Submitted:
22 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Preparing the INT 407 Cell Line for the Invasion Assay
2.3. Infection of INT 407 with the C. jejuni Strain CjTD-199
2.4. Isolation of RNA from the INT 407
2.5. Whole Transcriptome Analysis
2.6. Bioinformatics - Heatmap
3. Results
3.1. Comparison of Expression Changes of Genes Associated with Immune Functions
3.2. Affected Genes Related to Metabolic Functions
3.3. Comparison of Expression Changes of Genes Associated with Stress Responses
| Gene | Function | 1vs3 | Ref. |
|---|---|---|---|
| VNN1 | response to oxidative stress, pantothenate metabolic process | 379.08 | [58] Zhang et al. 2017 |
| CHAC1 | apoptosis in response to endoplasmic reticulum stress | 8.84537 | [59] Mungrue et al. 2009 |
| LPO | response to oxidative stress | 5.05 | [60] Kovács et al. 1996 |
| ADCYAP1R1 | multicellular organismal response to stress | 3.791 | [61] Ressler et al. 2011 |
| RGCC | positive regulation of stress fiber formation, cell cycle regulation | 3.791 | [62] Wang et al. 2011 |
| HSPA12B | response to stress | -2.374122 | [63] Zouein et al. 2013 |
| SCAMP5 | response to endoplasmic reticulum stress | -3.165496 | [57] Noh et al. 2009 |
3.4. Affected Genes Related to Apoptosis
| Genes. | 1vs3 | Funciton | References |
|---|---|---|---|
| SERPINB9 | 11.3726 | negative regulation of apoptosis by inhibiting granzyme B | [67] Bird et al. 2014 [68] Kaiserman et al. 2010 |
| ACVR1C | 10.1089 | regulation of apoptosis | [69] Asnaghi et al. 2019 |
| CHAC1 | 8.8454 | apoptosis in response to endoplasmic reticulum stress | [70] Zhou et al. 2023 |
| FNDC1 | 7.8517 | positive regulation of cardiac muscle cell apoptosis | [71] Das et al. 2017 [72] Yunwen et al. 2021 |
| G0S2 | 6.9499 | positive regulation of apoptosis | [73] Heckmann et al. 2013 |
| NFATC4 | 6.31812 | positive regulation of apoptosis | [74] Mognol et al. 2016 |
| HIC1 | 5.8969 | signal transduction resulting in induction of apoptosis | [75] Wang et al. 2017 |
| DCC | 5.6863 | regulation of apoptosis | [76] Mehlen et al. 1998 |
| DLC1 | 5.0545 | induction of apoptosis | [77] Zhang et Li 2020 [78] Ullmannova et al. 2007 |
| CD27 | 3.79087 | induction of apoptosis | [79] Prasad et al. 1997 |
| CASP3 | 2.7851 | nuclear fragmentation during apoptosis | [80] Porter et Jänicke 1999 |
3.5. Genes Involved in the Potential Development of Chronic Conditions
3.6. Markedly Affected Genes with Unknown Functions
| Gene name | Fold change |
|---|---|
| ST20-MTHFS | 18.95437 |
| PRR4 (NW_003571047 44554..48182) | 11.3726 |
| C12orf55 | 10.109 |
| NDUFA3(NW_003571054 77272..81394) | 10.109 |
| COL11A2 (NT_167245 4411775..4441552) | 7.581748 |
| ADCK5 (NT_037704 165142..185869) | 3.79087 |
3.7. Heat Map Analysis
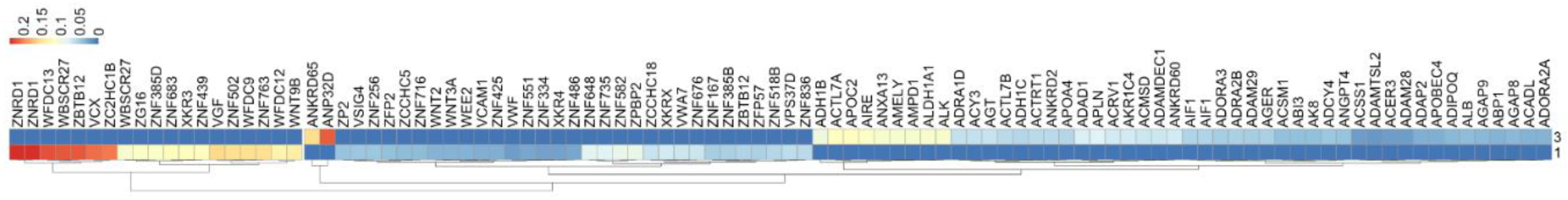
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sher, A.A.; Ashraf, M.A.; Mustafa, B.E.; Raza, M.M. Epidemiological trends of foodborne Campylobacter outbreaks in the United States of America, 1998–2016. Food Microbiology 2021, 97, 103751. [Google Scholar] [CrossRef] [PubMed]
- Melby, K.; Dahl, O.P.; Crisp, L.; Penner, J.L. Clinical and serological manifestations in patients during a waterborne epidemic due to Campylobacter jejuni. Journal of Infection 1990, 21, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.G.; Price, L.; Ahmed, R.; Woodward, D.L.; Melito, P.L.; Rodgers, F.G.; Jamieson, F.; Ciebin, B.; Li, A.; Ellis, A. Characterization of waterborne outbreak–associated Campylobacter jejuni, Walkerton, Ontario. Emerging infectious diseases 2003, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Revez, J.; Llarena, A.-K.; Schott, T.; Kuusi, M.; Hakkinen, M.; Kivistö, R.; Hänninen, M.