1. Introduction
Coffee is believed to have been discovered in the 9th-century in the region of East Africa, particularly Ethiopia, where it grew naturally. The legend of the discovery of coffee often involves a goat herder named Kaldi. The goat herder noticed that the goats became more energetic after eating the berries from a certain tree, which we now know as the coffee plant (Pendergrast, 1999). Coffee was introduced to Europe in the 17th century, with the first coffeehouse opening in Venice in 1645 (Pendergrast, 2010). The coffee market is currently valued at USD 15.1 billion and is expanding. This market consists primarily of roasted, instant, and ready-to-drink coffee (Seninde & Chambers 2020). With 5.7 million tons of Coffea arabica (Arabica coffee) and 4.3 million tons Coffea canephora (Robusta coffee), world coffee production is set to reach 10.2 million tons in 2020 (Velásquez et al., 2022). Caffeine is the primary compound in coffee, so it is not surprising that about two thirds of the daily caffeine consumption of adults over the age of ten in the U.S comes from coffee. Caffeine use has been linked to several health advantages, including improved anti-inflammatory response, enhanced cognitive function, and hypoalgesic effects (Celli et al., 2019). Packaged coffee refers to coffee beans or ground coffee that has been processed, roasted, and sealed in a container for commercial distribution and consumer purchase. The quality and flavor of packaged coffee are influenced by factors such as the coffee bean origin, roasting methods, packaging materials, and storage conditions. During the roasting process, several intricate reactions take place, including the Maillard reactions, breakdown of sugars, oxidation of lipids, and pyrolysis (Kocadağlı et al., 2012). Coffee bean roasting occurs at temperatures of 180–250 °C. Depending on the desired roast level (light, medium or dark), the majority of chemical compounds related to aroma and flavor are created during the roasting prosses due to degradation and the Maillard reaction. In addition, more than 950 post-roasting compounds have been found in coffee (Sualeh et al., 2020). The caramelization stage of roasting accounts for coffee's hue, whereas pyrolysis reactions yield both volatile and non-volatile compounds that play a critical role in the aroma and flavor characteristics of coffee (Tarigan et al., 2022). Certain bioactive compounds, specifically total phenolic compounds (TPC) and chlorogenic acids (CGA), undergo a progressive degradation as a result of the roasting process, which is influenced by both the temperature level and the roasting intensity (Herawati et al., 2019). Coffee is also rich in bioactive compounds such as chlorogenic acids (CGAs), caffeine, as well as various phenolic compounds such as caffeoylquinic acids, dicaffeoylquinic acids, and feruloylquinic acids. These chlorogenic acid compounds, and particularly the chloroquinic acids, contribute to coffee's bitterness and are recognized for their antioxidant properties, which could potentially offer health advantages to coffee consumers (Rao et al., 2020). Basically, organic acids (OAs) and chlorogenic acids (CGAs) are the two types of acids found in coffee. In addition, 38 organic acids have been identified and measured in roasted coffees, with the most noticeable ones in green coffee being citric, malic, and quinicacids. As the coffee fruit ages, bioactive substances accumulate naturally in the bean and consist of various quinic acid esters or series of esters, collectively known as CGAs (Yeager et al., 2023). This accumulation undergoes significant changes in the acid content of coffee beans during the roasting process. For example, green coffee has lower acid levels than roasted coffee, with a pH between 5.8 – 6.1 (Araújo et al., 2020; Bicho et al., 2013). During roasting, chlorogenic, citric, and malic acids are broken down, whereas quinic acid concentration increases as a result of the breakdown of chlorogenic acid (Rune et al., 2023). As a secondary effect, Trugo et al. (1984) demonstrated that higher roasting temperatures led to the breakdown of chlorogenic acids (CGA), consequently yielding reduced extraction concentrations and leading to a reduction in acidity levels. This result was corroborated by Rao et al (2020) who found a higher concentration of CGA in medium roast coffee than in dark roast. This higher CGA concentration caused the dark roasted coffee to be slightly less acidic than the medium roast. However roasted coffee with a pH range between 4.8-5.2, is significantly more acidic than green coffee, whose pH ranges between 5.8 - 6.1.
