1. Introduction
Cellulose, whose structure consists of β-1,4-linked anhydro-D-glucose units, is a naturally occurring polymer in plants and the most abundant source of lignocellulosic biomass, [
1,
2]. This biopolymer is a valuable resource for many types of applications, due to its low cost, high abundance, great biocompatibility, biodegradability, and non-toxic nature [
1,
3]. Thus, many efforts have been made to develop efficient extraction methodologies to obtain cellulose to be further transformed in high-value products, such as viscose rayon, cellulose nitrate, cellulose acetate and nanocrystalline cellulose (reviewed in [
4]). However, the extraction of this polymer from raw materials with a high degree of purity, represents a big challenge due to the presence of other major components in the biomass, mainly lignin [
5] and hemicellulose [
6]. The lignin content depends on the natural source but ranges from approximately 10 to 25% [
7] while hemicellulose is present within the interval 20-40% [
8].
Several chemical and thermal methods have been reported for the removal of these components, such as organo-solvent extraction or enzymatic hydrolysis. Even though some of these methods are easy and low-cost options, they are time-consuming, have poor extraction yield, and use a great volume of solvent, most of them from non-benign nature, as well as high temperature and pressure conditions, as referred in [
2,
3,
4].
Microwave-assisted extraction (MAE) methods have been explored in the last decades as a way to develop sustainable, faster and more efficient approaches for cellulose extraction [
3]. The use of microwave radiation in the heating process offers many advantages in extraction processes, which include lower reaction time, the use of high dielectric constant / polar solvents to improve energy absorption, and lower solvent consumption [
9,
10]. Particularly for plant subtracts, MAE procedures can improve cell wall disruption, thus allowing a better extraction of the components, such as lignin and hemicellulose, [
2].
The most rudimentary studies were performed considering the extraction of cellulose by using simple domestic microwave equipment, eventually in combination with conventional heating methods [
3,
11]. Some other investigations employed more advanced laboratorial devices in which the developed MAE methodologies for the cellulose extraction considered single vessel (monomode) microwave equipment, usually using close vessels to increase the range of temperature and pressure applied. From these approaches, some were exclusively performed using microwave radiation [
12] or in combination with traditional heating methods [
13,
14].
The use of monomode equipment can limit the scalability of the process due to reduced volume of solvents that can be used (mL scale), in comparison to the alternative multimode apparatus that offer the possibility of using volumes up to few litres [
15]. Considering this, some studies have been reported such as the work developed by Kusumattaqiin and Chonkaew [
16] in which the authors proposed a method for cellulose extraction from cotton wool using MAE technique done in a multimode equipment. Other reports described similar approaches considering different cellulose sources and in combination with conventional heating methods [
2] or other techniques such as ultrasound [
17].
In addition to various types of extraction methods, also different cellulose sources can be considered mainly comprising plants or algae feedstocks [
1,
2,
13]. Many studies focused on sustainable methods to the valorisation of plants, particularly from fruits [
12], or by-products and waste generated from fruit processing [
3,
18]. Amongst other sources, wood waste from trees has been largely explored as a valuable source for the extraction of this bio-polymer, as evidenced by many reports in the literature [
5,
6,
19,
20,
21]. From the large variety of wood that can be used for cellulose extraction, various studies have reported eucalyptus and pine trees as interesting biomass feedstocks [
22,
23,
24]. Some of the referred studies include MAE techniques to obtain the desired polymer, as the work developed by Hou et al. [
4] in which eucalyptus wood was considered as a natural source for cellulose extraction and MAE as part of the process. In another study, pine and eucalyptus wood were selected as cellulose sources and the proposed extraction process involved the use of microwave heating methods together with the use of ionic liquids to improve the efficacy of the extraction process [
21]. However, this methodology includes a less sustainable purification process since the authors considered the use of non-environmentally friendly solvents, namely dimethylsulfoxide (DMSO).
The use of such type of cellulose sources also contributes to a better use of wood feedstocks usually considered as waste.
Eucalyptus and pine tree wood samples were selected as a lignocellulosic source, since they have a high cellulose content and are present in great abundance in Portugal. Pine and eucalyptus tree wood differs in nature and composition since the first is considered as a softwood [
19] and eucalyptus classified as hardwood [
25]. This aspect can be relevant for the efficiency of the extraction process since it is reported that softwood is harder to be delignified than hardwood in general [
26,
27].
Thus, the main aim of the present work consists in the evaluation of the applicability of the new developed approach to extract cellulose from eucalyptus and pine tree wood samples. Additionally, it is also an objective of this work to verify if the protocol is valid for the different categories of wood (soft and hard) considered herein.
To accomplish these objectives, a comparison of the extracts obtained in each step of the methodology have been performed, considering structural and thermal analysis of the samples from both sources, and comparing them with commercially available microcrystalline cellulose and an industrial paper pulp.
The herein proposed extraction protocol is one of the very few considering soft and hard wood using mild extraction conditions and benign purification solvents. Moreover, the methodology exclusively using MAE technique in a multimode apparatus, thus offering a great advantage of a future scale up study to potentially apply the develop method to the extraction of cellulose in industrial scale.
2. Materials and Methods
2.1. Materials and Instrumentation
NaOH, H2O2 (30% w/w, Perdrogen), H2SO4, and 1-Ethyl-3-methylimidazolium chloride were acquired from Sigma-Aldrich (St. Louis, MO, USA). Wood samples (small branches from eucalyptus and pine tree) were collected from urban gardens in the Porto region (Gondomar, Portugal). Industrial paper pulp was gently given by a paper company.
Extractions have been processed using a multi-vessels MARS 6™ microwave-heating equipment (CEM Corporation, Matthews, NC, Canada), using glass containers. Fourier Transform Infrared Spectroscopy (FTIR) spectra were recorded in a PerkinElmer Frontier (Beaconsfield, UK) equipped with an attenuated total reflectance (ATR) accessory with a zinc selenite crystal. The ATR accessory as a pressure arm to control the applied force and reduce sample-to-sample variability. Thermogravimetric analysis (TGA) was performed at FCUP|DQB - Lab&Services, using a Hitachi STA7200RV equipment (Tokyo, Japan). Differential Scanning Calorimetry (DSC) studies were performed in a DSC 200 F3 Maia equipment with an automatic sample changer (Netzsch, Germany) and Powder X-ray Diffraction studies were performed at UniNova, Nova School of Science and Technology, Lisbon, Portugal, using a Malvern Panalytical X'Pert PRO MPD equipment (Malvern, United Kingdom).
