Submitted:
21 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
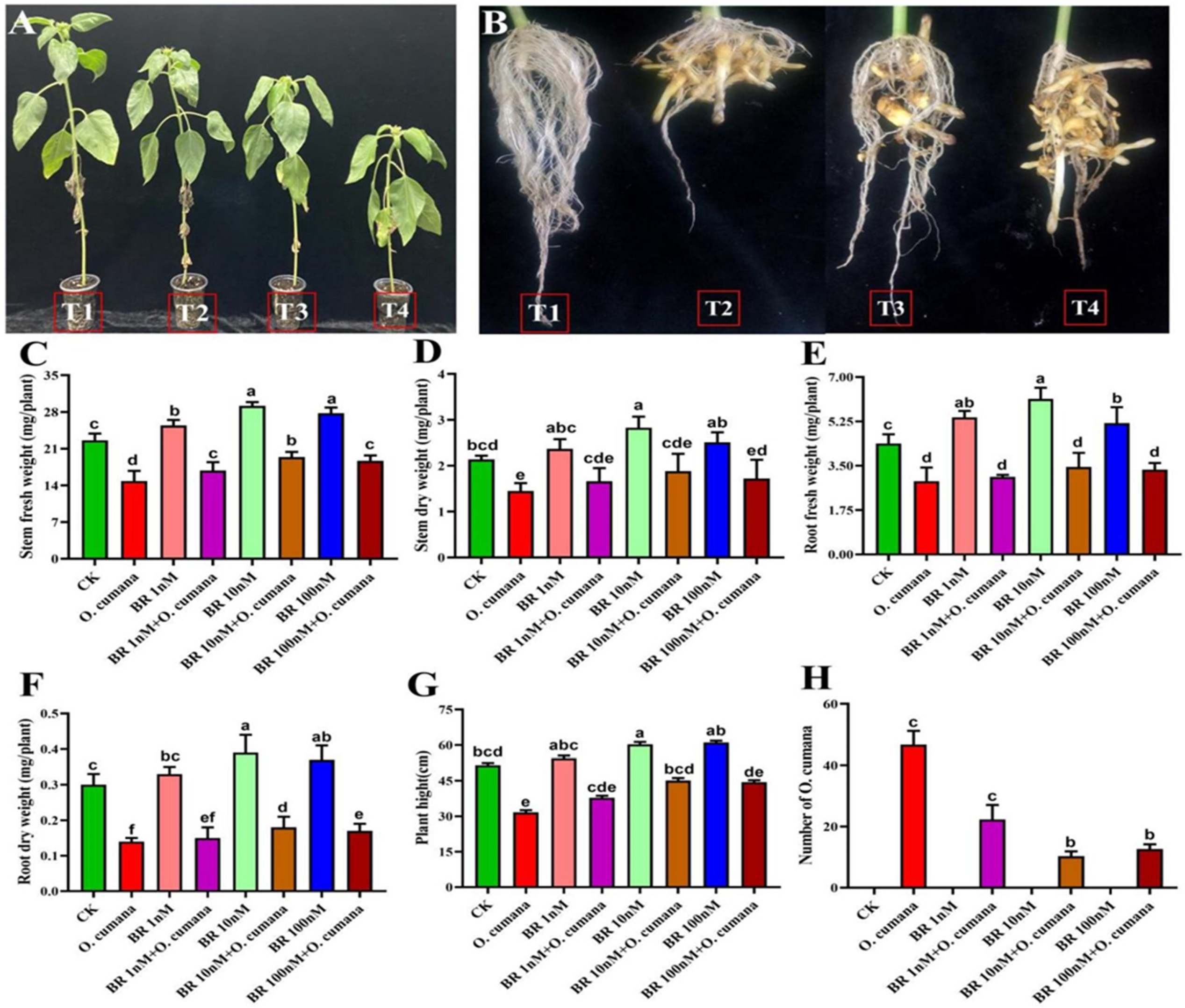
2.1. Exogenous BR Improved the Plant Phenotype and Growth Attributes against O. cumana Infection
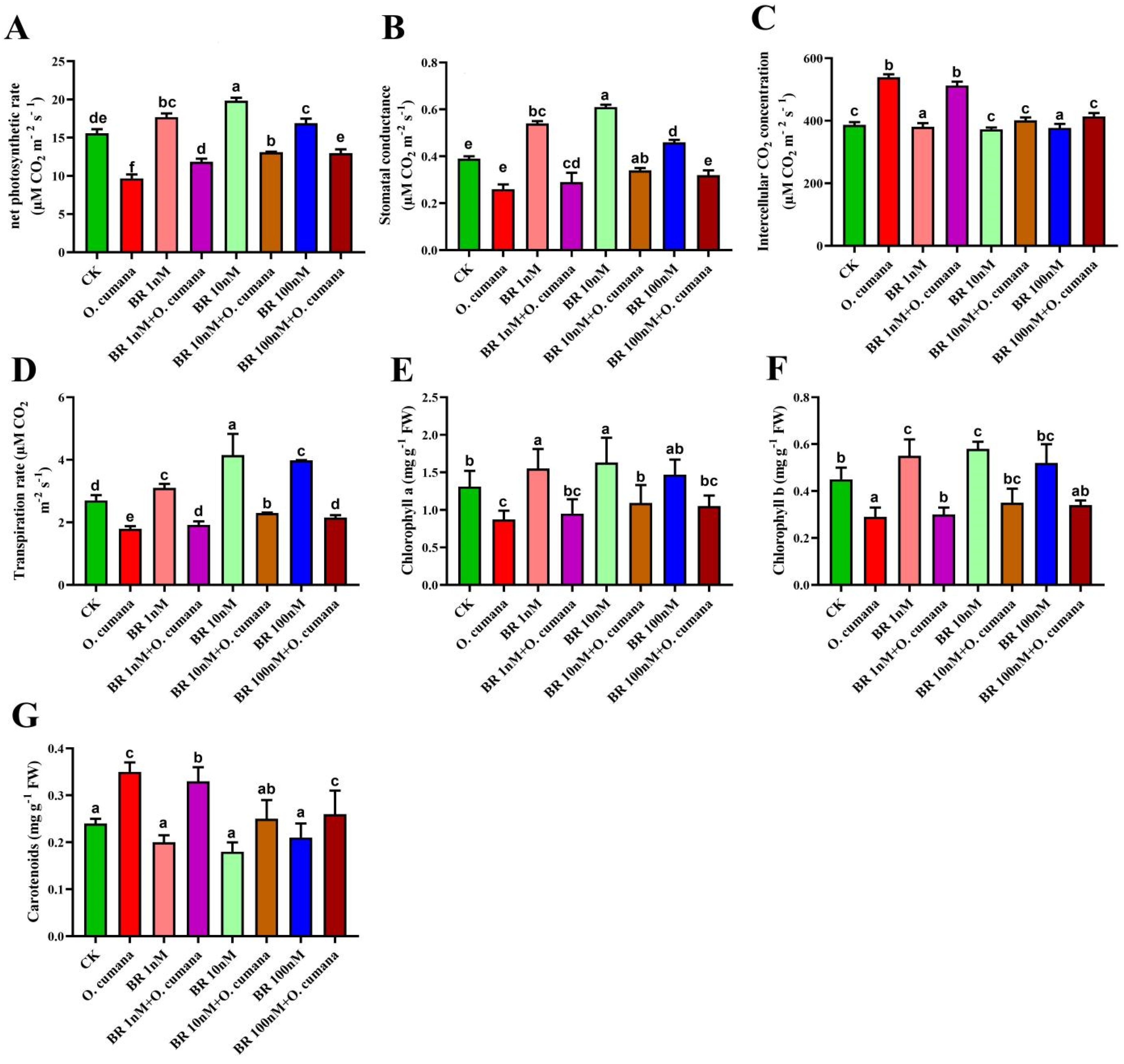
2.2. Exogenous BRs Improved the Chlorophyll Contents