-L.; Rossi, M. Genome analysis of Campylobacter jejuni strains isolated from a waterborne outbreak. BMC genomics 2014, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gahamanyi, N.; Mboera, L.E.; Matee, M.I.; Mutangana, D.; Komba, E.V. Prevalence, risk factors, and antimicrobial resistance profiles of thermophilic Campylobacter species in humans and animals in sub-saharan Africa: A systematic review. International Journal of Microbiology 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Hlashwayo, D.F.; Sigauque, B.; Noormahomed, E.V.; Afonso, S.M.; Mandomando, I.M.; Bila, C.G. A systematic review and meta-analysis reveal that Campylobacter spp. and antibiotic resistance are widespread in humans in sub-Saharan Africa. PLoS One 2021, 16, e0245951. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clinical microbiology reviews 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Moropeng, R.C.; Tanih, N.F.; Dillingham, R.; Guerrant, R.; Bessong, P.O. Epidemiology of Campylobacter infections among children of 0–24 months of age in South Africa. Archives of Public Health 2022, 80, 107. [Google Scholar] [CrossRef]
- Damborg, P.; Olsen, K.E.; Møller Nielsen, E.; Guardabassi, L. Occurrence of Campylobacter jejuni in pets living with human patients infected with C. jejuni. Journal of Clinical Microbiology 2004, 42, 1363–1364. [Google Scholar] [CrossRef]
- Ghosh, R.; Uppal, B.; Aggarwal, P.; Chakravarti, A.; Jha, A.K. Increasing antimicrobial resistance of Campylobacter jejuni isolated from paediatric diarrhea cases in a tertiary care hospital of New Delhi, India. Journal of clinical and diagnostic research: JCDR 2013, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ramamurthy, T.; Bhattacharya, M.K.; Rajendran, K.; Mukhopadhyay, A.K. Campylobacter jejuni in hospitalized patients with diarrhea, Kolkata, India. Emerging Infectious Diseases 2013, 19, 1155. [Google Scholar] [CrossRef] [PubMed]
- Budge, S.; Barnett, M.; Hutchings, P.; Parker, A.; Tyrrel, S.; Hassard, F.; Garbutt, C.; Moges, M.; Woldemedhin, F.; Jemal, M. Risk factors and transmission pathways associated with infant Campylobacter spp. prevalence and malnutrition: a formative study in rural Ethiopia. PLoS One 2020, 15, e0232541. [Google Scholar] [CrossRef] [PubMed]
- Konkel, M.E.; Mead, D.J.; Hayes, S.F.; Cieplak Jr, W. Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. Journal of Infectious Diseases 1992, 166, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ketley, J.M. Pathogenesis of enteric infection by Campylobacter. Microbiology 1997, 143, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Allos, B.M. Campylobacter jejuni Infections: Update on Emerging Issues and Trends. Clinical Infectious Diseases 2001, 32, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Kovács, J.K.; Cox, A.; Schweitzer, B.; Maróti, G.; Kovács, T.; Fenyvesi, H.; Emődy, L.; Schneider, G. Virulence traits of inpatient Campylobacter jejuni isolates, and a transcriptomic approach to identify potential genes maintaining intracellular survival. Microorganisms 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Amill, V.; Konkel, M.E. Secretion of Campylobacter jejuni Cia proteins is contact dependent. In Mechanisms in the Pathogenesis of Enteric Diseases 2, Springer: 1999; pp. 225–229. [CrossRef]
- Rivera-Amill, V.; Kim, B.J.; Seshu, J.; Konkel, M.E. Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. The Journal of infectious diseases 2001, 183, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Kopecko, D.J.; Hu, L.; Zaal, K.J. Campylobacter jejuni–microtubule-dependent invasion. TRENDS in Microbiology 2001, 9, 389–396. [Google Scholar] [CrossRef]
- Hendrixson, D.R.; DiRita, V.J. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Molecular microbiology 2004, 52, 471–484. [Google Scholar] [CrossRef]
- Reid, A.N.; Pandey, R.; Palyada, K.; Naikare, H.; Stintzi, A. Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit. Applied and environmental microbiology 2008, 74, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Baqar, S.; Bourgeois, A.L.; Ewing, C.P.; Guerry, P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infection and immunity 1999, 67, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Ingmer, H.; Madsen, M.; Bang, D.D. Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence-associated genes. BMC microbiology 2008, 8, 1–10. [Google Scholar] [CrossRef]