2.2. Wood Samples Pre-Treatment
Wood samples (
Figure 1 A) were cut in small pieces of approximately 0.5 cm length and then washed in deionised water and subjected to an ultrasonic bath for 1 hour to remove soil and other residues (
Figure 1 B). After the wash pre-treatment, samples were let to dry (
Figure 1 C) in a hoven at 50 °C, overnight. The dried wood samples were then milled in an Ultra Centrifugal Mill ZM 200 processor, operating at 8000 rpm (Retsch, Germany). Pulverized samples (
Figure 1 D) were then considered for the alkaline treatment.
2.3. Alkaline Treatment
Pulverized eucalyptus and pine wood samples (1 g) were firstly treated in an alkaline aqueous solution of NaOH (10 mL, 2% w/v) and the reaction was carried out during 1 hour, at 120 °C with a maximum power of 600 W in the multimode microwave equipment (glass containers) [
14]. In the end of reaction, samples were filtrated where the solid part was recovered and the supernatant, the black liquor, rejected. The solid was washed with water and ethanol until the remaining solution become clean with a neutral pH. Samples were then dried overnight in a drying vacuum pump at 65 °C and 35 mBar and stored in a desiccator at room temperature, until needed.
2.4. Bleaching I
To perform the first step of the bleaching treatment, 250 mg of each sample from the alkaline treatment were mixed with 10 mL of the bleaching agent (H
2O
2, 30% w/w) [
2]. The reaction mixture was subjected to microwave radiation at 50 °C for 1 hour using a maximum power of 600 W. In the end of the reaction, the resultant solid material was filtered under vacuum and the material washed four times with water and ethanol. The product was then dried and stored as described for the alkaline step of reaction.
2.5. Bleaching II
The second step of the bleaching process consisted in the reaction of 200 mg of each sample from bleaching I with 10 mL of an aqueous solution of H
2SO
4 (1 M) and the ionic liquid, 1-Ethyl-3-methylimidazolium chloride, (1 M, 10% v/v ) [
13]. The reaction was placed in the glass vessel for the microwave reaction which occurred at 95 °C, during 1 hour with a maximum power of 600 W. After, the products were washed as described for the previous steps and dried in similar conditions.
2.6. Characterization of the Extracted Samples
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of the wood samples subjected to the different chemical treatments were obtained placing the samples directly in the ATR accessory. Spectra were recorded between 4000 cm−1 and 600 cm−1 with a resolution of 4 cm−1 and 16 co-added scans. Background was obtained with the ATR accessory empty. Microcrystalline cellulose, industrial paper pulp, and the starting materials from both eucalyptus and pine tree wood were also analysed as controls.
For the PCA analysis the FTIR spectra was pre-processed with standard normal variate (SNV) and mean centered (MNCN). PCA was performed using the software Matlab 2022a (MathWorks, Natick, USA) and the PLS Toolbox version 9.2.1 (2023) (Eigenvector research Inc., Manson, WA, USA).
2.6.2. Powder X-ray Diffraction (PXRD)
Powder X-ray diffractograms were obtained considering the following measurement conditions: Theta angles from 5º to 70º; step size of 0.033º 2Theta; counts/step*= 45 s (it is an 1D detector); total scan time of 10 min. The instrumental settings were: tube settings of 45 KV & 40 mA; Divergent Slit -1/2º; AntiScatter Slit- 1 º; nickel filter on detector side. Samples were mounted on a Si-0 background holder to avoid background signals on the diffractograms. The same control samples described in
Section 2.6.1 were considered for these studies.
2.6.3. Thermogravimetric Analysis (TGA)
For the TGA analysis the amount of sample used for each measurement was approximately 20 mg. All measurements were carried out under a nitrogen atmosphere with a flow rate of 200 mL.min−1 by heating the material from 20 to 800 °C at a heating rate of 10 °C. min−1. The same control samples described in the previous sections were considered for these studies.
2.6.4. Differential Scanning Calorimetry (DSC)
DSC thermograms of wood samples subjected to the different chemical treatments as well as of microcrystalline cellulose, the industrial paper pulp, and the starting materials from both trees were obtained. Accurately weighed samples (1-2 mg) were poured into aluminium pans and hermetically sealed. The samples were scanned from 30 to 450 °C, at 10 °C min−1. Nitrogen gas was used as purging gas, at 50 mL.min−1. The onset temperature was calculated using the (NETZSCH Proteus® Software – Thermal Analysis – Version 6.1) software.
3. Results and Discussion
3.1. Development of the Cellulose Extraction Protocol from Wood Samples
In the present work, eucalyptus and pine tree wood wastes were used as starting materials to implement a new protocol for cellulose extraction using a simple, and sustainable method. The applicability of the developed approach was evaluated by qualitative analysis of the obtained samples in each reaction step, based on the information acquired from the spectroscopic and thermal characterization techniques. The analysis herein performed also considered the comparison of the structural and thermal features of the obtained products with that of industrial paper pulp and commercial microcrystalline cellulose.
The new methodology presented in this work includes a pre-treatment followed by 3 main steps: the alkaline treatment (A); the first bleaching step (B1); and the second bleaching step (B2).
The pre-treatment was employed to remove soil and other residues present in the wood samples. As can be seen in
Figure 1 B, samples were cleaned in the wash in an ultrasound bath where the residues were retained in the water, as it is possible to visualize directly. The pre-treated samples were then dried and milled (
Figure 1 C and D, respectively) to be further considered for the following step.
The first step consists on the alkaline treatment (
Figure 1 E) in which a alkaline solution of NaOH 2% (v/v) [
12,
13,
14] was used targeting the removal of hemicellulose commonly present in the wood samples [
2]. The second step (
Figure 1 F), bleaching I, comprise the use of H
2O
2, which is considered as a chlorine Gfree green bleaching agent [
12], aimed the delignification of the samples [
13]. The last step (
Figure 1 E), bleaching II was based on literature reports which considered acid treatments [
11,
14,
28] as an additional stage to improve the lignin removal, particularly including the use of an aqueous solution of H
2SO
4 [
3,
13,
28] and ionic liquids [
13]. Of note, ionic liquids have drawn much attention in cellulose extraction processes, as referred by different authors [
3,
11,
29,
30], since their cations and anions components can disrupt the linkages in cellulosic biomass thus enhancing the removal of lignin and hemicellulose. Particularly, Hou and colleagues [
4] demonstrated that the combination of microwave radiation and ionic liquids improved the cellulose extraction from eucalyptus wood. For this reason, the use of this type of solvent were also considered in the herein developed methodology.
The herein establish protocol differs from other in the literature since it considers easy purification steps, which consists only in the filtration of the samples using benign mixture of solvents, particularly water/ethanol, instead of less environmentally friendly solvents such as the DMSO used in the methodology developed by for cellulose extraction from eucalyptus and pine tree samples [
21].
To evaluate the success of the applied treatments, the extraction fractions from each step were characterized by different techniques as previously described.
