the outpatient department) based on the absence of vertigo and nystagmus while performing the Dix-Hallpike test. Inclusion criteria were the presence of dizziness (RD) eventually associated to neurovegetative symptoms (mainly nausea) after canalolithiasis od PSC successfully treated with CRP. We excluded patients with secondary BPPV (post-traumatic BPPV, BPPV following an acute vestibular loss), patients with central nervous system diseases, alcohol or psychoactive substance abuse, uncontrolled hypertension, hypotension, thyroid diseases, severe coronary artery disease, severe renal or hepatic failure, pregnancy or breast-feeding, chronic instability pre-existing at the onset of BPPV, known intolerance to polyphenol compound supplementation and poor compliance
.
2.2. Methods
The subjective symptomatology at diagnosis (T0) and its modifications after polyphenol compound therapy (1 tablet 2 times a day for 60 days) were studied by means of the Visuo-Analog Scale (VAS) [
20] and of the Italian version of the Dizziness Handicap Inventory (DHI) [
21]. The Visuo-Analog Scale (VAS) is aimed at quantifying the intensity of dizziness and nausea. Patients were asked to indicate on a 100 mm-long line their subjective “level” of intensity of symptoms (dizziness and nausea respectively) at T0 (basal), T30 (after 30 days of treatment) and at T60 (after 60 days of treatment). The Dizziness Handicap Inventory (DHI) is a self-administered questionnaire that estimates the impact of balance disorders on the quality of life (QoL). It consists of 25 questions regarding the emotional (9 questions), functional (9 questions) and physical (7 questions) domains of daily life. To each question the patient could answer with a number going from 0 to 4, with 0 meaning “No”, 2 meaning “Sometimes” and 4 meaning “Yes”. As the VAS Dizziness and Nausea scales, also the DHI was administered during the initial patient evaluation (T0-basal), and then again after 30 (T30) and 60 (T60) days of treatment with polyphenol compound supplementation (Vertigoval®). For each medical examination results were collected and mean, and standard deviation were calculated (mean ± sd). For T30 and T60, mean values were correlated with basal (T0) mean values by calculating their percentual variation (Δ%). Comparison of T30 and T60 with respect to their basal values was performed by means of the Student “T”-test for paired data.
For each medical examination the numerical answers to all the 25 questions were added, and the mean and standard deviation were calculated. Moreover, mean, and standard deviation for the questions relative to each of the three domains (emotional, functional and physical) were calculated. The mean values recorded at T30 and T60 were also compared to the basal (T0) values by calculating their percentual variation (Δ%). Comparison of T30 and T60 values with respect to their basal values was performed by means of the Student “T”-test for paired data. Patients were also asked about efficacy and tolerance to the treatment as excellent, good, sufficient, or poor.
2.3. Urine samples preparation and 1H-NMR analysis
Urine collection was done at T0, T30 and T60 with clinical evaluation. An aliquot of 800 µL of urine was transferred into an Eppendorf tube with 8 µL of a 1% aqueous solution of NaN3 to inhibit bacteria growth and stored at -80 °C. Before the analysis, the sample was centrifuged at 12000g for 10 min at 4 °C to remove solid particles. Then, 630 µL of the supernatant was mixed with 70 µL of potassium phosphate buffer in D2O (1.5 M, pH 7.4) containing sodium 3-trimethylsilyl-propionate-2,2,3,3,-d4 (TSP) as an internal standard (98 atom% D, Sigma-Aldrich, Milan). Finally, an aliquot of 650 µL was transferred to 5-mm NMR glass tubes for 1H-NMR analysis. NMR analysis was carried out using a Varian UNITY INOVA 500 spectrometer operating at 499.839 MHz for proton and a 5 mm double resonance probe (Agilent Technologies, CA, USA). One-dimensional proton NMR spectra were obtained using a 1D Nuclear Overhauser Enhancement Spectroscopy (NOESY) standard pulse sequence to suppress water signal with a relaxation delay of 3 sec. For each sample, 256 free induction decays (FIDs) were collected into 64K data points with a spectral width of 6000 Hz with a 90° pulse, an acquisition time of 2 sec, and a mixing time of 100 ms. The FIDs were weighted by an exponential function with a 0.5-Hz line-broadening factor prior to Fourier transformation.
2.4. 1H-NMR Data Preprocessing
NMR spectra were phased, and baseline corrected using an ACD/lab Processor Academic Edition (Advanced Chemistry Development, 12.01, 2010) and chemical shifts referenced internally to TSP at d= 0.0 ppm. Next, the spectral region comprising the signal of residual water (4.7–4.9 ppm) was removed. The final spectral regions considered were between 0.7-4.7 ppm and 4.9-9.5 ppm. The ACD Labs intelligent bucketing method was used for spectral integration (20). A 0.04 ppm bucket width was defined with an allowed 50% looseness. The degree of looseness allows the bucket width to vary over a particular value from the set bucket value. The intelligent bucket method identifies local minima in the spectra and sets the buckets accordingly. In this manner, a peak is integrated into one bucket, although there may be minor chemical shift differences due to pH, for instance.
The area of bucketed regions was normalized using Median Fold Change Normalization (MFC) [
22], primarily preferred to total sum normalization when studying urine samples. Finally, the spectral data were imported into the SIMCA software (Version 16.0, Sartorius Stedim Biotech, Umea, Sweden) for multivariate statistical analysis. All imported data were then pre-processed using Pareto scaling [
23].
2.5. Statistical Analysis on urine metabolomics data
The multivariate statistical analysis was based on principal component analysis (PCA) and orthogonal partial least square discriminant analysis (OPLS-DA). The PCA, an unsupervised pattern recognition method, was performed to evaluate the samples' homogeneity at T0, T30, and T60 time and identified any possible trend and outlier (defined as observations located outside the 95% confidence region of the model) [
23].
