1. Introduction
Grapevine (
Vitis vinifera L.) is one of the most commonly cultivated plant species in several countries like Australia, France, Greece, Italy, South Africa, Spain, and USA, and has a significant social, economic, and environmental impact [
1]. Greece is belonging to the top 20
th leading grapevine-growing countries of the world in terms of production quantity [
2], while viticulture is essential for the economy and rural development of the country, covering an area of 89.230 ha with an annual yield of 818.860 tons in 2021 [
3]
In recent decades, grapevine trunk diseases (GTDs) have emerged as the most severe threat to viticulture sustainability worldwide [
4]. GTDs are a cluster of diseases incited by a vast number of fungal pathogens (mostly ascomycetes) that colonize and infect wood tissues, causing internal symptoms such as wood streaking and necrosis, vascular browning, and white rot [
5,
6]. Consequential external symptoms include leaf chlorosis, yellowing and wilting, wood cankers, and dieback of spurs, canes, and cordons, leading to stunted plant growth, decline, reduced longevity and productivity, and occasionally plant death.
Increased incidence of GTDs globally has been attributed to several factors like the withdrawal of effective chemicals (i.e. sodium arsenate, benzimidazole fungicides), alterations in cultivation practices, globalization in the dissemination of plant material contaminated with latent pathogens, and climate change [7-10]. In particular, climate change and global warming can increase plant stress and make vines vulnerable to various biotic stresses, including GTD-inciting pathogens, in a synergistically interactive manner [10-11]. On the other hand, rapid alterations of wet and dry cycles along with extremely low and/or high temperatures, as a consequence of climate change, may favor pathogen dispersal from their original hosts and adaption to
Vitis, resulting in the emergence of new GTD-associated fungal species as it has been speculated for other woody hosts [
8,
12,
13]. This possibly explains the increased number of fungal pathogens identified as causal agents of GTDs nowadays compared to the past. Additionally, the availability of high-resolution molecular tools has also provided a deeper insight into the GTD-associated microbiome in recent years compared to previous ones and has enriched, so far, our knowledge of microbial species that contribute to the GTD complex [14-18].
To date, more than 140 fungal species belonging to 35 genera have been involved in GTDs complex worldwide, although the pathogenic potential has not been confirmed for all of them in pathogenicity trials [7,19-21]. Interestingly, recent surveys conducted in marginal temperate regions in Cyprus and southern Italy revealed several lesser-known and/or novel species associated with GTDs, like Cadophora luteo-olivacaea, Colletotrichum fioriniae, Seimatosporium vitis-vinifera, Seim. cyprium sp. nov., Sporocadus kurdistanicus, Spo. rosigena and Truncatella angustata, and proved their pathogenic potential [20-21].
Spatial determination of the phytopathogenic status of GTDs in grapevine-growing countries following a regional-scale mapping is important for planning and implementing effective management practices targeting specific diseases. Furthermore, accurate estimation of disease parameters in distinct viticultural zones may provide valuable information on the susceptibility level for widely used genotypes and thus, improved disease management through risk assessment [
13].
Although GTDs incidence and implicated microorganisms have extensively been investigated for most grapevine-growing countries, only crude estimations have been conducted in Greece. In particular, Rumbos and Rumbou [
22], determined specific fungal genera (i.e.
Phaeomoniella,
Fomitiporia,
Stereum,
Phaeoacremonium,
Cylindrocarpon, and
Botryosphaeria) isolated from different-aged vine samples in Greece following a culture-depended and morphological identification approach. Due to their low isolation frequency, authors suggested incorrectly that these fungi could not be the cause of young grapevine decline. In a recent study, Bekris et al. [
14] determined the fungal and bacterial microbiome in symptomatic and asymptomatic grapevines of three important cultivars grown in geographically distinct viticultural zones in Greece following a metagenomic approach and revealed several lesser-known fungal genera related to GTD affected vines. Both studies are inadequately informative about the incidence and severity of GTDs as well as the phytopathogenic role of certain fungal species identified in the GTD-associated microbiome in the country. Therefore, the main objectives of the present study were to: i) estimate disease parameters and identify fungi associated with GTDs in Greece following a culture-dependent approach, ii) determine the population structure of GTDs-inciting fungi in distinct viticultural zones, iii) investigate the phytopathogenic potential of lesser-known fungal species on grapevine, and iv) identify the potential associations of disease indices with vine age, genotype and the prevailed meteorological conditions in discrete viticultural zones.
2. Materials and Methods
2.1. Field Surveys and Disease Incidence Assessment
In total, 310 vineyards in three (3) geographically distinct viticultural zones in northern (n=234), central (n=28), and southern (n=48) Greece were surveyed during July and August of the years 2018-2020. The number of vineyards surveyed for the assessment of GTDs incidence in each grapevine-growing region and their age scale are shown in
Table 1. The geographical distribution of the surveyed locations is shown in
Supplementary Figure S1. For each vineyard, either all or at least 100 vines in randomly selected lines in the vineyard were surveyed, and the percentage of diseased vines (including dead and non-dead vines), symptomatic but non-dead vines, and dead/apoplectic vines, was measured. Additional information about each vineyard's age, genotype (cultivar/rootstock), training-pruning system, and geographical position was also recorded.
2.2. Sampling and fungal isolation
Wood samples from 41 vineyards in southern (Heraklion), 16 vineyards in central (Nemea), and 19 vineyards in northern Greece (9 vineyards in Amyntaio and 10 vineyards in Kavala) were collected and analyzed. In each vineyard, 3 to 9 representative grapevines with typical GTDs symptoms were sampled. Overall, 252 vines were sampled from northern (n=60), central (n=69), and southern (n=129) Greece. Wood samples from the trunk and the cordon of each vine (approximately 30-cm-length each) were cut and visually inspected to verify the occurrence of internal wood symptoms. Samples were transferred to the laboratory and cut crosswise and lengthwise, the phloem was removed, and wood fragments (approx. 10-cm-length) were surface sterilized by soaking them in 93% ethanol and passing through a flame, thrice. Xylem chips taken from symptomatic wood tissue were aseptically placed onto acidified potato dextrose agar (PDA, Merck, Germany). Plates were incubated at 24±0.2 °C in the dark and observed daily for at least two weeks. The emerging fungal colonies that grew out of tissue excisions were examined visually and under a light microscope, and the fungi were transferred onto new PDA plates. For short-term storage, all fungal isolates were maintained on PDA at 4 oC, whereas for long-term they were stored at -80 oC in a 25% (v/v) aqueous glycerol solution.
2.3. Morphological and Cultural Characterization of Isolates
To estimate mycelial growth of fungal isolates, mycelial-colonized PDA agar discs (5 mm in diameter) was transferred into the center of new 92 mm-diameter PDA plates (one disc per plate, three per isolate). Plates were incubated at 24 oC in the dark, and the colonies’ diameter was measured periodically for up to three weeks or stopped earlier when the fungus completely covered the plate surface. The growth rate of fungal isolates was expressed in mm/day. At the end of the incubation period, colony characteristics (colour, mycelium, colony texture, and shape) were observed. Dimensions of available conidia (30 readings per isolate), carpophores, and hyphal features (color, shape, presence or absence of septum, clump, and chlamydospores) were also recorded.