and Photosynthetic Apparatus against O. cumana Infection
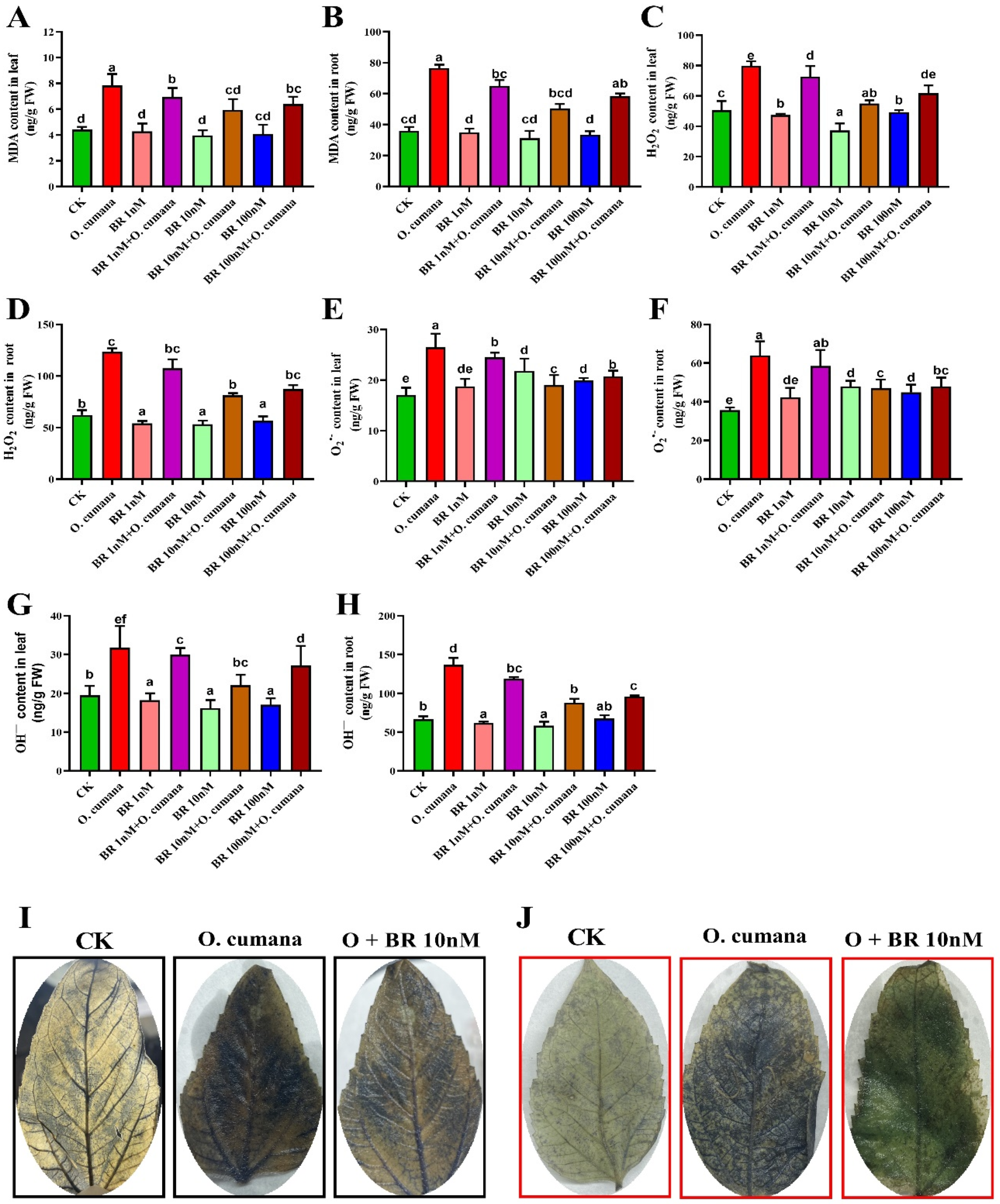
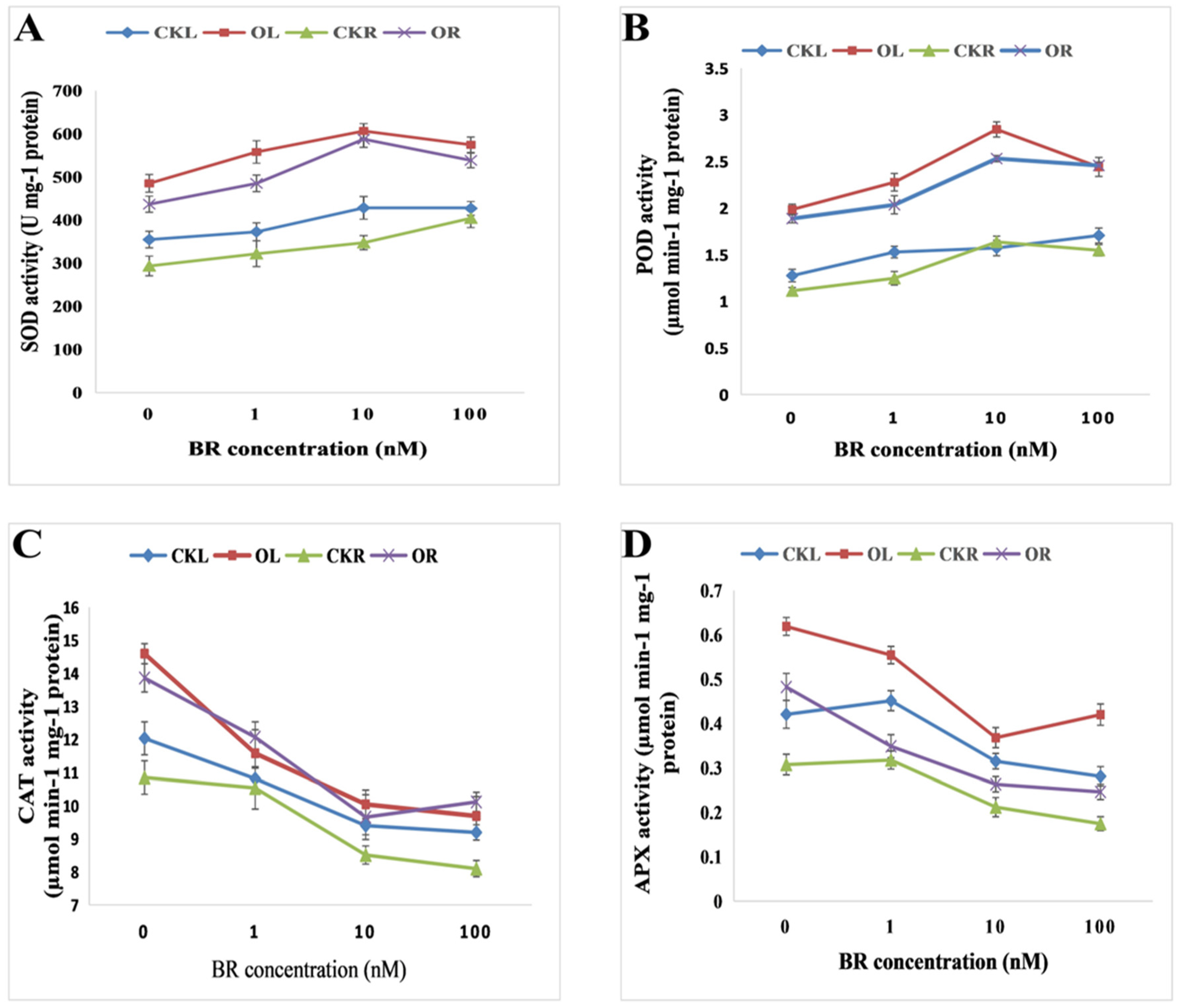
2.3. Exogenous BRs Reduce the Oxidative Damages Induced by O. cumana Infection
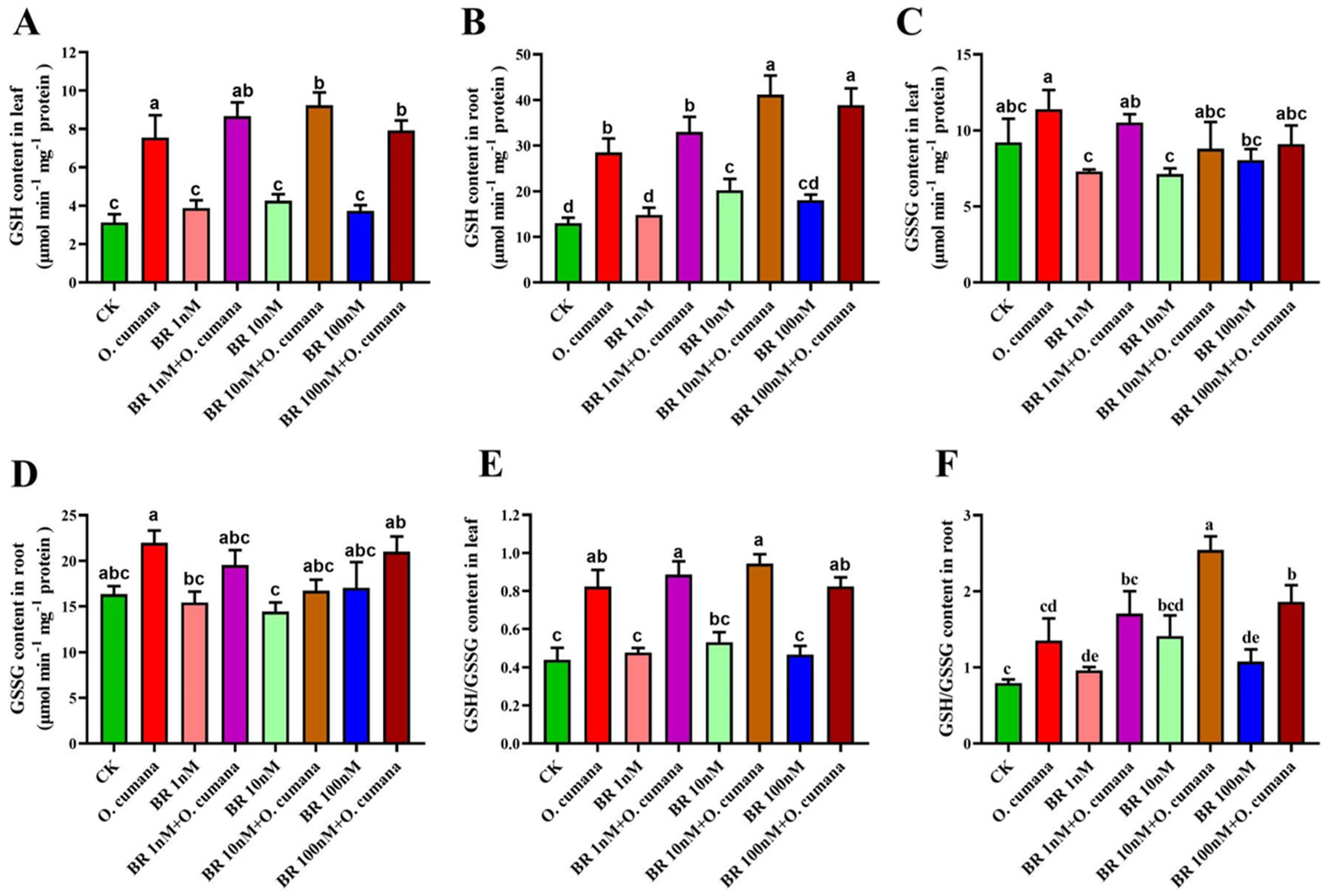