OPLS-DA, a supervised method, was used to reduce model complexity and highlight sample discrimination [
8]. The quality of the OPLS-DA model was evaluated using a 7-fold cross-validation and permutation test (500 times). The permutation test was calculated by randomizing the Y-matrix (class assignment or continuous variables) while the X-matrix (peak intensity in NMR spectra) was kept constant. The permutation plot then displays the correlation coefficient between the original y-variable and the permuted y-variable on the x-axis versus the cumulative R2 and Q2 on the y-axis and draws the regression line. The intercept is a measure of the overfit, Q2Y intercept value less than 0.05 indicates a valid model. The estimated predictive power of the models was expressed by R2Y and Q2Y, which represent the fraction of the variation of the Y-variable and the predicted fraction of the variation of the Y-variable, respectively. A good prediction model is achieved when Q2 ≥ 0.5. One-way analysis of variance (ANOVA) and Kruskal-Wallis tests was performed to reveal the significance of differences between groups. To control the false-discovery rate, the Benjamini and Hochberg's procedure was applied (with the Benjamini-Hochberg FDR of 0.05).
3. Results
3.1. Visuo-Analog Scales (VAS) of Dizziness
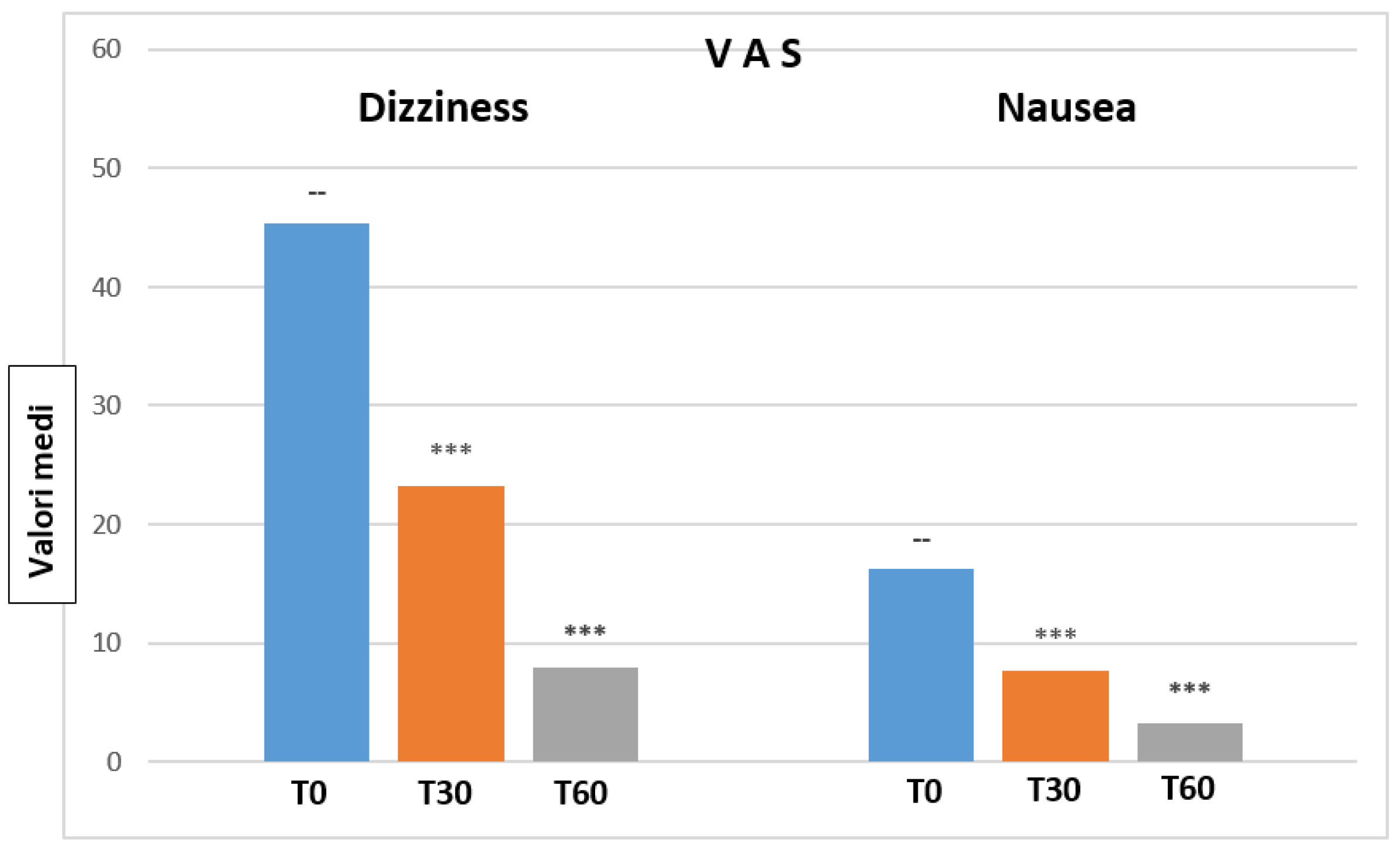
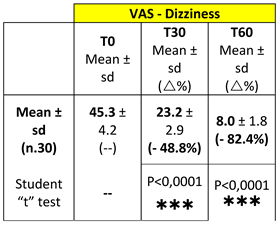
Mean VAS Dizziness values are reported in
Table 1.
Figure 1 depicts mean value changes on VAS Dizziness and Nausea scales at T0, T30 and T60, (at 30 and 60 days of treatment with Vertigoval, respectively) in comparison with the basal level (T0). Data show a progressive and significant reduction of dizziness and nausea at T30 and T60.