2.4. DNA Extraction, PCR Amplification and Sequencing
Molecular identification of fungal isolates was carried out by extracting fungal DNA and sequencing their internal transcribed spacer regions of ribosomal DNA (
rDNA-ITS) gene. In addition, rDNA large subunit (
LSU), translation elongation factor 1-alpha (
tef1-α), β-tubulin (
tub2) and actin
(act) genes of isolates HOURD2.1AVR1, PEROG2.1YP2, SAROG1.3AVR10, SAROG1.3AVR13, SAROG1.2AKO1, and SAROG1.3AVR7 were sequenced. Fungal DNA was extracted from 100 mg mycelium (fresh weight), scraped from the surface of 2 to 3-week-old PDA cultures by using a sterilized scalpel, according to Cary et al. [
23]. The quantity and quality of the obtained DNA’s were determined using a Q5000 UV-Vis Spectrophotometer (Quawell, San Jose, CA, USA). Final DNA concentration of each isolate was adjusted to 20 ng ml
-1 and stored at -20 °C until use. The primer pairs used were ITS1/ITS4 for the
rDNA-ITS region, LROR/Un-Lo28S1220 for
LSU [
24,
25], EF1-728F/EF1-986R for
tef1-α [
26], T1/Bt2b-R for
tub2 [
26,
27] and ACT-512F/ACT-783R for
act [
26] regions of the six isolates mentioned above. All PCR assays were carried out in an FG-TC01 FastGene
® Gradient thermocycler (NIPPON Genetics EUROPE), by the use of BK 1003 KAPA Taq PCR kit (KAPABIOSYSTEMS, Wilmington, Massachusetts, USA). PCR assay for
rDNA-ITS included an initial denaturation at 95
oC for 3 min; followed by 35 cycles of 30 sec of denaturation at 94
oC, 30 sec of annealing at 54
oC, and 50 sec of extension at 72
oC; and a final extension step at 72
oC for 5 min. LSU amplification included initial denaturation at 95
oC for 3 min; followed by 35 cycles of 30 sec of denaturation at 94
oC, 30 sec of annealing at 50
oC, and 1 min of extension at 72
oC; and a final extension step at 72
oC for 10 min. Cycling conditions for
tef1-α,
tub2 and
act were similar to those of
rDNA-ITS but with annealing temperatures of 53
oC, 51
oC, and 57
oC, respectively. Amplified products were purified with the “NuncleoSpin® Gel and PCR Clean-up” kit (MACHEREY-NAGEL, Düren, Germany) and sequenced by both forward and reverse primers at Macrogen Europe B.V., Amsterdam, the Netherlands. The “BioEdit 7.0.1” software was used to edit the raw sequencing data [
28]. For isolates HOURD2.1AVR1, PEROG2.1YP2, SAROG1.3AVR10, SAROG1.3AVR13, SAROG1.2AKO1, and SAROG1.3AVR7 the assembled sequences of their
rDNA-ITS, LSU,
tef1-α,
tub2, and
act regions were deposited in GenBank under the accession numbers shown in
Supplementary Table S1.
2.5. Identification and Characterization of Isolates
The fungal isolates obtained from diseased wood tissues were characterized using morphological and physiological features, along with
rDNA-ITS gene sequences. Molecular identification of the wood-inhabiting fungi was conducted by utilizing the "blastn" option at NCBI and comparing their gene sequences with those in the GenBank database. Additionally, for six (6) selected lesser-known fungal isolates (HOURD2.1AVR1, PEROG2.1YP2, SAROG1.3AVR10, SAROG1.3AVR13, SAROG1.2AKO1, and SAROG1.3AVR7), their
LSU, tef1-α, tub2, and
act gene sequences were employed. Phylogenetic analysis was performed to assess the relationships of these isolates within the genera
Neosetophoma,
Didymosphaeria,
Seimatosporium, and
Kalmusia (
Supplementary Table S2). Phylogenetic analysis was carried out for each respective genus using two or more genomic regions:
Seimatosporium (
rDNA-ITS, LSU, tef1-α, and
tub2),
Didymosphaeria (
rDNA-ITS, LSU, tub2),
Kalmusia, and
Neosetophoma (
rDNA-ITS, LSU). A combined dataset of aligned multi-locus sequences was constructed using "ClustalW," and evolutionary analyses was conducted in Geneious Prime® 2022.1.1 software by employing the Neighbor-Joining method (NJ) and Tamura-Nei model [
29]. The NJ consensus trees were based on 1000 bootstrap replications.
2.6. Correlation between Meteorological Data and GTDs Pathogens` Frequency
The potential associations of GTD-incited population structure with the prevailed meteorological conditions in different viticultural zones in Greece were investigated by inquiring and obtaining meteorological data over the last decade (2010-2020) in the regions of interest from the Hellenic National Meteorological Service. In detail, a Principle Component Analysis (PCA) was conducted to assess the correlation between the frequency of GTD pathogens and endophytic fungi isolated from each region and the average values of air temperature, relative humidity, and rainfall prevailed. PCA analysis was performed using GraphPad Prism Software Version 10.0.0 for Windows, Boston, Massachusetts, USA.
2.7. Pathogenicity Tests
In April 2022, a field trial was set up to confirm pathogenicity of four isolates, representative of Seimatosporium vitis (isolate SARO1.3AVR10), Didymosphaeria variabile (isolate SARO1.2AKO1), Kalmusia variispora (isolate HOURD2.1AVR1) and Neosetophoma italica (isolate SARO1.3AVR7), identified by morphological and molecular analyses. The former three species have not been identified previously as causal agents of GTDs in Greece; whereas the latter species has never been reported infecting grapevine worldwide. Isolates were used to inoculate 2-year-old canes of the vine cv. Soultanina grafted onto the 110 Richter rootstock. Pathogenicity trials were conducted in a 15-year-old vineyard located in Hellenic Mediterranean University, Crete, southern Greece. Only vines that remained visibly healthy for at least the last 3 years were included in the experiment.
Artificial inoculation was performed, according to Markakis et al. [
12,
13]. In brief, a 6.0-mm-diameter and 10.0-mm-length hole was opened into the cane using a Black & Decker EPC 12 drill, and one 5-mm diameter mycelial disc taken from a 2-week-old PDA culture was inserted into the hole. Then, the hole was sealed with cellophane membrane and covered with adhesive paper tape to protect the inoculum. In total, twenty canes (10 vines having had two canes each) were inoculated with each isolate. Additionally, a set of twenty canes (ten vines with two canes each) were similarly treated with sterilized PDA discs and served as controls. Treated canes remained under ambient conditions and were inspected periodically for foliar symptom development.
All the inoculated and control canes were collected in October 2022 (6 months post-inoculation), their leaves were removed, and longitudinal and transverse sections were performed to estimate the extension of wood tissue symptoms above and below the inoculation point. Five representative canes per inoculated fungus were randomly selected to verify the presence of the applied fungi in wood tissues, and pathogen re-isolations onto APDA were conducted as mentioned above.
2.8. Statistical Analysis
Data were checked for normality of distribution using the Kolmogorov-Smirnov and Shapiro-Wilk tests, and for homogeneity using the Levene’s test. Data on disease indices in the field surveys were analyzed using the Kruskal-Wallis (non-parametric) test, followed by Dunn’s posthoc multiple comparison test of average rankings (P ≤ 0.05). The percentage of vines and vineyards infected by individual fungal genera in three viticultural zones were analyzed using the Chi-square test by pair-wise comparisons (at P ≤ 0.05). In pathogenicity trials, analysis of variance (ANOVA) was employed to evaluate the pathogenic effects of the fungal strains on wood discoloration length in canes. When a significant F test was obtained for treatments (P ≤ 0.05), the data were subjected to means separation by Tukey’s HSD test. Standards errors of means were also calculated.
4. Discussion
In the last two decades, increased incidence of grapevine trunk diseases (GTDs) has been reported in numerous studies, and their detrimental effect on grapevine industry has raised awareness globally [
4,
7,
10]. In the present study, a three-year survey (from 2018 to 2020) was carried out to investigate the incidence and identify fungi associated with GTDs in Greece. In total, 310 vineyards in different geographical regions with variable pedoclimatic conditions in northern, central, and southern Greece were surveyed, and 533 fungal strains were isolated from diseased vines and identified.
