1. Introduction
Water reservoirs are crucial in sustaining ecosystems and supporting human activities, providing a number of ecosystem services like water supply, flood prevention, and recreational etc. [
1,
2]. However, lentic aquatic ecosystems are often subject to degradation by environmental stressors, such as pollution, eutrophication, and habitat loss. Effective and sustainable revitalisation methods are essential to address these challenges and restore the ecological balance of water reservoirs [
3,
4].
In recent years, biotechnological approaches, particularly utilizing microbiological interventions, have emerged as promising strategies for water reservoir revitalization [
5,
6]. Microorganisms, with their remarkable ability to degrade pollutants and enhance nutrient cycling, offer great potential to remediate environmental issues in a natural and eco-friendly manner [
7]. In response to these challenges, increasing attention has been given to the application of microbiological biopreparations, particularly Effective Microorganisms (EM), as potential remediation tools [
8,
9,
10]. Microbiological biopreparations with EM, represent a compelling approach to the remediation of water bodies for their capacity to support natural biodegradation processes and ecosystem restoration. EM are a unique combination of beneficial microorganisms, including bacteria, yeast, and fungi, which synergistically interact, exerting a positive influence on the aquatic environment [
11]. The scientific literature [
6,
9,
12,
13]. Numerous reports on the potential benefits offered by these biopreparations, such as water quality improvement, pollution reduction, inhibition of cyanobacterial and algal growth, biodiversity restoration, and revitalization of aquatic ecosystems, emphasize on the success and advantages of using such techniques [
6,
9,
12,
13,
14]. Be that as it may, the successful application of microbiological biotechnology in water reservoirs heavily relies on understanding and addressing the complex environmental factors that influence its efficiency [
14].
As the global need for sustainable water management intensifies, mastering the complexities of microbiological biotechnology in the context of water reservoir revitalization becomes increasingly vital, if we are to comply with the Ecological Assessment of Streams and Reservoirs suggested by the Environment Action Programme 2030, protecting, preserving and restoring ecosystems and biodiversity, and enhancing natural capital [
15].
A brief overview of the application area of the microbiological remediation method for lakes:
The assessment encompassed 34 water bodies located in Poland, spanning a wide range of geographical and geomorphological regions. Water bodies shared comparable conditions for vegetative seasonal patterns and pathologies within lake ecosystems, the temperate climate surrounding Poland influences the ecosystem’s dynamic aquatic vegetative period, governed by a myriad of complex and diverse factors spanning from spring to autumn. It is worthwhile examining in detail the factors that influence duration and intensity, in particular those influencing reservoirs that frequently display uncommon seasonal patterns.
Water temperature plays a fundamental role in the development of aquatic organisms and is one of the main determinants of vegetative seasonal length. Literature reports by Prejs et al. [
16] and more contemporary analyses by Bielczyńska [
17], Pełechata et al. [
18], Napiórkowski et al. [
19] demonstrated that yearly seasonal temperature variability significantly affects the vegetative period timespan. Water Temperature rise during spring stimulates aquatic organisms’ activity, driving the onset of the vegetative season, conversely, late summer and autumn cool temperatures, decrease aquatic organism’s function who enter a dormant phase, marking the conclusion of this period [
20,
21,
22]. The presence of nutrients, such as nitrates and phosphates from many sources, hold essential significance for the duration of the vegetative season. Water enriched with these nutrients can favour the vigorous growth of aquatic organisms, which can either shorten or prolong the vegetative season, depending on the extent of nutrient availability [
23,
24].
Regrettably, the state of water quality in Poland is far from the EU Water Framework Directive’s desired objectives. According to the latest research data [
25,
26], most Polish lakes (approximately 89%) exhibit a low ecological status, and over 90% of water bodies have their chemical conditions rated below good, at least once a year. Consequently, there is an urgent need for remedial actions and the elimination of pollution sources to restore ecological balance in Poland's surface waters. The requirement for comprehensive remediation efforts encompassing both small and large water reservoirs require the use of methods and tools characterized by a wide-ranging efficacy, minimal perturbation to the aquatic environment, and a sound economic justification. In Poland, only one biotechnological company ACS Holding (group) engages in large-scale initiatives related to the reclamation of water reservoirs through biological methods. This company exclusively employs biological methods, avoiding chemical approaches, while collaborating with numerous scientific research centers. Since 2014, this consortium of entrepreneurs and water biotechnology experts has successfully executed over 200 microbiological remediation processes for degraded water reservoirs all over Poland.
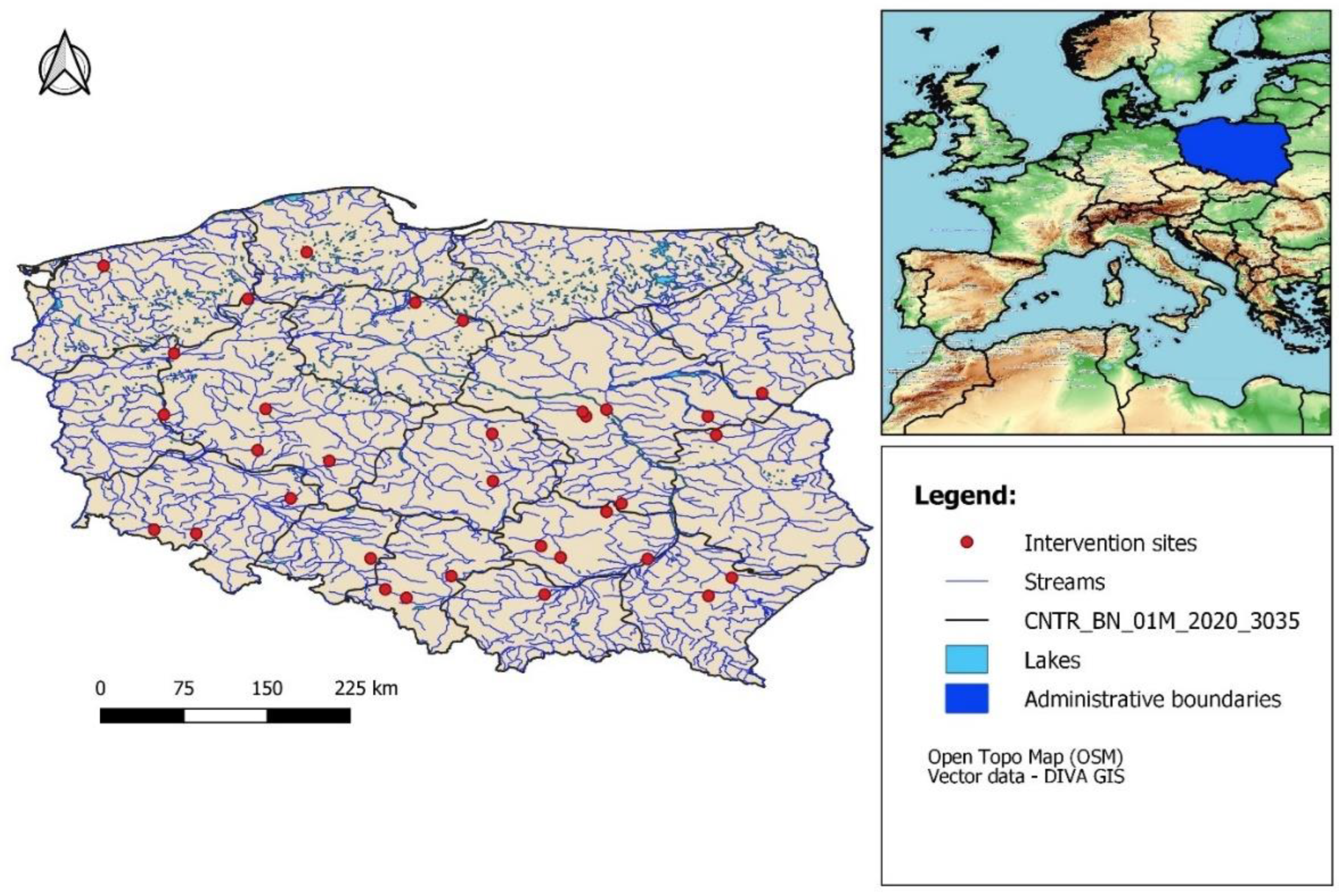
The aim of this article is to present the results of changes in the reduction of bottom sediments, water transparency, dissolved oxygen concentration (at the bottom and near the surface), and water turbidity for 34 small and large reservoirs, subject to reclamation efforts from 2014 to 2023 (
Figure 1). All processes of water reservoir reclamation were carried out for public entities managed by local government units. Annually, the holding conducts approximately 30 reclamation projects for various water reservoirs in Poland. Sites were selected randomly and according to data monitoring availability for all parameters.
2. Materials and Methods
Lake reclamation using biopreparates application involves road transportation to the application site in volumes appropriate to the reservoir reclamation plan (ranging from a couple to several dozen cubic meters) (
Figure 2).
The application of biopreparations can begin when the water temperature exceeds 5°C, though the optimal temperature range is 10 - 25°C, within this range, microorganisms awaken from a dormant state and initiate active metabolism in the new aquatic environment. The cocktail of biopreparates also stimulates the growth of autochthonous microorganisms and colonizes the aquatic macrophyte root radicular zone. The application of biopreparations for the reduction of soft organic sediment fractions is carried out with gentle flow injector streams, so as not to disturb the bottom sediments and cause increased turbidity in the water, thus reducing the efficiency of the process.
Water Surface application is conducted with high-flow injector streams, thereby ensuring good mixing in water and improved oxygenation (stimulating better adaptation of consortia to the new critical environment). Biomixtures were intensively introduced into the macrophyte zone, which provides a very safe area for their development and protects against the negative effects of sunlight UV radiation (
Figure 3). The application is carried out by several technical groups in predesignated quadrants of the reservoir.
Before commencing the biopreparations application process, a comprehensive monitoring of water quality parameters is conducted with the participation of project’s inspectors.
The main types of biopreparations used were primarily ODO1 for sediment treatment and ODO2 for the reduction of organic pollutants in surface water.