3.2. Dizziness Handicap Inventory (DHI)
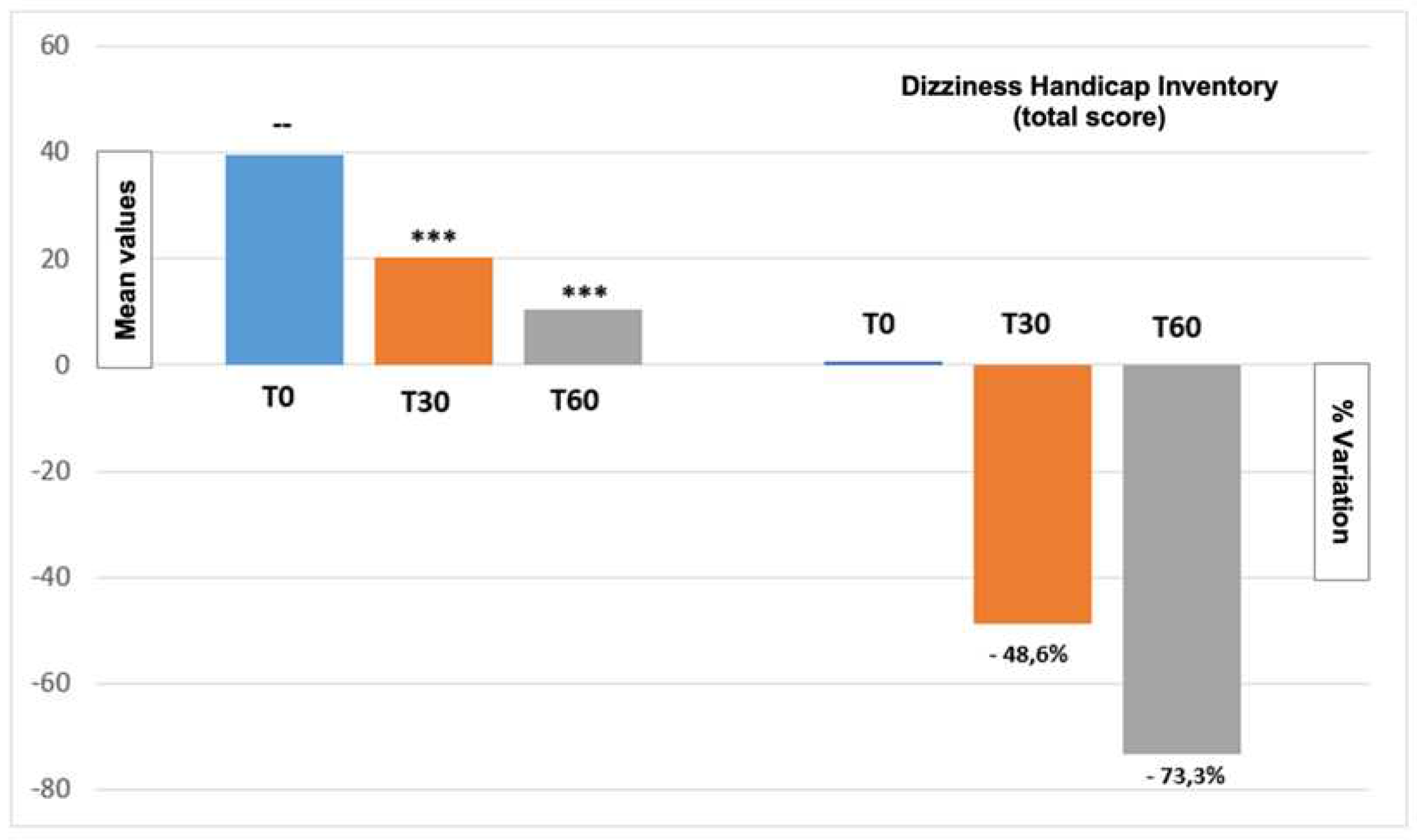
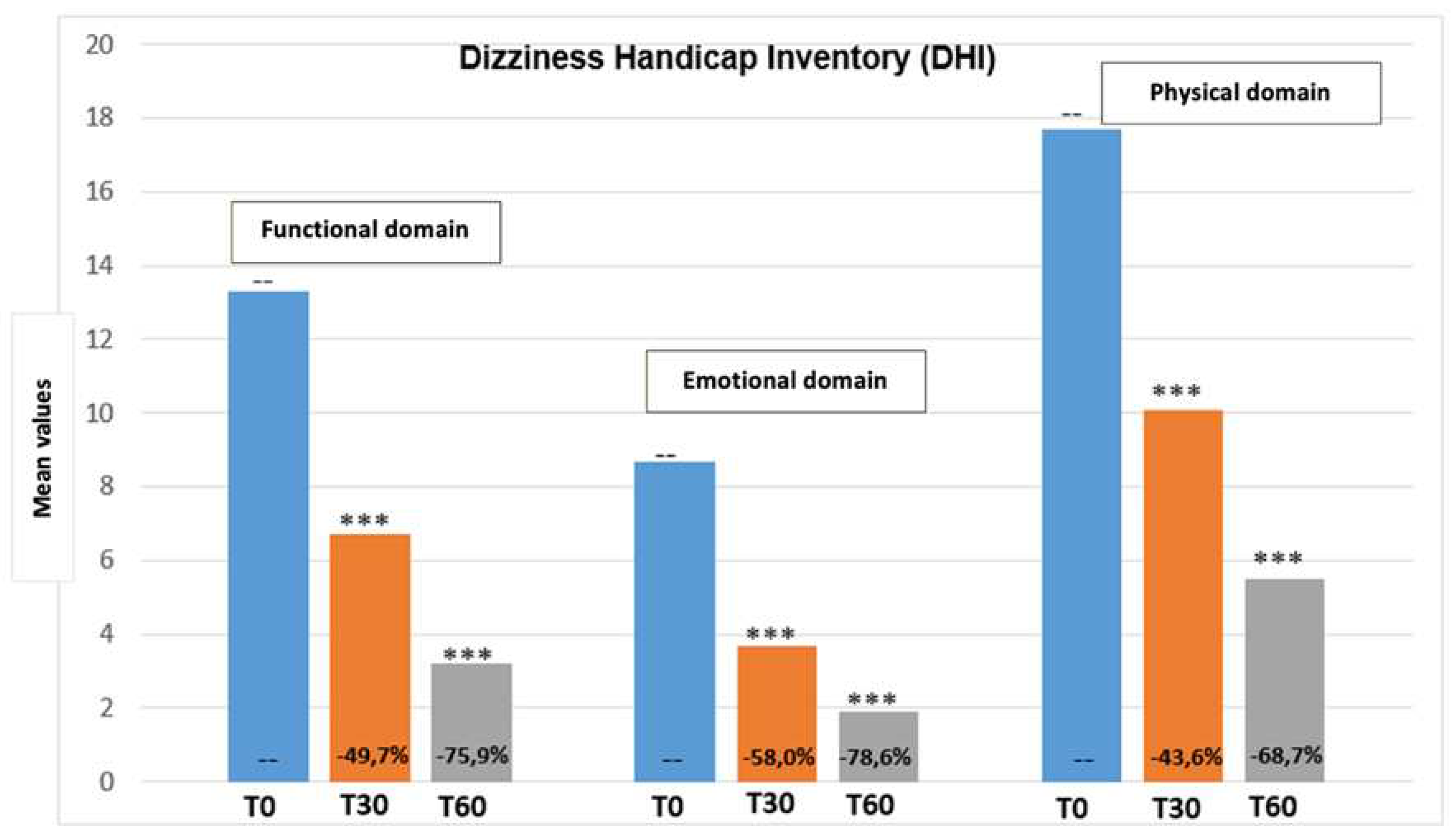
Figure 2 report the DHI score total variation from the beginning until the end of the treatment period. Moreover,
Figure 3 show values regarding the three DHI domains (emotional, functional, and physical). Even in this case results show a progressive reduction of DHI value, demonstrating an improvement of quality of life.