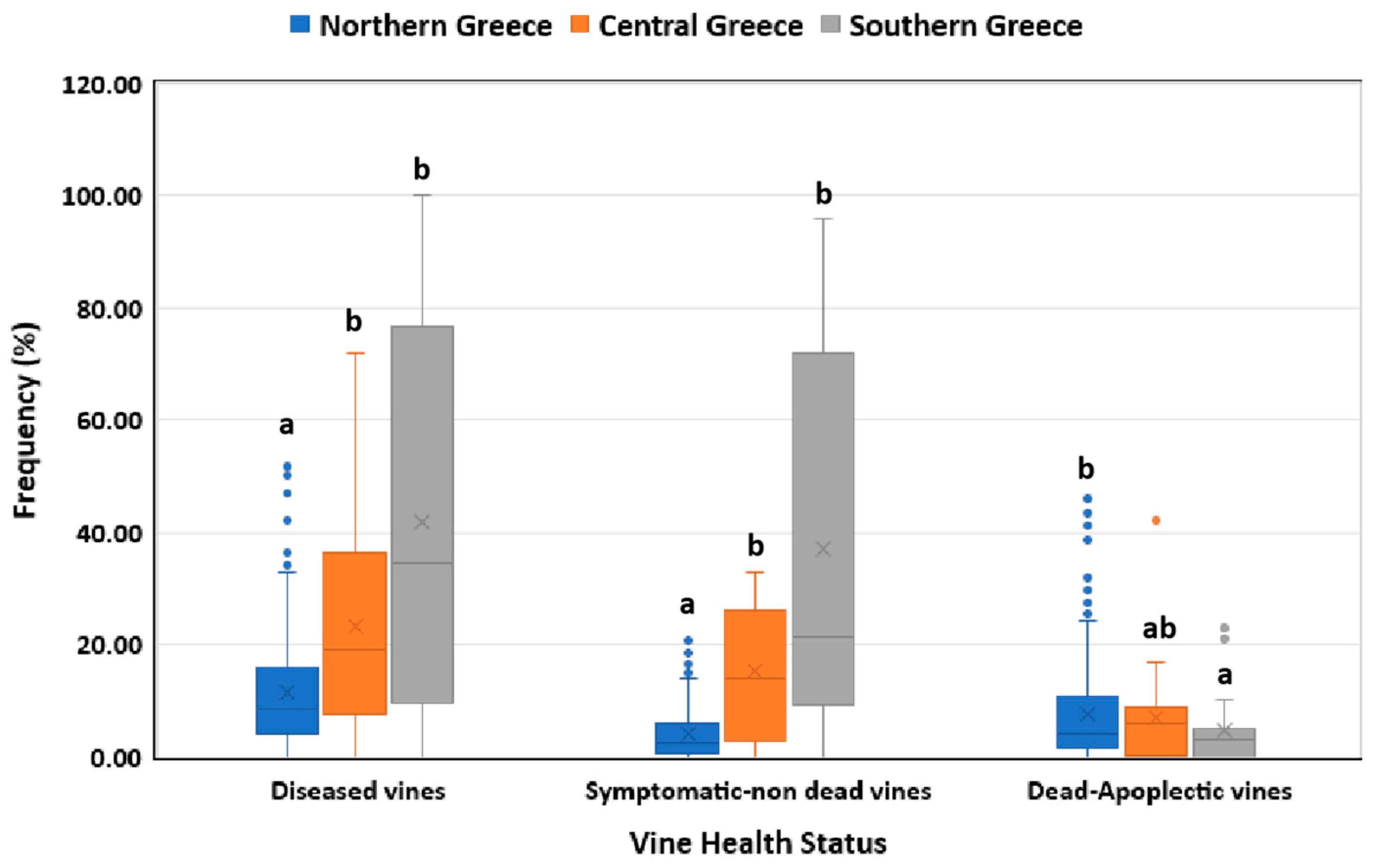
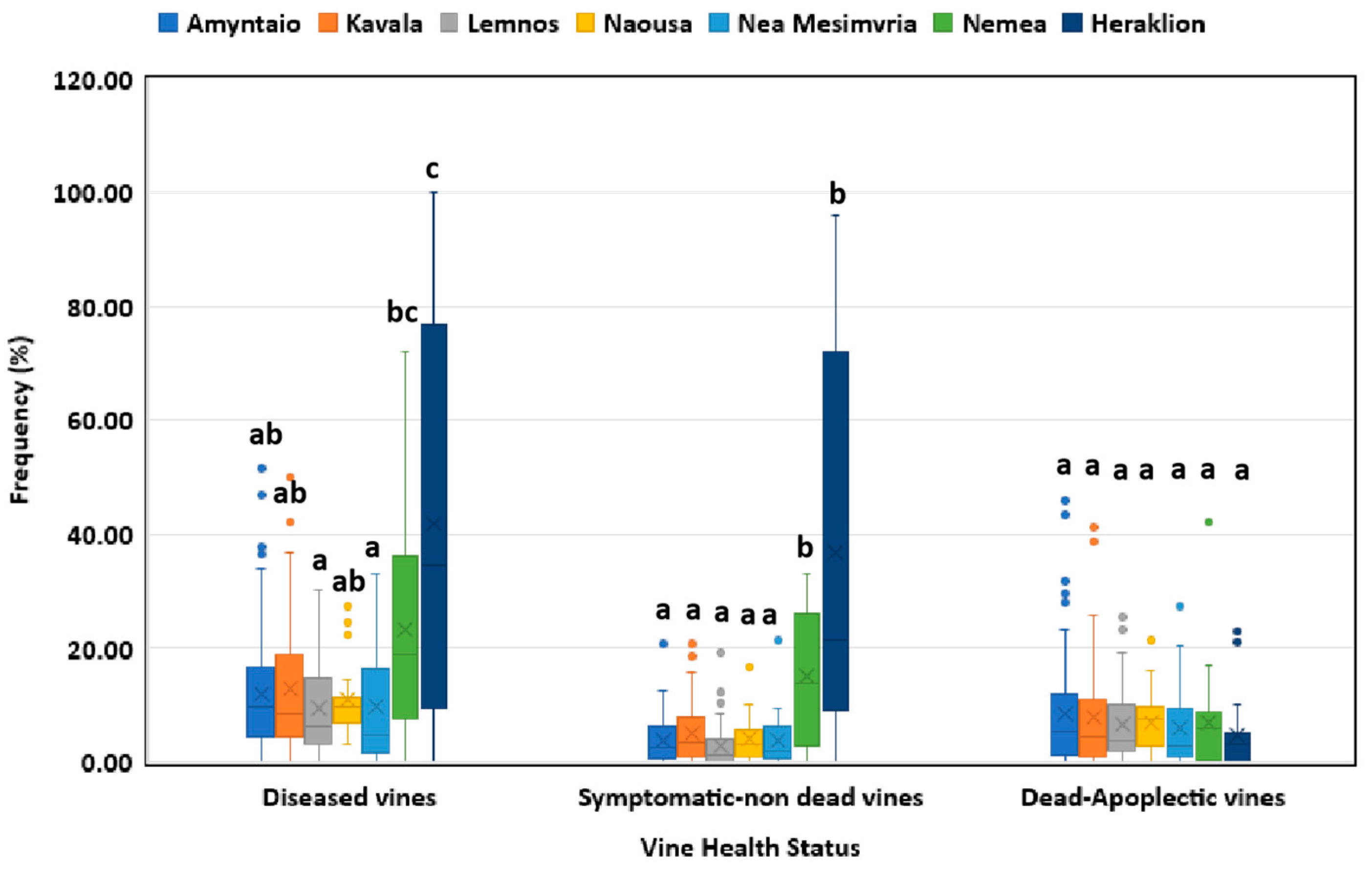
Measurements of GTDs incidence in geographically distinct regions in Greece, revealed higher frequency of symptomatic plants in vineyards in the warm and dry region of Heraklion (southern Greece) compared to those in cold and wet regions in northern Greece. Vineyards in central Greece (Nemea), characterized by moderate rainfall levels during the period of increased grapevine water requirements (in spring and summer months), indicated intermediate disease incidence. Differences in GTD symptom expression between vineyard regions can be attributed to variable climatic conditions [
30]. Although correlation between climatic or environmental conditions and GTD symptom severity is not always clear, the combined effect of heat and water stresses on grapevine has been speculated to favor initiation and progress of GTDs [
10,
11].