2.4. Influence of BR on GSH, GSSG and GSH/GSSG Ratio against O. cumana Infection
2.5. Exogenous BR Activate the Antioxidant Defence System against O. cumana Infection
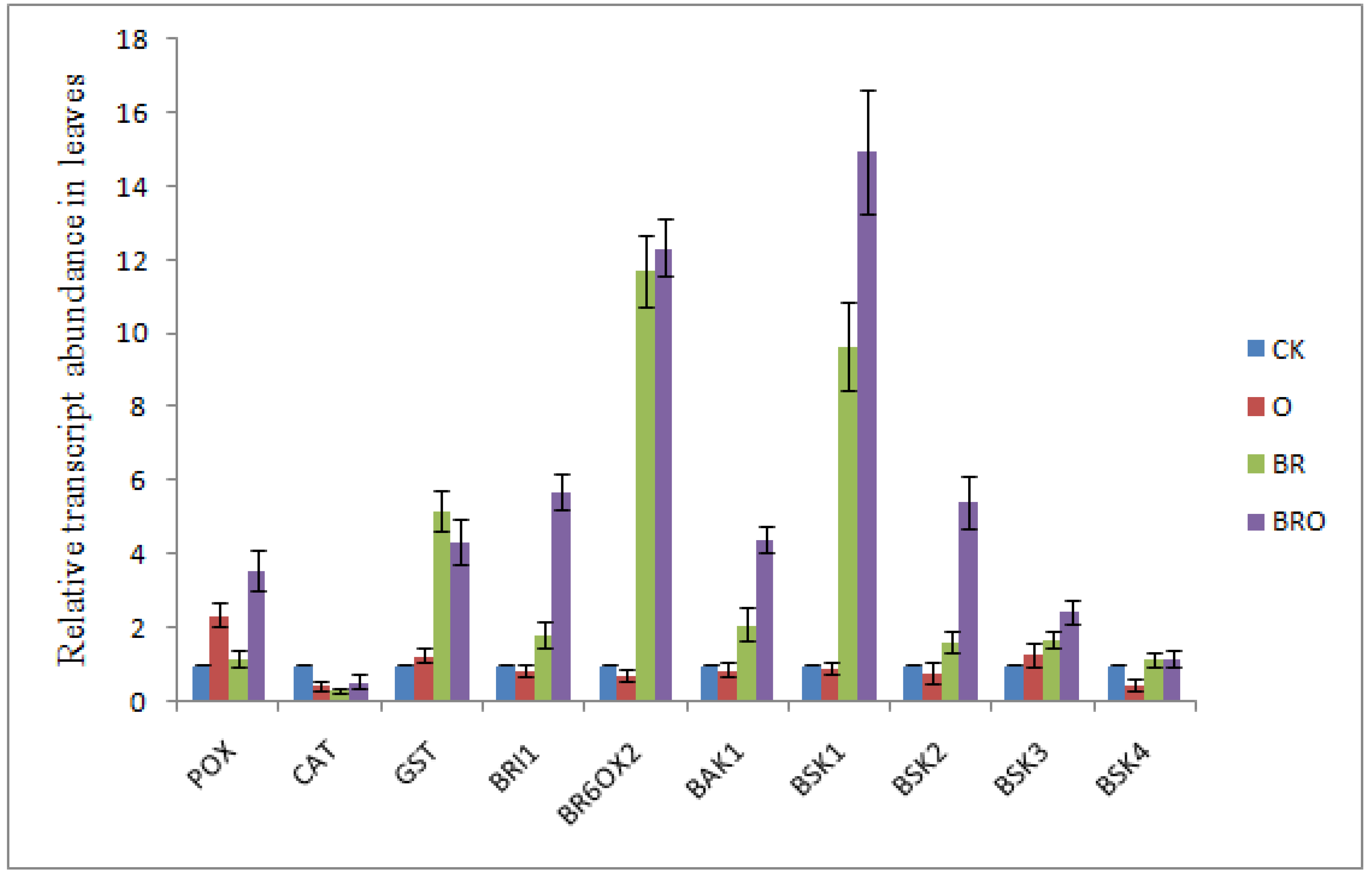
2.6. Influence of BR in Regulating Different Transcript Levels against O. cumana Infection