3.3. Efficacy and tolerability evaluation
After 30 and 60 days of treatment both the clinician and the patient were asked to express their opinion regarding the overall efficacy and tolerability of the medication. Efficacy and tolerance to the polyphenol compound were reported as excellent or good in 100 % of the patients at T30 and T60.
3.4. Urine metabolomics
The urine metabolomics profile of samples at T0, T30, and T60 time was investigated using
1H-NMR spectroscopy coupled with multivariate statistical analysis. Metabolits were identified based on literature [
24] and using a dedicated library, such as the Human Metabolome Database (HMDB,
http://www.hmdb. ca) and the 500 MHz libraries from Chenomx NMR suite 7.1. A first PCA (data not shown) analysis was conducted to identify potential outliers (outside the 95% confidence limit) and to evaluate the samples' homogeneity. Only one sample at a time, T60, was excluded from subsequent analyses.
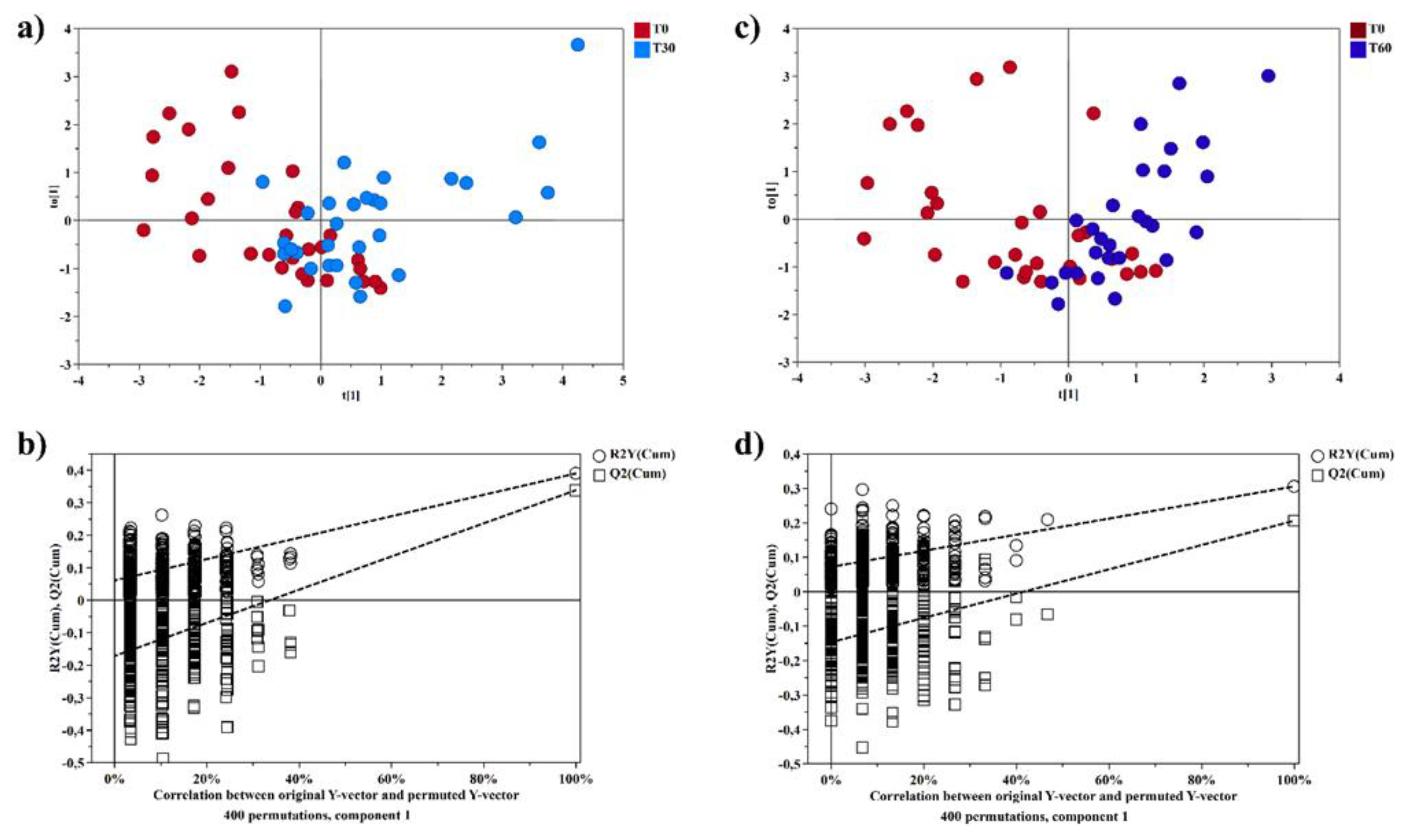
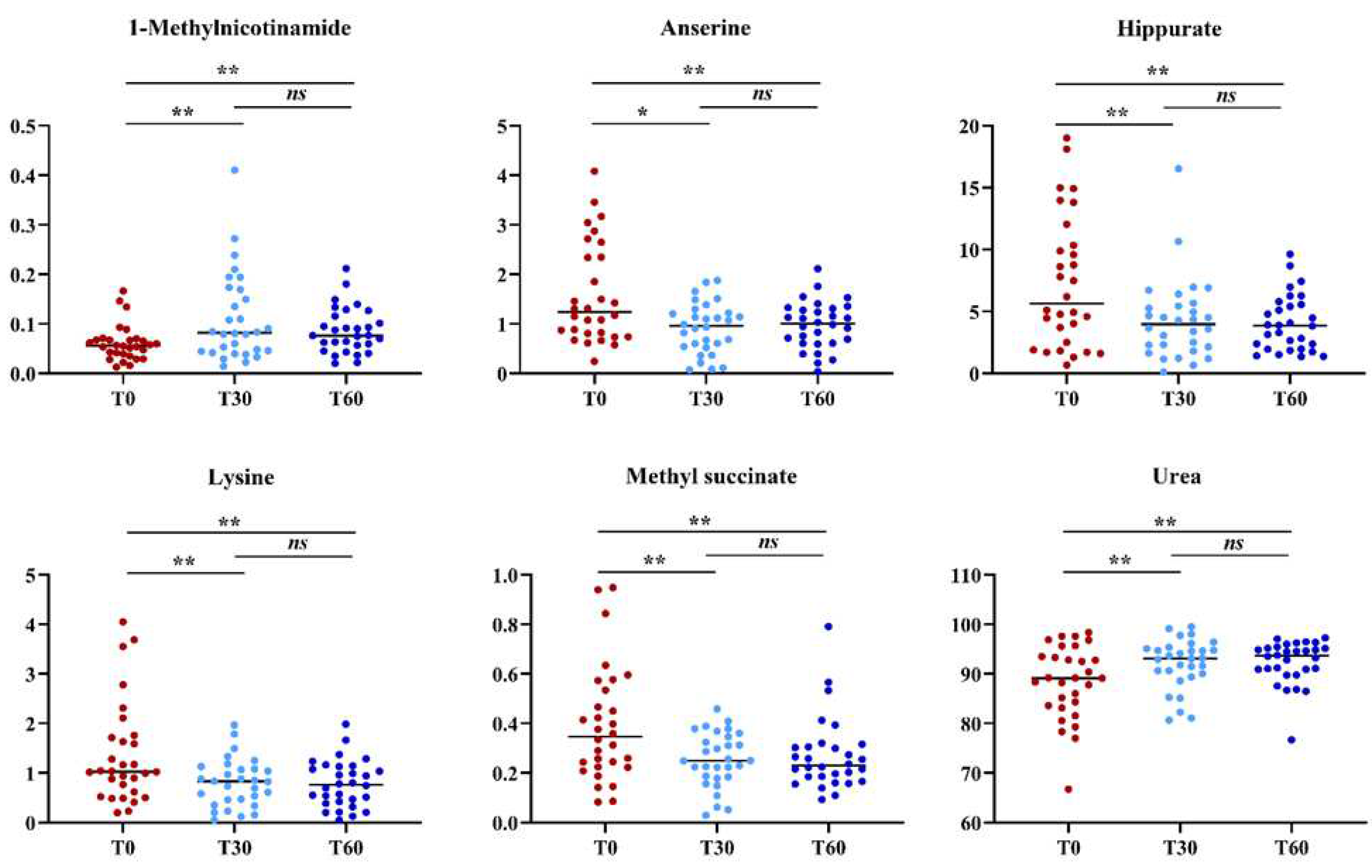
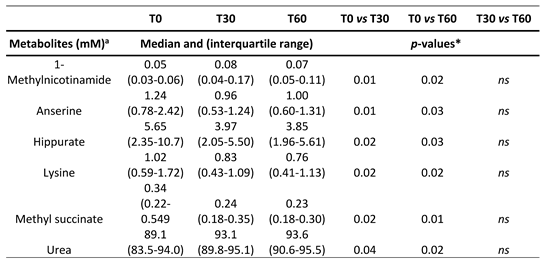
Then, a supervised statistical analysis was performed on the same dataset. The statistical analysis on the whole urinary profile was not able to discriminate the samples analyzed at T0 with respect to the subsequent analysis times. This may be due to an unidentified number of disturbing variables and/or noise variables that characterize the NMR spectra. Thus, a univariate statistical analysis was performed on the different spectral regions identified with the intelligent bucket method to remove potential information unrelated to the pathology. Only the spectral regions with a p-value <0.05 were quantified with Chenomx NMR suite 7.1. The spectral regions with a p-value <0.05 were characterized by the metabolites such as 1-Methylnicotinamide, Anserine, Hippurate, Lysine, Methyl succinate, and Urea. Then, a new statistical supervised model was created using only the six identified metabolites. In
Figure 4a, the OPLS-DA model shows how the samples at time T0 are partly separated along t[
1] with respect to T30, but a large number of samples overlap in the intersection of t[
1] with t[
2]. The OPLS-DA model was established with one predictive and one orthogonal component and showed values of R2X, R2Y, and Q2 of 0.613, 0.306, and 0.206, respectively. The validity of the OPLS-DA model was tested through a permutation test (
Figure 4b) using 400 times. R2 and Q2 intercept values of 0.07 and -0.14, respectively, indicate a valid model. The OPLS-DA model in
Figure 4c shows how the samples at time T60 are more separated along t[