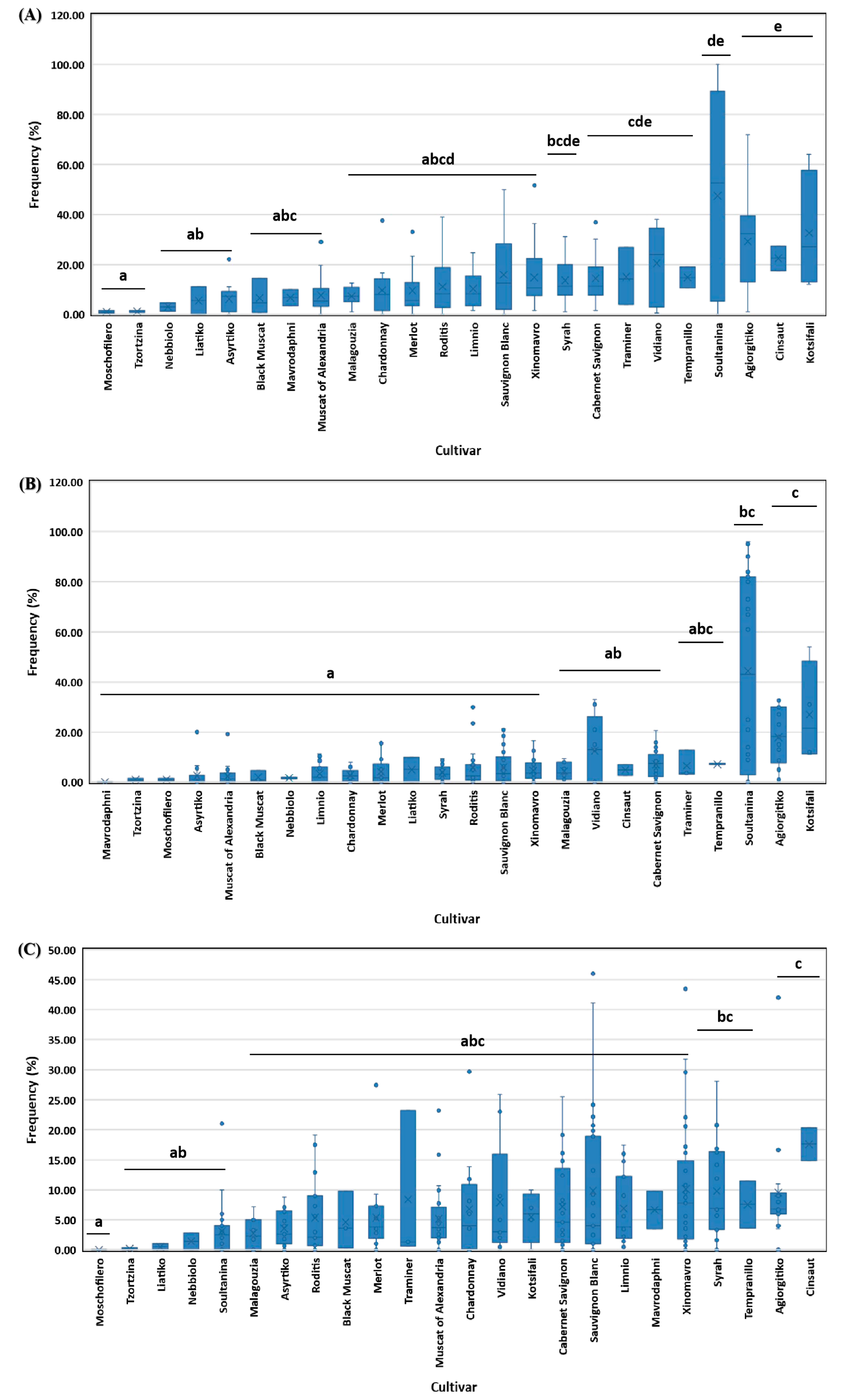
In addition, differences in the disease incidence between regions may be correlated with differences in the level of susceptibility to GTDs of the vine cultivars, grown in each region. Knowledge of susceptibility for widely used plant genotypes is essential for estimating disease risk and planning effective management strategies [
13]. The present study's data suggests that grapevine cultivars grown in Greece are differentially affected by GTDs, thus demonstrating variable resistance. Considerably increased disease incidence (in terms of diseased vines and symptomatic-non dead vines) was recorded on ‘Agiorgitiko’ grown primarily in central, and ‘Kotsifali’ and ‘Soultanina’ grown primarily in southern Greece. Apart from Kotsifali, these cultivars have been mentioned among the most susceptible to various GTD-pathogens in several studies as defined by artificial inoculation experiments and field observations [
31]. On the other hand, recent unpublished data of our group suggest that cultivars, such as Xinomavro or Limnio, which are among the most commonly used cultivars in northern Greece are highly resistant to GTDs (Testempasis, Unpublished data) and this could explain the lower disease incidence measured in northern Greece.
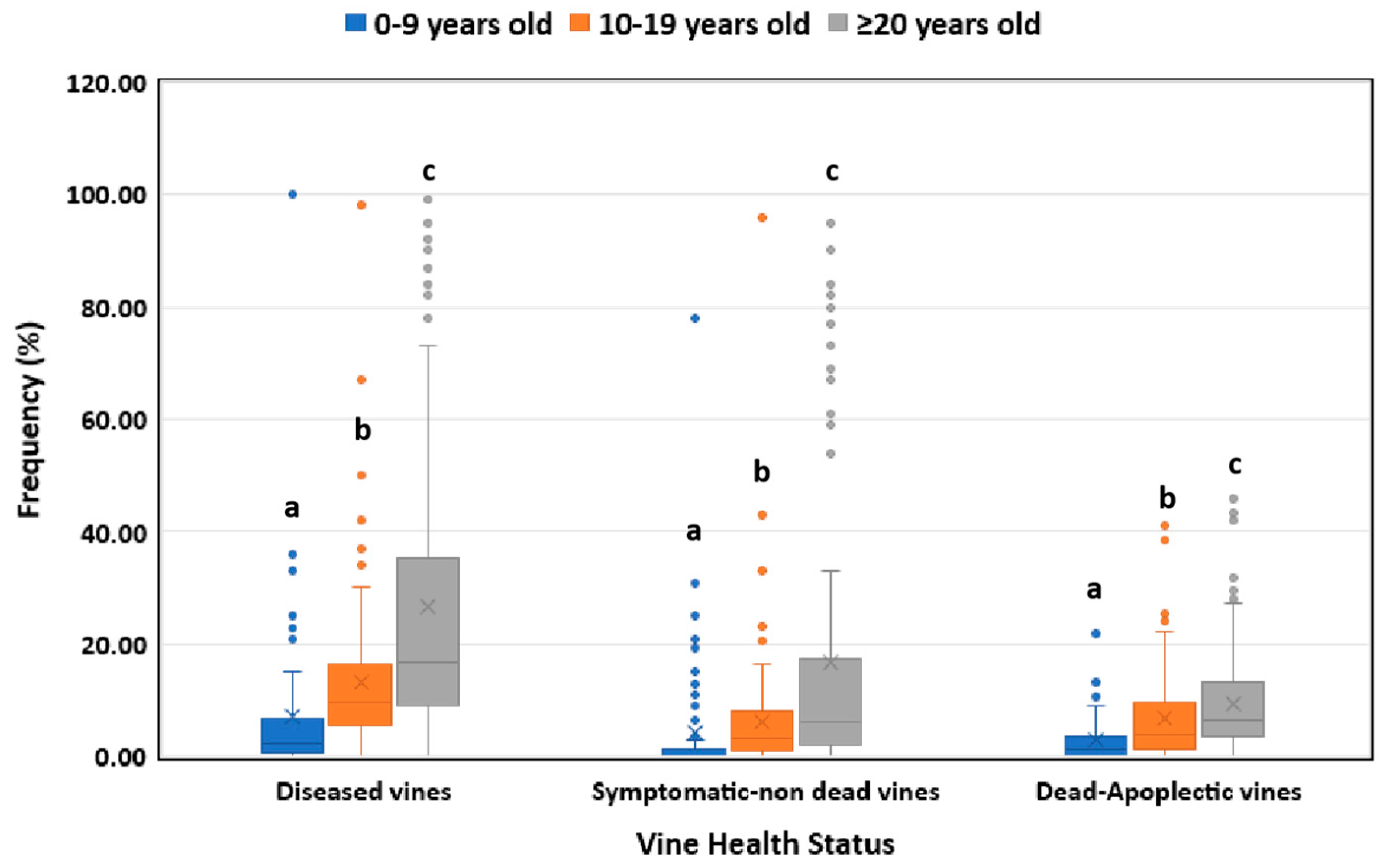
The occurrence of GTDs exhibits a positive correlation with the age of vines, displaying an exponential-related pattern. Our research findings indicate a heightened prevalence of GTDs in older vines (≥20 years old), followed by intermediate and lower incidence rates in middle-aged (10-19 years old) and younger (0-9 years old) vines, respectively. These outcomes align with earlier studies that observed increased frequencies of diseased vines in middle-aged and older categories compared to younger ones [
32,
33]. The variations in disease incidence across age groups were, to some extent, linked to the frequencies of GTD-inducing fungal genera isolated from each vine age category. Notably, the majority of GTD-inciting fungi were predominantly harbored in middle-aged and elder vines. Previous research has similarly documented a correlation between vine's age and the presence of specific GTDs, including White rot, Botryosphaeria and Eutypa dieback [
7,
34,
35].
Climatic conditions, including heat and water stress, influence the prevalence and distribution of GTDs fungi [
11]. Indeed, in the case of geographically distinct viticultural regions studied here, the population structure of GTD-related fungi differed significantly. Indicatively, the most predominant genera/species in regions of southern Greece (e.g.
P. chlamydospora and
Diaporthe spp.) were completely absent or found at very low frequencies in northern and/or central Greece and vice versa. These findings are strongly supported by a recent grapevine wood microbiome study conducted in Greece [
14] pointing out that certain GTD-associated pathogens like
P. chlamydospora and
Diaporthe spp. were consistently present in cultivar ‘Vidiano’ collected from vineyards in southern (Heraklion, Crete) but not in ‘Xinomavro’ collected from vineyards in northern Greece (Amyntaio). On the contrary,
Diplodia spp. (mostly
D. seriata) were the most frequently isolated species in northern and central Greece but considerably less frequent in southern Greece. Furthermore,
Lasiodiplodia spp. (mostly
L. viticola and
L. theobromae) were found in northern Greece, exclusively. Likewise, Hernandez and Alchala [
32], reported higher abundancies of Botryosphaeria dieback pathogens in the northern regions of Oregon compared to the southern ones. Additionally, the elevated incidence of Botryosphaeria dieback complex pathogens in the northern regions of Greece might be associated with the prevalence of these pathogens in temperate regions or those with cold winters [
36].