The strength of the beverage and the yield of soluble material extracted from the bed of dry coffee grounds are two fundamental and related brewing indicators that help to define the coffee brewing process. Strength is often measured using the total dissolved solids (TDS) stated as a mass percent, and brewing yield, also known as percent extraction (PE), is the mass of soluble material extracted from the coffee grounds per the original dry mass of grounds. (Frost et al., 2020).
High-acid coffee can cause stomach discomfort and potential health problems in people who are acid-sensitive. Low acid coffee was designed to have less acidity and cause less stomach discomfort due to its gentler acidity profile. Whereas the average pH of roasted coffee sits below the critical pH line of 5.5 for beverages, coffee causes the acidification and discoloration of teeth and is associated with being a leading cause of gastral issues for many consumers (Ranjitkar et al., 2012). Numerous coffee products are marketed as low-acid coffee, and yet only a few studies have investigated the acidity levels of hot-brewed coffees that claim to be low in acidity. Additionally, some coffee packaging labels include claims of the product being acid-free. In this context, the objective of the present study was to examine and compare the differences in acidity levels across various packaged coffee products. Additionally, the results will serve as a benchmark to support enhanced decision-making in coffee product development and a resultant standard for use on packaging labels of low acid or acid-free coffee.
2. Materials and Methods
2.1. Sampling:
Eleven commercial products of higher quality ground dark roasted coffee were purchased from a local grocery store (Greensboro, North Carolina) and online store (Amazon). The coffee samples were given codes as XL and stored in a dry environment at room temperature.
2.2. Brewing Coffee Samples
The coffee samples were brewed using a drip coffee maker (Mr. Coffee, 12 Cup Switch Coffee Maker) per guidelines for coffee grounds to water ratio for each sample (
Table 1). The measured coffee grounds were placed in a clean paper filter (coffee filters, Harris Teeter, unbleached) after filling the coffee maker reservoir with cold deionized water (DI). The pot of coffee and filter basket were washed and rinsed with DI water after each coffee making cycle.
2.3. Determination of pH Values
The pH readings were recorded using a pH meter (Accumet AB 150, Fisher Scientific, Pittsburgh, PA) after calibration with standard pH buffers (4.0, 7.0, and 10). The pH values of each coffee sample were measured in triplicate by placing the pH electrode into the sample after cooling down the sample to room temperature (~25 ℃). The pH electrode was rinsed thoroughly with DI water and wiped clean between each measurement.
2.4. Determining total Dissolved Solids (TDS)
TDS refers to the total concentration of dissolved substances, including minerals, salts, ions, and other organic and inorganic compounds. In order to measure the TDS using a refractometer (Boss refractometers, National Industrial Supply), the sample should be at room temperature. A few drops of the coffee samples were placed onto the refractometer's prism surface after which the prism cover was gently closed to ensure that there were no air bubbles or gaps between the prism and the liquid. The reading of TDS (%) was taken in triplicate by looking through the eyepiece of the refractometer until a clear line or boundary could be seen between the light and dark areas.
2.5. Statistical Analysis
Each experiment was performed in triplicate, (n = 3). For each experiment, the mean and standard deviation of the result was recorded, and analysis of variance using one-way ANOVA was conducted (SAS version 9.4). Differences in the mean values of the results of experiments were calculated using Tukey’s test, and the criterion for labeling a difference as significant was p < 0.05.