1] than at T0, indicating how the supplement of polyphenol compound after two months of treatment has a more significant impact on the metabolomic profile. The OPLS-DA model was established with one predictive and one orthogonal component and showed values of R2X, R2Y, and Q2 of 0.598, 0.390, and 0.339, respectively. The validity of the OPLS-DA model was tested through a permutation test (
Figure 4d) using 400 times. R2 and Q2 intercept values of 0.06 and -0.17, respectively, indicate a valid model. The concentration variation of the six discriminant metabolites in the samples at different times was evaluated with one-way analysis of variance (ANOVA) and Kruskal-Wallis tests. The univariate statistical analysis results were shown using box-and-whisker plots (
Figure 5). The subjects at T30 and T60 times were characterized by a higher level of 1-Methylnicotinamide and Urea and lower levels of Anserine, Hippurate, Lysine, and Methyl succinate compared to T0 time (
Table 2).
4. Discussion
RD is a common experience that manifests as persistent imbalance after successful CRP for BPPV. These symptoms may affect the quality of life and prevent carrying out daily activities. The decreasing postural control can contribute to falling and psychological problems [
25]. The prevalence of these residual symptoms varies and depends on the patient’s description of their symptoms and the physical handicap perceived by the patient. Previous studies reported an association with a condition of stress of the subject that may be correlated with the duration of the BPPV and number of recurrences [
26,
27]. Some authors reported the duration of the residual symptoms of about 6–20 days [
9,
11,
26,
27,
28]. However, some patients recover later than others and some of these still present symptoms after 1 and 2 months [
11,
26,
28]. The decreasing postural control can contribute to falling and psychological problems [
29]. Although many hypotheses have been postulated, the origin of this disturbance is not yet clear [
10,
11,
30]. Several medications have been proposed to approach these symptoms [
31,
32,
33] but nothing has yet proved to provide relief for residual dizziness compared to placebo. The most used molecule to approach residual dizziness after BPPV is betahistine dihydrochloride, but its results are conflicting [
34,
35]. Recently, a multicentric non-double- blinded designed study reported good results using a supplementation with the polyphenol compound that proved to be able to reduce subjective symptoms and improve instability earlier, decreasing the risk of potential complications without any significant side effects [
19].
