Submitted:
23 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
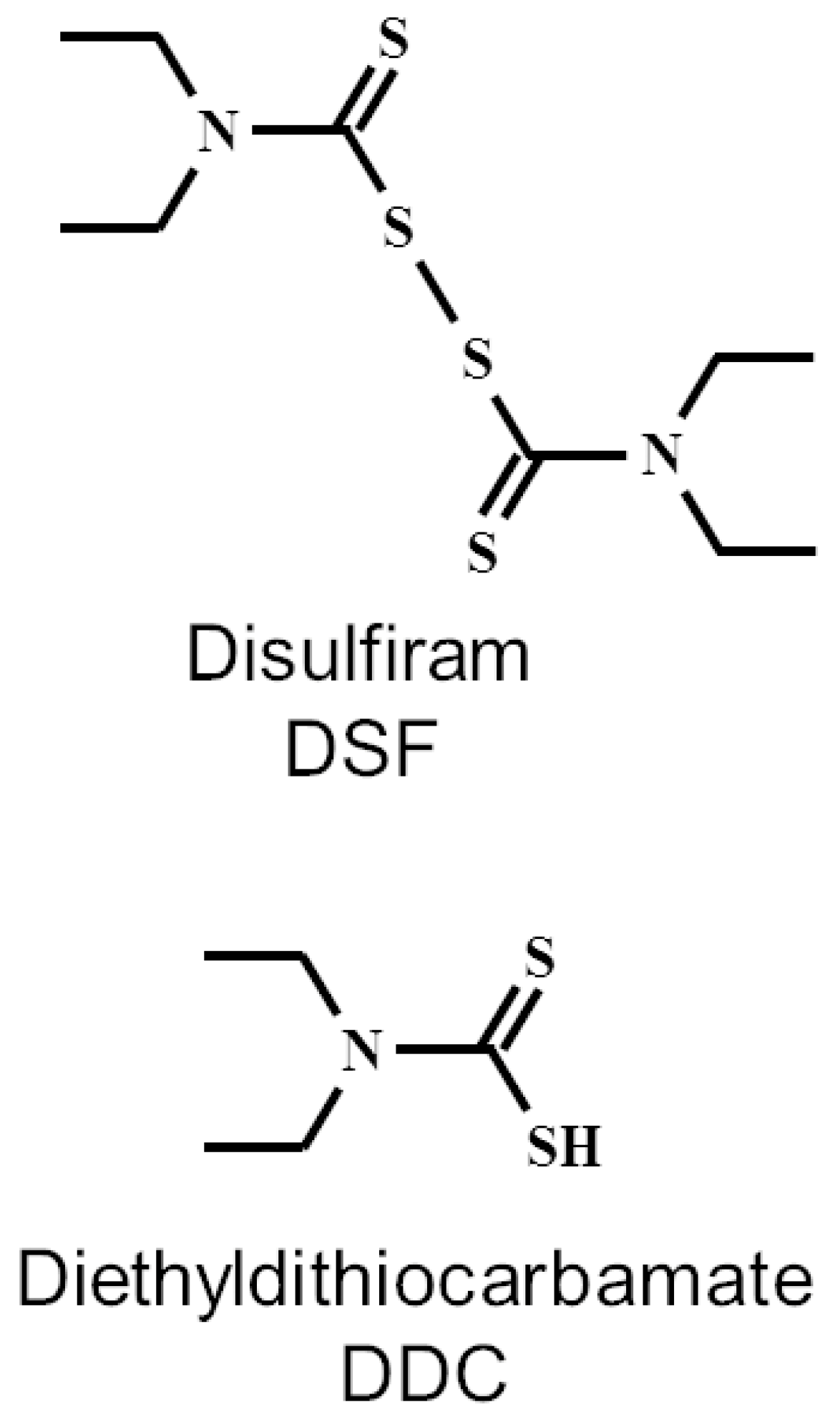
1. Introduction
2. Material and Methods
2.1. General
2.2. Detection and identification of Ureaplasma spp
2.3. The effect of DDC on Ureaplasma spp
2.4. Bacteriostatic effect of DDC on Ureaplasma cultures
2.5. Bactericidal activity of DDC
2.6. Statistical analysis
3. Results
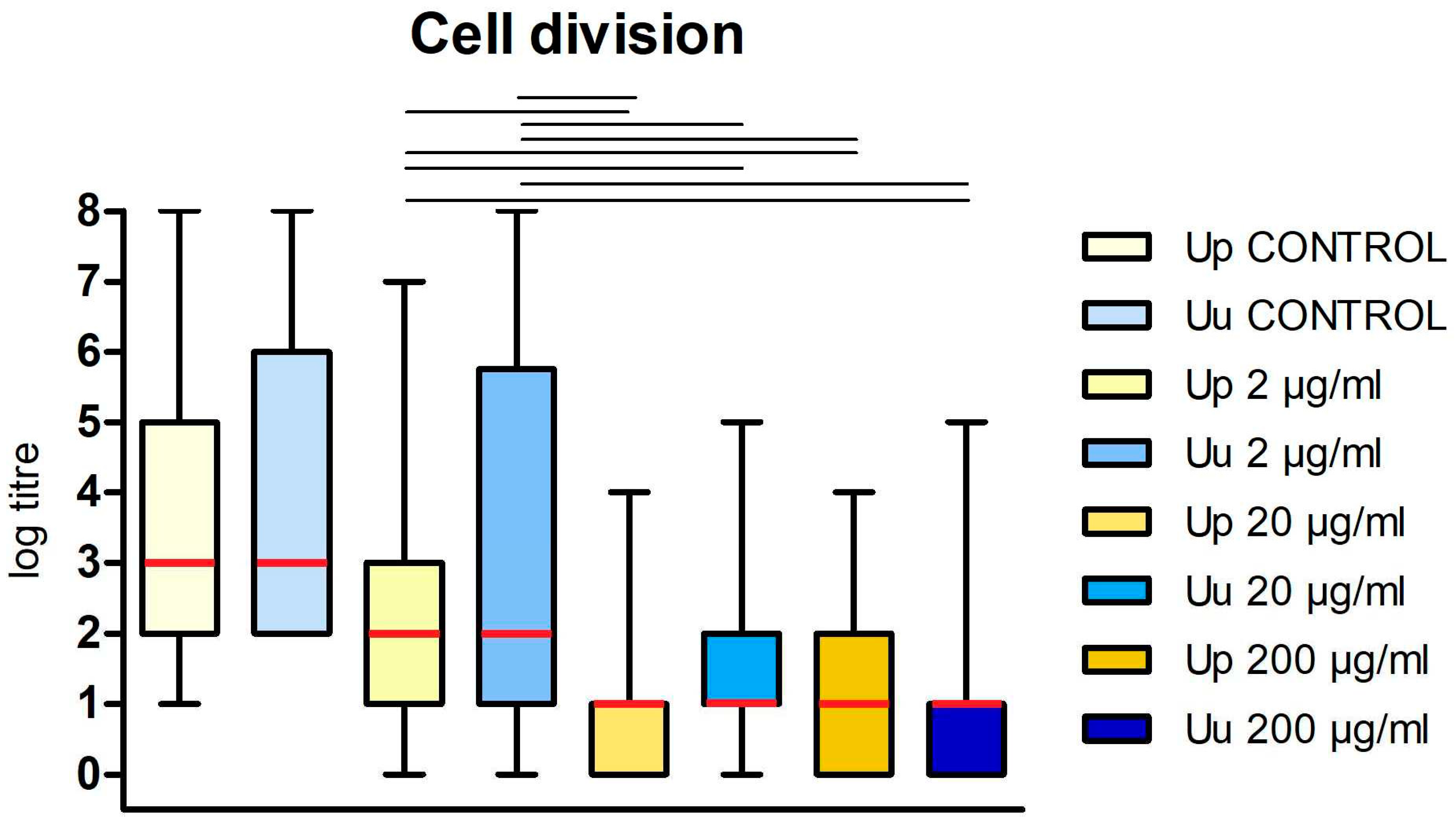
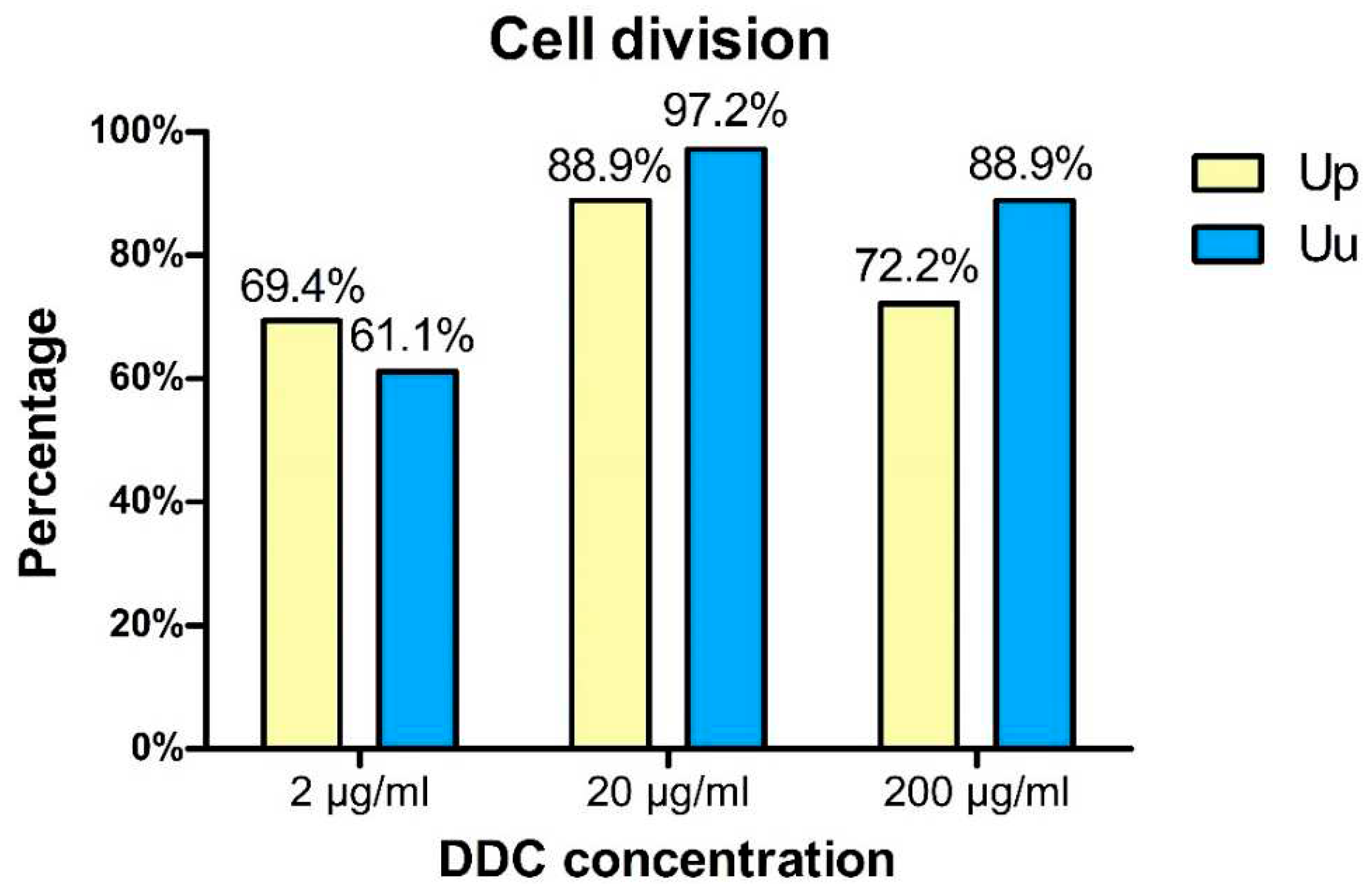
3.1. Bacteriostatic effect of DDC on Ureaplasma cultures
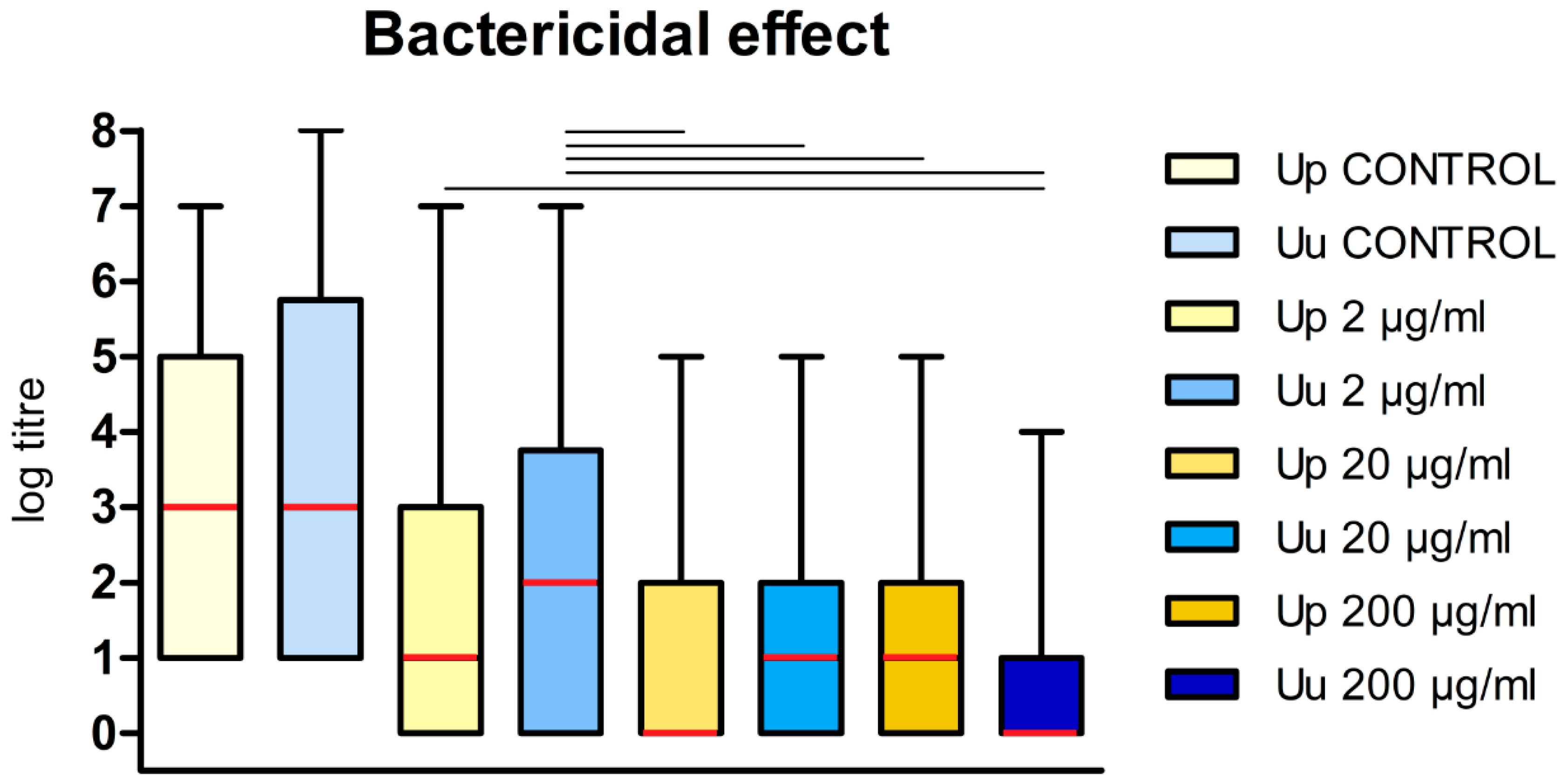
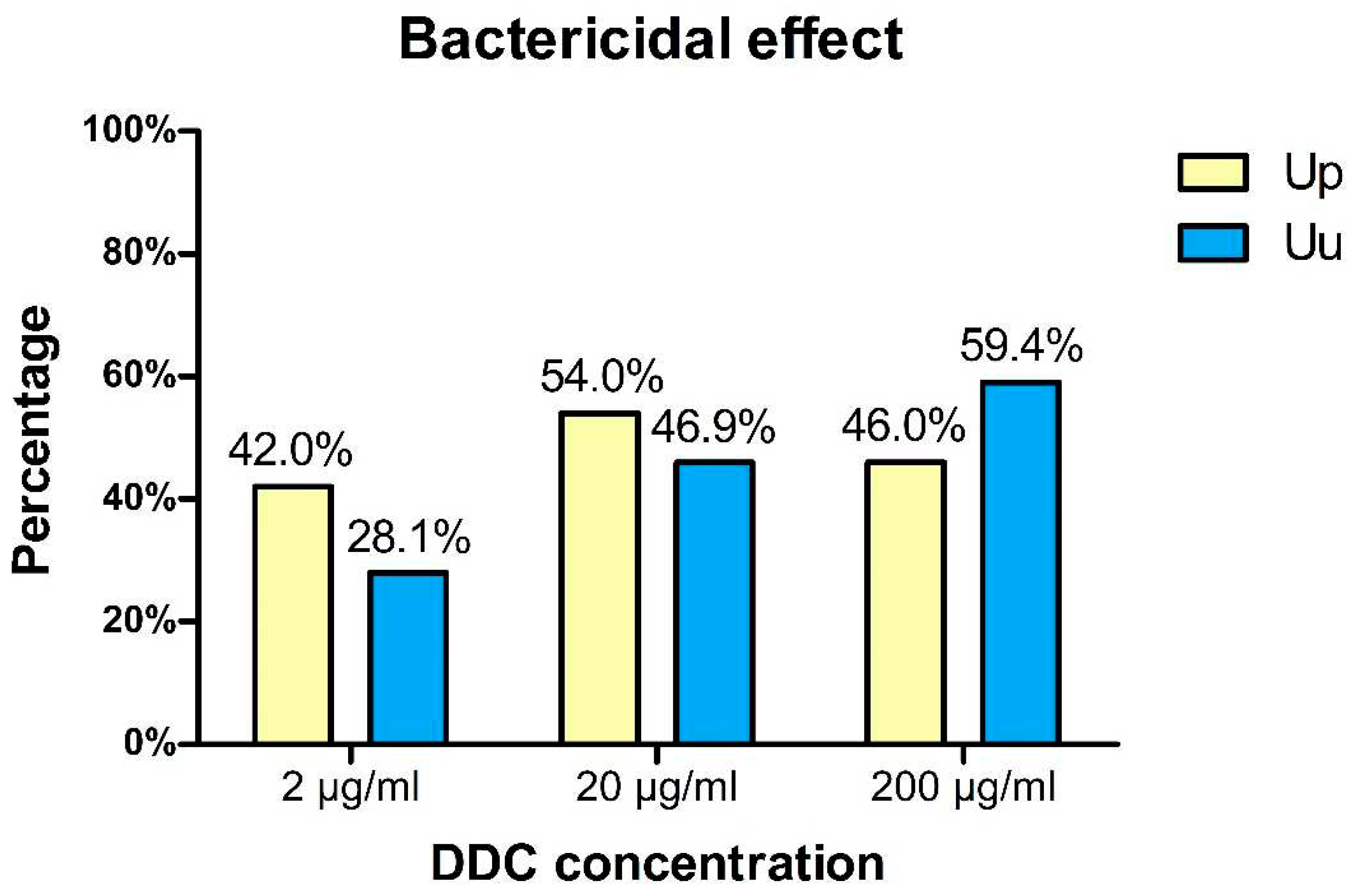
3.2. Bactericidal activity of DDC on Ureaplasma cultures
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Deetjen:, P.; Maurer, C.; Rank, A.; Berlis, A.; Schubert, S.; Hoffmann, R. Brain abscess caused by Ureaplasma urealyticum in an adult patient. J Clin Microbiol 2014, 52, 695–698. [Google Scholar] [CrossRef]
- Vittecoq, O.; Schaeverbeke, T.; Favre, S.; Daragon, A.; Biga, N.; Cambon-Michot, C.; Bébéar, C.; Le Loët, X. Molecular diagnosis of Ureaplasma urealyticum in an immunocompetent patient with destructive reactive polyarthritis. Arthritis Rheum 1997, 40, 2084–2089. [Google Scholar] [CrossRef]
- Shepard, M.C. The recovery of pleuropneumonia-like organisms from Negro men with and without nongonococcal urethritis. Am J Syph Gonorrhea Vener Dis 1954, 38, 113–124. [Google Scholar]
- Crăcea, E.; Constantinescu, S.; Lazar, M. Serotypes of Ureaplasma urealyticum isolated from patients with nongonococcal urethritis and gonorrhea and from asymptomatic urethral carriers. Sex Transm Dis. 1985, 12, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Shepard, M.C.; Lunceford, C. D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol 1978, 8, 566–574. [Google Scholar] [CrossRef]