ACS ODO-1 biopreparation: Comprises water, a blend of lactic acid bacteria, phototrophic bacteria, yeast, ecological molasses derived from sugar cane, fermented wheat bran, and minerals. In addition, the micro-level ingredients of this biopreparation include phytosterols (such as sitosterol and taraxasterol), phytohormones, triterpenes (like lupeol, betulin, and betulinic acid), flavonoids (including hyperoside, quercetin, and kaempferol), ellagic acid, pyrocatechic acid, brevofolin (an ellagic acid derivative), vitamins (C, PP, P, B3, B5, B8, B11, B1, B2, A, E, F), and tannins.
ACS aqua 2 biopreparation: Comprises water, sugar cane molasses, and effective microorganisms, including the primary strains of effective microorganisms: Lactobacillus casei, Lactobacillus plantarum (at a concentration of 5.0 × 106 cfu·mL−1), and Saccharomyces cerevisiae (at a concentration of 5.0 × 103 cfu·mL−1).
Water monitoring.
Measurements were conducted at multiple points within each of the analysed reservoirs, following the established research plan before commencing the reclamation process, and in the subsequent season. The research included a minimum of 6 measurement points. In the case of interconnected reservoirs, 6 measurement points were utilized for each reservoir. Monitoring studies were conducted at corresponding times in both seasons (before revitalization and after). Water quality was monitored during rain-free periods as well as during periods of algal blooms. The measured parameters and methods/tools used for water monitoring are shown in
Table 1.
The data was averaged for each lake, and comparative statistical analyses were performed for the selected sites using the statistical significance analyses of differences with SPSS 25 software for the examined water quality parameters before reclamation and in the subsequent vegetative season after bioremediation treatments. Statistical non-parametric Wilcoxon paired t-tests were employed to verify data significance.
Environmental parameters of investigated water reservoirs
Most of the analysed water reservoirs are classified as shallow, with only three cases considered to have a moderate depth (
Table 2). The Pniowiec Dam Reservoir and Niskie Lake have zones where a thermocline is present (
Table 2). The others belong to polymictic reservoirs, which respond rapidly and vigorously to changes in water trophic status and the influence of water pollution. These reservoirs are predominantly located throughout the territory of Poland (
Figure 1), except mountainous regions.
3. Results and Discussion
3.1. Reduction of Soft Organic Sediment Fractions (SOSF)
The sedimentation process of suspended particles, predominantly consisting of organic fractions, leads to an increase in bottom sediments during the spring-summer and autumn periods in eutrophicated and highly polluted reservoirs [
27,
28,
29,
30]. The rise in lake trophic levels, coupled with intense algal blooms, intensifies the summer sedimentation processes, resulting in significant increments of organic fractions, thereby affecting the variability of sediment thickness [
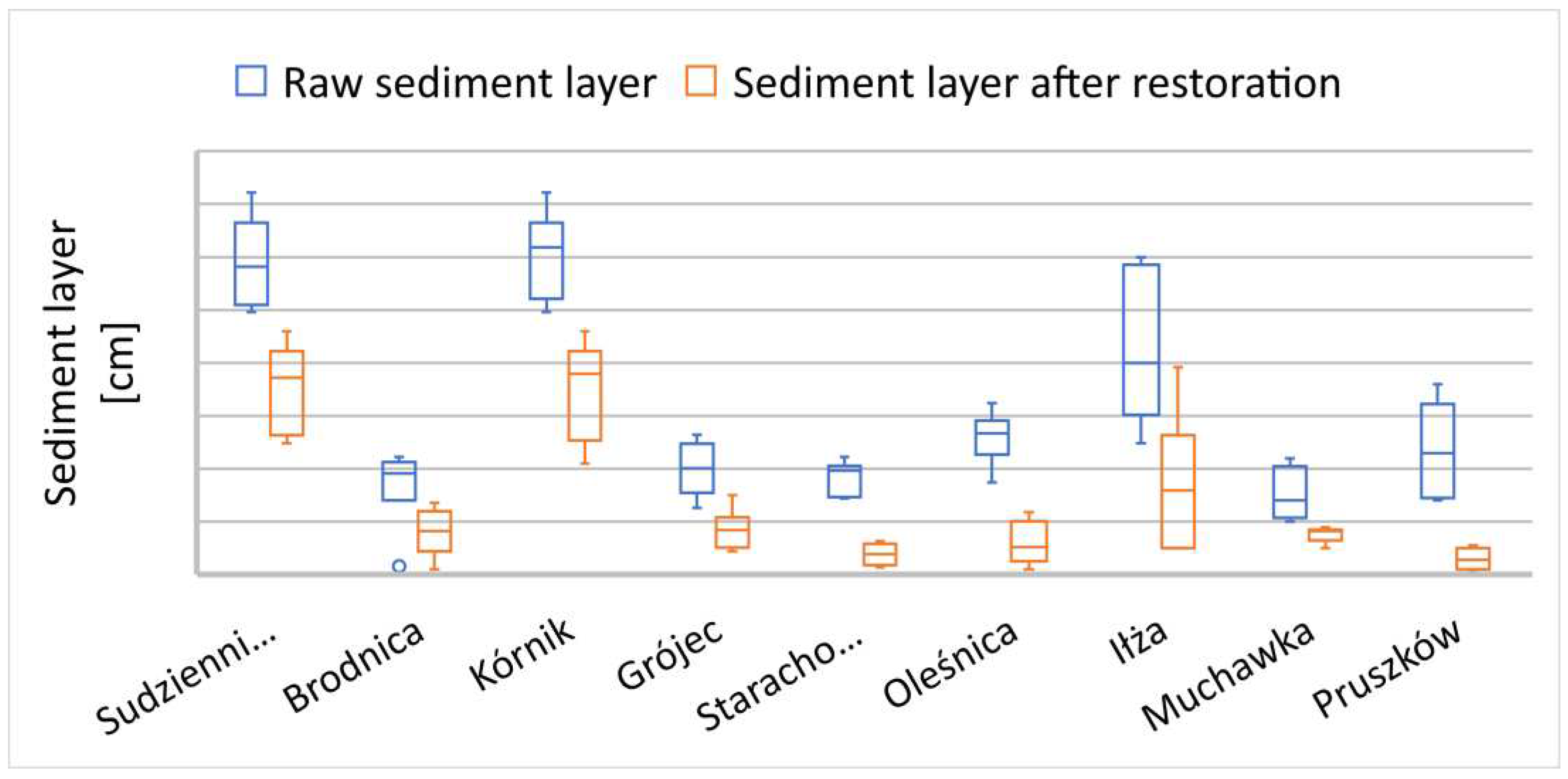
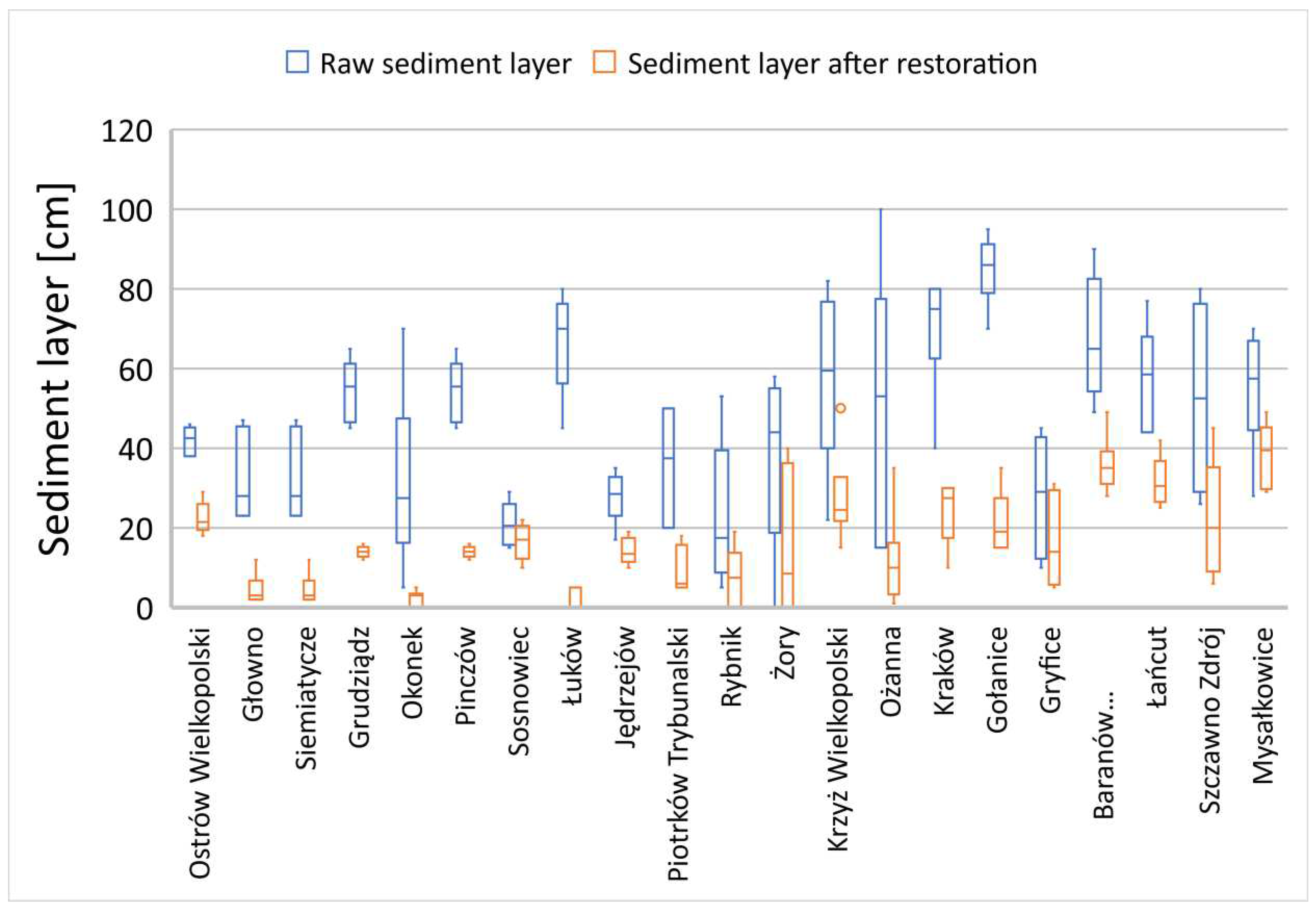
29]. In nine of the assessed rehabilitated reservoirs, a layer of accumulated organic sediment fractions exceeding 1 meter in thickness was observed (
Figure 4). These reservoirs were characterized by pronounced algal blooms and hypertrophy due to the inflow and accumulation of significant nutrients in the water. In the remaining surveyed reservoirs, the layers of organic sediments did not exceed 1 meter in thickness; nevertheless, still a considerable burden for these water bodies. The biological decomposition of organic material in the bottom layer, once dissolved oxygen is depleted, occurs under strongly oxygen-deprived conditions through fermentation, generating a range of toxic substances and environmental nuisances for such degraded lakes [
31].