In order to confirm the effective validity of the treatment of RD we propose, we studied the metabolomic aspects in a group of patients treated with the polyphenol supplementation trying to correlate the positive clinical results with those of the metabolomic study. Using a metabolomics approach, it is possible to obtain detailed information to understand the molecular phenotype of a particular disease. This approach generates a plethora of data, which can help to understand the mechanisms underlying biological processes and molecular functions [
12,
13]. Phenomena in vestibular and auditory neuroscience, as in other areas of neuroscience, involve the complex interaction of multiple variables. MVAs and data mining methods have been successfully used in metabolomics studies to predict the progression of diseases [
12,
14,
36,
37,
38]. Thus, applying a 1H-NMR analysis coupled with an MVA approach on BPPV patients treated for 60 days with a polyphenol supplementation, we aimed to identify biomarkers related to the treatment. The metabolomics analysis identified six significant metabolites: 1-methylnicotinamide, anserine, Hippurate, lysine, methyl succinate, and urea. Urea and 1-methylnicotinamide showed an increasing trend from T0 to T60d, while anserine, hippurate, lysine, and methyl succinate resulted in reduced. Urea is synthesized in the urea cycle from ammonia or by oxidation of amino acids, thus is the main product of protein catabolism. Urea production occurs mainly in the liver and, to a lesser extent, in the kidneys, and an increased concentration may reflect protein catabolism which may increase due inflammatory processes [
39]. In contrast, the increased concentration of 1-methyl-nicotinamide at 30 and 60 days appears related to the anti-inflammatory property of the molecule. Lakshmi and Bamji [
40] have suggested the evaluation of the urinary 1-methylnicotinamide measurement to indirectly determine niacin (vitamin B3) status, which is of great importance for monitoring the health of energy metabolism. Normal adults excrete more than 17 μmol 1-methyl-nicotinamide per day, whereas during deficiency, its excretion is less than 5.8 μmol per day. Patients treated for two months with a polyphenol compound supplementation had a statistically increased level of 1-methyl-nicotinamide, which could be interpreted as a restoration of the level of niacin and nicotinamide adenine dinucleotide (NAD+) levels. [
41]. Niacin level is also related to lysine metabolism. Lysine is an alpha-amino acid, and its catabolism occurs through several biochemical pathways. A recent phase 4 clinical study analyzed the correlation between vertigo and lysine, underlying a solid connection (
https://www.ehealthme.com/ds/l-lysine/vertigo/). In particular, a high lysine concentration is related to the presence of vertigo, especially in females older than 60 years. Therefore, a significant reduction of lysine concentration after 60 days of Vertigoval could be interpreted as an ameliorant indicator. Anserine is a dipeptide containing beta-alanine and 3-methylhistidine, it is the most common variant of methylated carnosine analogs. Carnosine has several antioxidant properties and has been proven to scavenge ROS and alpha–beta-unsaturated aldehydes formed from peroxidation of cell membrane fatty acids during oxidative stress [
42]. Patients treated for 60 days with Vertigoval showed a reduction of anserine, which may be interpreted as a reduction of ROS presence. In agreement with this, the glutamine level has been shown to be related to a reduction in neuronal swelling caused by acoustic trauma [
43] via 5-aminovalerate transaminase. A reduction of Hippurate, the glycine conjugate of benzoic acid, is interpreted as a plausible hallmark of changes in gut microflora [
44].
These results are absolutely in agreement with the favorable clinical outcome. VAS and DHI values showed a progressive and significant reduction of RD at T30 and T60. The congruence between the metabolomics approach and the clinical results can be easily supported analyzing the characteristics of the components of the polyphenol compound we used. Citicoline might facilitate the function of the central vestibular system having a protective effect on micro- vascular circulation [
45]. However, Melissa seems to be able to potentiate the gamma-aminobutyric acid (GABA) receptors alleviating the stress, which has been counted among the conditions below the residual instability after BPPV, as previously reported [
46]. The antiemetic power of ginger, on the other hand, has been known since antiquity. The last component is ViNitrox, a synergistic combination of apple and grape poly- phenols that have been demonstrated to have a remarkable vasodilator effect through endothelial nitric oxide synthase (NOS) and antioxidant activity [
47]. The combination seems effective in at least partially restoring the microvascular impairment in these patients and also improving cognitive function. Usefully, a recent article published by Ulivi et al [
48] reports that the supplementation for 60 days with the polyphenol compound we studied reduces oxidative stress load in patients with a pre-existing imbalance, improving dizziness symptoms. Even if the study has been carried out on patients suffering from chronic dizziness because of the underlying small vessel disease, the mechanisms below the symptom’s improvement in this category of patients can be the same ability to take relief to the patients recruited in our study.
5. Conclusions
Our results confirm the positive activity of a polyphenol compound supplementation in improving the symptomatology of residual dizziness after successful treatment of BPPV, and the metabolomics analysis provides excellent scientific support for this evidence. This is the first preliminary approach to BPPV and RD with metabolomics. The preliminary data from the present study suggest that supplementation with a polyphenol compound cold induces some metabolic changes that can help recover residual dizziness. However, the future steps will require confirmation with a more significant cohort of patients and an extension of the metabolomic evaluation to other problems concerning the different clinical aspects of BPPV, such as the high relapse found in a high percentage of patients [
5].