3.2. Characterization of the Extracted Products
3.2.1. Fourier Transform Infrared Spectroscopy
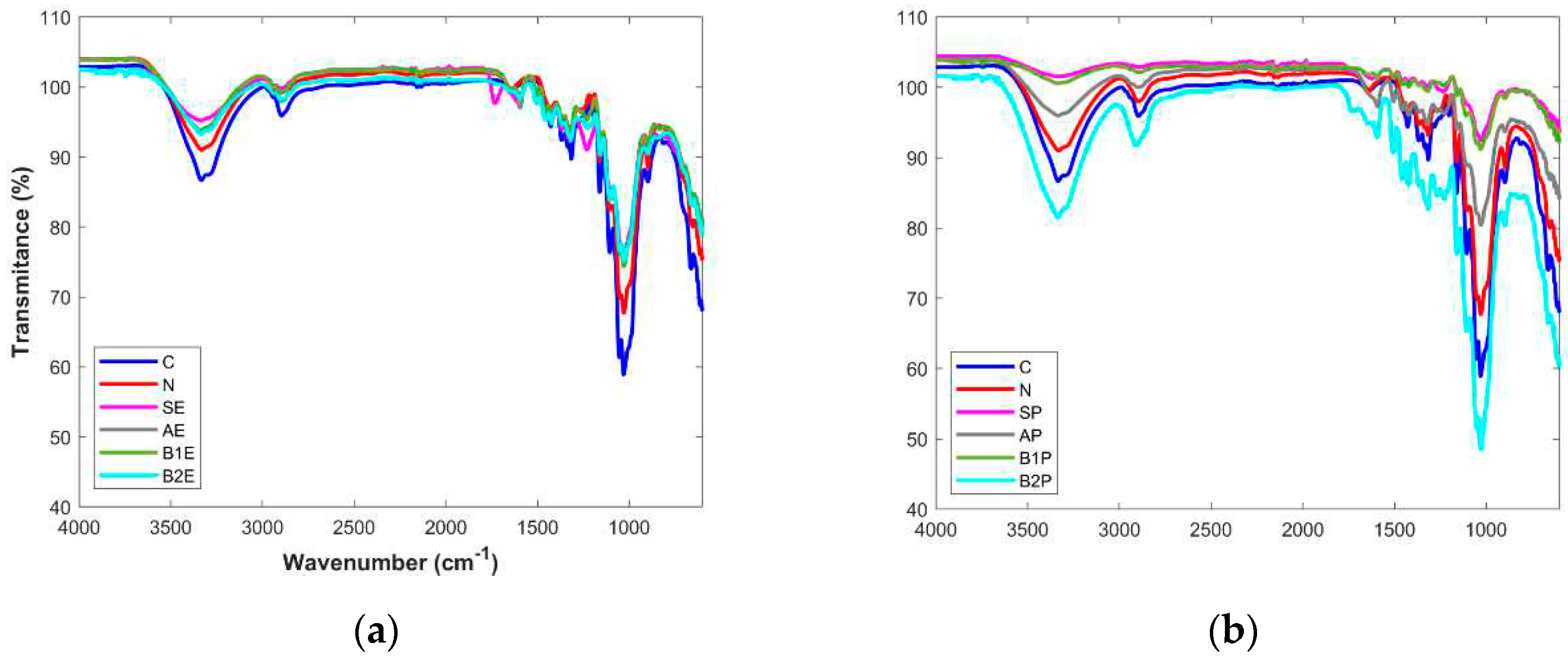
The extracted products obtained in each reaction step were analysed by FTIR spectroscopy and compared to the starting materials (pre-treated wood, SE, SP), as well as with the commercially available microcrystalline cellulose (C) and the industrial pulp paper (N).
Figure 2 (a and b) summarizes the results obtained for eucalyptus and pine tree wood, respectively.
FTIR spectrum of the industrial pulp paper sample (N) shows a great similarity with the spectrum obtained for the pure cellulose (C), revealing the characteristic cellulosic peaks at 1160 cm
-1, 1050 cm
-1, 1030 cm
-1, and 895 cm
-1 [
12,
13,
14]. Regarding the starting materials from both types of wood (SE, SP), it was possible to conclude that, in addition to the characteristic bands from cellulose polymer, other bands are present, in particular, a band at 1230 cm
-1, a set of bands from 1320-1600 cm
-1, and two others appearing at 1620 cm
-1 and 1720 cm
-1, form the other wood components manly hemicellulose, and lignin. After the alkaline treatment and for both type of wood (AE and AP), it can be seen in the spectra that the band at 1720 cm
-1 almost disappeared. Peaks appearing at 1620 cm
-1 and 1720 cm
-1 were totally extinct and a decrease of bands between 1320-1600 cm
-1 was also verified.
There are many reports in the literature suggesting that the both prominent peaks at 1732 cm
-1 and 1235 cm
−1 are assigned to the C=O linkage of the acetyl and uronic ester groups present on hemicellulose structure or to the ester linkage of the carboxyl groups of lignin and/or hemicellulose [
14,
20,
28]. Therefore, the decrease of the wavelength of the peaks at 1720 cm
-1 and 1230 cm
-1 suggests the removal of hemicellulose after the alkaline treatment. Another study referred that the vibration at 1628 cm
-1 is due to the presence of proteins [
18] and that this band was totally removed after the alkaline treatment. Therefore, it is, it is plausible to conclude that this extraction step was also efficient in the removal of the protein content present in the wood samples. According to the literature reports, lignin presented characteristic peaks in the range 1450 to 1600 cm
−1, which are due to the aromatic skeletal vibration [
12,
14,
20,
28]. Since the bands in this range decreased after the first step of the chemical treatment applied to the wood samples, it was possible to suggest that lignin was partially removed from the starting materials.
The subsequent chemical treatment steps originated slight differences in the characteristic of all peaks in the FTIR spectra.