All rehabilitated reservoirs, prior to treatment, exhibited odours and other environmental and socio-social nuisances that hindered their proper exploitation and exhibited an overall negative impact on the quality of aquatic ecosystems. In all studied reservoirs, a successive reduction of soft organic fractions in bottom sediments was observed in the following vegetative season after the application of directional Biomixtures (
Figure 4–6).
In reservoirs where the accumulated thickness of organic sediment fractions (SOSF) exceeded 1 meter, there was a pronounced dynamic reduction observed in subsequent seasons during corresponding research periods, clearly observed in Gotanice, Tuków and Baranów (
Figure 4). The reduction processes occurred slightly slower in water bodies with sediment layers less than 1 meter thick, Sosnowiec, Jedrzejów and Rybnik (
Figure 5).
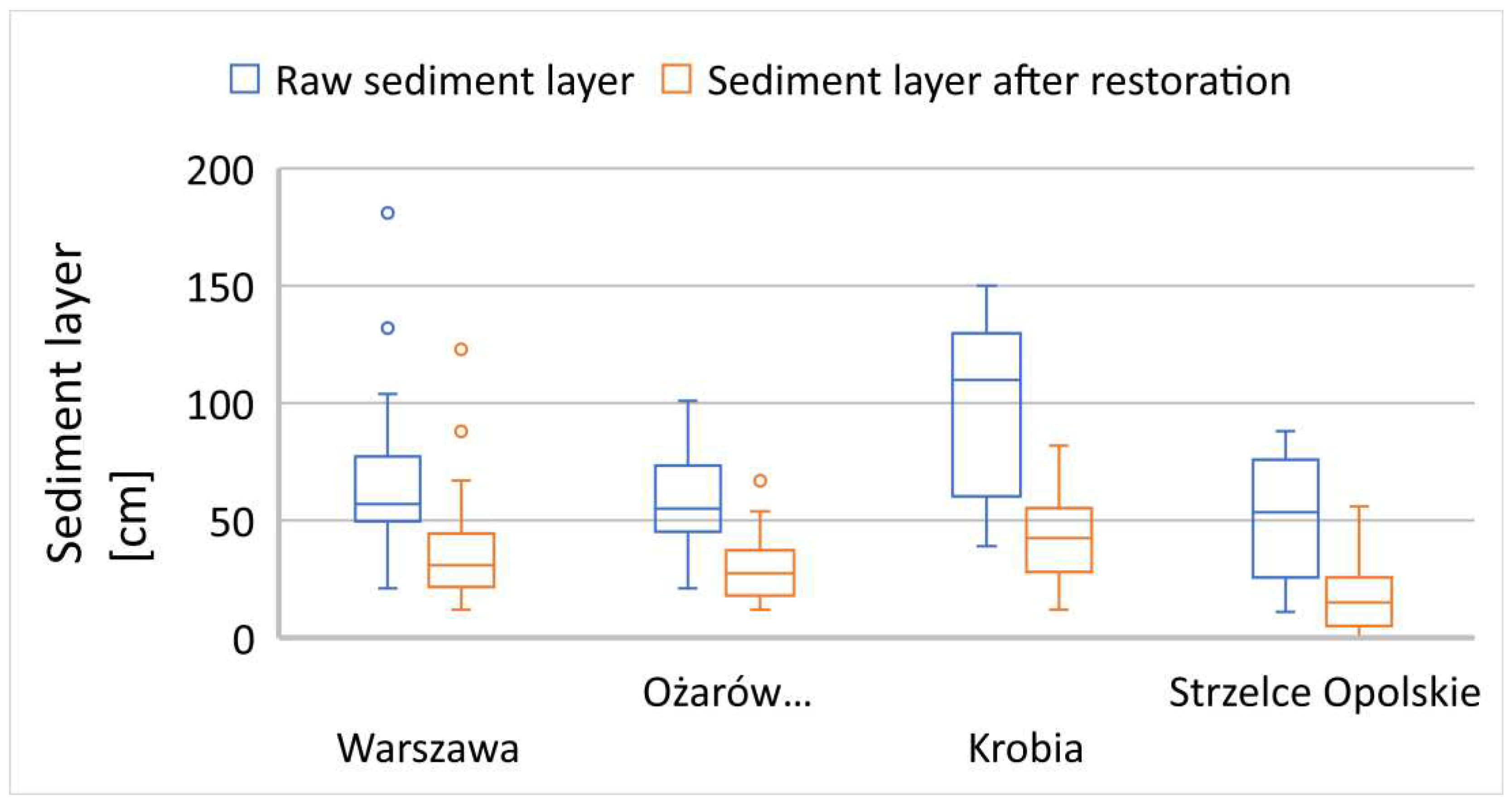
The analysis of result distribution demonstrated a highly satisfactory decomposition process, with, in most cases, a twofold or greater reduction compared to the previous season before the start of the rehabilitation efforts. Additionally, the interconnected reservoirs in the analysed areas exhibited a highly satisfactory decrease in SOSF (
Figure 6). Due to the local conditions, the individual interconnected ponds had varying thicknesses of bottom sediment layers, with the initial reservoirs having thicker sediment layers and the final ones significantly thinner. This is evident in the distribution of SOSF results, both for the original layers and post-rehabilitation (
Figure 6).
Observations conducted after the application of biopreparations to rehabilitate water bodies revealed morphological changes in SOSF. Accumulated primary layers were characterized by significantly compact and dense consistency. Following the introduction of biopreparations, a gradual softening of these fractions was observed, making them further hydrated and easier to be carried away by the flowing water. In reservoirs where these fractions reached thicknesses of 2 to 3 meters (Studniczno, Iłżanka, Kórnik reservoirs -
Figure 4), an additional factor enhancing the dynamics of their reduction could have been erosion, especially in the case of flow-through lakes. Besides the microbiological remediation method, the only effective and rapid removal of sediment from silted and polluted lakes was determined by mechanical sediment removal processes [
32,
33,
34,