- Al-Salloom, F.S.; Al Mahmeed, A.; Ismaeel, A.; Botta, G.A.; Bakhiet, M. Campylobacter-stimulated INT407 cells produce dissociated cytokine profiles. Journal of Infection 2003, 47, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, E.; Berndt, A.; Hänel, I.; Köhler, H. Campylobacter-induced interleukin-8 responses in human intestinal epithelial cells and primary intestinal chick cells. Veterinary microbiology 2007, 124, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio 2014, 5, e01364-14. [Google Scholar] [CrossRef]
- Al-Amri, A.I.; Botta, G.A.; Tabbara, K.S.; Ismaeel, A.Y.; Al-Mahmeed, A.E.; Qareeballa, A.Y.; Dayna, K.M.B.; Bakhiet, M.O. Campylobacter jejuni induces diverse kinetics and profiles of cytokine genes in INT-407 cells. Saudi medical journal 2008, 29, 514. [Google Scholar]
- Nyati, K.K.; Prasad, K.N.; Rizwan, A.; Verma, A.; Paliwal, V.K. TH1 and TH2 response to Campylobacter jejuni antigen in Guillain-Barre syndrome. Archives of neurology 2011, 68, 445–452. [Google Scholar] [CrossRef]
- Sun, X.; Threadgill, D.; Jobin, C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology 2012, 142, 86–95.e5. [Google Scholar] [CrossRef]
- Nyati, K.K.; Nyati, R. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: an update. BioMed research international 2013, 2013. [Google Scholar] [CrossRef]
- Lo, Y. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle & nerve 2007, 36, 615–627. [Google Scholar]
- Java, A.; Liszewski, M.K.; Hourcade, D.E.; Zhang, F.; Atkinson, J.P. Role of complement receptor 1 (CR1; CD35) on epithelial cells: A model for understanding complement-mediated damage in the kidney. Molecular immunology 2015, 67, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Das, N. Complement Receptor 1: disease associations and therapeutic implications. Molecular immunology 2009, 46, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Na, N.; Zhang, X.; Zhao, Y. The biological function and significance of CD74 in immune diseases. Inflammation Research 2017, 66, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Liu, M.; Ji, B.; Bai, B.; Cheng, B.; Wang, C. Role of the extracellular signal-regulated kinase 1/2 signaling pathway in ischemia-reperfusion injury. Frontiers in physiology 2019, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Klimov, E.; Novitskaya, E.; Koval’chuk, S. CD209 (DC-SIGN) – role in the work of the innate immunity and pathogen penetration. Veterinariya, Zootekhniya i Biotekhnologiya 2020, 1, 64–71. [Google Scholar] [CrossRef]
- Shouval, D.S.; Biswas, A.; Goettel, J.A.; McCann, K.; Conaway, E.; Redhu, N.S.; Mascanfroni, I.D.; Al Adham, Z.; Lavoie, S.; Ibourk, M. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014, 40, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.D. Structure and function of natural-killer-cell receptors. Immunologic research 2003, 27, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Fällman, M.; Andersson, R.; Andersson, T. Signaling properties of CR3 (CD11b/CD18) and CR1 (CD35) in relation to phagocytosis of complement-opsonized particles. The Journal of Immunology 1993, 151, 330–338. [Google Scholar] [CrossRef]
- Lupardus, P.J.; Garcia, K.C. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. Journal of molecular biology 2008, 382, 931–941. [Google Scholar] [CrossRef]
- Grimshaw, M.J.; Wilson, J.L.; Balkwill, F.R. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. European journal of immunology 2002, 32, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- DeDiego, M.L.; Martinez-Sobrido, L.; Topham, D.J. Novel functions of IFI44L as a feedback regulator of host antiviral responses. Journal of Virology 2019, 93. [Google Scholar] [CrossRef]
- Starlets, D.; Gore, Y.; Binsky, I.; Haran, M.; Harpaz, N.; Shvidel, L.; Becker-Herman, S.; Berrebi, A.; Shachar, I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 2006, 107, 4807–4816. [Google Scholar] [CrossRef]
- Urban, B.C.; Willcox, N.; Roberts, D.J. A role for CD36 in the regulation of dendritic cell function. Proceedings of the National Academy of Sciences 2001, 98, 8750–8755. [Google Scholar] [CrossRef]