3. Results and Discussion
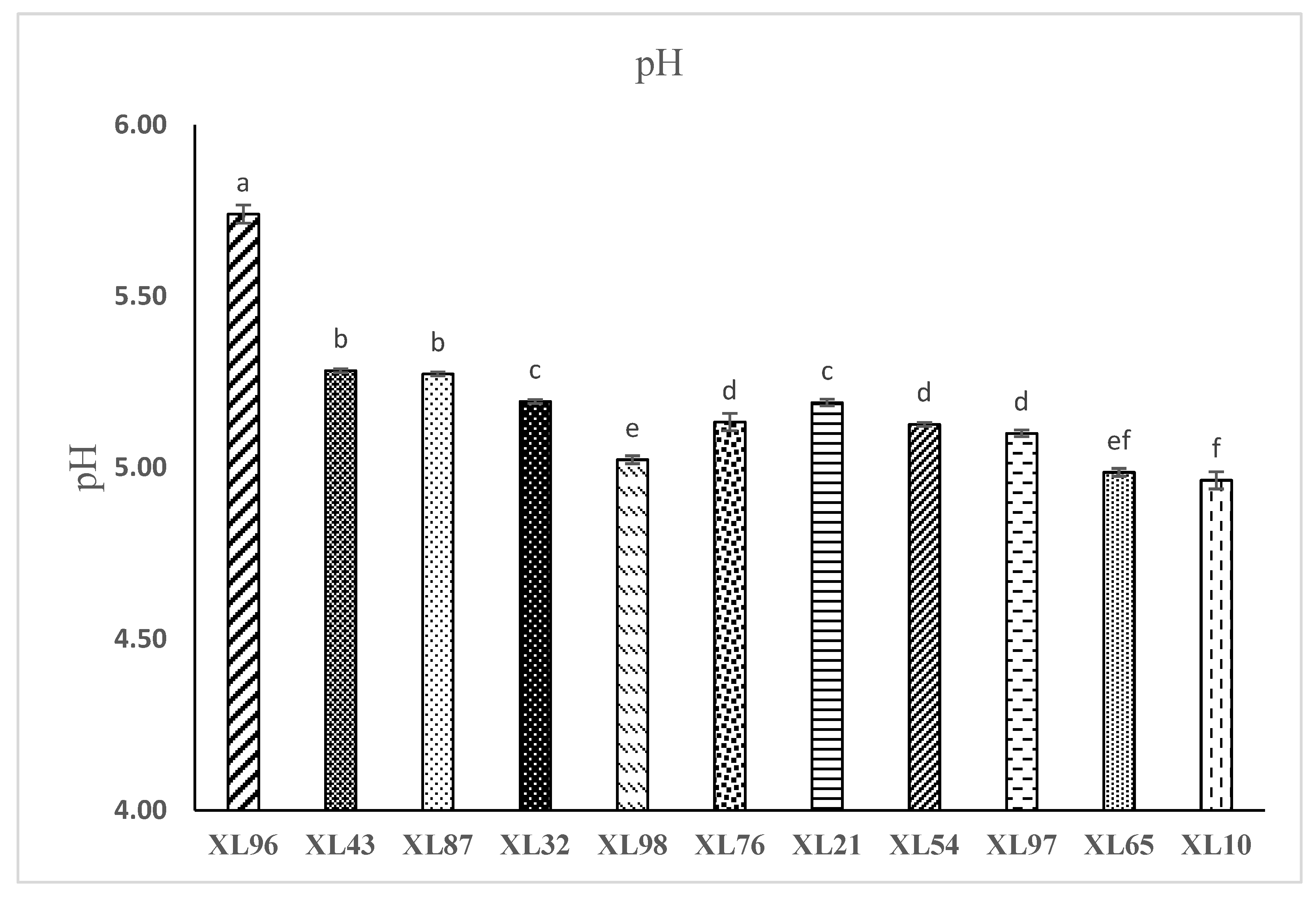
Figure 1 shows the pH values of brewing coffee samples. All coffee samples tested in the experiment were dark roasted. The pH measurements for coffee samples showed a low acidity in sample XL96 (pH 5.74) which was significantly different (P < 0.05) from other samples. The higher acidity was observed in samples XL10, XL65, XL98 (pH 4.96, 4.99, and 5.02) respectively. The pH values for the rest of samples ranged between (pH 5.10 - 5.28) and this is within the expected average pH range of the regular commercial coffee. Many factors can affect the final pH level of brewing coffee including the roasting temperature. Heat initiates a series of chemical changes in the coffee beans during the roasting process, which alters the flavor profile of the coffee. Moreover, the acidity of coffee can also be affected by the type of roast. The acid composition of coffee beans changes dramatically during roasting. In addition, chlorogenic, citric, and malic acid are degraded, although quinic acid concentration increases due to the presence of chlorogenic acid degradation (
Rune et al., 2023). Some bioactive chemicals, such as total phenolic compounds and chlorogenic acids, break down gradually throughout the roasting process, which is influenced by the roasting temperature and intensity process (
Tarigan et al., 2022).