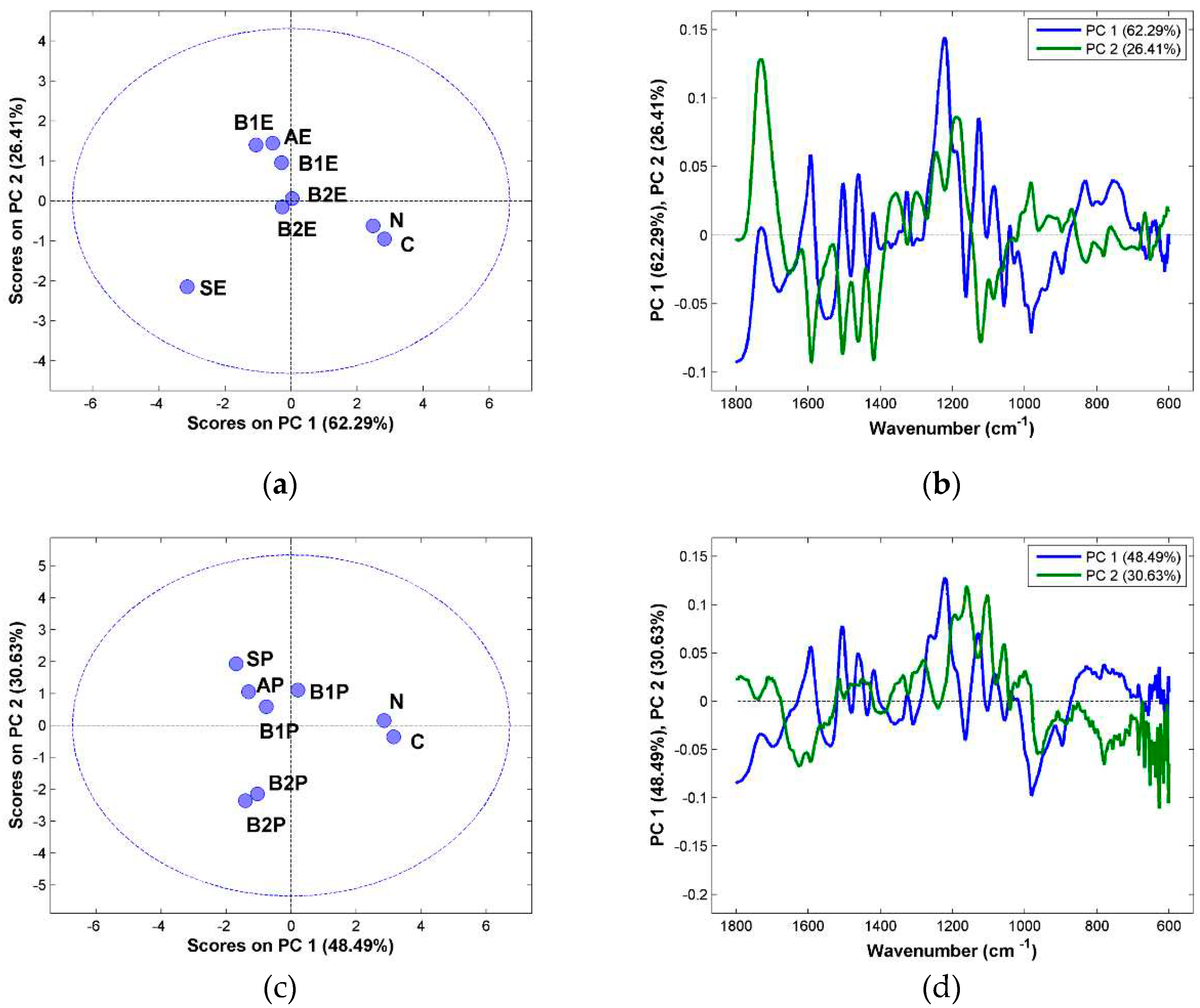
For a more detailed examination of the data, Principal Component Analysis (PCA) was performed to get a deeper insight on the effect of each chemical treatment (
Figure 3). Before the PCA, the FTIR spectra was pre-processed with SNV and analysed between the range 1800 cm
-1 and 600 cm
-1. The pre-processing was preformed to remove some effects (physical and instrumental) not related with the chemical process.
In the PCA score plot for the eucalyptus samples (
Figure 3, a), it can be seen that microcrystalline cellulose sample (C) and the paper pulp sample (N) are separated in the first component from the rest of the samples, in particularly form the starting material (SE). This separation is an indication of the increase degree of purity in terms of cellulose content from the starting material (SE) until the microcrystalline cellulose (C). In the second component the samples are separated according to the amount of hemicellulose present in the samples. The sample after the alkaline treatment (AE) has similar bands to hemicellulose and to the ones after bleaching I (B1E), however the samples after the second bleaching are more like pure cellulose and therefore with a lower hemicellulose content. These conclusions can be confirmed by the analysis of the loadings (
Figure 3, b) in which it can be seen for both the first component that the bands due to cellulose (1160 cm
-1, 1050 cm
-1, 1030 cm
-1) and for the second component the bands due to hemicellulose (1320 – 1600 cm
-1 1620 – 1720 cm
-1) are responsible for the separation seen in the score plot.
For the pine samples a similar analysis can be done. The differences in the first component of the score plot (
Figure 3, c) are due to differences in the degree of purity in the cellulose content, The removal of hemicellulose can be seen in the second component, with the starting material (SP) near the alkaline treatment sample (AP) and Bleaching I (B1P), indicating that the first steps were not efficient in removing hemicellulose. The loadings (
Figure 3, d) confirm this analysis with the bands due to cellulose in the first component around 1000 cm
-1 and 1200 cm
-1, and in the second component with the bands due to hemicellulose around 1200 cm
-1 and between 1600 cm
-1 and 1700 cm
-1, being the main responsible for the separation seen in the score plot.