- Kishi, Y.; Kondo, T.; Xiao, S.; Yosef, N.; Gaublomme, J.; Wu, C.; Wang, C.; Chihara, N.; Regev, A.; Joller, N. Protein C receptor (PROCR) is a negative regulator of Th17 pathogenicity. Journal of Experimental Medicine 2016, 213, 2489–2501. [Google Scholar] [CrossRef]
- Preza, G.C.; Tanner, K.; Elliott, J.; Yang, O.O.; Anton, P.A.; Ochoa, M.-T. Antigen-presenting cell candidates for HIV-1 transmission in human distal colonic mucosa defined by CD207 dendritic cells and CD209 macrophages. AIDS research and human retroviruses 2014, 30, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, S.; Ho, A.; de Waal Malefyt, R.; Moore, K.W. Expression cloning and characterization of a human IL-10 receptor. Journal of Immunology (Baltimore, Md.: 1950) 1994, 152, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, S.; Ruiz, M.; Camps, C.; Schluter, A.; Houten, S.M.; Mooyer, P.A.; Pampols, T.; Dacremont, G.; Wanders, R.J.; Giros, M. A key role for the peroxisomal ABCD2 transporter in fatty acid homeostasis. American Journal of Physiology-Endocrinology and Metabolism 2009, 296, E211–E221. [Google Scholar] [CrossRef]
- Chubanov, V.; Gudermann, T. Trpm6. Mammalian Transient Receptor Potential (TRP) Cation Channels: Volume I 2014, 503-520. [CrossRef]
- van der Wijst, J.; Bindels, R.J.; Hoenderop, J.G. Mg2+ homeostasis: the balancing act of TRPM6. Current opinion in nephrology and hypertension 2014, 23, 361–369. [Google Scholar] [CrossRef]
- Tsai, S.H.; Kinoshita, M.; Kusu, T.; Kayama, H.; Okumura, R.; Ikeda, K.; Shimada, Y.; Takeda, A.; Yoshikawa, S.; Obata-Ninomiya, K. The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity 2015, 42, 279–293. [Google Scholar] [CrossRef]
- Wang, Q.; Karvelsson, S.T.; Kotronoulas, A.; Gudjonsson, T.; Halldorsson, S.; Rolfsson, O. Glutamine-fructose-6-phosphate transaminase 2 (GFPT2) Is upregulated in breast epithelial–mesenchymal transition and responds to oxidative stress. Molecular & Cellular Proteomics 2022, 21. [Google Scholar]
- Signorini, C.; De Felice, C.; Durand, T.; Oger, C.; Galano, J.-M.; Leoncini, S.; Pecorelli, A.; Valacchi, G.; Ciccoli, L.; Hayek, J. Isoprostanes and 4-hydroxy-2-nonenal: markers or mediators of disease? Focus on Rett syndrome as a model of autism spectrum disorder. Oxidative Medicine and Cellular Longevity 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers–assembly, dynamics and biological roles. Journal of cell science 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Hu, G.; Tang, J.; Zhang, B.; Lin, Y.; Hanai, J.-i.; Galloway, J.; Bedell, V.; Bahary, N.; Han, Z.; Ramchandran, R. A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. Journal of cell science 2006, 119, 4117–4126. [Google Scholar] [CrossRef]
- Noh, J.-Y.; Lee, H.; Song, S.; Kim, N.S.; Im, W.; Kim, M.; Seo, H.; Chung, C.-W.; Chang, J.-W.; Ferrante, R.J. SCAMP5 links endoplasmic reticulum stress to the accumulation of expanded polyglutamine protein aggregates via endocytosis inhibition. Journal of Biological Chemistry 2009, 284, 11318–11325. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Gao, G.; Wei, G.; Zheng, Y.; Wang, C.; Gao, N.; Zhao, Y.; Deng, J.; Chen, H. Elevation of GPRC5A expression in colorectal cancer promotes tumor progression through VNN-1 induced oxidative stress. International journal of cancer 2017, 140, 2734–2747. [Google Scholar] [CrossRef]
- Mungrue, I.N.; Pagnon, J.; Kohannim, O.; Gargalovic, P.S.; Lusis, A.J. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. The Journal of Immunology 2009, 182, 466–476. [Google Scholar] [CrossRef]
- Kovacs, P.; Juranek, I.; Stankovicova, T.; Svec, P. Lipid peroxidation during acute stress. Die Pharmazie 1996, 51, 51–53. [Google Scholar] [PubMed]
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011, 470, 492–497. [Google Scholar] [CrossRef]
- Wang, J.-N.; Shi, N.; Xie, W.-B.; Guo, X.; Chen, S.-Y. Response gene to complement 32 promotes vascular lesion formation through stimulation of smooth muscle cell proliferation and migration. Arteriosclerosis, thrombosis, and vascular biology 2011, 31, e19–e26. [Google Scholar] [CrossRef]