35]. However, mechanical methods are extremely costly and invasive for the aquatic environment, making them nearly impossible to implement in the case of large water reservoirs. The reduction dynamics characteristic of other methods is not as effective when compared to this biotechnological method based on microbial consortia utilizing SOSF as a source of organic carbon [
9]. Changes in bottom sediment layer outside the park pond in Sosnowiec exhibited statistically significant differences in both periods before reclamation and in the subsequent season after bioremediation treatments were conducted (
Table A1). Watercourses supplying the Leśny pond in Sosnowiec continued to supply a substantial amount of sediment, contributing to turbidity, during the reclamation period, disrupting the purification processes. Since this reservoir is non-flowing, the excessive accumulation of introduced sediments had a detrimental impact on the rate of their reduction.
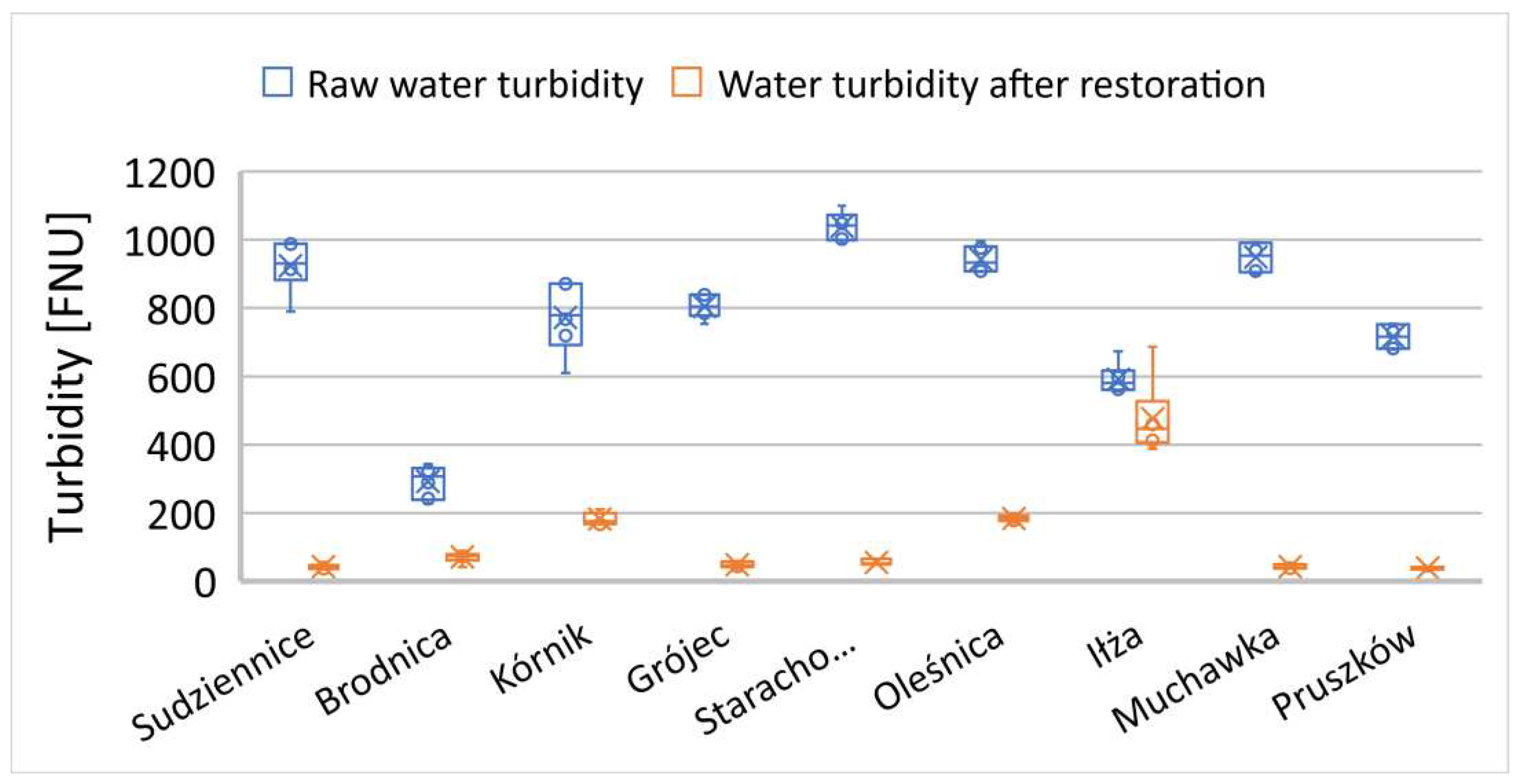
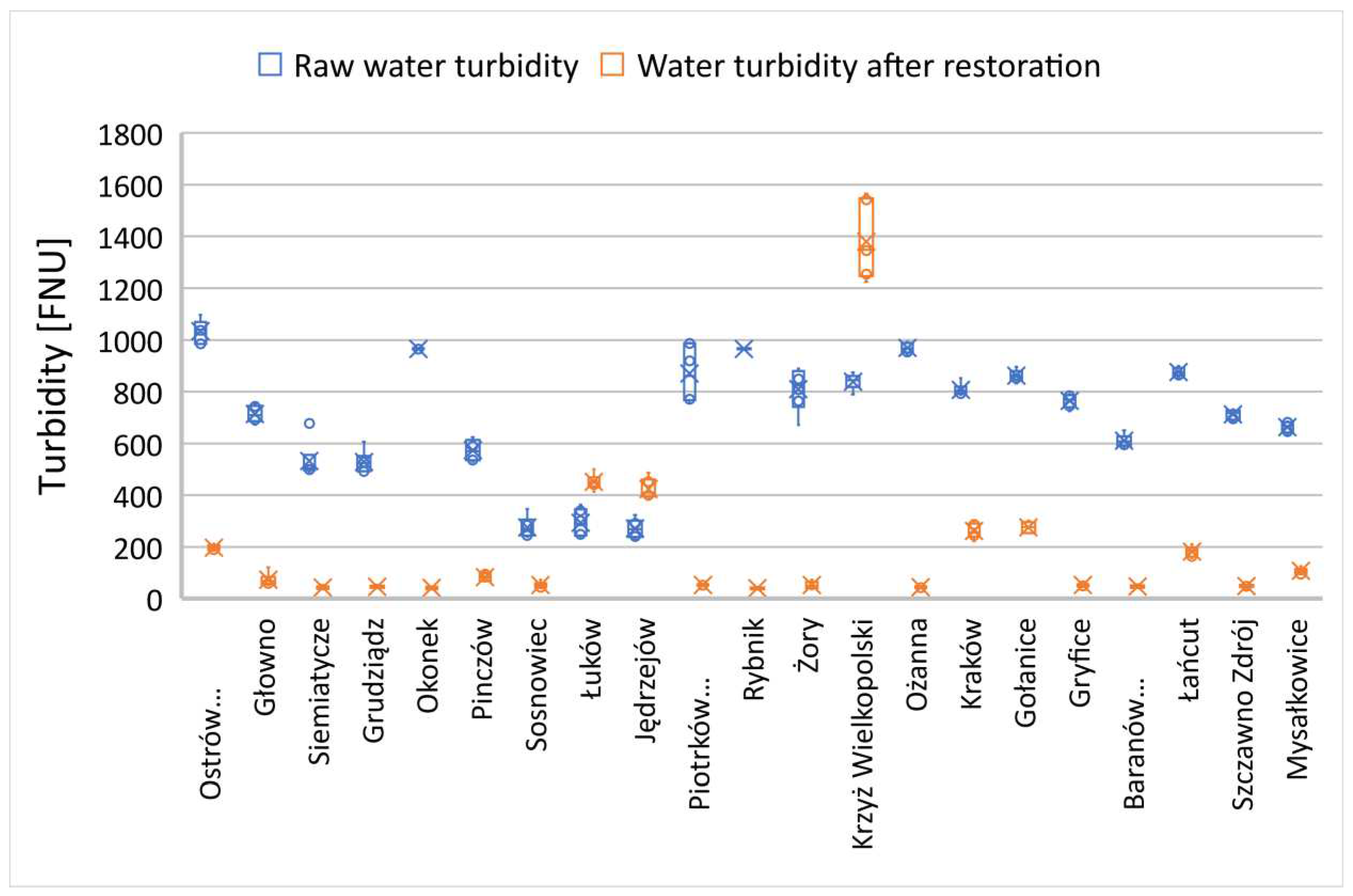
3.2. Changes in water transparency and turbidity
Water transparency and water turbidity are inversely proportional phenomena, resulting from a variety of environmental factors. The process of eutrophication and algal blooms leads to a significant decrease in water transparency and a reduction in the penetration of light into the aquatic environment [
36,
37,
38]. However, water turbidity may result from causes other than the development of planktonic algae, such as heavy rainfall or snowmelt, which result in turbulent flows responsible for persistent turbidity due to an increase in suspended mineral particles in the water [
39]. Water of all the rehabilitated reservoirs, prior to treatment, was characterized by significant eutrophication and intense algal blooms during the growing season. In most cases, the predominant form of water turbidity is long-term reconstructed turbidity resulting from the development of planktonic algae.
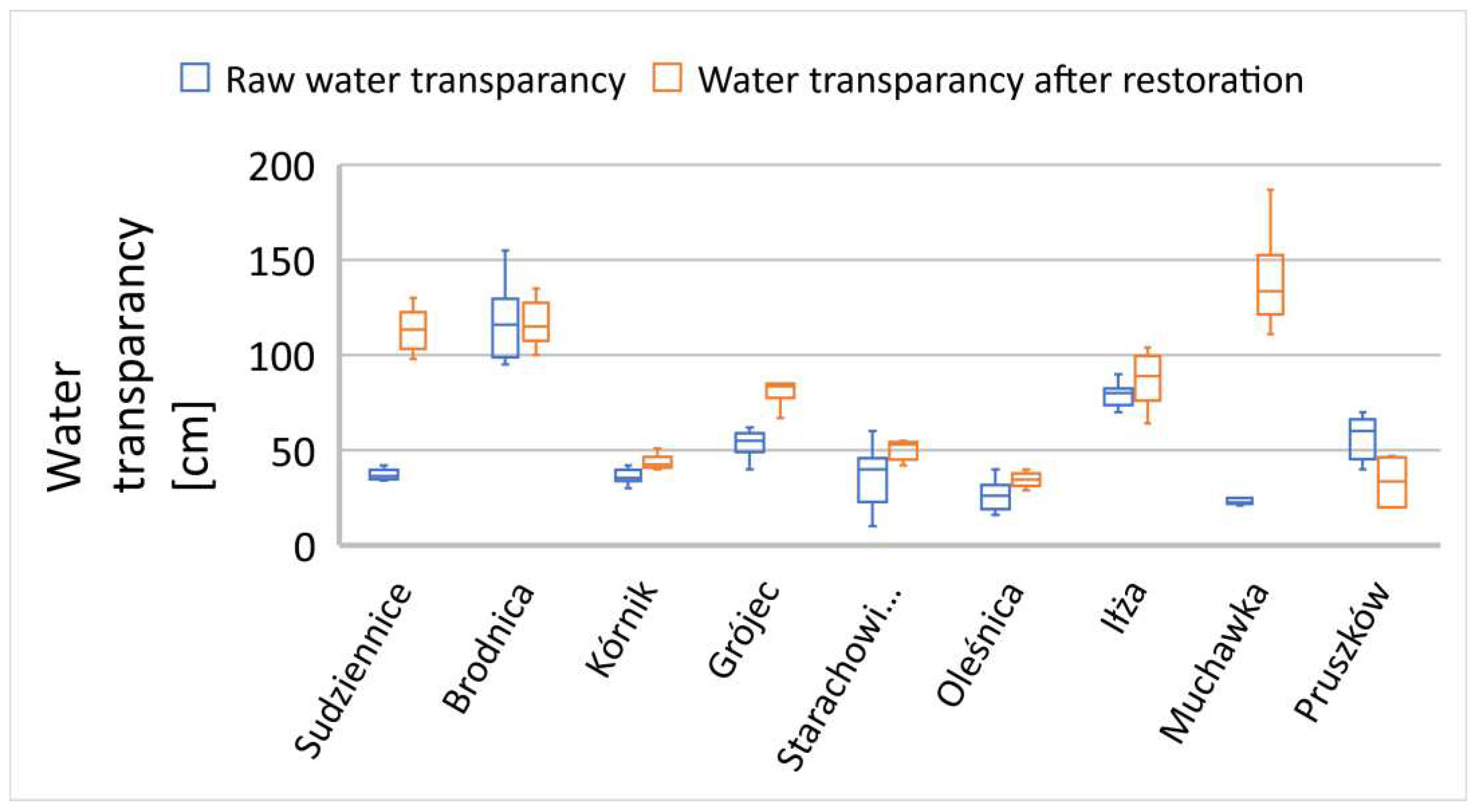
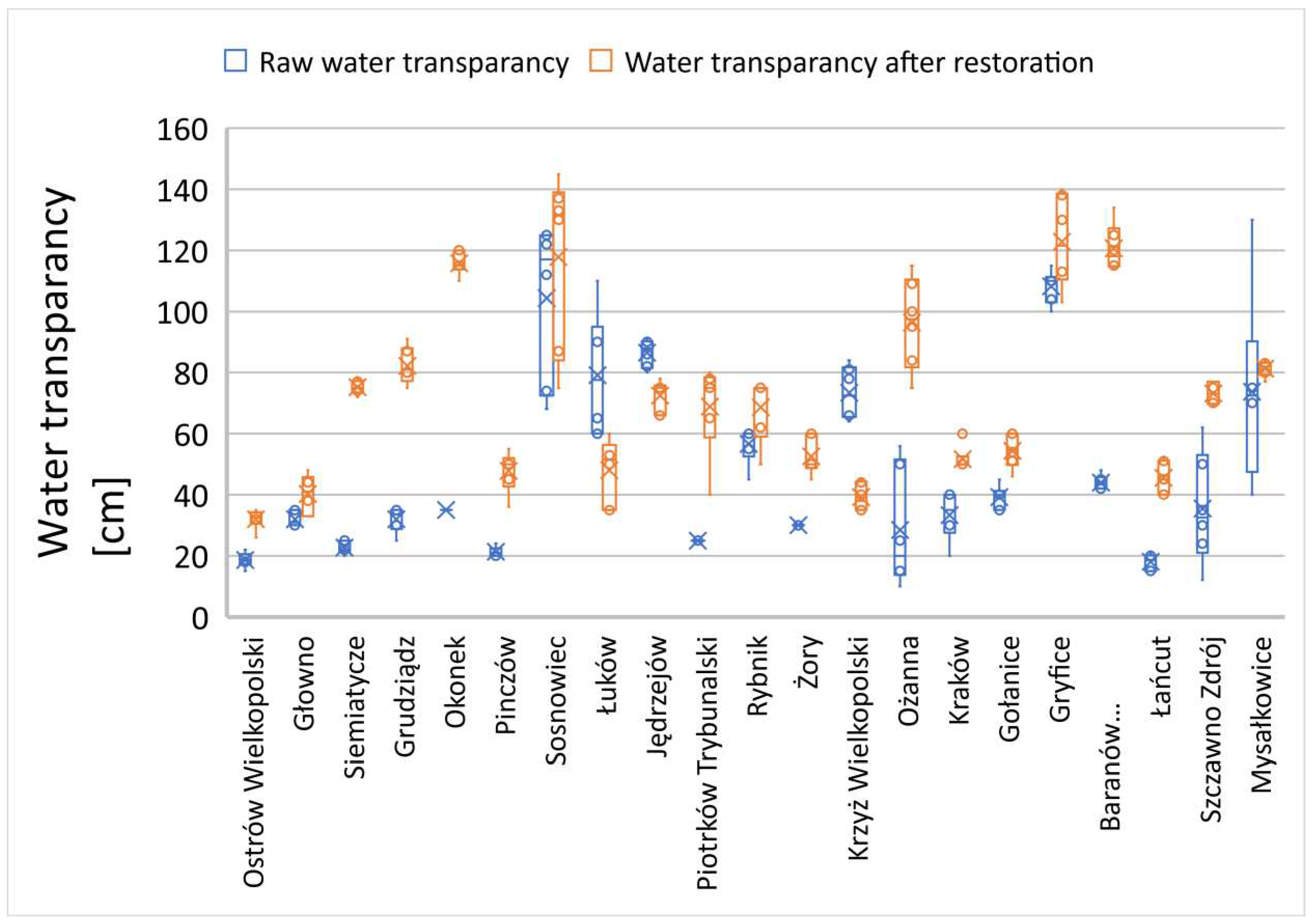
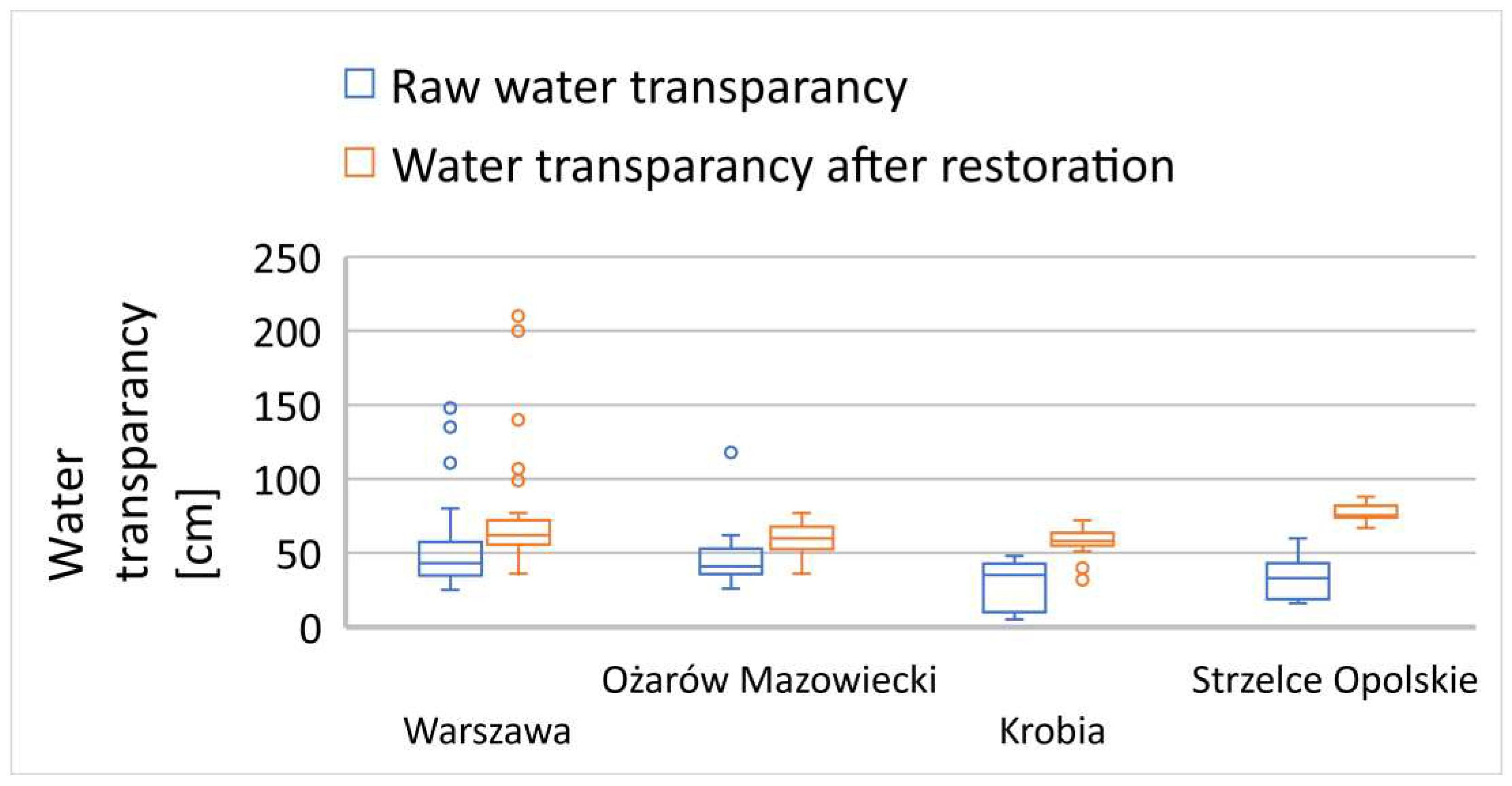
In many of the examined reservoirs, the initial transparency value did not exceed 60 cm, with only a few characterized by a transparency zone of 60 cm or more (
Figure 7,
Figure 8 and
Figure 9). There is no clear correlation between the thickness of sediment layers and the light penetration zone in the reservoirs subjected to reclamation processes. In the following growing season, a significant improvement in the euphotic zone was observed in most of the examined cases (
Figure 7,
Figure 8 and
Figure 9), except for the Jędrzejów reservoir (