- Maeda, S.; Deguchi, T.; Ishiko, H.; Matsumoto, T.; Naito, S.; Kumon, H.; Tsukamoto, T.; Onodera, S.; Kamidono, S. Detection of Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma parvum (biovar 1) and Ureaplasma urealyticum (biovar 2) in patients with non-gonococcal urethritis using polymerase chain reaction-microtiter plate hybridization. Int J Urol 2004, 11, 750–754. [Google Scholar] [CrossRef]
- Povlsen, K.; Bjørnelius, E.; Lidbrink, P.; Lind, I. Relationship of Ureaplasma urealyticum biovar 2 to nongonococcal urethritis. Eur J Clin Microbiol Infect Dis 2002, 21, 97–101. [Google Scholar] [CrossRef]
- Deguchi, T.; Yoshida, T.; Miyazawa, T.; Yasuda, M.; Tamaki, M.; Ishiko, H.; Maeda, S. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis 2004, 31, 192–195. [Google Scholar] [CrossRef]
- Weidner, W.; Brunner, H.; Krause, W. Quantitative culture of ureaplasma urealyticum in patients with chronic prostatitis or prostatosis. J Urol 1980, 124, 622–625. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Lee, K.A. Prevalence of sexually transmitted infections among healthy Korean women: implications of multiplex PCR pathogen detection on antibiotic therapy. J Infect Chemother 2014, 20, 74–76. [Google Scholar] [CrossRef]
- Kafetzis, D.A.; Skevaki, C.; Skouteri, V.; Gavrili, S.; Peppa, K.; Kostalos, C.; Petrochilou, V.; Michalas, S. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery. Association of the respiratory colonization of the premature infants with chronic lung disease and increased mortality. Clin Infect Dis 2004, 39, 1113–1122. [Google Scholar] [CrossRef]