Out of 35 fungal genera identified in total, 25 were found in the warm and dry region in southern Greece, whereas 15 and 19 out of 35 genera were found in the comparatively cooler regions in northern and central Greece, respectively. Amongst them, 18 fungal genera are well-known (e.g.
Botryosphaeria dothidea,
Diaporthe spp.,
Diplodia seriata,
Eutypa sp.,
Fomitiporia mediterranea,
Phaeoacremonium spp.,
Phaeomoniella clamydospora) or lesser-known (i.e.
Fusarium sp.,
Seimatosporium sp.,
Didymosphaeria sp.,
Kalmusia sp.) GTDs-inducing agents [
7,
16,
19,
20,
21]. The rest 17 determined genera include wood canker agents or saprophytes (e.g.
Alternaria sp.,
Aspergillus sp.,
Penicillium sp.,
Rhizopus sp.), endophytic symbionts with potential biocontrol activity (e.g.
Clonostachys sp.,
Epicoccum sp.,
Trichoderma sp.), or genera that have not been reported previously as wood tissue colonizers (e.g.
Lopadostroma sp.,
Monocillium sp.,
Neosetophoma sp.) [14, 37-41]. The increased number of fungal genera originated from GTD-affected vines in southern Greece could be partially attributed to the thermophilic nature (within a certain limit) of most GTD-associated fungi [
11]. Besides, the higher number of samples collected and analyzed from southern Greece may also play a role in the increased fungal diversity identified there.
Sequencing of
rDNA-ITS,
LSU,
tef1-α,
tub2, and
act genes of selected isolates, coupled with pathogenicity tests, revealed that
N.
italica,
S.
vitis,
D.
variabile and
K.
variispora caused wood infection on grapevine canes, with the former species being the most virulent. Although these fungi were identified (at the genus level) in a grapevine wood microbiome study conducted recently, their pathogenic potential was not examined [
14]. Therefore, this is the first record of
K. variispora,
S. vitis and
D. variabile associated with GTDs in Greece.
Seimatosporium vitis has never been isolated in Greece previously, whereas
K. variispora and
D. variabile have been reported as causal agents of fruit rot on apples [
42] and leaf spot on
Phoenix theophrasti [
43], respectively. Τhis is also the first report of
N. italica associated with GTDs worldwide.
Neosetophoma italica was first found as saprobic on dead leaves of
Iris germanica L. in Italy and typified in 2015 [
44]. Its close relative,
N. samararum has been reported as a pathogen causing leaf spots of various hosts [
45], but up to now,
N. italica has never been shown to infect plant hosts. The increased virulence of
N. italica revealed here, may suggest the predominant role of this species in GTD-associated microbiome, and should be taken into consideration in future surveys in Greece and elsewhere.
Given all the above, the data from the present study suggest an overall increased incidence of GTDs in Greece. Differences in disease incidence and population structure of the GTD-associated microbiome were observed among the discrete viticultural zones. The increased incidence of GTDs and the high variability of fungal genera implicated with the disease complex, recorded in the driest and warmest region of Heraklion (southern Greece) may portend even more ominous scenarios for viticulture sustainability globally, against climate change. Apart from the different climatological conditions, these variances may be reasonably attributed to the differential susceptibility level of grapevine genotypes cultivated in geographically distinct viticultural regions. Herein, we record for the first time K. variispora, S. vitis and D. variabile as GTD-associated pathogens in Greece, and N. italica as GTDs-inducing pathogen worldwide. This highly virulent species may have a predominant role in GTDs complex, and its biological cycle and epidemiology should be further investigated. Regional-scale mapping conducted here will provide valuable support in planning and implementing effective management practices targeting specific diseases.
Author Contributions
Conceptualization, E.A.M, G.S.K., E.P., A.K.T.; methodology, E.A.M, G.S.K, E.P, S.I.T, D.G, A.K.T.; software, E.A.M., S.I.T; validation, E.A.M., G.S.K., A.K.T., E.P., S.I.T.; investigation, S.I.T., E.A.M., G.I.T., S.K.S., C.T., D.G., A.K.T., E.P., G.S.K.; resources, E.A.M., S.I.T., G.S.K.; writing—original draft preparation, E.A.M., S.I.T.; writing—review and editing, E.A.M, S.I.T., G.S.K., E.P., A.K.T., D.G.; visualization, E.A.M., S.I.T; supervision, E.A.M, G.S.K., E.P., A.K.T.
Figure 1.
Grapevine Trunk Diseases incidence (symptomatic-non dead and dead-apoplectic vines) in vineyards located in northern, central and southern Greece. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Within each group of plants, columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 1.
Grapevine Trunk Diseases incidence (symptomatic-non dead and dead-apoplectic vines) in vineyards located in northern, central and southern Greece. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Within each group of plants, columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 2.
Grapevine Trunk Diseases incidence (symptomatic-non dead and dead-apoplectic vines) for young (0-9 years old), middle-aged (10-19) and elder (≥20 years old) vineyards. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Within each group of plants, columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 2.
Grapevine Trunk Diseases incidence (symptomatic-non dead and dead-apoplectic vines) for young (0-9 years old), middle-aged (10-19) and elder (≥20 years old) vineyards. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Within each group of plants, columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 3.
Grapevine Trunk Diseases incidence (diseased, symptomatic-non dead and dead/apoplectic vines) for vineyards located in different regions in Greece. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Within each group of plants, columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 3.
Grapevine Trunk Diseases incidence (diseased, symptomatic-non dead and dead/apoplectic vines) for vineyards located in different regions in Greece. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Within each group of plants, columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 4.
Frequency (%) of diseased (A), symptomatic-non dead (B) and dead/apoplectic (C) vines on 24 grapevine cultivars grown in Greece. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
Figure 4.
Frequency (%) of diseased (A), symptomatic-non dead (B) and dead/apoplectic (C) vines on 24 grapevine cultivars grown in Greece. Within the box, the median and the mean are represented by the solid line and the ‘X’, respectively. Top and bottom lines of the box correspond to the 25th and 75th percentiles of the data, respectively. Error bars represent the 10th and 90th percentiles. Columns with the same letter do not differ significantly according to Kruskal-Wallis and the Dunn’s test for multiple comparisons (p ≤ 0.05).
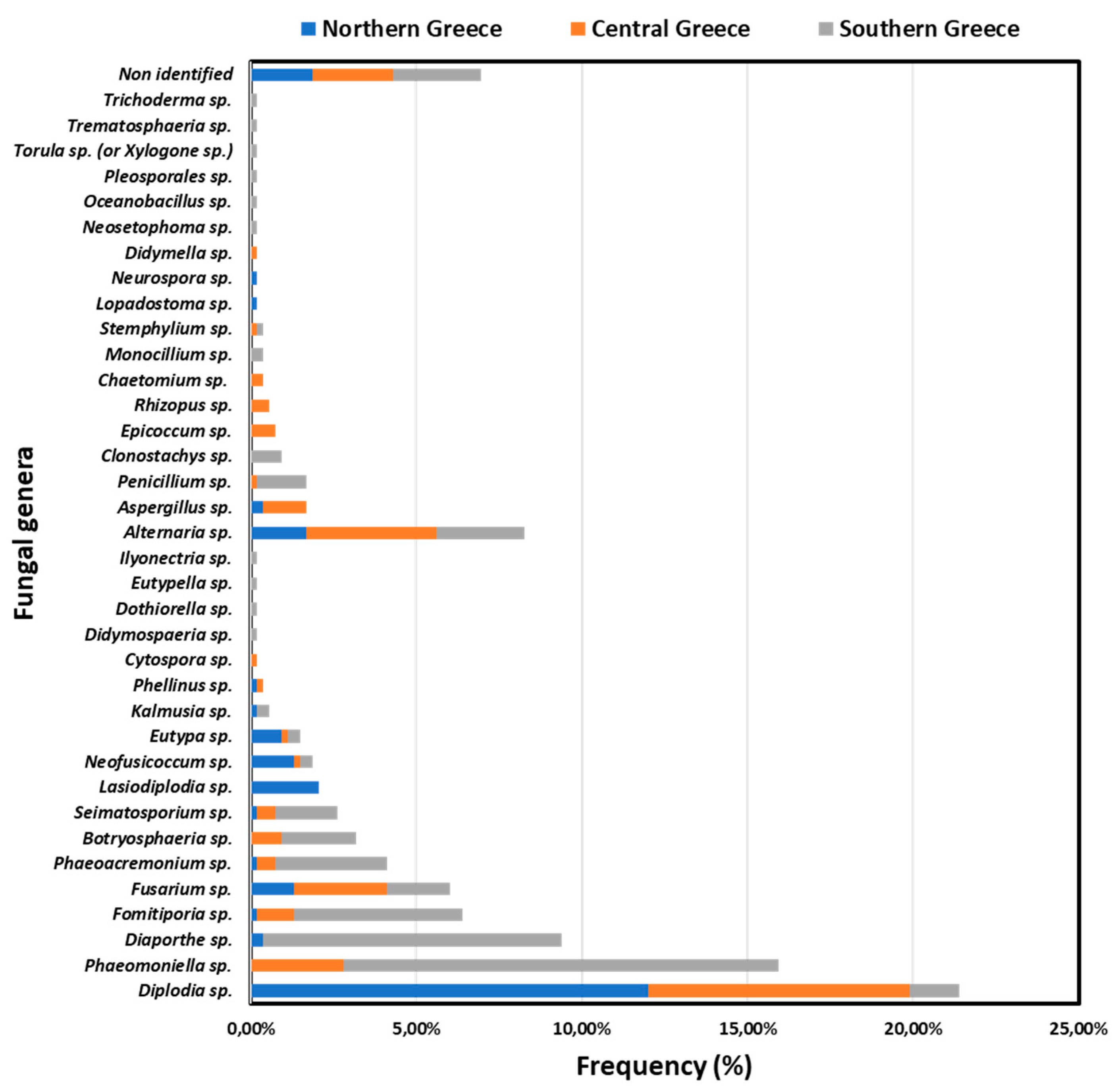
Figure 5.