Figure 8). This was a case in which the commission's investigations took place during a rainy period with persistent mineral suspensions affecting water transparency.
The reservoirs in the towns of Brodnica, Krzyż Wielkopolski, Pruszków, Sosnowiec, and Mysłakowice (
Figure 7 and 8) also exhibited low or poor water transparency due to stagnant water conditions and the release of nutrients from the biological degradation of organic carbon in the sediment layers. In the remaining reservoirs, water flow and a well-developed zone of aquatic macrophytes buffer zone stabilized the supply of nutrients, preventing algal blooms in the subsequent season after reclamation. In the reservoirs that showed improved transparency, there was also a trend of reduced summer sedimentation rates. A strong inverse correlation was observed between transparency and water turbidity results (Table 3). In five cases (
Table A2), statistical analyses did not allow for such conclusions to be drawn for the majority of the examined reservoirs. Statistically significant differences in water transparency improvement were clearly demonstrated in the subsequent season following microbiological bioremediation processes. The research results were influenced by local weather conditions or lake parameters that disrupted natural transparency or slowed down the improvement process. Sediment analysis for these reservoirs with disturbed euphotic zones nonetheless indicated that biological decay processes are effective (
Table A1).
Reservoirs that exhibited increased turbidity levels in the subsequent season after reclamation also had a poorer euphotic zone. In these cases, these relationships are closely intertwined (
Figure 10 and
Figure 11). Turbidity in most cases was induced by planktonic algae, though when employing microbiological reclamation methods, the improvements in the studied parameters become evident in the following season. Enhancing the euphotic zone plays a crucial role in the development of aquatic organisms and achieving the appropriate ecological diversity for this type of ecosystem [
40]. The improvement of the euphotic zone and the reduction in turbidity constitute the second important criterion achieved using such biopreparations in the reclamation process of degraded water reservoirs.
Changes in water turbidity level (FTU) and dissolved oxygen concentration near the bottom in all cases showed statistically significant differences (
Table A3). Improvements in these parameters in the subsequent vegetative season after bioremediation treatments were demonstrated in all reservoirs at 34 locations.