3.2.2. Powder X-ray Diffraction
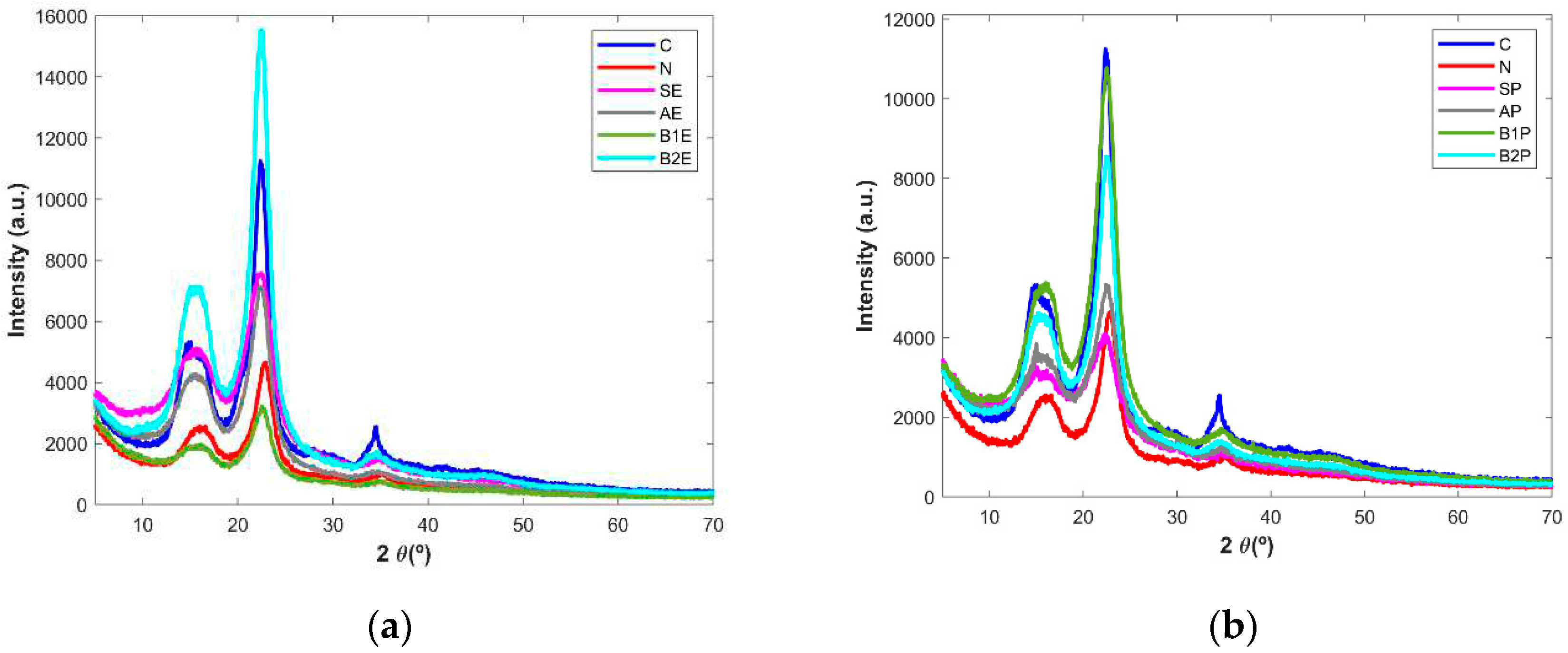
Powder X-ray diffraction analysis was performed to evaluate the crystallinity of the wood samples after different chemical treatment stages since cellulose may have a crystalline or amorphous structures [
13,
31]. The diffraction patterns obtained for the starting materials, alkaline-treated, and for bleached samples as well as for the commercially microcrystalline cellulose and paper pulp are show in
Figure 4 (a and b), for eucalyptus and pine tree samples, respectively. Three peaks can be observed in the powder X-ray diffractograms of all samples: a main peak at 2 Ɵ = 22.5°, a smaller pick at 2 Ɵ = 15.5° and a more discrete peak at 2 Ɵ = 34.5°. The last signal was clearly present in the microcrystalline cellulose and it was mainly observed in the wood fractions obtained after the second step of bleaching treatment (B2E and B2P). This peak is also present in the other samples but generally presenting lower intensities. Those peaks are typical of cellulose structure [
14,
28]. The amorphization of the samples can be seen by the formation of bands instead of well define peaks. For example, the sharp peak at 34.5º in microcrystalline cellulose diffractogram is a broad band in the other diffractograms showing that these samples are less crystalline than microcrystalline cellulose. [
2,
3,
11,
13]. Additionally, the peaks become more defined upon chemical treatments, a fact that is more evident for pine tree derived samples which means that the crystallinity is increasing. Nevertheless, in the last step of the extraction process, both B2E and B2P presented X-ray diffraction patterns closely related to the pure cellulose thus pointing out the success of the extraction process in which concerns to the obtention of fraction with structural features similarly to that characteristic of the commercially available cellulose as well as with that found in the diffractogram of paper pulp.
3.2.3. Thermogravimetric Analysis
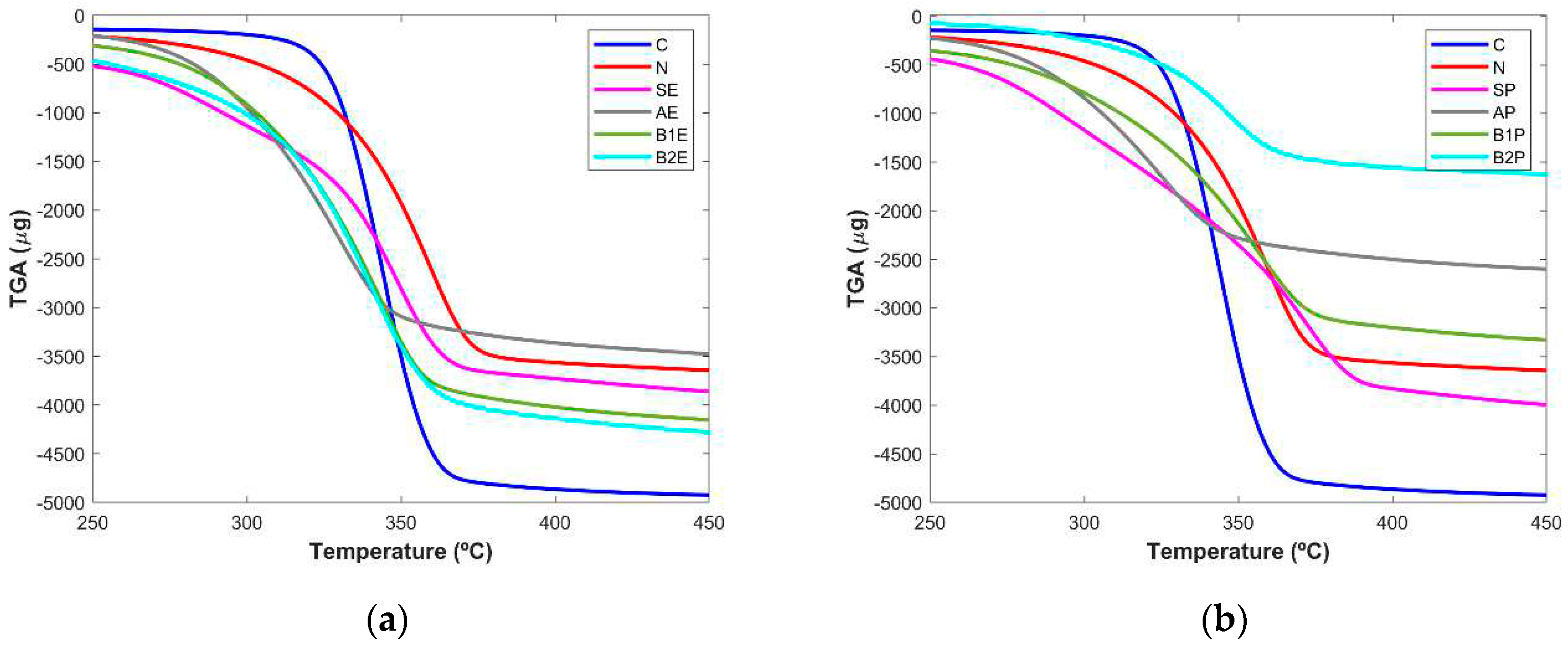
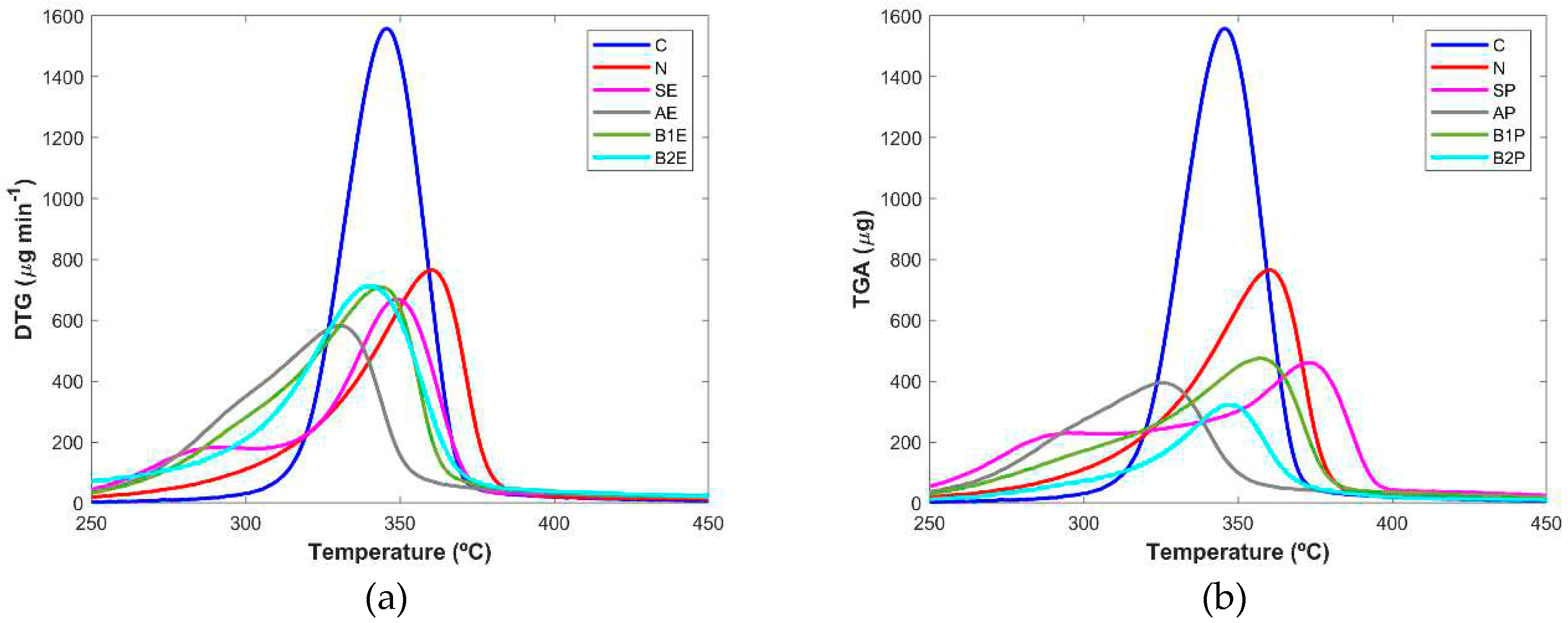
The purity of samples obtained from the microwave extraction in terms of cellulose content was also analysed based on the changes in the thermal behaviour of the samples from the entire process as well as the two controls (C, N). The TGA curves depicted in (
Figure 5, a and b) showed the differences in thermal stability of the sample and based on this analysis it was possible to infer about the efficacy of each step. TGA data revealed that cellulose degradation began around 315°C, being the sample with the higher rate of degradation, reaching around 90% of weight loss for a temperature value up to 450°C. The reference sample obtained from the paper company started to loss mass at approximately 290°C until 378°C, when it reached the maximum percentage of decomposition of ~85%. The starting materials from eucalyptus (SE) and pine (SP) tree wood presented a slow rate of mass loss which starts from 260/350°C until 380/400°C, respectively. These samples showed a bigger decay in the weight loss at higher temperature values than those registered for the extracts of the following steps and for cellulose control. This fact is more evident for eucalyptus samples.