Frequencies of isolates belonging to discrete fungal genera in northern (Amyntaio and Kavala), central (Nemea) and southern (Heraklion) Greece.
Figure 5.
Frequencies of isolates belonging to discrete fungal genera in northern (Amyntaio and Kavala), central (Nemea) and southern (Heraklion) Greece.
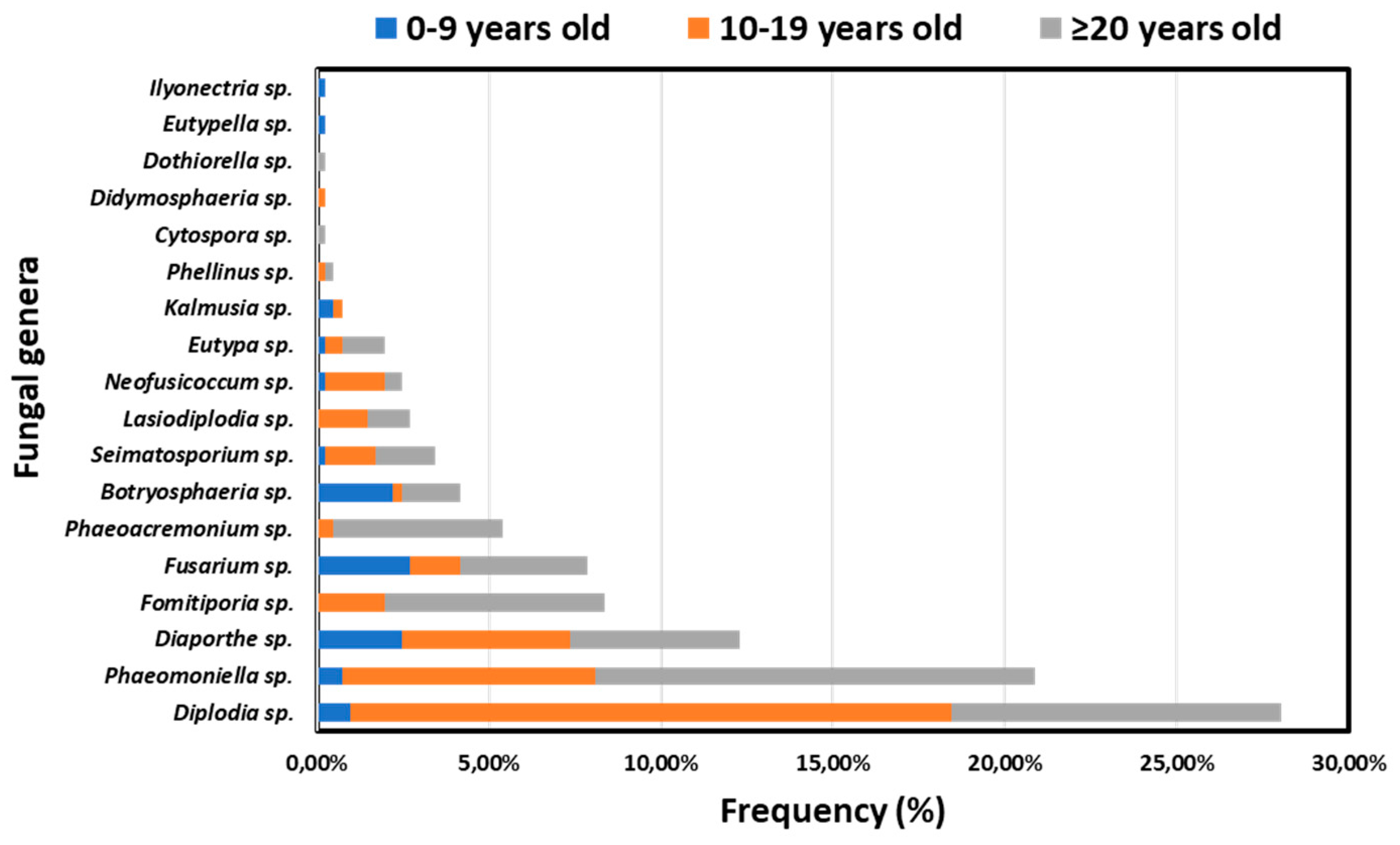
Figure 6.
Frequencies of isolates belonging to 18 discrete fungal genera associated with grapevine trunk diseases, originated from young (0-9 years old), middle-aged (10-19) and elder (≥20 years old) vineyards in Greece.
Figure 6.
Frequencies of isolates belonging to 18 discrete fungal genera associated with grapevine trunk diseases, originated from young (0-9 years old), middle-aged (10-19) and elder (≥20 years old) vineyards in Greece.
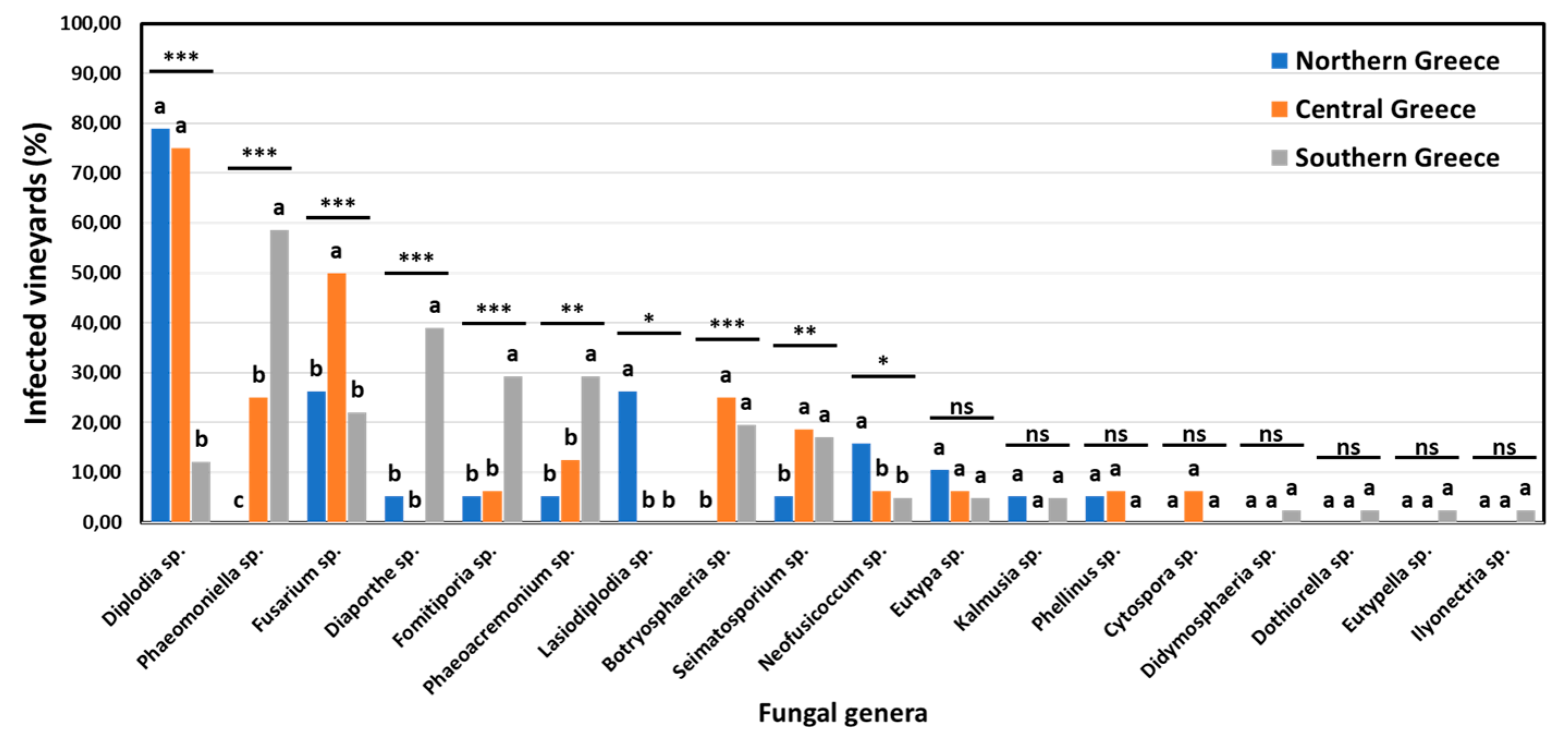
Figure 7.