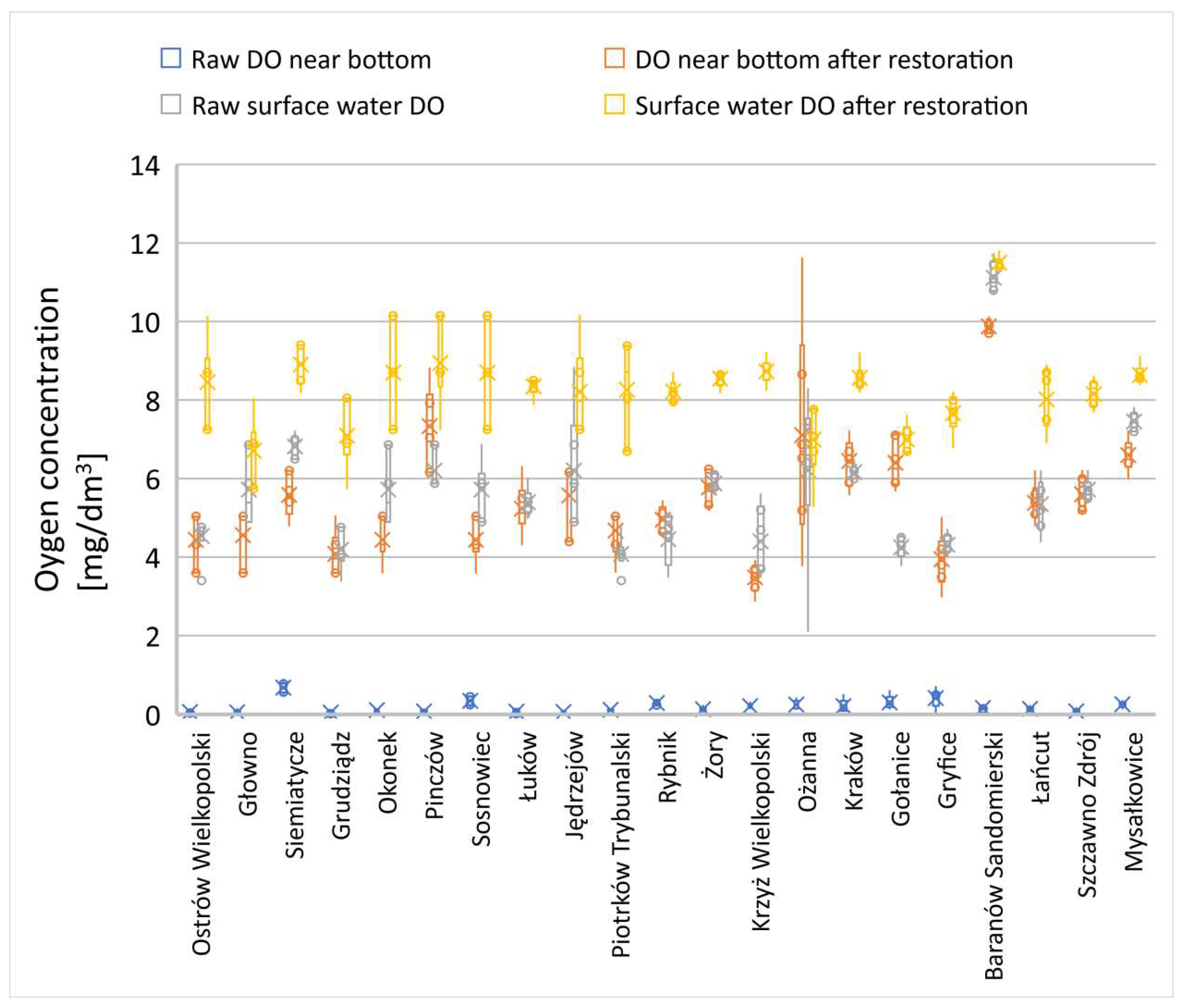
3.3. Changes in the concentration of dissolved oxygen (DO) in a water column
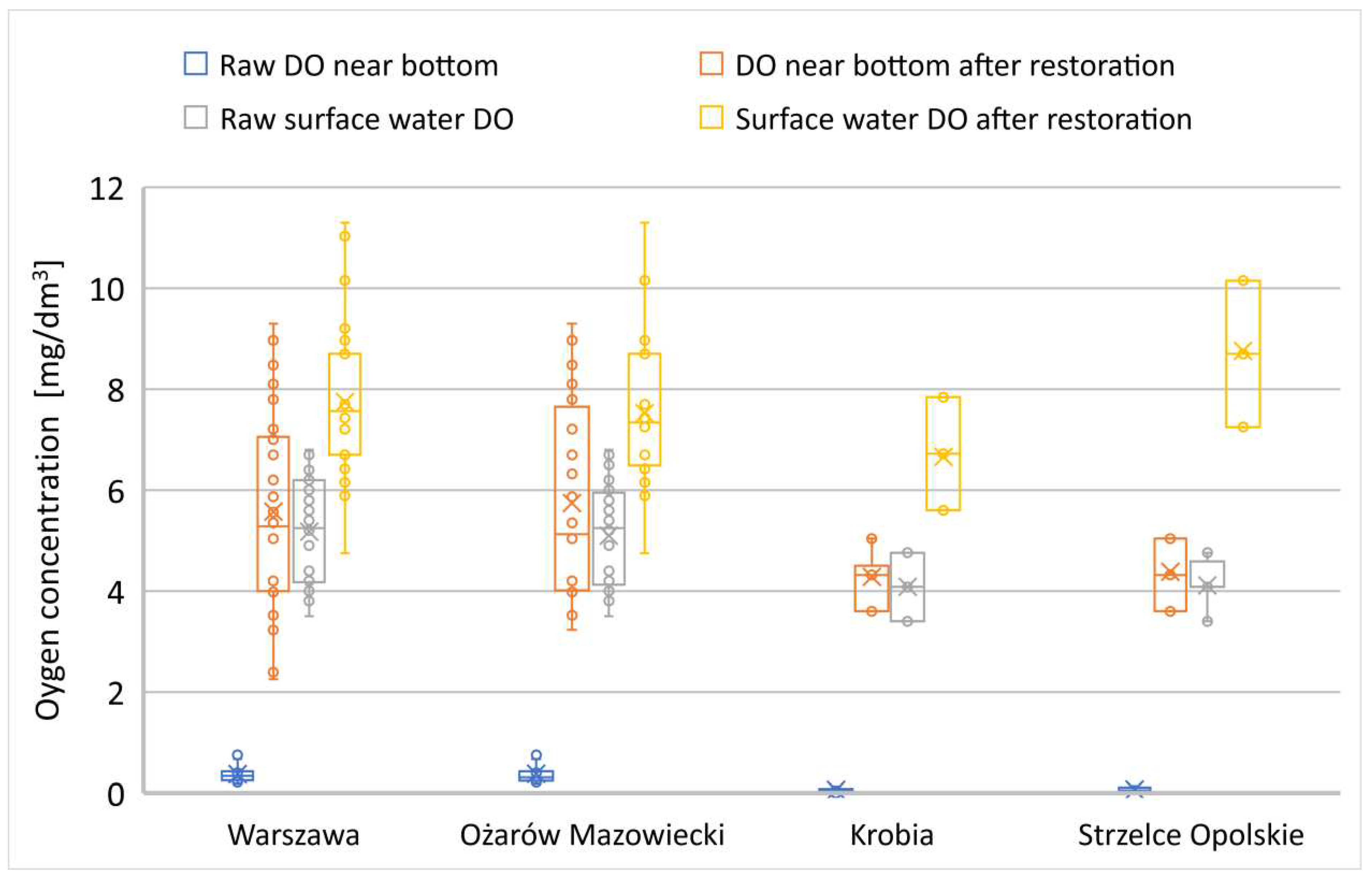
In the examined reservoirs, a trend associated with a strong hypoxic zone near the bottom was observed in all rehabilitated reservoirs. The decomposition of organic matter by autochthonous microorganisms rapidly leads to oxygen depletion in this zone [
41]. In all the examined reservoirs, dissolved oxygen (DO) near the bottom approached values close to 0 (
Figure 13-15), whether the surface water zone also had reduced levels of dissolved oxygen or levels appropriate for the specific type of reservoir (
Figure 13-15).
For dissolved oxygen in the surface water layer, statistically significant differences were not observed in two water reservoirs: Mrożyczka and Ożanna dam reservoirs (
Table A4). In Lake Ożanna, preliminary research conducted shortly after heavy rainfall events and turbulent surface water flows showed non-specific oxygenation in this layer (significant variability in different measurement points). In the Mrożyczka Dam Reservoir in Głowna, due to non-specific weather conditions, the dissolved oxygen level during the initial research period was very similar to the concentration after bioremediation treatments. In both periods, the water exhibited very good oxygen conditions in the surface layer.
Changes in dissolved oxygen (DO) in the surface layer were variable and mostly dependent on environmental conditions. Deoxygenation was not observed in any of the reservoirs within this zone (
Figure 13,
Figure 14 and
Figure 15).
The results also indicate an improvement in the surface oxygen profile in the following growing season after the application of microbiological biopreparations. The extent of these changes depended on the initial state and the degree of pollution of the respective reservoir (
Figure 13–15). However, the changes were not as effective as the increase in DO in the anoxic layer. Due to the continuous sediment decomposition process promoting a considerable oxygen demand zone, the DO levels in the bottom zone remained significantly lower than in the surface zone, which is in contact with atmospheric air [
42,
43]. Dissolved oxygen serves as a key indicator for eutrophication in freshwater lakes, playing a crucial role in water trophy assessment [
43]. Similar to the depth of the euphotic zone, the appropriate DO level provides the necessary conditions for the proper functioning of aquatic ecosystems and the maintenance of biodiversity specific to a given climate and lake type [
44]. The microbiological reclamation method, in all examined lakes, demonstrated an improvement in the oxygen profile in both the bottom and surface layers, regardless of the other parameters examined (
Figure 13–15).
5. Conclusions
Though there has been much debate on the subject, it is clear that results are notoriously positive over the one-season analysis period. This is made evident by the fact that general conditions of bottom dissolved oxygen, transparency and sediment thickness improved considerably after treatment with biopreparates. Additionally, there is a growing emphasis on ecosystem services, and this methodology can contribute to that. Despite the very favourable results, the application involves costs which may be considerably reduced if long-term monitoring verifies that no recurrent treatment is necessary, and a healthy bacterial ecosystem is restored.
The most relevant conclusions from the analyses carried out on the 34 water reservoirs subjected to remediation with biopreparations are:
1. After the application of directional biopreparations, a reduction of soft organic fractions in the bottom sediments of all reservoirs was observed in the following growing season. Sediment reduction studies showed a highly satisfactory decomposition process. In most cases, a twofold or greater reduction was observed compared to the season before rehabilitation measures began.
2. Morphological changes in the soft organic sediment fractions were observed after the application of biopreparations for water body restoration. The gradual softening of these fractions helped them to become more hydrated and more easily transported by flowing water.
3. Significant eutrophication and intense algal blooms during the growing season characterised the waters of all rehabilitated reservoirs before the treatment. An improvement in water transparency was shown in the subsequent season after microbial bioremediation.
4. In all studied reservoirs, an improvement of the oxygen profile in both the bottom and surface layers was observed after the application of the microbial restoration method.
5. The use of biopreparations for the reclamation of water bodies is an alternative to mechanical methods, which are extremely expensive and invasive to the aquatic environment. Biopreparation technology can be particularly suitable for large water bodies, where technical methods are almost impossible to implement.