- Zouein, F.A.; Kurdi, M.; Booz, G.W. HSPA12B and repairing the heart: beauty in simplicity. Cardiovascular Research 2013, 99, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, T.; Wizenty, J.; Quint, J.; Spitz, W.; Bosma, M.; Becker, O.; Adler, A.; Veltzke-Schlieker, W.; Stockmann, M.; Weiss, S. Expression analysis of fibronectin type III domain-containing (FNDC) genes in inflammatory bowel disease and colorectal cancer. Gastroenterology research and practice 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gao, W.; Lin, S.; Chen, H.; Du, B.; Liu, Q.; Lin, X.; Chen, Q. FNDC1 promotes the invasiveness of gastric cancer via Wnt/β-catenin signaling pathway and correlates with peritoneal metastasis and prognosis. Frontiers in Oncology 2020, 10, 590492. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Wang, J.; Ouyang, C.; Chen, C.; Xu, X.-F.; Ye, X.-Q. Overview of serpin B9 and its roles in cancer. Oncology Reports 2021, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.H.; Christensen, M.E.; Mangan, M.; Prakash, M.D.; Sedelies, K.A.; Smyth, M.; Harper, I.; Waterhouse, N.J.; Bird, P.I. The granzyme B-Serpinb9 axis controls the fate of lymphocytes after lysosomal stress. Cell Death & Differentiation 2014, 21, 876–887. [Google Scholar]
- Kaiserman, D.; Bird, P.I. Control of granzymes by serpins. Cell Death & Differentiation 2010, 17, 586–595. [Google Scholar]
- Asnaghi, L.; White, D.T.; Key, N.; Choi, J.; Mahale, A.; Alkatan, H.; Edward, D.P.; Elkhamary, S.M.; Al-Mesfer, S.; Maktabi, A. ACVR1C/SMAD2 signaling promotes invasion and growth in retinoblastoma. Oncogene 2019, 38, 2056–2075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, H. CHAC1 exacerbates LPS-induced ferroptosis and apoptosis in HK-2 cells by promoting oxidative stress. Allergologia et Immunopathologia 2023, 51, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Ogunwobi, O.O. A novel microRNA-1207-3p/FNDC1/FN1/AR regulatory pathway in prostate cancer. RNA & disease (Houston, Tex.) 2017, 4. [Google Scholar]
- Yunwen, C.; Shanshan, G.; Zhifei, B.; Saijun, C.; Hua, Y. The silencing of FNDC1 inhibits the tumorigenesis of breast cancer cells via modulation of the PI3K/Akt signaling pathway. Molecular Medicine Reports 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Zhang, X.; Xie, X.; Liu, J. The G0/G1 switch gene 2 (G0S2): regulating metabolism and beyond. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 2013, 1831, 276–281. [Google Scholar] [CrossRef]
- Mognol, G.P.; Carneiro, F.R.G.; Robbs, B.K.; Faget, D.V.; Viola, J.P.d.B. Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player. Cell death & disease 2016, 7, e2199–e2199. [Google Scholar]
- Wang, Y.; Liang, H.; Zhou, G.; Hu, X.; Liu, Z.; Jin, F.; Yu, M.; Sang, J.; Zhou, Y.; Fu, Z. HIC1 and miR-23~ 27~ 24 clusters form a double-negative feedback loop in breast cancer. Cell Death & Differentiation 2017, 24, 421–432. [Google Scholar]
- Mehlen, P.; Rabizadeh, S.; Snipas, S.J.; Assa-Munt, N.; Salvesen, G.S.; Bredesen, D.E. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998, 395, 801–804. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G. A tumor suppressor DLC1: The functions and signal pathways. Journal of Cellular Physiology 2020, 235, 4999–5007. [Google Scholar] [CrossRef]
- Ullmannova, V.; Popescu, N.C. Inhibition of cell proliferation, induction of apoptosis, reactivation of DLC1, and modulation of other gene expression by dietary flavone in breast cancer cell lines. Cancer detection and prevention 2007, 31, 110–118. [Google Scholar] [CrossRef]
- Prasad, K.; Ao, Z.; Yoon, Y.; Wu, M.X.; Rizk, M.; Jacquot, S.; Schlossman, S.F. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proceedings of the National Academy of Sciences 1997, 94, 6346–6351. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell death & differentiation 1999, 6, 99–104. [Google Scholar]