Author Contributions
Conceptualization, A.P.C, R.A., and V.F.; methodology, A.P.C., C.P., A.N., M.M., and V.F.; software, C.P., A.N, N.D., and L.A.; validation, A.P.C., M.M., and V.F.; formal analysis, C.P., A.A., A.N., N.D., L.A., and S.L.; investigation, A.A., A.N., N.D., L.A., S.L., and M.M.; data curation, C.P., A.N., N.D., and S.L.; writing—original draft preparation, A.P.C., R.A., M.M., and V.F.; writing—review and editing, A.P.C., R.A., A.N., and V.F.; supervision, A.P.C., R.A., and V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University of Turin (and subsequentially confirmed by the Ethics Committee of the University of Pisa) (protocol code 00031/2021, date of approval: October 4th, 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhattacharyya, N.; Gubbels, S. P.; Schwartz, S. R.; Edlow, J. A.; El-Kashlan, H.; Fife, T.; Holmberg, J. M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; Seidman, M.D.; Steiner, R.W.; Do, B.T.; Voelker, C. C.; Waguespack, R.W.; Corrigan, M.D. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Head Neck Surg. 2017, 536, S1–S47. [Google Scholar] [CrossRef]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: a population based study. J. Neurol. Neurosurg. Psychiatry. 2007, 78, 710–15. [Google Scholar] [CrossRef]
- Nuti, D.; Zee, D.S.; Mandalà, M. Benign paroxysmal positional vertigo: what we do and do not know. Semin. Neurol. 2020, 40, 49–58. [Google Scholar] [CrossRef]
- You, P.; Instrum, R.; Parnes, L. Benign paroxysmal positional vertigo Laryngoscope. Investig. Otolaryngol. 2019, 4, 116–123. [Google Scholar]
- Casani, A.P.; Gufoni, M. Recurring benign paroxysmal positional vertigo after successful canalith repositioning manoeuvers. Acta Otorhinolaryngol. Ital. 2023, 43, S61–S66. [Google Scholar] [CrossRef]
- Mandalà, M.; Santoro, G. P.; Asprella Libonati, G.; Casani, A. P.; Faralli, M.; Giannoni, B.; Gufoni, M.; Marcelli, V.; Marchetti, P.; Pepponi, E.; Vannucchi, P.; Nuti, D. Double-blind randomized trial on short-term efficacy of the Semont maneuver for the treatment of posterior canal benign paroxysmal positional vertigo. J. of neurol. 2012, 259, 882–5. [Google Scholar] [CrossRef]
- Casani, A.P.; Nacci, A.; Dallan, I.; Panicucci, E.; Gufoni, M.; Sellari-Franceschini, S. Horizontal semicircular canal benign paroxysmal positional vertigo: effectiveness of two different methods of treatment. Audiol. Neurootol. 2011, 16, 175–84. [Google Scholar] [CrossRef]
- Albera, A.; Boldreghini, M.; Canale, A.; Albera, R.; Gervasio, C.F. Vertigo returning to the sitting position after the Semont manoeuvre. Is it a prognostic symptom? Acta Otorhinol. Ital. 2018, 38, 145–150. [Google Scholar] [CrossRef]
- Teggi, R.; Giordano, L.; Bondi, S.; Fabiano, B.; Bussi, M. Residual dizziness after successful repositioning maneuvers for idiopathic benign paroxysmal positional vertigo in the elderly. Eur. Arch. Otorhinolaryngol. 2011, 68, 507–11. [Google Scholar] [CrossRef]
- Ke, Y.; Ma, X.; Jing, Y.; Diao, T.; Yu, L. Risk factors for residual dizziness in patients with benign paroxysmal positional vertigo after successful repositioning: a systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 3237–56. [Google Scholar] [CrossRef]
- Giommetti, G.; Lapenna, R.; Panichi, R.; Mobaraki, P. D.; Longari, F.; Ricci, G.; Faralli, M. Residual Dizziness after Successful Repositioning Maneuver for Idiopathic Benign Paroxysmal Positional Vertigo: A Review. Audiol. Res. 2017, 7, 178. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Piras, C.; Atzori, L.; Fanos, V. Slotting metabolomics into routine precision medicine. J. Exprt. Rev. Precisin. Med. Drug. Develpmnt. 2021, 6, 173–87. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Metabolomics: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999, 29, 1181–9. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fanos, V. Metabolomics of autism spectrum disorders: early insights regarding mammalian-microbial cometabolites. Expert Rev Mol Diagn 2016, 16, 869–81. [Google Scholar] [CrossRef]
- Sugimoto Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010, 6, 78–95. [Google Scholar] [CrossRef]
- Noto, A.; Piras, C.; Atzori, L.; Mussap, M.; Albera, A.; Albera, R.; Casani, A.P.; Capobianco, S.; Fanos, V. Metabolomics in Otorhinolaryngology. Front. Mol. Biosci. 2022, 9, 934311. [Google Scholar] [CrossRef]
- Carta, F.; Lussu, M.; Bandin, F.; Noto, A.; Peppi, M.; Chuchueva, N.; Atzori, L.; Fanos, V.; Puxeddu, R. Metabolomic analysis of urine with Nuclear Magnetic Resonance spectroscopy in patients with idiopathic sudden sensorineural hearing loss: A preliminary study. Auris Nasus Larynx. 2017, 44, 381–9. [Google Scholar] [CrossRef]
- Di Berardino, F.; Zanetti, D.; Ciusani, E.; Caccia, C.; Leoni, V.; De Grazia, U.; Filipponi, E.; Elli, L. Intestinal permeability and Ménière's disease. Am. J. Otolaryngol. 2018, 39, 153–6. [Google Scholar] [CrossRef]
- Casani, A. P.; Navari, E.; Albera, R.; Agus, G.; Asprella Libonati, G.; Chiarella, G.; Lombardo, N.; Marcelli, V.; Ralli, G.; Scotto di Santillo, L.; Teggi, R.; Viola, P.; Califano, L. Approach to residual dizziness after successfully treated benign paroxysmal positional vertigo: effect of a polyphenol compound supplementation. Clin. Pharmacol. 2019, 11, 117–25. [Google Scholar] [CrossRef]
- Toupet, M.; Ferrary, E.; Grayeli, A.B. Visual analog scale to assess vertigo and dizziness after repositioning maneuvers for benign paroxysmal positional vertigo. J. Vest. Res. 2011, 2, 235–41. [Google Scholar] [CrossRef]