Author Contributions
Conceptualization, R.M., M.J. and L.S.; methodology, R.M.; software, R.M. and L.S.; validation R.M., M.J. and L.S.; formal analysis, R.M.; investigation, R.M.; resources, R.M.; data curation, R.M.; writing—original draft preparation, R.M., M.J. and L.S.; writing—review and editing, R.M., M.J. and L.S.; visualization, R.M. and L.S.; supervision, R.M., and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Statistical differences for changes in the bottom sediment layer (SOSF) before and after reclamation, in the case of the studied water reservoirs.
Table A1.
Statistical differences for changes in the bottom sediment layer (SOSF) before and after reclamation, in the case of the studied water reservoirs.
| Pair of variables |
Wilcoxon paired t-test |
| N significant |
T |
Z |
p |
| Sediments layer before & after restoration for reservoirs group area * |
6 |
0,00 |
2,201398 |
0,027709 |
| Sosnowiec 1 & Sosnowiec 2 |
6 |
1,5 |
1,886913 |
0,059173 |
| Rybnik 1 & Rybnik 2 |
6 |
1 |
1,991741 |
0,0464 |
| Żory 1 & Żory 2 |
5 |
0 |
2,0226 |
0,043115 |
| Mysłakowice 1 & Mysłakowice 2 |
6 |
1 |
1,991741 |
0,0464 |
| Warszawa 1 & Warszawa 2 |
30 |
0 |
4,782139 |
0,000002 |
| Ożar. Maz. 1 & Ożar. Maz. 2 |
24 |
0 |
4,285714 |
0,000018 |
| Krobia 1 & Krobia 2 |
18 |
0 |
3,723555 |
0,000196 |
| Strz. Opol. 1 & Strz. Opol. 2 |
24 |
0 |
4,285714 |
0,000018 |
| * Ostrów Wielk.,Siemiatycze, Głowno, Grudziądz, Okonek, Pinczów, Łuków, Jędrzejów, Piotrków Tryb., Krzyż Wlk., Ożanna, Kraków, Gołanice, Gryfice Garanów Sand., Łańcut, Szczawno Zdr., Studziennice, Bronica, Kórnik, Grójec,Starachowice, Oleśnica, Iłza, Muchawka, Pruszków. |
Table A2.
Statistical differences for changes in the water transparency before and after reclamation, in the case of the studied water reservoirs.
Table A2.
Statistical differences for changes in the water transparency before and after reclamation, in the case of the studied water reservoirs.
| Pair of variables |
Wilcoxon paired t-test |
| N significant |
T |
Z |
p |
| Transparency before & after restoration for reservoirs group area ** |
6 |
0,00 |
2,201398 |
0,027709 |
| Głowno 1 & Głowno 2 |
6 |
1,00 |
1,991741 |
0,046400 |
| Rybnik 1 & Rybnik 2 |
6 |
1 |
1,991741 |
0,0464 |
| Gryfice 1 & Gryfice 2 |
6 |
2 |
1,782084 |
0,074736 |
| Mysłakowice 1 & Mysłakowice 2 |
6 |
6 |
0,943456 |
0,345448 |
| Brodnica 1 & Brodnica 2 |
6 |
10 |
0,104828 |
0,916512 |
| Starachowice 1 & Starachowice 2 |
6 |
1 |
1,991741 |
0,0464 |
| Oleśnica 1 & Oleśnica 2 |
5 |
1 |
1,75292 |
0,079617 |
| Iłża 1 & Iłża 2 |
6 |
5 |
1,153113 |
0,248865 |
| Warszawa 1 & Warszawa 2 |
30 |
38 |
4,000542 |
0,000063 |
| Ożar. Maz. 1 & Ożar. Maz. 2 |
24 |
27 |
3,514286 |
0,000441 |
| Krobia 1 & Krobia 2 |
17 |
0 |
3,621365 |
0,000293 |
| Strz. Opol. 1 & Strz. Opol. 2 |
24 |
0 |
4,285714 |
0,000018 |
| * Ostrów Wielk.,Siemiatycze, Grudziądz, Okonek, Pinczów, Sosnowiec, Łuków, Jędrzejów, Piotrków Tryb., Żory, Krzyż Wlk., Ożanna, Kraków, Gołanice, Baranów Sand., Łańcut, Szczawno Zdr., Studziennice, Kórnik, Grójec, Muchawka, Pruszków. |
Table A3.
Statistical differences for changes in the dissolved oxygen (and turbidity) in water near the bottom before and after reclamation, in the case of the studied water reservoirs.
Table A3.
Statistical differences for changes in the dissolved oxygen (and turbidity) in water near the bottom before and after reclamation, in the case of the studied water reservoirs.
| Pair of variables |
Wilcoxon paired t-test |
| N significant |
T |
Z |
p |
| DO near bottom (and water turbidity) before & after restoration for reservoirs group area *** |
6 |
0,00 |
2,201398 |
0,027709 |
| Warszawa 1 & Warszawa 2 |
30 |
0 |
4,782139 |
0,000002 |
| Ożar. Maz. 1 & Ożar. Maz. 2 |
24 |
0 |
4,285714 |
0,000018 |
| Krobia 1 & Krobia 2 |
18 |
0 |
3,723555 |
0,000196 |
| Strz. Opol. 1 & Strz. Opol. 2 |
24 |
0 |
4,285714 |
0,000018 |
| ***Ostrów Wielk.,Siemiatycze, Głowno, Grudziądz, Okonek, Pinczów, Sosnowiec, Łuków, Jędrzejów, Piotrków Tryb., Rybnik, Żory, Krzyż Wlk., Ożanna, Kraków, Gołanice, Gryfice Garanów Sand., Łańcut, Szczawno Zdr., Mysłakowice, Studziennice, Bronica, Kórnik, Grójec,Starachowice, Oleśnica, Iłza, Muchawka, Pruszków. |
Table A4.
Statistical differences for changes in the dissolved oxygen in surface water before and after reclamation, in the case of the studied water reservoirs.
Table A4.
Statistical differences for changes in the dissolved oxygen in surface water before and after reclamation, in the case of the studied water reservoirs.
| Pair of variables |
Wilcoxon paired t-test |
| N significant |
T |
Z |
p |
| DO surface before & after restoration for reservoirs group area **** |
6 |
0,00 |
2,201398 |
0,027709 |
| Głowno 1 & Głowno 2 |
6 |
5,00 |
1,153113 |
0,248865 |
| Jędrzejów 1 & Jędrzejów 2 |
6 |
1,00 |
1,991741 |
0,046400 |
| Ożanna 1 & Ożanna 2 |
6 |
5,00 |
1,153113 |
0,248865 |
| Studziennice 1 & Studziennice 2 |
6 |
1,00 |
1,991741 |
0,046400 |
| Warszawa 1 & Warszawa 2 |
30 |
0 |
4,782139 |
0,000002 |
| Ożar. Maz. 1 & Ożar. Maz. 2 |
24 |
0 |
4,285714 |
0,000018 |
| Krobia 1 & Krobia 2 |
18 |
0 |
3,723555 |
0,000196 |
| Strz. Opol. 1 & Strz. Opol. 2 |
24 |
0 |
4,285714 |
0,000018 |
| **** Ostrów Wielk.,Siemiatycze, Grudziądz, Okonek, Pinczów, Sosnowiec, Łuków, Piotrków Tryb., Rybnik, Żory, Krzyż Wlk., Kraków, Kórnik, Gołanice, Gryfice Garanów Sand., Łańcut, Szczawno Zdr., Mysłakowice, Bronica, Grójec,Starachowice, Oleśnica, Iłza, Muchawka, Pruszków. |
References
- Yusoff, F. M.; Shariff, M.; Gopinath, N. Diversity of Malaysian aquatic ecosystems and resources Aquat. Ecosyst. Health Manag 2006, 9(2), 119–135. [Google Scholar] [CrossRef]
- Jakubiak, M.; Chmielowski, K. Identification of urban water bodies ecosystem services. Acta Sci. Pol., Form. Circumiectus, 2020; 19, 2, 73–82. [Google Scholar] [CrossRef]
- Baron, J. S.; Poff, N. L.; Angermeier, P. L.; Dahm, C. N.; Gleick, P. H.; Hairston Jr, N. G.; Jackson, R.B.; Johnston, C.A.; Richter, B.D.; Steinman, A. D. Meeting ecological and societal needs for freshwater. Ecol Appl 2002, 12(5), 1247–1260. [Google Scholar] [CrossRef]
- Alahuhta, J.; Erős, T.; Kärnä, O. M.; Soininen, J.; Wang, J.; Heino, J. Understanding environmental change through the lens of trait-based, functional, and phylogenetic biodiversity in freshwater ecosystems. Environ. Rev. 2019, 27(2), 263–273. [Google Scholar] [CrossRef]
- Das, S.; Ward, L. R.; Burke, C. Prospects of using marine actinobacteria as probiotics in aquaculture. Appl. Microbiol. Biotechnol. 2008, 81, 419–429. [Google Scholar] [CrossRef]
- Hou, D.; Al-Tabbaa, A.; O’Connor, D.; Hu, Q.; Zhu, Y. G.; Wang, L. ... Rinklebe, J. Sustainable remediation and redevelopment of brownfield sites. Nat. Rev. Earth Environ. 2023, 4(4), 271–286. [Google Scholar] [CrossRef]
- Mehmood, T.; Gaurav, G. K.; Cheng, L.; Klemeš, J. J.; Usman, M.; Bokhari, A.; Lu, J. A review on plant-microbial interactions, functions, mechanisms and emerging trends in bioretention system to improve multi-contaminated stormwater treatment. J. Environ. Manage. 2021, 294, 113108. [Google Scholar] [CrossRef]
- Dondajewska, R.; Kozak, A.; Rosińska, J.; Gołdyn, R. Water quality and phytoplankton structure changes under the influence of effective microorganisms (EM) and barley straw–Lake restoration case study. Sci. Total Environ. 2019, 660, 1355–1366. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Mazur, A.; Mazur, R.; Chmielowski, K.; Czekała, W.; Janczak, D. The process of microbiological remediation of the polluted Słoneczko reservoir in Poland: for reduction of water pollution and nutrients management. Water, 2020; 12, 3002. [Google Scholar]
- Simon, M.; Joshi, H. A review on green technologies for the rejuvenation of polluted surface water bodies: Field-scale feasibility, challenges, and future perspectives. J. Environ. Chem. Eng. 2021, 9(4), 105763. [Google Scholar] [CrossRef]
- Velmurugan, L.; Pandian, K. D. Recycling of wet grinding industry effluent using effective Microorganisms™(EM). Heliyon, 2023; 9. [Google Scholar] [CrossRef]
- Zakaria, Z.; Gairola, S.; Shariff, N. M. Effective microorganisms (EM) technology for water quality restoration and potential for sustainable water resources and management. 5th International Congress on Environmental Modelling and Software, Ottawa, Canada, 01.07.2010.