Percentage of vineyards infested by each fungal genera in northern (Amyntaio and Kavala), central (Nemea) and southern (Heraklion) Greece. Within each genus, columns with the same letter do not differ significantly according to Chi-square test and a pair-wise comparison, whereas asterisks (*, ** and ***) indicate significance at p ≤ 0.05 , 0.01 and 0.001, respectively.
Figure 7.
Percentage of vineyards infested by each fungal genera in northern (Amyntaio and Kavala), central (Nemea) and southern (Heraklion) Greece. Within each genus, columns with the same letter do not differ significantly according to Chi-square test and a pair-wise comparison, whereas asterisks (*, ** and ***) indicate significance at p ≤ 0.05 , 0.01 and 0.001, respectively.
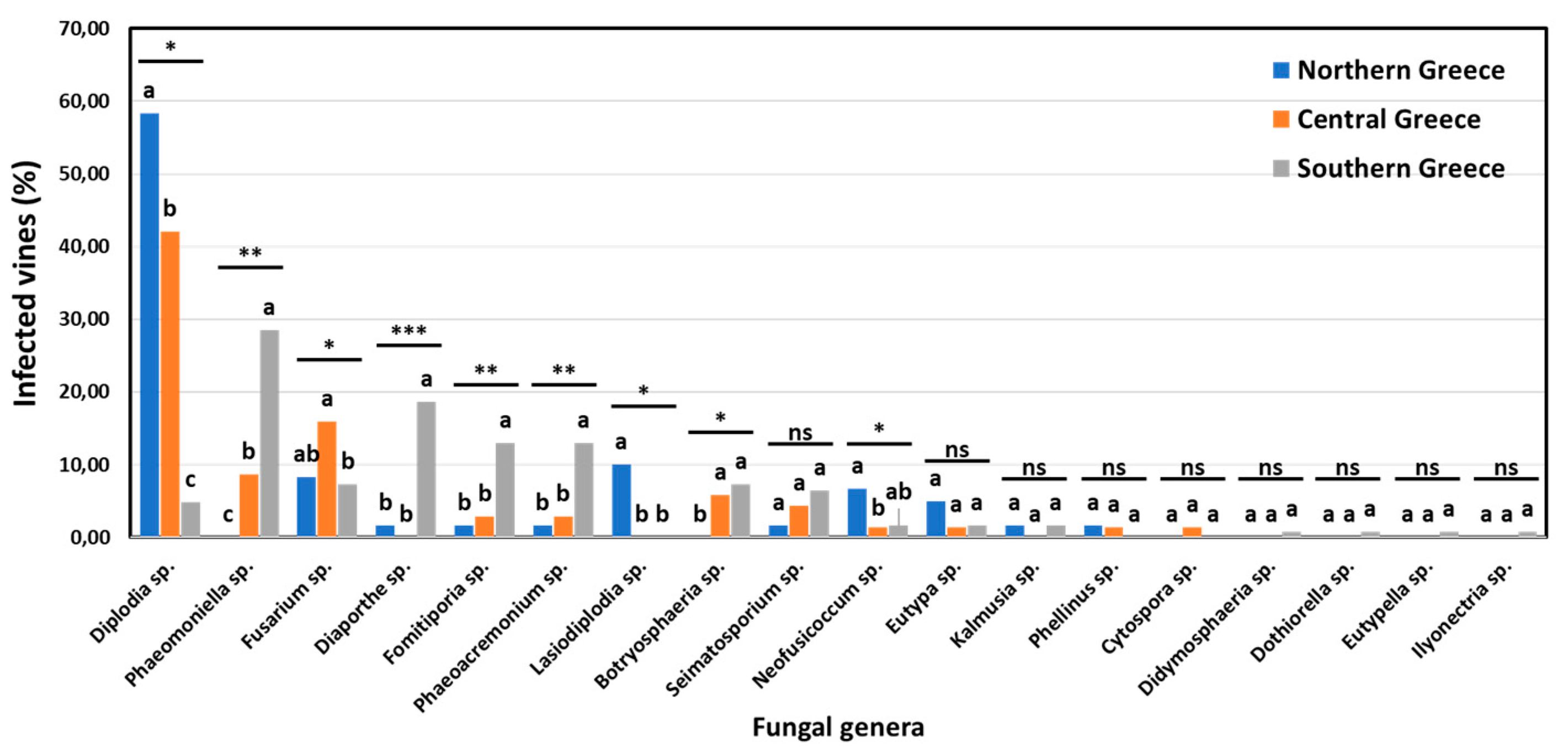
Figure 8.
Percentage of vines infested by each fungal genera in northern (Amyntaio and Kavala), central (Nemea) and southern (Heraklion) Greece. Within each genus, columns with the same letter do not differ significantly according to Chi-square test and a pair-wise comparison, whereas asterisks (*, ** and ***) indicate significance at P ≤ 0.05, 0.01 and 0.001, respectively.
Figure 8.
Percentage of vines infested by each fungal genera in northern (Amyntaio and Kavala), central (Nemea) and southern (Heraklion) Greece. Within each genus, columns with the same letter do not differ significantly according to Chi-square test and a pair-wise comparison, whereas asterisks (*, ** and ***) indicate significance at P ≤ 0.05, 0.01 and 0.001, respectively.
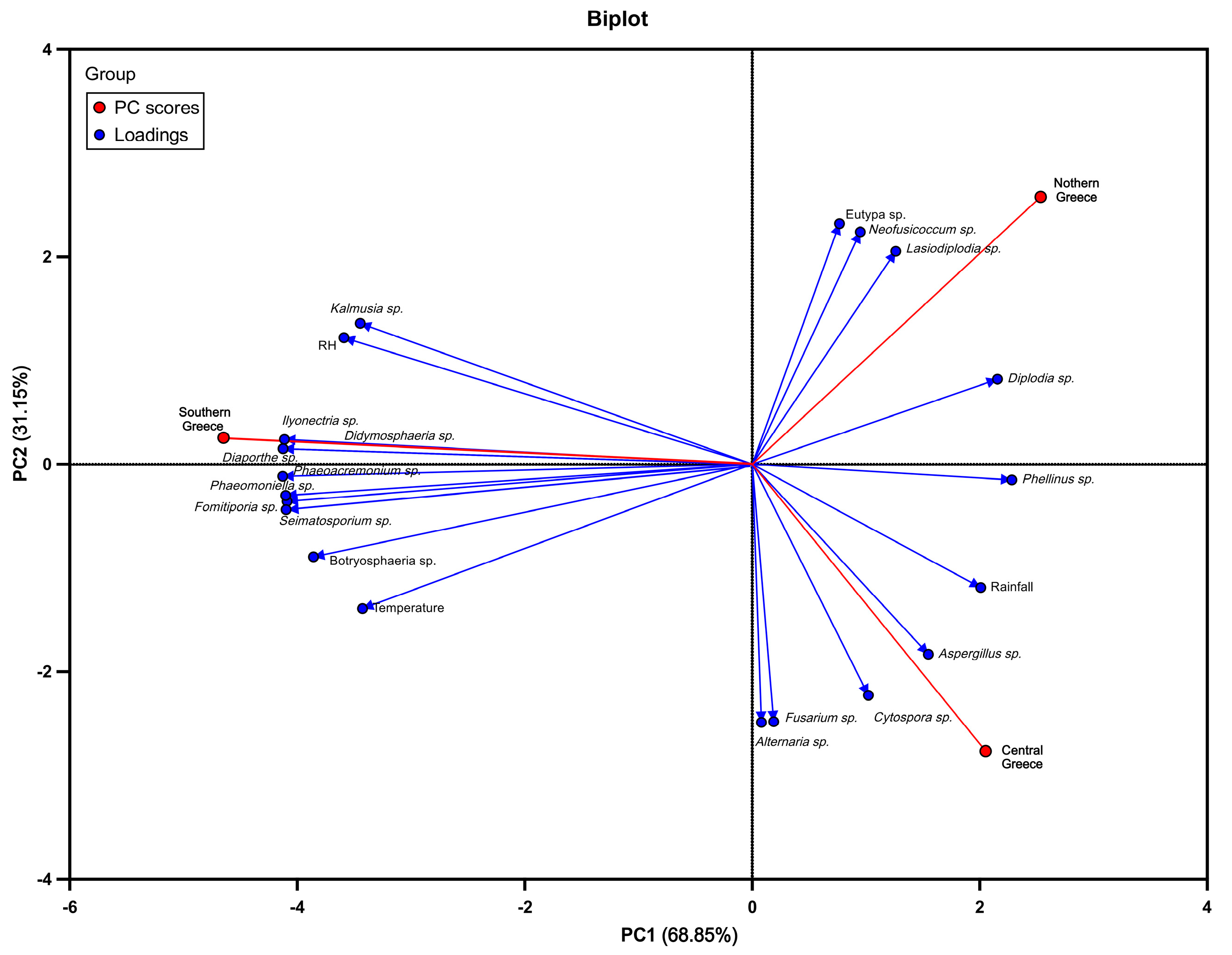
Figure 9.