Extracts obtained after the alkaline treatment (AE and AP) showed a similar thermal stability profile and the weight loss was verified for a lower temperature range, from 260 to 365°C. This fact can be explained due to the presence of different wood components, namely hemicellulose and lignin, in agreement with the results obtained by Ndruru
et al. [
12], since these components started their decomposition for lower temperature values than that described for the pure cellulose.
B1E and B2E products showed a similar decomposition profile and bigger differences were verified for B1P and B2P samples, in which the extract resultant from the last step of the methodology loses a small weight, being almost totally degraded (~83%) at 370°C.
Other studies performed by Morán and co-workers [
28] also demonstrated that cellulose started its decomposition for higher temperature values (~315°C) while hemicellulose and lignin started the weight loss at lower values of approximately 220 and 200°C, respectively. This information is in agreement with the results herein reported since the extracts showed in general, their decomposition in a temperature range lower than that found for the pure cellulose.
From the derivative thermogravimetric (DTG) curves (
Figure 5, c and d), a more evident evaluation of the success of the extraction steps was inferred. It was possible to visualize a well-defined and sharper peak for the pure cellulose (345°C) while the curves from the starting materials and from alkaline samples seem to comprise two bands at approximately 283/287°C and 348/373°C (SE/SP) or 293/292°C and 330/325°C (AE/AP), respectively for each step. The presence of the two bands is probably due to the cellulose and other wood components. Interestingly, the DTG curves clearly evidence that these extra components were successfully removed during the following steps since, the curves for B2E and B2P do not present a defined second peak (
Figure 5, c and d). For eucalyptus samples, no major differences (~ 3°C) were found between B1E (344°C) and B2E (
Figure 5, c) thus pointing out that the most relevant step to the removal of hemicellulose and lignin in this type of wood was the first bleaching treatment. A small difference of approximately 10°C was found for the band’s correspondent to B1P and B2P extract (
Figure 5, d), which appeared at 357 and 347 °C, respectively, thus suggesting that the last extraction step had still a slightly contribution to the removal of non-cellulosic components in the pine tree samples. This conclusion was already obtained by the PCA analysis of the FTIR spectra.
The obtained results are in good agreement with those reported for the thermogravimetric analysis of products obtained by the other cellulose extraction methodologies [
2,
3,
13,
28]. Globally, TGA studies demonstrated a clear evolution in the cellulose isolation along the extraction steps, mainly evidenced by the great similarity of the DTG curves from the bleaching II step and the pure cellulose.