- Sanchez, P.J.; Regan, J.A. Vertical transmission of Ureaplasma urealyticum from mothers to preterm infants. Pediatr Infect Dis J 1990, 9, 398–401. [Google Scholar] [CrossRef]
- Katz, B.; Patel, P.; Duffy, L.; Schelonka, R.L.; Dimmitt, R.A.; Waites, K.B. Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J Clin Microbiol 2005, 43, 4852–4854. [Google Scholar] [CrossRef]
- Heggie, A.D.; Bar-Shain, D.; Boxerbaum, B.; Fanaroff, A.A.; O'Riordan, M.A. , Robertson, J.A. Identification and quantification of ureaplasmas colonizing the respiratory tract and assessment of their role in the development of chronic lung disease in preterm infants. Pediatr Infect Dis J 2001, 20, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Abele-Horn, M.; Wolff, C.; Dressel, P.; Pfaff, F.; Zimmermann, A. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol 1997, 35, 1199–1202. [Google Scholar] [CrossRef]
- Pinna, G.S.; Skevaki, C.L.; Kafetzis, D.A. The significance of Ureaplasma urealyticum as a pathogenic agent in the paediatric population. Curr Opin Infect Dis 2006, 19, 283–289. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; de Castro Silva, M.; Velayo, C.L. The role of genital mycoplasma infection in female infertility: A systematic review and meta-analysis. Am J Reprod Immunol 2021, 85, e13390. [Google Scholar] [CrossRef]
- Busolo, F.; Zanchetta, R. The effect of Mycoplasma hominis and Ureaplasma urealyticum on hamster egg in vitro penetration by human spermatozoa. Fertil Steril 1985, 43, 110–114. [Google Scholar] [CrossRef]