2.7. Effects of BRs and O. cumana on Lignin, Phenolics and Endogenous BRs Contents
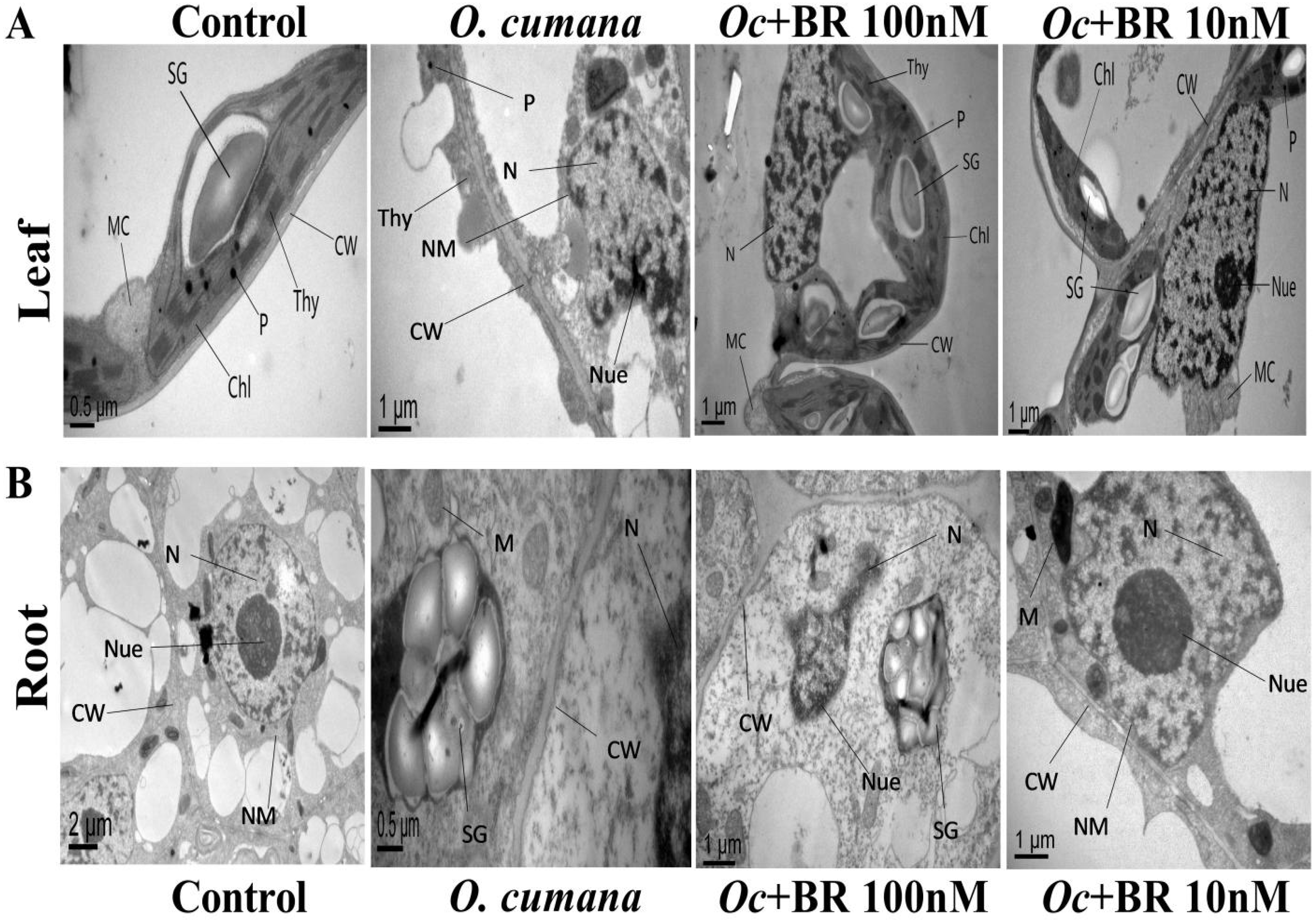
2.8. Exogenous BRs Minimize the O. cumana Induced Cellular Ultrastructural Damages
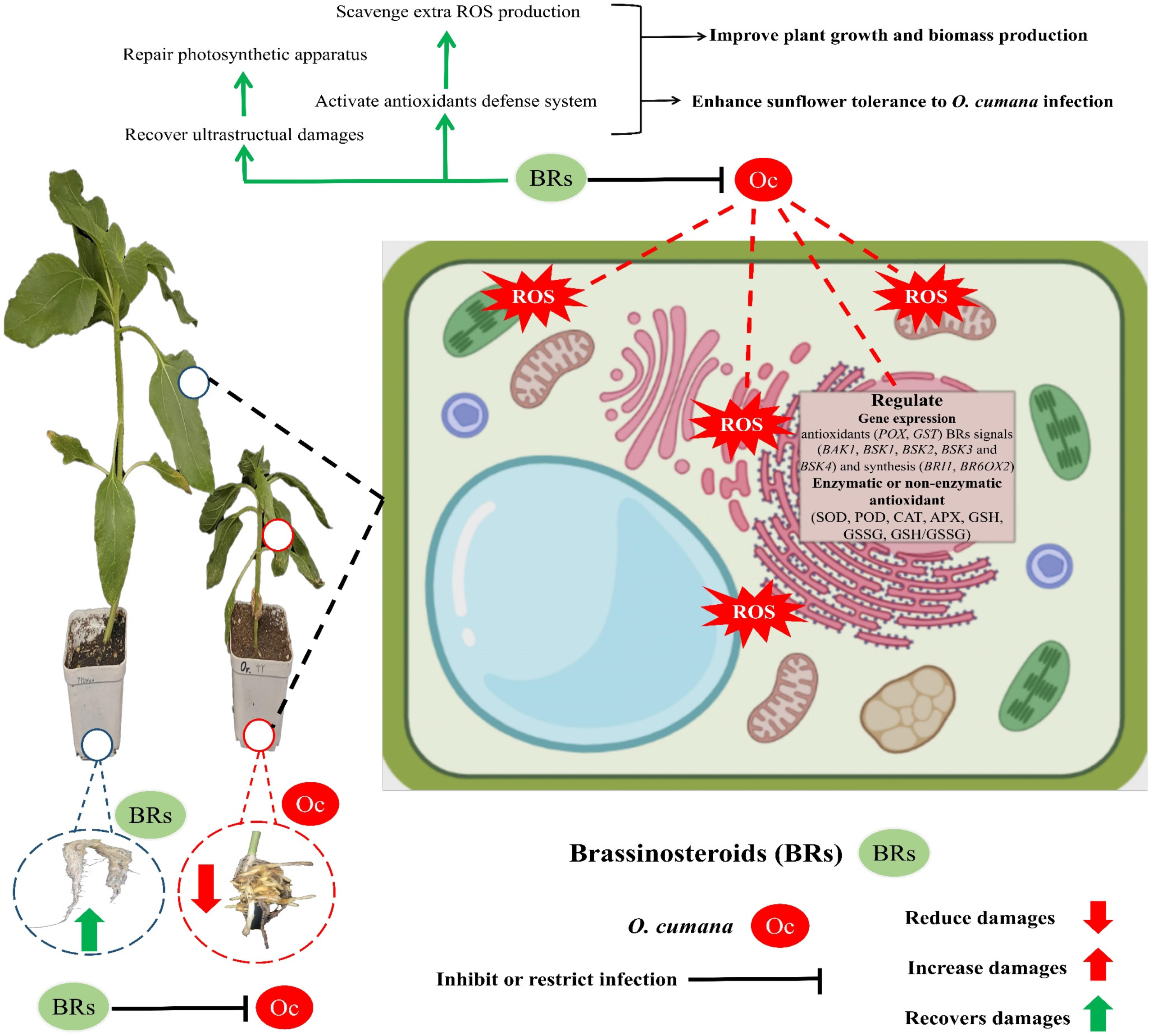
3. Discussion
4. Materials and Methods
4.1. Experiment Design and Growth Conditions
4.2. Measurement of Agronomic Parameters and Photosynthesis Traits
4.3. Estimation of Lipids Peroxidation and Reactive Oxygen Species
4.4. Determination of Antioxidant and Non-enzymatic Antioxidants
4.5. Gene Expression Analysis
4.6. Determination of Phenolics, Lignin and Endogenous Brassinosteroids (BRs) Levels
4.7. Transmission Electron Microscopy (TEM)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Taher, M.; Javanİ, M.; Beyaz, R.; & Yİldİz, M. A new environmental friendly production method in sunflower for high seed and crude oil yields. Fresenius Environ. Bullet. 2017, 26, 4004–4010.