Principal component analysis (PCA) biplot illustrating the correlation between meteorological data (air temperature, RH, and rainfall) from past decades (2010-2020) and the identified fungal genera isolated from symptomatic grapevines in the viticultural zones of southern, central, and northern Greece.
Figure 9.
Principal component analysis (PCA) biplot illustrating the correlation between meteorological data (air temperature, RH, and rainfall) from past decades (2010-2020) and the identified fungal genera isolated from symptomatic grapevines in the viticultural zones of southern, central, and northern Greece.
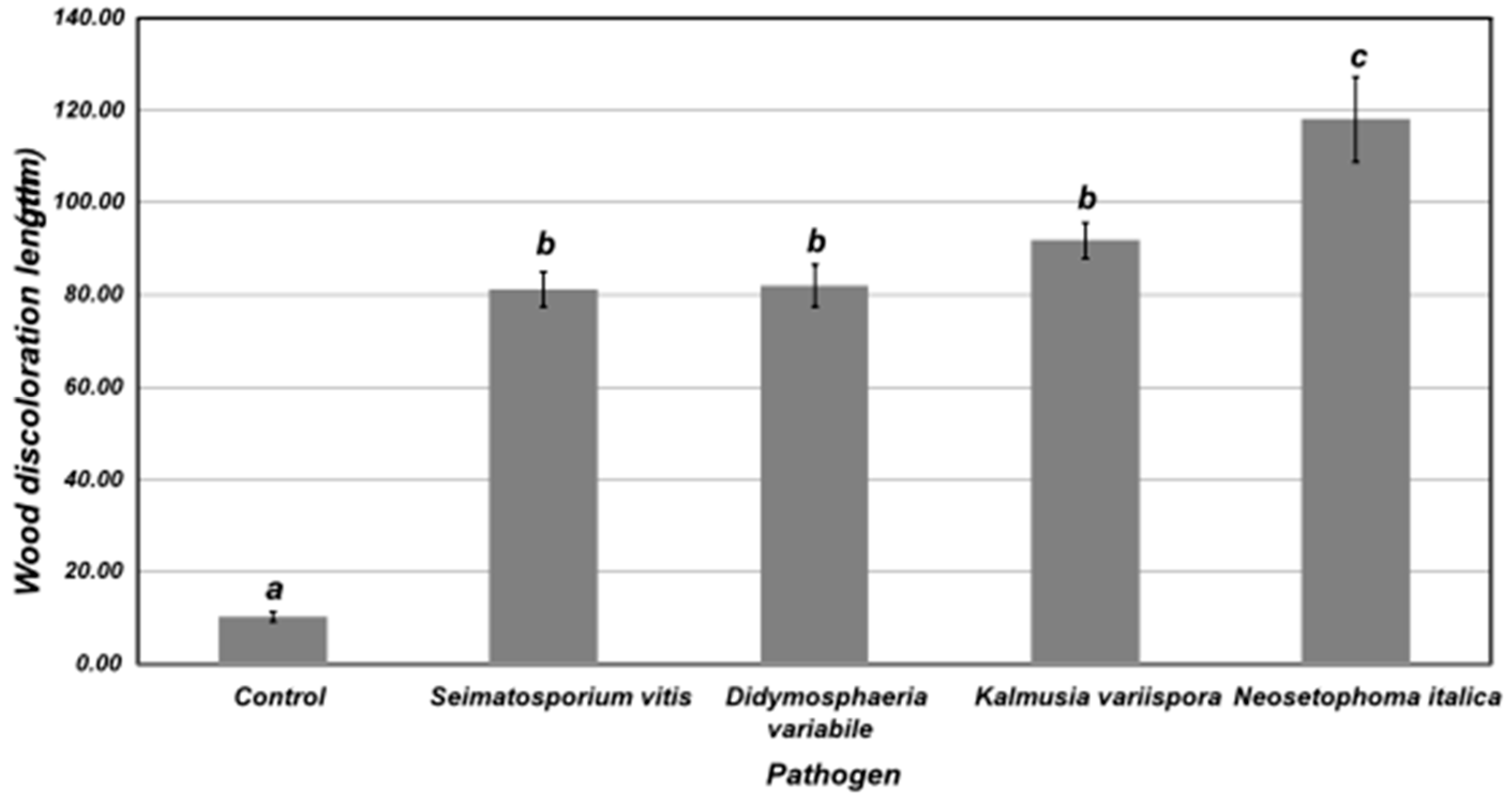
Figure 10.
Length of vascular tissue discoloration in 2-year-old canes of cv. Soultanina, 6 months after artificial inoculation with Seimatosporium vitis (isolate SARO1.3AVR10), Didymosphaeria variabile (isolate SARO1.2AKO1), Kalmusia variispora (isolate HOURD2.1AVR1) and Neosetophoma italica (isolate SARO1.3AVR7). Control canes (C-) were mock-inoculated with sterilized PDA discs. Columns followed by the same letter do not differ significantly according to Tukey honestly significant difference test (P ≤ 0.05). Each column represents the mean of 20 canes and vertical bars indicate standard errors.
Figure 10.
Length of vascular tissue discoloration in 2-year-old canes of cv. Soultanina, 6 months after artificial inoculation with Seimatosporium vitis (isolate SARO1.3AVR10), Didymosphaeria variabile (isolate SARO1.2AKO1), Kalmusia variispora (isolate HOURD2.1AVR1) and Neosetophoma italica (isolate SARO1.3AVR7). Control canes (C-) were mock-inoculated with sterilized PDA discs. Columns followed by the same letter do not differ significantly according to Tukey honestly significant difference test (P ≤ 0.05). Each column represents the mean of 20 canes and vertical bars indicate standard errors.
Table 1.
Numerical distribution of vineyards located in seven (7) important grapevine-growing regions in three geographically distinct viticultural zones (northern, central and southern Greece) falling into three (3) age-scales, surveyed for the assessment of GTD disease parameters.
Table 1.
Numerical distribution of vineyards located in seven (7) important grapevine-growing regions in three geographically distinct viticultural zones (northern, central and southern Greece) falling into three (3) age-scales, surveyed for the assessment of GTD disease parameters.
| Viticultural zone |
Region |
Number of vineyards |
Vineyard age (years) |
| |
|
<10 |
10-19 |
≥20 |
| Northern Greece |
Amyntaio |
78 |
10 |
39 |
29 |
| Kavala |
60 |
8 |
27 |
25 |
| Lemnos |
51 |
21 |
14 |
16 |
| Naousa |
20 |
0 |
17 |
3 |
| Nea Mesimvria |
25 |
9 |
7 |
9 |
| Central Greece |
Nemea |
28 |
3 |
8 |
17 |
| Southern Greece |
Heraklion |
48 |
19 |
4 |
25 |
| Total |
|
310 |
70 |
116 |
124 |