- Abdulrazzak, A.A.; Bakr, S.S. Role of mycoplasma in male infertility. East Mediterr Health J 2000, 6, 149–155. [Google Scholar] [CrossRef]
- Beeton, M.L.; Spiller, O.B. Antibiotic resistance among Ureaplasma spp. isolates: cause for concern? J Antimicrob Chemother 2017, 72, 330–337. [Google Scholar] [CrossRef]
- Khosropour, C.M.; Manhart, L.E.; Gillespie, C.W.; Lowens, M.S.; Golden, M.R.; Jensen, N.L.; Kenny, G.E.; Totten, P.A. Efficacy of standard therapies against Ureaplasma species and persistence among men with non-gonococcal urethritis enrolled in a randomised controlled trial. Sex Transm Infect 2015, 91, 308–313. [Google Scholar] [CrossRef]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Drzewiecki, A.; Lauterbach, R. Antimicrobial susceptibility of Ureaplasma urealyticum and ureaplasma parvum isolated from premature infants with respiratory disorders. Przegl Epidemiol 2007, 61, 371–376, Polish. [Google Scholar]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat Anti-Cancer Drug Discov 2019, 14, 113–132. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska-Wilkosz, A.; Kozdrowicki, M.; Górny, M. Reactive Sulfur Compounds in the Fight against COVID-19. Antioxidants (Basel) 2022, 11, 1053. [Google Scholar] [CrossRef]
- Lajarin-Reinares, M.; Pena-Rodríguez, E.; Cañellas-Santos, M.; Rosell-Vives, E.; Cortés, P.; Casas, M.L.; Calvo, M.À.; Fernandez-Campos, F. Repurposing Disulfiram as an Antimicrobial Agent in Topical Infections. Antibiotics (Basel) 2022, 11, 1752. [Google Scholar] [CrossRef]
- Shirley, D.-A.; Sharma, I.; Warren, C.A.; Moonah, S. Drug Repurposing of the Alcohol Abuse Medication Disulfiram as an Anti-Parasitic Agent. Front Cell Infect Microbiol 2021, 11, 165. [Google Scholar] [CrossRef]