- Kieseier, B.; Clements, J.; Pischel, H.; Wells, G.; Miller, K.; Gearing, A.; Hartung, H.P. Matrix metalloproteinases MMP-9 and MMP-7 are expressed in experimental autoimmune neuritis and the Guillain-Barre syndrome. Annals of neurology 1998, 43, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Newsom-Davis, J.; Perkin, G.; Pierce, J. Controlled trial of prednisolone in acute polyneuropathy. The Lancet 1978, 312, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Gu, W.; McLandsborough, L. Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food microbiology 2012, 29, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Youssef, S.; Hur, E.M.; Ho, P.P.; Han, M.H.; Lanz, T.V.; Phillips, L.K.; Goldstein, M.J.; Bhat, R.; Raine, C.S. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1-and TH17-mediated autoimmunity. Proceedings of the National Academy of Sciences 2009, 106, 14948–14953. [Google Scholar] [CrossRef] [PubMed]
- Breuer, K.; Foroushani, A.K.; Laird, M.R.; Chen, C.; Sribnaia, A.; Lo, R.; Winsor, G.L.; Hancock, R.E.; Brinkman, F.S.; Lynn, D.J. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic acids research 2013, 41, D1228–D1233. [Google Scholar] [CrossRef]
- Connell, S.; Meade, K.G.; Allan, B.; Lloyd, A.T.; Kenny, E.; Cormican, P.; Morris, D.W.; Bradley, D.G.; O'Farrelly, C. Avian resistance to Campylobacter jejuni colonization is associated with an intestinal immunogene expression signature identified by mRNA sequencing. 2012. [CrossRef]
- Wang, B.G.; Yi, D.H.; Liu, Y.F. TLR3 gene polymorphisms in cancer: a systematic review and meta-analysis. Cancer Communications 2015, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y. CD36 tango in cancer: signaling pathways and functions. Theranostics 2019, 9, 4893. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Pan, Y.; Jin, M.; Zhang, M.; Zhang, S.; Li, Q.; Jiang, X.; Liu, H.; Guo, J.; Liu, H. Association of genetic variants in tachykinins pathway genes with colorectal cancer risk. International journal of colorectal disease 2012, 27, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Jianfeng, W.; Yutao, W.; Jianbin, B. TACR2 is associated with the immune microenvironment and inhibits migration and proliferation via the Wnt/β-catenin signaling pathway in prostate cancer. Cancer Cell International 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, L.; Shi, B.; Bao, J.; Zheng, D.; Zhou, B.; Shi, J. GALNT5 uaRNA promotes gastric cancer progression through its interaction with HSP90. Oncogene 2018, 37, 4505–4517. [Google Scholar] [CrossRef] [PubMed]
- Marín, F.; Bonet, C.; Munoz, X.; García, N.; Pardo, M.L.; Ruiz-Liso, J.M.; Alonso, P.; Capella, G.; Sanz-Anquela, J.M.; González, C.A. Genetic variation in MUC1, MUC2 and MUC6 genes and evolution of gastric cancer precursor lesions in a long-term follow-up in a high-risk area in Spain. Carcinogenesis 2012, 33, 1072–1080. [Google Scholar] [CrossRef]
- Chang, Y.J.; Bae, J.; Zhao, Y.; Lee, G.; Han, J.; Lee, Y.H.; Koo, O.J.; Seo, S.; Choi, Y.-K.; Yeom, S.C. In vivo multiplex gene targeting with Streptococcus pyogens and Campylobacter jejuni Cas9 for pancreatic cancer modeling in wild-type animal. Journal of Veterinary Science 2020, 21. [Google Scholar] [CrossRef]
- Xia, X.; Wu, W.; Huang, C.; Cen, G.; Jiang, T.; Cao, J.; Huang, K.; Qiu, Z. SMAD4 and its role in pancreatic cancer. Tumor Biology 2015, 36, 111–119. [Google Scholar] [CrossRef]
- Naderi, A.; Couch, F.J. BRCA2 and pancreatic cancer. International journal of gastrointestinal cancer 2002, 31, 99–106. [Google Scholar] [CrossRef]
- Olakowski, M.; Tyszkiewicz, T.; Jarzab, M.; Król, R.; Oczko-Wojciechowska, M.; Kowalska, M.; Kowal, M.; Gala, G.M.; Kajor, M.; Lange, D. NBL1 and anillin (ANLN) genes over-expression in pancreatic carcinoma. Folia histochemica et cytobiologica 2009, 47, 249–255. [Google Scholar] [CrossRef]
- Singh, A.P.; Chaturvedi, P.; Batra, S.K. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer research 2007, 67, 433–436. [Google Scholar] [CrossRef]