3.2.4. Differential Scanning Calorimetry
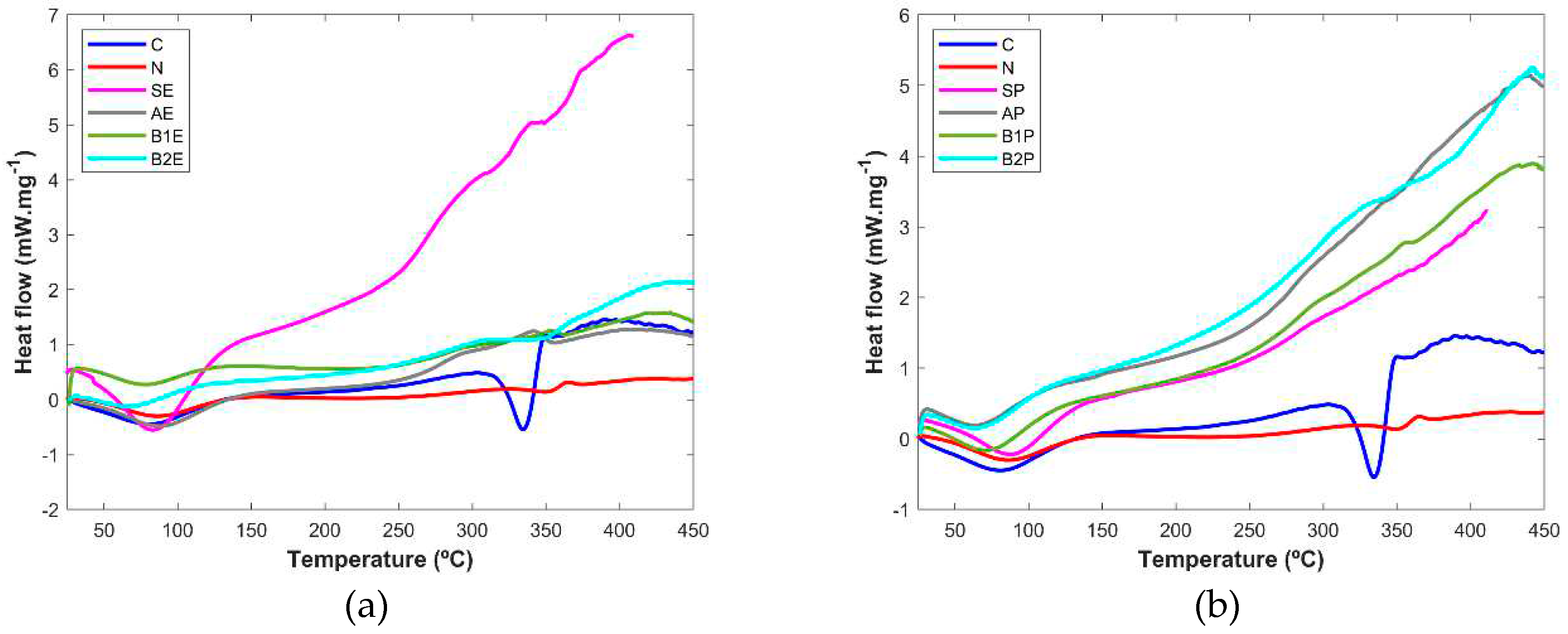
DSC was also considered as another technique to fully characterize the extraction fractions obtained in each step and their comparison with the pure microcrystalline cellulose and the sample resultant from the paper industry processing (
Figure 6). The results have shown in all samples, a first endothermic event at around 100°C in both pine and eucalyptus extracts, which is due to water evaporation [
3,
28]. A melting at approximately 300°C in the cellulose control (C) revealed the higher degree of crystallinity of this sample. In particular, the starting material from eucalyptus wood (SE) showed 3 endothermic events (at around 250, 300, and 350 °C), which can possibly be the melting of the main components of the wood, cellulose, hemicellulose, and lignin [
3,
28]. All the other samples, including the one obtained from the industrial processing (N), have very low crystallinity, and the endothermic peak due to cellulose melting cannot be seen (pine extracts) or is very small (eucalyptus samples). The lack of crystallinity showed in the DSC thermograms is in accordance with the XRPD results.
4. Conclusion
The results of the present work allowed the establishment of a new, simple, and sustainable microwave-assisted protocol for cellulose extraction from eucalyptus and pine tree wood waste.
FTIR studies, particularly PCA data, corroborate the conclusions from TGA studies and XRPD diffractograms confirmed the presence of cellulose in all extracts. It can also be concluded that in the last beaching step the removal of hemicellulose is more efficient. The analysis of DTG curves has shown that the starting materials and the samples from the first extraction step are composed by more than one component. This fact pointed out the benefit of the following extraction steps since the final products, especially those from bleaching II, are closer to cellulose, being more evident for pine samples. The results confirm that for pine, a softwood, the delignification process is more difficult than for a hardwood as eucalyptus, since hemicellulose was easily removed in eucalyptus products in the earlier extraction steps. Nevertheless, after all the steps considered in the protocol, it was possible to obtain cellulose in a similar way for both type of samples.
The overall results reveal the applicability of the new approach as a sustainable strategy based on the use of green methodologies such as MAE and mild extraction/purification conditions. The established protocol enables the obtention of cellulose from softwood and hardwood, particularly from pine and eucalyptus waste products and it offers the possibility to be further tested as a valuable tool to extract this biopolymer from other natural sources, such as algae and many different types of plants’ feedstocks. Moreover, the fact that the methodology was exclusively developed using a multimode microwave equipment offers the great opportunity to scale up the process to be eventually considered in the industry.
Author Contributions
Conceptualization, M.S., S.V. and T.M.; methodology, M.S., S.V. and T.M.; validation, M.S., T.M., S.R. and M.R.; formal analysis, M.S., and T.M.; investigation, M.S., S.V. and T.M.; resources, M.S., S.R., M.R. and T.M.; data curation, M.S. and T.M.; writing—original draft preparation, M.S., S.V. and T.M.; writing—review and editing, S.R. and M. R.; supervision, T.M., S.R. and M.R.; project administration, T.M., S.R. and M.R.; funding acquisition, T.M., S.R. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
T. Moniz also thanks the financial support from National Funds (FCT, Fundação para a Ciência e Tecnologia) through projects UIDB/50006/2020 and UIDP/50006/2020.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data Available upon request.