TDS is a measurement of the concentration of dissolved solids in a liquid, typically expressed in parts per million (ppm) or as a percentage (Jung et al., 2021). In the context of brewing coffee, TDS is often used to assess the strength and flavor of the coffee. A higher TDS reading generally indicates a stronger and more concentrated coffee with bolder flavors, while a lower TDS reading suggests a milder and potentially under-extracted brew.
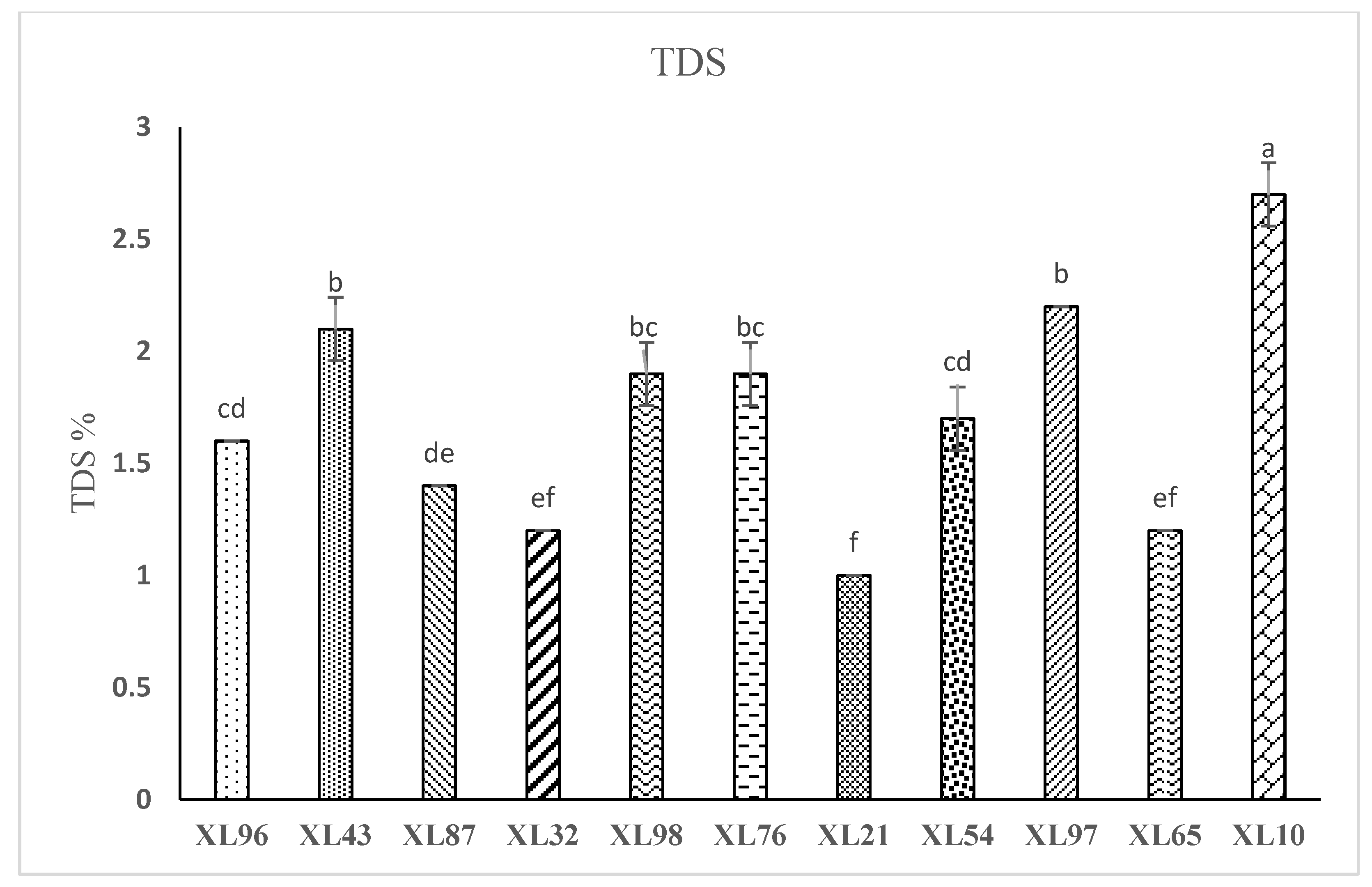
Figure 2 shows the TDS of brewing coffee samples. The highest TDS was observed in sample XL10 with a value of (2.7%), and the lower TDS was in sample XL21 with a value of (1%). Most coffee samples had a TDS range between (1.4-2%). In an analysis of how TDS affected the sensory qualities of drip coffee, Frost et al. (2019) came to the conclusion that coffee with a lower TDS was sweeter while coffee with a greater TDS was more acidic. The degree of grinding has a significant impact on physicochemical characteristics of brewing coffee such as pH and TDS. In order to manage extraction and dispersion, roasted beans must be ground into a smaller size; as a result, TDS decreases with increasing particle size (
Várady et al., 2022). The TDS in drip coffee is influenced by factors such as the composition of coffee beans, size of the coffee grounds, roasting temperature, the temperature of the water used for extraction, and extraction time. Jung et al (2021) indicated that the TDS decreased as the roasting time increased.
4. Conclusion
In the present study, the pH and TDS values of different commercial packaged coffee products sold in retail stores in North Carolina were evaluated in order to investigate the accuracy of the information on some coffee products labeled as low acid or acid-free. Our results showed a significant difference in pH measurements among coffee samples with the lowest acidity shown in sample XL 96 pH (5.74) and the highest acidity noted in sample XL 10 pH (4.96). Additionally, two additional samples, XL 65 and XL 98, exhibited high acidity levels