- Sisou, D.; Tadmor, Y.; Plakhine, D.; Ziadna, H.; Hübner, S.; Eizenberg, H. Biological and Transcriptomic Characterization of Pre-Haustorial Resistance to Sunflower Broomrape (Orobanche cumana W.) in Sunflowers (Helianthus annuus). Plants 2021, 10, 1810. [Google Scholar] [CrossRef] [PubMed]
- Parker, C. The parasitic weeds of the Orobanchaceae. In: Parasitic Orobanchaceae. Springer, Berlin, Heidelberg 2013, 313–344.
- Duca, M. Historical aspects of sunflower researches in the republic of moldova. Helia. 2015, 38, 79–92. [Google Scholar] [CrossRef]
- Louarn, J.; Boniface, M.C.; Pouilly, N.; Velasco, L.; Pérez-Vich, B.; Vincourt, P.; Muños, S. Sunflower resistance to broomrape (Orobanche cumana) is controlled by specific QTLs for different parasitism stages. Front. Plant Sci. 2016, 7, 590. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Liu, H.; Cao, M.; Yan, G.; Si, P.; Zhou, W.; & Xu, L. 5-Aminolevolinic acid enhances sunflower resistance to Orobanche cumana (broomrape). Ind. Crops Prod. 2019, 140, 111467.
- Yang, C.; Fu, F.; Zhang, N.; Wang, J.; Hu, L.; Islam, F.; Bai, Q.; Yun, X.; & Zhou, W.J. Transcriptional profiling of underground interaction of two contrasting sunflower cultivars with the root parasitic weed Orobanche cumana. Plant and Soil 2020, 450, 303–321.
- Nadler-Hassar, T.; Shaner, D.L.; Nissen, S.; Westra, P.; & Rubin, B. Are herbicide-resistant crops the answer to controlling Cuscuta? Pest Manag. Sci.: formerly Pesticide Science 2009, 65, 811–816.
- Hosni, T.; Abbes, Z.; Abaza, L.; Medimagh, S.; Ben, S.H.; Kharrat, M. Effect of broomrape (Orobanche cumana) on some aggro-morphological and biochemical traits of Tunisian and some reference sunflower (Helianthus annuus L.) accessions. J. Plant Diseases Protect. 2020, 127, 831–841. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Nomura, T.; Takahashi, I.; Asami, T.; Mori, N.; Akiyama, K.; Kusajima, M.; Nakashita, H. Regulation of biosynthesis, perception, and functions of strigolactones for promoting arbuscular mycorrhizal symbiosis and managing root parasitic weeds. Pest Manag. Sci. 2019, 75, 2353–2359. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Huang, L.F.; Cheng, F.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. Brassinosteroids accelerate recovery of the photosynthetic apparatus from cold stress by balancing electron partitioning, carboxylation, and redox homeostasis in cucumber. Physiol. Plant. 2013, 24, 1399–3054. [Google Scholar] [CrossRef]
- Loake, G.; Grant, M. Salicylic acid in plant defence the players and protagonists. Current Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: pigments of photosynthetic biomemranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Velikova, V., Yordanov, I., Edreva, A., 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151, 59–66.
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Zhang, W.F.; Zhang, F.; Raziuddin, R.; Gong, H.J.; Yang, Z.M.; Lu, L.;... & Zhou, W.J. Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. Plant Growth Regul. 2008, 27, 159–169.