Acknowledgments
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects EXPL/QUI-QIN/0411/2021, UIDB/50006/2020 and UIDP/50006/2020. S. Vinhas thanks national funding from FCT/MEC (NORTE-08-5369-FSE-000050. M. Sarraguça thanks FCT for funding through the Individual Call to Scientific Employment Stimulus 2022.01388.CEECIND/CP1724/CT0003. To all financing sources, the authors are greatly indebted. A special acknowledgement to Manuela Barros and Vânia Dias for all the technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kadir, N.H.A.; Mohammad, M.; Alam, M.; Torkashvand, M.; Silvaragi, T.G.B.; Gururuloo, S.L. Chapter 19 - Utilization of nanocellulose fibers, nanocrystalline cellulose and bacterial cellulose in biomedical and pharmaceutical applications. In Nanotechnology in Paper and Wood Engineering, Bhat, R., Kumar, A., Nguyen, T.A., Sharma, S., Eds.; Elsevier: 2022; pp. 409-470. [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durrani, A.I.; Naseem, S. Low temperature green extraction of Acer platanoides cellulose using nitrogen protected microwave assisted extraction (NPMAE) technique. Carbohydr. Polym. 2021, 272, 118465. [Google Scholar] [CrossRef]
- Azlan, N.S.M.; Yap, C.L.; Gan, S.; Rahman, M.B.A. Effectiveness of various solvents in the microwave-assisted extraction of cellulose from oil palm mesocarp fiber. Materials Today: Proceedings 2022, 59, 583–590. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Sun, J.; Li, M.; Wang, S.; Chen, K.; Gao, Z. A microwave-assisted aqueous ionic liquid pretreatment to enhance enzymatic hydrolysis of Eucalyptus and its mechanism. Bioresource Technology 2019, 272, 99–104. [Google Scholar] [CrossRef]
- Yadav, V.; Kumar, A.; Bilal, M.; Nguyen, T.A.; Iqbal, H.M.N. Chapter 12 - Lignin removal from pulp and paper industry waste streams and its application. In Nanotechnology in Paper and Wood Engineering, Bhat, R., Kumar, A., Nguyen, T.A., Sharma, S., Eds.; Elsevier: 2022; pp. 265-283. [CrossRef]
- Froschauer, C.; Hummel, M.; Iakovlev, M.; Roselli, A.; Schottenberger, H.; Sixta, H. Separation of hemicellulose and cellulose from wood pulp by means of ionic liquid/cosolvent systems. Biomacromolecules 2013, 14, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. Journal of Materials Research and Technology 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Ruhan Askin, U. Microwave-Assisted Green Extraction Technology for Sustainable Food Processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing, Kok Yeow, Y., Ed.; IntechOpen: Rijeka, 2018. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, S.; Shi, S.Q.; Cai, L. Microwave-assisted formic acid extraction for high-purity cellulose production. Cellulose 2019, 26, 5913–5924. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Green simple microwave-assisted extraction (MAE) of cellulose from Theobroma cacao L. (TCL) husk. In Proceedings of the IOP Conference Series: Materials Science and Engineering; 2019. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Park, S.-I.; Lee, Y.S. Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. International Journal of Biological Macromolecules 2017, 99, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.d.; Bukzem, A.d.L.; Ascheri, D.P.R.; Signini, R.; Aquino, G.L.B.d. Microwave-assisted carboxymethylation of cellulose extracted from brewer's spent grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Antonio De La, H.; Jesús, A.; José, C.; María, A.H.; Juan De, M.M.; Pilar, P.; Abel De, C.; Angel, D.-O. Reproducibility and Scalability of Microwave-Assisted Reactions. In Microwave Heating, Usha, C., Ed.; IntechOpen: Rijeka, 2011; p. Ch. 7. [Google Scholar] [CrossRef]
- Kusumattaqiin, F.; Chonkaew, W. Preparation and Characterization of Microcrystalline Cellulose (MCC) by Acid Hydrolysis Using Microwave Assisted Method from Cotton Wool. Macromolecular Symposia 2015, 354, 35–41. [Google Scholar] [CrossRef]
- Yan, D.; Ji, Q.; Yu, X.; Li, M.; Abiola Fakayode, O.; Yagoub, A.E.A.; Chen, L.; Zhou, C. Multimode-ultrasound and microwave assisted natural ternary deep eutectic solvent sequential pretreatments for corn straw biomass deconstruction under mild conditions. Ultrasonics Sonochemistry 2021, 72, 105414. [Google Scholar] [CrossRef]
- Jodeh, S.; Hu, M.; Hamed, O.; Salghi, R.; Abidi, N.; Hattb, R. Extraction and characterization of cellulose from agricultural waste argan press cake. Cellulose Chemistry and Technology 2017, 51. [Google Scholar]
- Silvy, N.; Reza, S.; Uddin, N.; Akther, M. Comparison between Different Components of Some Available Hardwood and Softwood in Bangladesh. Journal of Biotechnology and Biochemistry 2018, 4. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, W.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green Chemistry 2016, 18, 1010–1018. [Google Scholar] [CrossRef]
- Casas, A.; Alonso, M.V.; Oliet, M.; Santos, T.M.; Rodriguez, F. Characterization of cellulose regenerated from solutions of pine and eucalyptus woods in 1-allyl-3-methilimidazolium chloride. Carbohydr. Polym. 2013, 92, 1946–1952. [Google Scholar] [CrossRef]
- Duarte Urueña, G.; Ribeiro, K.C.; Prestes, E.; Pinheiro, L.A.; Carvalho, B.M. Extraction of Cellulose Nanocrystal from Multilayer Packaging Residues Composed of a Mixture of Eucalyptus and Pine Fibers. Waste and Biomass Valorization 2021, 12, 5763–5777. [Google Scholar] [CrossRef]
- Besbes, I.; Vilar, M.R.; Boufi, S. Nanofibrillated cellulose from Alfa, Eucalyptus and Pine fibres: Preparation, characteristics and reinforcing potential. Carbohydr. Polym. 2011, 86, 1198–1206. [Google Scholar] [CrossRef]
- Fall, A.B.; Burman, A.; Wågberg, L. Cellulosic nanofibrils from eucalyptus, acacia and pine fibers. Nordic Pulp & Paper Research Journal 2014, 29, 176–184. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y. High energy oxidation and organosolv solubilization for high yield isolation of cellulose nanocrystals (CNC) from Eucalyptus hardwood. Sci. Rep. 2018, 8, 16505. [Google Scholar] [CrossRef]
- Bajpai, P. Chapter Two - General Background. In Environmentally Benign Approaches for Pulp Bleaching (Second Edition), Bajpai, P., Ed.; Elsevier: Boston, 2012; pp. 5–18. [Google Scholar]
- Bajpai, P. Chapter One Introduction. In Environmentally Benign Approaches for Pulp Bleaching (Second Edition), Bajpai, P., Ed.; Elsevier: Boston, 2012; pp. 1–4. [Google Scholar]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Zetty Shafiqa, O.; Nur Hasyareeda, H.; Saiful Irwan, Z. Imidazolium-Based Ionic Liquid Binary Solvent System as an Extraction Medium in Enhancing the Rotenone Yield Extracted from Derris elliptica Roots. In Ionic Liquids, Scott, H., Ed.; IntechOpen: Rijeka, 2017; p. Ch. 21. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnology and Bioengineering 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).