- Colnaghi, S.; Rezzani, C.; Gnesi, M.; Manfrin, M.; Quaglieri, S.; Nuti, D.; Mandalà, M.; Monti, M.C.; Versino, M. Validation of the Italian Version of the Dizziness Handicap Inventory, the Situational Vertigo Questionnaire, and the Activity-Specific Balance Confidence Scale for Peripheral and Central Vestibular Symptoms. Front. Neurol. 2017, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, L. Sample normalization methods in quantitative metabolomics. J. Chromatogr. A. 2016, 1430, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; Van der Werf, M.J. (). Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC. Genomics 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Pintus, R.; Pruna, D.; Dessì, A.; Atzori, L.; Fanos, V. Pediatric acute-onset neuropsychiatric syndrome and mycoplasma pneumoniae infection: a case report analysis with a metabolomics approach. Curr. Pediatr. Rev. 2020, 16, 183–93. [Google Scholar] [PubMed]
- Vaduva, C.; Estéban-Sánchez, J.; Sanz-Fernández, R.; Martín-Sanz, E. Prevalence and management of post-BPPV residual symptoms. Eur. Arch. Otorhinolaryngol. 2018, 275, 1429–37. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.I.; Lee, H.M.; Yoo, J.H.; Lee, D.K. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J. Clin. Neurol. 2008, 4, 107–10. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Lapenna, R.; Giommetti, G.; Pellegrino, C.; Ricci, G. Residual dizziness after the first BPPV episode: role of otolithic function and of a delayed diagnosis. Eur. Arch. Otorhinolaryngol. 2016, 273, 3157–65. [Google Scholar] [CrossRef]
- Martellucci, S.; Pagliuca, G.; De Vincentiis, M.; Greco, A.; De Virgilio, A.; Nobili Benedetti, F. M.; Gallipoli, C.; Rosato, C.; Clemenzi, V.; Gallo, A. Features of Residual Dizziness after Canalith Repositioning Procedures for Benign Paroxysmal Positional Vertigo. Otolaryngol. Head Neck Surg. 2016, 154, 693–701. [Google Scholar] [CrossRef]
- Casani, A.P.; Gufoni, M.; Capobianco, S. Current insights into treating vertigo in older adults. Drugs Aging. 2021, 38, 655–70. [Google Scholar] [CrossRef]
- Wu, P.; Yang, J.; Huang, X.; Ma, Z.; Zhang, T.; Li, H. Predictors of residual dizziness in patients with benign paroxysmal positional vertigo after successful repositioning: a multi-center prospective cohort study. J. Vestib. Res. 2021, 31, 119–29. [Google Scholar] [CrossRef]
- Acar, B.; Karasen, R.M.; Buran, Y. Efficacy of medical therapy in the prevention of residual dizziness after successful repositioning maneuvers for benign paroxysmal positional vertigo (BPPV). B-ENT. 2015, 11, 117–21. [Google Scholar]
- Kim, M.B.; Lee, H.S.; Ban, J.H. Vestibular suppressants after canalith repositioning in benign paroxysmal positional vertigo. Laryngos. 2014, 124, 2400–03. [Google Scholar] [CrossRef]
- Jung, H.J.; Koo, J.W.; Kim, C.S.; Kim, J.S.; Song, J.J. Anxiolytics reduce residual dizziness after successful canalith repositioning maneuvers in benign paroxysmal positional vertigo. Acta Otolaryngol. 2012, 132, 277–84. [Google Scholar] [CrossRef]
- Guneri, E.A.; Kustutan, O. The effects of betahistine in addition to Epley maneuver in posterior canal benign paroxysmal positional vertigo. Otolaryngol. Head Neck Surg. 2012, 146, 104–8. [Google Scholar] [CrossRef]
- Jalali, M.M.; Gerami, H.; Saberi, A.; Razaghi, S. The impact of Betahistine versus dimenhydrinate inthe resolution of residual dizziness in patients with benign paroxysmal positional vertigo: a randomized clinical trial. Ann. Otol. Rhinol. Laryngol. 2020, 129, 434–40. [Google Scholar] [CrossRef]
- Piras, C.; Mussap, M.; Noto, A.; De Giacomo, A.; Cristofori, F.; Spada, M.; Fanos, V.; Atzori, L.; Francavilla, R. Alterations of the Intestinal Permeability are Reflected by Changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites. 2022, 12, 104. [Google Scholar] [CrossRef]
- Piras, C.; Pintus, R.; Pruna, D.; Dessì, A.; Atzori, L.; Fanos, V. Pediatric acute-onset neuropsychiatric syndrome and mycoplasma pneumoniae infection: a case report analysis with a metabolomics approach. Curr. Pediatr. Rev. 2020, 16, 183–93. [Google Scholar]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods. 2014, 6, 2812–31. [Google Scholar] [CrossRef]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, A.V.; Bamji, M.S. Nutritional Assessment: Biochemical Tests for Vitamins and Minerals. In: Caballero B (ed) Encyclopedia of Food Sciences and Nutrition. 2nd edn. Academic Press-Elsevier Amsterdam, 4184. [Google Scholar]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Ann. Rev. Nutr. 2008, 28, 115–30. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Gabriel, R. Neuropeptides, trophic factors, and other substances providing morphofunctional and metabolic protection in experimental models of diabetic retinopathy. Int. Rev. Cell. Mol. Biol. 2014, 311, 1–121. [Google Scholar]
- Lingchao, J.; Lee, H.J.; Wan, G.; Zhang, L.; Sajjakulnukit, P.; Schacht, J.; Lyssiotis, C.A.; Corfas, G. Auditory metabolomics, an approach to identify acute molecular effects of noise trauma. Sci. Rep. 2019, 9, 9273. [Google Scholar]
- Jennings, A.; Mohney, R. P.; MacGregor, A.; Steves, C. J.; Cassidy, A.; Spector, T. D.; Menni, C. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 20, 13670. [Google Scholar]
- Secades, J.J.; Gareri, P. Citicoline: pharmacological and clinical review, update. Rev. Neurol. 2022, 75, S1–S73. [Google Scholar]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–28. [Google Scholar] [CrossRef]
- Prakas, D.; Sharma, G. Phytochemicals of Nutraceutical Importance. CABI. 2014, 1, 1–19. [Google Scholar]
- Ulivi, L.; Maccarrone, M.; Giannini, N.; Ferrari, E.; Caselli, M. C.; Montano, V.; Chico, L.; Casani, A.P.; Navari, E.; Cerchiai, N.; Siciliano, G.; Bonuccelli, U.; Mancuso, M. Oxidative Stress in Cerebral Small Vessel Disease Dizziness Patients, Basally and After Polyphenol Compound Supplementation. Curr. Mol. Med. 2018, 18, 160–5. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).