- Khan, S.; Singhal, S.; Mathur, T.; Upadhyay, D.J.; Rattan, A. Antifungal potential of disulfiram. Nihon Ishinkin Gakkai Zasshi 2007, 48, 109–113. [Google Scholar] [CrossRef]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Lauterbach, R. Assesment of various diagnostic methods of ureaplasma respiratory tract infections in newborns. Acta Biochim Pol 2006, 53, 609–611. [Google Scholar] [CrossRef]
- Poveda, J.B.; Nicholas, R. Serological identification of mycoplasmas by growth and metabolic inhibition tests. Methods Mol Biol 1998, 104, 105–111. [Google Scholar] [CrossRef]
- Agarwal, R.P.; McPherson, R.A.; Phillips, M. Rapid degradation of disulfiram by serum albumin. Res Commun Chem Pathol Pharmacol 1983, 42, 293–310. [Google Scholar]
- Cobby, J.; Mayersohn, M.; Selliah, S. The rapid reduction of disulfiram in blood and plasma. J Pharmacol Exp Ther 1977, 202, 724–731. [Google Scholar] [PubMed]
- Marshall, M.J.; Harris, A.M.; Horne, J.E. The bacteriological and clinical assessment of a new preparation for the treatment of otitis extema in dogs and cats. J Small Anim Pract 1974, 15, 401–410. [Google Scholar] [CrossRef]
- Scheibel, L.W.; Adler, A.; Trager, W. Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA 1979, 76, 5303–5307. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Ceri, H. A subpopulation of Candida albicans and Candida tropicalis biofilm cells are highly tolerant to chelating agents. FEMS Microbiol Lett 2007, 272, 172–181. [Google Scholar] [CrossRef]