with pH values (4.99, and 5.02) respectively. The remainder of the samples revealed pH values between (5.10, and 5.28). The results also indicate that only 1 of the 7 low acid coffee brands tested was in fact significantly less acidic than average commercial coffee and above the critical pH line. Moreover, in contrast to the 4 regular coffees that we tested, all of the other low acid-labeled coffee was either as acidic or more acidic than the regular coffees. None of the coffees were acid-free. This would suggest a perceived commercial liberty that has resulted in misleading consumers with regard to the labeling of low-acid coffee. The more recent arrival of numerous low acid coffee brands on the market serves to highlight consumer concern regarding the high acid levels in regular commercial coffee and its adverse effect on digestive health. As a result, customers will have access to more accurate information when purchasing roasted coffee that has been labeled as low acid. Our results thus serve to emphasize the need to establish a reliable set of standards for the labeling of low acid coffee in packaged coffee products. This effort will help the coffee industry itself to be better informed with regard to understanding how coffee processing can impact the acidity of the final product This new standard for low acid coffee should reference the medical definition of critical pH, include a standardized method for measuring pH, and clear information regarding acidity levels on all packaging labels as well as demonstrable assurance from the industry regarding compliance with this new standard.
Acknowledgment
This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture (NIFA) and in part by the Department of Family and Consumer Sciences and the Agriculture Research Station at North Carolina Agriculture and Technical State University (Greensboro, NC 27411). The authors would also like to acknowledge the financial support provided by Puroast Coffee Company, Inc., High Point, NC. The authors hereby declare that there are no conflicts of interest.