- Pinho, R.M.; Garas, L.C.; Huang, B.C.; Weimer, B.C.; Maga, E.A. Malnourishment affects gene expression along the length of the small intestine. Frontiers in Nutrition 2022, 9, 894640. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Qin, W.; Buya, M.; Dong, X.; Zheng, W.; Lu, W.; Chen, J.; Guo, Q.; Wu, Y. VNN1, a potential biomarker for pancreatic cancer-associated new-onset diabetes, aggravates paraneoplastic islet dysfunction by increasing oxidative stress. Cancer Letters 2016, 373, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhou, W.; Wu, X.; Hu, Z.; Qiu, L.; Zhang, H.; Chen, X.; Zhang, S.; Lu, Z. CTLs: Killers of intracellular bacteria. Frontiers in Cellular and Infection Microbiology 2022, 1627. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Mangan, N.E.; Cumming, H.; Horvat, J.C.; Mayall, J.R.; Stifter, S.A.; De Weerd, N.; Roisman, L.C.; Rossjohn, J.; Robertson, S.A. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science 2013, 339, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Hamza, E.; Kittl, S.; Kuhnert, P. Temporal induction of pro-inflammatory and regulatory cytokines in human peripheral blood mononuclear cells by Campylobacter jejuni and Campylobacter coli. PloS one 2017, 12, e0171350. [Google Scholar] [CrossRef]
- Kvansakul, M. Viral infection and apoptosis. MDPI: 2017; p 356. [CrossRef]
- Shannon, M.; Kim, J.; Ashworth, L.; Branscomb, E.; Stubbs, L. Tandem zinc-finger gene families in mammals: insights and unanswered questions. DNA Sequence 1998, 8, 303–315. [Google Scholar] [CrossRef]
- Li, W.; Gong, M.; Park, Y.P.; Elshikha, A.S.; Choi, S.-C.; Brown, J.; Kanda, N.; Yeh, W.-I.; Peters, L.; Titov, A.A. Lupus susceptibility gene Esrrg modulates regulatory T cells through mitochondrial metabolism. JCI insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Yamada, N.; Yokoyama, S.; Houjou, I.; Higashi, M.; Yonezawa, S. Promoter hypomethylation contributes to the expression of MUC3A in cancer cells. Biochemical and biophysical research communications 2010, 397, 333–339. [Google Scholar] [CrossRef]
- Sheng, Y.H.; Hasnain, S.Z.; Florin, T.H.; McGuckin, M.A. Mucins in inflammatory bowel diseases and colorectal cancer. Journal of gastroenterology and hepatology 2012, 27, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Praharaj, A.B.; Dehury, B.; Negi, S. Exploring the role and diversity of mucins in health and disease with special insight into non-communicable diseases. Glycoconjugate journal 2015, 32, 575–613. [Google Scholar] [CrossRef]
- Pinto, M.; Máximo, V. NDUFA13 (NADH: ubiquinone oxidoreductase subunit A13). Atlas of Genetics and Cytogenetics in Oncology and Haematology 2018, 8. [Google Scholar]
- Taugbøl, A.; et al. Small changes in gene expression of targeted osmoregulatory genes when exposing marine and freshwater threespine stickleback (Gasterosteus aculeatus) to abrupt salinity transfers. PLoS One 2014, 9, e106894. [Google Scholar] [CrossRef]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Kowalsky, A.H.; Namkoong, S.; Mettetal, E.; Park, H.-W.; Kazyken, D.; Fingar, D.C.; Lee, J.H. The GATOR2–mTORC2 axis mediates Sestrin2-induced AKT Ser/Thr kinase activation. Journal of Biological Chemistry 2020, 295, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Korenbaum, E.; Olski, T.M.; Noegel, A.A. Genomic organization and expression profile of the parvin family of focal adhesion proteins in mice and humans. Gene 2001, 279, 69–79. [Google Scholar] [CrossRef]
- Sargolzaei, J.; Chamani, E.; Kazemi, T.; Fallah, S.; Soori, H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clinical Biochemistry 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Finsterer, J. Triggers of Guillain–Barré syndrome: campylobacter jejuni predominates. International Journal of Molecular Sciences 2022, 23, 14222. [Google Scholar] [CrossRef]
- Chang, K.-H.; Chuang, T.-J.; Lyu, R.-K.; Ro, L.-S.; Wu, Y.-R.; Chang, H.-S.; Huang, C.-C.; Kuo, H.-C.; Hsu, W.-C.; Chu, C.-C. Identification of gene networks and pathways associated with Guillain-Barre syndrome. PloS one 2012, 7, e29506. [Google Scholar] [CrossRef]