- Khouri, R.; Novais, F.; Santana, G.; de Oliveira, C.I.; Vannier dos Santos, M.A.; Barral, A.; Barral-Netto, M.; Van Weyenbergh, J. DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: a promising therapeutic alternative in Leishmaniasis. PLoS One 2010, 5, e14394. [Google Scholar] [CrossRef]
- Celes, F.S.; Trovatti, E.; Khouri, R.; Van Weyenbergh, J.; Ribeiro, S.J.; Borges, V.M.; Barud, H.S.; de Oliveira, C.I. DETC-based bacterial cellulose biocuratives for topical treatment of cutaneous leishmaniasis. Sci Rep 2016, 6, 38330. [Google Scholar] [CrossRef]
- Taylor, E.H.; Walker, E.M. Jr.; Bartelt, M.; Day, S.; Pappas, A.A. In-vitro antimicrobial activity of diethyldithiocarbamate and dimethyldithiocarbamate against methicillin-resistant staphylococcus. Ann Clin Lab Sci 1987, 17, 171–177. [Google Scholar] [PubMed]
- Phillips, M.; Malloy, G.; Nedunchezian, D.; Lukrec, A.; Howard, R.G. Disulfiram inhibits the in vitro growth of methicillin-resistant staphylococcus aureus. Antimicrob Agents Chemother 1991, 35, 785–787. [Google Scholar] [CrossRef]
- Long, T.E. Repurposing Thiram and Disulfiram as Antibacterial Agents for Multidrug-Resistant Staphylococcus aureus Infections. Antimicrob Agents Chemother 2017, 61, e00898–17. [Google Scholar] [CrossRef]
- Kaul, L.; Abdo, A.I.; Coenye, T.; Krom, B.P.; Hoogenkamp, M.A.; Zannettino, A.C.W.; Süss, R.; Richter, K. The combination of diethyldithiocarbamate and copper ions is active against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro and in vivo. Front Microbiol 2022, 13, 999893. [Google Scholar] [CrossRef]