References
- Araújo CD, S.; Macedo, L.L.; Vimercati, W.C.; Ferreira, A.; Prezotti, L.C.; Saraiva, S.H. Determination of pH and acidity in green coffee using near-infrared spectroscopy and multivariate regression. J. Sci. Food Agric. 2020, 100, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Bicho, N.C.; Leitão, A.E.; Ramalho, J.C.; de Alvarenga, N.B.; Lidon, F.C. Identification of chemical clusters discriminators of Arabica and Robusta green coffee. Int. J. Food Prop. 2013, 16, 895–904. [Google Scholar] [CrossRef]
- Celli, G. B. , & de Camargo, A. C. What is in a “Cup of Joe”? From green beans to spent grounds: a mini-review on coffee composition and health benefits. J. Food Bioact. 2019, 6. [Google Scholar]
- Frost, S. C. , Ristenpart, W. D., & Guinard, J. X. Effect of basket geometry on the sensory quality and consumer acceptance of drip brewed coffee. J. Food Sci. 2019, 84, 2297–2312. [Google Scholar] [PubMed]
- Frost, S. C. , Ristenpart, W. D., & Guinard, J. X. Effects of brew strength, brew yield, and roast on the sensory quality of drip brewed coffee. J. Food Sci. 2020, 85, 2530–2543. [Google Scholar] [PubMed]
- Herawati, D. , Giriwono, P. E., Dewi, F. N. A., Kashiwagi, T., & Andarwulan, N. Critical roasting level determines bioactive content and antioxidant activity of Robusta coffee beans. Food Sci. Biotechnol. 2019, 28, 7–14. [Google Scholar] [PubMed]
- Jung, S. , Gu, S., Lee, S. H., & Jeong, Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee extracted from Coffea arabica cv. Java. Appl. Sci. 2021, 11, 7025. [Google Scholar] [CrossRef]
- Kocadağlı, T. , Göncüoğlu, N., Hamzalıoğlu, A., & Gökmen, V. In depth study of acrylamide formation in coffee during roasting: role of sucrose decomposition and lipid oxidation. Food Funct. 2012, 3, 970–975. [Google Scholar] [PubMed]
- Moon, J. K. , Yoo, H. S., & Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [PubMed]
- Pendergrast, M. The History of Coffee and How it Transformed our World; Perseus Bo-oks Group: New York, NY, USA, 1999. [Google Scholar]
- Pendergrast, M. Uncommon Grounds: The history of coffee and how it transformed our world; Basic Books: 2010.
- Ranjitkar, S. , Kaidonis, J. A., & Smales, R. J. Gastroesophageal reflux disease and tooth erosion. Int. J. Dent. 2012, 2012, 479850. [Google Scholar] [CrossRef]
- Rao, N. Z. , Fuller, M., & Grim, M. D. Physiochemical characteristics of hot and cold brew coffee chemistry: The effects of roast level and brewing temperature on compound extraction. Foods 2020, 9, 902. [Google Scholar] [PubMed]
- Rune, C. J. B. , Giacalone, D., Steen, I., Duelund, L., Münchow, M., & Clausen, M. P. Acids in brewed coffees: Chemical composition and sensory threshold. Curr. Res. Food Sci. 2023, 6, 100485. [Google Scholar] [PubMed]
- Seninde, D. R. , & Chambers IV, E. Coffee flavor: A review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Sualeh, A. , Tolessa, K., & Mohammed, A. Biochemical composition of green and roasted coffee beans and their association with coffee quality from different districts of southwest Ethiopia. Heliyon 2020, 6, e05812. [Google Scholar] [CrossRef]
- Tarigan, E. B. , Wardiana, E., Hilmi, Y. S., & Komarudin, N. A. The changes in chemical properties of coffee during roasting: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012115. [Google Scholar] [CrossRef]
- Trugo, L. C. , & Macrae, R. A study of the effect of roasting on the chlorogenic acid composition of coffee using HPLC. Food Chem. 1984, 15, 219–227. [Google Scholar] [CrossRef]
- Várady, M. , Tauchen, J., Klouček, P., & Popelka, P. Effects of total dissolved solids, extraction yield, grinding, and method of preparation on antioxidant activity in fermented specialty coffee. Fermentation 2022, 8, 375. [Google Scholar] [CrossRef]
- Velásquez, S.; Banchón, C. Influence of pre-and post-harvest factors on the organoleptic and physicochemical quality of coffee: a short review. J. Food Sci. Technol. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yeager, S. E. , Batali, M. E., Guinard, J. X., & Ristenpart, W. D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2023, 63, 1010–1036. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).