- He, Z.; Gharaibeh, R.Z.; Newsome, R.C.; Pope, J.L.; Dougherty, M.W.; Tomkovich, S.; Pons, B.; Mirey, G.; Vignard, J.; Hendrixson, D.R. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 2019, 68, 289–300. [Google Scholar] [CrossRef]
- de Savornin Lohman, E.; Duijster, J.; Groot Koerkamp, B.; van der Post, R.; Franz, E.; Mughini Gras, L.; de Reuver, P. Severe Salmonella spp. Or Campylobacter spp. infection and the risk of biliary tract cancer: a population-based study. Cancers 2020, 12, 3348. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Man, S.M.; Mitchell, H.M. Is Campylobacter to esophageal adenocarcinoma as Helicobacter is to gastric adenocarcinoma? Trends in microbiology 2015, 23, 455–462. [Google Scholar] [CrossRef]
| Gene Name | Function | 1vs3 Experiment - Fold Change (normalized values) | Refer. |
|---|---|---|---|
| ULBP3 | regulation of immune response, natural killer cell activation | 10.1090 | [39] Sun 2013 |
| CR1 | innate immune response phagocytosis |
7.581748 | [40] Fällman et al. 1996 |
| IL23R | positive regulation of defense response to virus by host, inflammatory response | 6.31 | [41] Lupardus et Garcia 2008 |
| EDN2 | macrophage activation, signaling patway | 3.68557 | [42] Grimshaw et al 2002 |
| IFI44L | defense response to virus, immune response | 3.317 | [43] DeDiego et al.2019 |
| CD74 | negative regulation of mature B cell apoptosis, positive regulation of neutrophil chemotaxis, positive regulation of T-helper 2 type immune response, T cell selection, negative regulation of apoptosis, positive regulation of B cell proliferation, positive regulation of ERK1 and ERK2 cascade, antigen processing and presentation |
2.5272 | [35] Su et al.2017 [44] Starlets et al.2006 |
| CD36 | antigen processing and presentation of exogenous, peptide antigen via MHC class I, antigen processing and presentation of peptide antigen via MHC class I |
2.73785 | [45] Urban et al. 2001 |
| PROCR | antigen processing and presentation, PROCR acted as a negative regulator of Th17 pathogenicity | 2.5272 | [46] Kishi et al. 2016 |
| CD209 | regulation of T cell proliferation, antigen processing and presentation, innate immune response |
2.5272 | [47] Preza et al. 2014 |
| IL10RA | inhibits the synthesis of proinflammatoric cytokines | 2.5272 | [48] Liu et at. 1994 |
| Gene name | Function | Fold change | References |
|---|---|---|---|
| ABCD2 | very-long-chain fatty acid metabolic process | 13.89 | [49] Fourcade et al.2009 |
| TRPM6 | Mg2+ channel, and uptake regulator | 11.37 | [51] van der Wijst et al.2014 |
| ENPP3 | phosphate metabolic process, nucleoside triphosphate catabolic process | 8.84537 | [52] Tsai et al. 2015 |
| GFPT2 | glutamine metabolic process, fructose 6-phosphate metabolic process | 2.52 | [53] Wang et al.2022 |
| Gene name | 1vs3 | referenc. | |
|---|---|---|---|
| Guillan- Barré s. disease severity (GBS) | PTGS2 | 1.188331 | [83] Chang et al.(2012) |
| ANXA3 | 1.315609 | [82] Hughes et al. (1978) |
|
| CREB1 | 1.732517 | [82] Hughes et al. (1978) |
|
| Inflammatory | RELB | 1.624660 | [85] Breuer et al. 2013 |
| BIRC3 | 1.958618 | [85] Breuer et al. 2013 |
|
| NFKBIA | -2.553647 | [85] Breuer et al. 2013 |
|
| Autoimmune inflammation | ACE | 3.79087 |
[86] Connell et al. 2012 |
| General cancer markers | TLR3 | 3.79087 | [87] Wang et al. 2015 |
| CD36 | 2.73785 | [88] Wang et Li 2019 |
|
| Tumorgenesis | SERPINB9 | 11.37262 | Wang et al. 2021 |
| FNDC1 | 7.581749 | Jiang et al. 2020 | |
| TACR2 | 8.845373 | [89] Yu et al. 2012 [90] Jianfeng et al. 2021 |
|
| Gastric cancer | GALNT5 | 8.84537 | [65,91] Jiang et al.2020 Guo et al.2018 |
| MUC6 | 3.36 | [92] Marín et al. 2012 |
|
| Pancreatic cancer |
KRAS | 2.011985 | [93] Chang et al. 2020 |
| SMAD4 | 1.34157 | [94] Xia et al. 2015 |
|
| BRCA2 | 1.231316 | [95] Naderi et Couch. 2002 |
|
| NBL1 | 5.054499 | [96] Olakowski et al. 2009 |
|
| MUC4 | 3.15 | [97] Singh et al. 2007 |
|
| Oxidative stress in the intestine | VNN1 | 379.087 | [98] Pinho et al. 2022 [99] Kang et al. 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