- Kobatake, T.; Ogino, K.; Sakae, H.; Gotoh, K.; Watanabe, A.; Matsushita, O.; Okada, H.; Yokota, K. Antibacterial Effects of Disulfiram in Helicobacter pylori. Infect Drug Resist 2021, 14, 1757–1764. [Google Scholar] [CrossRef]
- Totten, A.H.; Crawford, C.L.; Dalecki, A.G.; Xiao, L.; Wolschendorf, F.; Atkinson, T.P. Differential Susceptibility of Mycoplasma and Ureaplasma Species to Compound-Enhanced Copper Toxicity. Front Microbiol 2019, 10, 1720. [Google Scholar] [CrossRef]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Bilska-Wilkosz, A. In-vitro activity of lipoic acid against Ureaplasma urealyticum and Ureaplasma parvum isolated from women with infections of the urogenital tract. A pilot study. Acta Biochim Pol 2020, 67, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Gajda, P.; Bilska-Wilkosz, A. Lipoic Acid Does Not Affect The Growth of Mycoplasma hominis Cells In Vitro. Pol J Microbiol 2021, 70, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.D.; Riedel, T.M.; Kesler, M.B.A.; Varney, M.E.; Long, T.E. Pharmacological evaluation of disulfiram analogs as antimicrobial agents and their application as inhibitors of fosB-mediated fosfomycin resistance. J Antibiot (Tokyo) 2022, 75, 146–154. [Google Scholar] [CrossRef]
- Messens, J.; Collet, J.F. Thiol-disulfide exchange in signaling: disulfide bonds as a switch. Antioxid Redox Signal 2013, 18, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Razin, S.; Kenny. G.E.; Barile, M.F. Characteristics of Ureaplasma urealyticum urease. J Bacteriol 1988, 170, 2692–2697. [Google Scholar] [CrossRef]
- Masover, G. K.; Hayflick, L. Growth of T-strain mycoplasmas in media without added urea. Ann N Y Acad Sci 1973, 225, 118–130. [Google Scholar] [CrossRef]