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotech. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Livak, K.J., Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 2001, 25, 402–408. [CrossRef]
- Kofalvi, S.A. Nassuth A. Influence of wheat streak mosaic virus infection on phenylpropanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiological and Molecular Plant Pathology 1995, 47, 365–377. [CrossRef]
- Cahill, D.M.; McComb, J.A. A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiological and Molecular Plant Pathology 1992, 40, 315–332. [Google Scholar] [CrossRef]
- Tarkowská, D.; Strnad, M. ; Protocol for Extraction and Isolation of Brassinosteroids from Plant Tissues. Methods Mol Biol. 2017, 1564, 1–7. [Google Scholar]
- Yang, C.; L. Y. Hu.; B. Ali.; F. Islam.; Q. J. Bai.; X. P. Yun.; K. Yoneyama.; and W. J. Zhou. "Seed treatment with salicylic acid invokes defence mechanism of Helianthus annuus against Orobanche cumana." Ann. Appl. Bio. 2016, 169, 40–422.
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.J.; Anjum, S.A.; Wang, L.; Song, J.X.; Zong, X.F.; Lv,J.; Zohaib, A.; Ali, I.; Yan, R.; Zhang, Y.; Wang, S.G. Effect of foliar application of brassinolide on photosynthesis and chlorophyll fluorescence traits of Leymus chinensis under varying levels of shade. Photosyn. 2018, 56, 873–883.
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinosteroids application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agro. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Briache, F.Z.; Ennami, M.; Mbasani-Mansi, J.; Lozzi, A.; Abousalim, A.; El Rodeny, W.; Amri, M.; Triqui, Z.E.A.; Mentag, R. Effects of salicylic acid and indole acetic acid exogenous applications on induction of faba bean resistance against Orobanche crenata. Plant Pathol. J. 2020, 36, 476. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Huang, Y.Y.; Wang, L.; Huang, L.F.; Yu, Y.L.; Zhou, Y.H.; & Yu, J.Q. Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pesticide Biochem. Physiol. 2006, 86, 42–48.
- Kothari, A.; Lachowiec, J. Roles of brassinosteroids in mitigating heat stress damage in cereal crops. Int. J. Mol. Sci. 2021, 22, 2706. [Google Scholar] [CrossRef] [PubMed]
- Ennami, M.; Mbasani-mansi, J.; Briache, F.Z.; Oussible, N.; Gaboun, F.; Ghaouti, L.; Belqadi, L.; Ghanem, M.E.; Aberkani, K.; Westwood, J.; Mentag, R. Growth-defense tradeoffs and source-sink relationship during both faba bean and lentil interactions with Orobanche crenata Forsk. Crop Prot. 2022, 127, 104924. [Google Scholar] [CrossRef]
- Madany, M.M.; Zinta, G.; Abuelsoud, W.; Hozzein, W.N.; Selim, S.; Asard, H.; Abd Elgawad, H. Hormonal seed-priming improves tomato resistance against broomrape infection. J. Plant Physiol. 2020, 250, 153184. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.S.C.; Delavault, P.; Letousey, P.; Thalouarn, P. Identification by suppression subtractive hybridization and expression analysis of Arabidopsis thaliana putative defence genes during Orobanche ramosa infection. Physiol. Mol. Plant Pathol. 2003, 62, 297–303. [Google Scholar] [CrossRef]
- Kusumoto, D.; Goldwasser, Y.; Xie, X;, Yoneyama, K.,; Takeuchi, Y.; Yoneyama, K. Resistance of red clover (Trifolium pratense) to the root parasitic plant Orobanche minor is activated by salicylate but not by jasmonate. Ann. Bot. 2007, 100, 537–544. [CrossRef]
- Wen, P.F.; Chen,J.Y.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [CrossRef]
- Pincovici, S.; Cochavi, A.; Karnieli, A.; Ephrath, J.; Rachmilevitch, S. Source-sink relations of sunflower plants as affected by a parasite modifies carbon allocations and leaf traits. Plant Sci. 2018, 271, 100–107. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Gene Name | Forward | Reverse |
|---|---|---|---|
| EF-1α | Elongation factor-1 alpha (reference gene) | GGATACAACCCCGACAAA | CCTGAAGTGGGAGACGAA |
| POX | Peroxidase | GGACCGTAAGACTTGGAG | ATAAGGCGACCATTTCTC |
| CAT | Catalase | CGTCTTGGACCGAACTATT | CAAACCACCCACAACTCTG |
| GST | Glutathione | GCATTCCCGCATTATTC | ACTTACTGCCTTTCACTTTC |
| BRI1 | Brassinosteroid homeostasis | GTGAAACTAAACTGGTCCACAC | GTTGAACACCTGAAACTTTGGT |
| BR6OX2 | Cytochrome p450 enzyme | GGGAGTTTCTTCAAGTCTCACA | GCAACAAGTCCTTTCGATTCAT |
| BAK1 | BRI1-associated receptor kinase | ACTTCTTTGATGTACCAGCTGA | CAACTTGTAGTTCACGCAATGA |
| BSK1 | BR-signaling kinase 1 | GATTTCAAAACAGCCATCGACT | AGTAAGTAGCATAGACTTCGCC |
| BSK2 | BR-signaling kinase 2 | AATACTACTCCAAGTTGGTGGG | TCATTAAGTAGGAGAAAGCCCG |
| BSK3 | BR-signaling kinase 3 | TGATGAAGAATAGTCGGGATGG | TCACTATCAGAAAACTGCCCAT |
| BSK4 | BR-signaling kinase 4 | GATCTTTCGTTTATGTGGAC | AGACTGGAGATACAATGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