- Romano, N.; La Licata, R.; Russo Alesi, D. Energy production in Ureaplasma urealyticum. Pediatr Infect Dis 1986, 5, S308–S312. [Google Scholar] [CrossRef]
- Smith, D.G.; Russell, W.C.; Ingledew, W.J.; Thirkell, D. Hydrolysis of urea by Ureaplasma urealyticum generates a transmembrane potential with resultant ATP synthesis. J Bacteriol 1993, 175, 3253–3258. [Google Scholar] [CrossRef]
- Dixon, N.E.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5.1.5). III. The involvement of active-site nickel ion in inhibition by beta-mercaptoethanol, phosphoramidate, and fluoride. Can J Biochem 1980, 58, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Benini, S.; Rypniewski, W. R.; Wilson, K. S.; Ciurli, S.; Mangani, S. The complex of Bacillus pasteurii urease with β-mercaptoethanol from X-ray data at 1.65-Å resolution. J Biol Inorg Chem 1998, 3, 268–273. [Google Scholar] [CrossRef]
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects, catalytic, and non-catalytic properties - A review. J Adv Res 2018, 2018 13, 3–17. [Google Scholar] [CrossRef]
- Zhang, L.; Mulrooney, S.B.; Leung, A.F.; Zeng., Y.; Ko, B.B.; Hausinger, R.P.; Sun, H. Inhibition of urease by bismuth(III): implications for the mechanism of action of bismuth drugs. Biometals 2006, 19, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Kaul, L.; Abdo, A.I.; Coenye, T.; Krom, B.P.; Hoogenkamp, M.A.; Zannettino, A.C.W.; Süss, R.; Richter, K. The combination of diethyldithiocarbamate and copper ions is active against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro and in vivo. Front Microbiol 2022, 13, 999893. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