- Safwat, S. M.; Matta, M. E. Environmental applications of Effective Microorganisms: a review of current knowledge and recommendations for future directions. J. Eng. Appl. Sci. 2021, 68(1), 1–12. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Kulkova, I.; Wierzchowski, P. S.; Wróbel, B. Response of Physicochemical and Microbiological Properties to the Application of Effective Microorganisms in the Water of the Turawa Reservoir. Water 2021, 14(1), 12. [Google Scholar] [CrossRef]
- European Commission, EU Biodiversity Strategy for 2030 - Bridging Nature Back into Our Lives (2021). [CrossRef]
- Prejs, A.; Koperski, P.; Prejs, K. Food-web manipulation in a small, eutrophic Lake Wirbel, Poland: The effect of replacement of key predators on epiphytic fauna. Hydrobiologia 1997, 342, 377–381. [Google Scholar] [CrossRef]
- Bielczyńska, A. Bioindication on the basis of benthic diatoms: Advantages and disadvantages of the Polish phytobenthos lake assessment method (IOJ–the Diatom Index for Lakes)/Bioindykacja na podstawie okrzemek bentosowych: Mocne i słabe strony polskiej metody oceny jezior na podstawie fitobentosu (IOJ–Indeks Okrzemkowy Jezior). Environmental Protection and Natural Resources 2015, 26(4), 48–55. [Google Scholar] [CrossRef]
- Pełechata, A.; Pełechaty, M.; Pukacz, A. Winter temperature and shifts in phytoplankton assemblages in a small Chara-lake. Aquat. Bot. 2015, 124, 10–18. [Google Scholar] [CrossRef]
- Napiórkowski, P.; Bąkowska, M.; Mrozińska, N.; Szymańska, M.; Kolarova, N.; Obolewski, K. The effect of hydrological connectivity on the zooplankton structure in floodplain lakes of a regulated large river (the Lower Vistula, Poland). Water 2019, 11(9), 1924. [Google Scholar] [CrossRef]
- Ptak, M.; Sojka, M.; Choiński, A.; Nowak, B. Effect of environmental conditions and morphometric parameters on surface water temperature in Polish lakes. Water 2018, 10(5), 580. [Google Scholar] [CrossRef]
- Martinsen, K. T.; Andersen, M. R.; Sand-Jensen, K. Water temperature dynamics and the prevalence of daytime stratification in small temperate shallow lakes. Hydrobiologia 2019, 826(1), 247–262. [Google Scholar] [CrossRef]
- Woolway, R. I.; Kraemer, B. M.; Lenters, J. D.; Merchant, C. J.; O’Reilly, C. M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ 2020, 1(8), 388–403. [Google Scholar] [CrossRef]
- Kennedy, R. H.; Walker, W. W. Reservoir nutrient dynamics. In Reservoir limnology: ecological perspectives, Thornton, K.W.; Kimmel, B.L.; Payne, F.E. Eds. John Wiley & Sons, Inc. New York, USA, 1990, pp.109-131.
- Berthold, M. Nutrient and Limitation Regimes in Coastal Water Ecosystems. In Southern Baltic Coastal Systems Analysis, Schubert, H.; Müller, F. Eds. Springer Ecological Studies 246. 2023, pp. 175-185.
- Gebler, D.; Kolada, A.; Pasztaleniec, A.; Szoszkiewicz, K. Modelling of ecological status of Polish lakes using deep learning techniques. Environ. Sci. Pollut. Res. 2021, 28, 5383–5397. [Google Scholar] [CrossRef] [PubMed]
- Ulańczyk, R.; Łozowski, B.; Woźnica, A.; Absalon, D.; Kolada, A. Water Quality and Ecosystem Modelling: Practical Application on Lakes and Reservoirs. In Quality of Water Resources in Poland, Zeleňáková, M; Kubiak-Wójcicka, K; Negm, A.M. Eds. Springer Water 2021, pp. 173-189. [CrossRef]
- Dean, W. E. Carbonate minerals and organic matter in sediments of modern north temperate hard-water lakes. In Recent and Ancient Nonmarine Depositional Environments: Models for Exploration, Ethridge, F.G.; R.M. Eds. SEPM Society for Sedimentary Geology 1981, pp. 213–231. [CrossRef]
- Dean, W. E. The carbon cycle and biogeochemical dynamics in lake sediments. J. Paleolimnol. 1999, 21, 375–393. [Google Scholar] [CrossRef]
- Anderson, N. J.; Bennion, H.; Lotter, A. F. Lake eutrophication and its implications for organic carbon sequestration in Europe. Glob Chang Biol 2014, 20(9), 2741–2751. [Google Scholar] [CrossRef]
- Ferland, M. E.; Prairie, Y. T.; Teodoru, C.; del Giorgio, P. A. Linking organic carbon sedimentation, burial efficiency, and long-term accumulation in boreal lakes. J. Geophys. Res. Biogeosci. 2014, 119(5), 836–847. [Google Scholar] [CrossRef]
- Bastviken, D.; Olsson, M.; Tranvik, L. Simultaneous measurements of organic carbon mineralization and bacterial production in oxic and anoxic lake sediments. Microb. Ecol. 2003, 46, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, J.; Verstraelen, P.; Boers, P.; Van Roestel, J.; Roijackers, R.; Moser, G. Lake restoration with and without dredging of phosphorus-enriched upper sediment layers. Hydrobiologia 1992, 233, 197–210. [Google Scholar] [CrossRef]
- Annadotter, H.; Cronberg, G.; Aagren, R.; Lundstedt, B.; Nilsson, P. Ǻ.; Ströbeck, S. Multiple techniques for lake restoration. In The Ecological Bases for Lake and Reservoir Management: Proceedings of the Ecological Bases for Management of Lakes and Reservoirs Symposium, 19–22 March 1996, Leicester, United Kingdom; Springer Netherlands, 1999; pp. 77–85. [Google Scholar]
- Hupfer, M.; Hilt, S.; Jørgensen, S. E.; Asolekar, S. R.; Kalbar, P. P.; Maillacheruvu, K. Y.; Abell, R.; Blanch, S.; Thieme, M.; Geller, W.; Koschorreck, M. 2008 Lake restoration. pp. 79. [CrossRef]
- Mazur, A.; Chmielowski, K. Innovative aeration and treatment technologies supporting the process of revitalization of degraded water reservoirs. Acta Sci. Pol., Form. Circumiectus, 2020; 19, 3, 15–28. [Google Scholar] [CrossRef]
- Asaeda, T.; Pham, H. S.; Priyantha, D. N.; Manatunge, J.; Hocking, G. C. Control of algal blooms in reservoirs with a curtain: a numerical analysis. Ecol. Eng. 2001, 16(3), 395–404. [Google Scholar] [CrossRef]
- Kratzer, S.; Håkansson, B.; Sahlin, C. Assessing Secchi and photic zone depth in the Baltic Sea from satellite data. Ambio 2003, 32(8), 577–585. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E. V.; Petrucio, M. M. Water level decreases and increased water stability promotes phytoplankton growth in a mesotrophic subtropical lake. Mar. Freshw. Res. 2015, 66(8), 711–718. [Google Scholar] [CrossRef]
- Abirhire, O.; Davies, J. M.; Guo, X.; Hudson, J. Understanding the factors associated with long-term reconstructed turbidity in Lake Diefenbaker from Landsat imagery. Sci. Total Environ. 2020, 724, 138222. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Beisner, B. E. Zooplankton biodiversity and lake trophic state: explanations invoking resource abundance and distribution. Ecology 2007, 88(7), 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Chen, X.; Jin, Y.; Xie, X.; Zhao, H.; Yu, L.; Lin, L.; Xu, S. ... Hypolimnetic oxygen depletion by sediment-based reduced substances in a reservoir formerly affected by acid mine drainage. Ecol. Indic. 2023, 151, 110301. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Liu, M.; He, J.; Shi, K.; Zhou, Y. . Liu, X. Dissolved oxygen stratification and response to thermal structure and long-term climate change in a large and deep subtropical reservoir (Lake Qiandaohu, China). Water Res. 2015, 75, 249–258. [Google Scholar] [CrossRef]
- Viet, N. D.; Bac, N. A.; Huong, H. T. T. Dissolved oxygen as an indicator for eutrophication in freshwater lakes. In Proceedings of the International Conference on Environmental Engineering and Management for Sustainable Development, Hanoi, Vietnam; 15.09.2016. [Google Scholar]
- Banerjee, A.; Chakrabarty, M.; Rakshit, N.; Bhowmick, A. R.; Ray, S. Environmental factors as indicators of dissolved oxygen concentration and zooplankton abundance: Deep learning versus traditional regression approach. Ecol. Indic. 2019, 100, 99–117. [Google Scholar] [CrossRef]
Figure 1.
Polluted water reservoirs restoration process on the territory of Poland by application of microbiological methods.
Figure 1.
Polluted water reservoirs restoration process on the territory of Poland by application of microbiological methods.
Figure 2.
Biopreparation (for water and sediments) transported on the banks of Ożanna Lake before the application process.
Figure 2.
Biopreparation (for water and sediments) transported on the banks of Ożanna Lake before the application process.
Figure 3.
Application process of biopreparation in Ożanna Lake (intensive saturation of macrophytes zone).
Figure 3.
Application process of biopreparation in Ożanna Lake (intensive saturation of macrophytes zone).
Figure 4.
Reduction of soft organic sediment fractions in reservoirs with a primary layer exceeding 1 meter.
Figure 4.
Reduction of soft organic sediment fractions in reservoirs with a primary layer exceeding 1 meter.
Figure 5.
Reduction of soft organic sediment fractions in reservoirs with a primary layer below 1 meter.
Figure 5.
Reduction of soft organic sediment fractions in reservoirs with a primary layer below 1 meter.
Figure 6.
Reduction of soft organic sediment fractions in interconnected small reservoirs.
Figure 6.
Reduction of soft organic sediment fractions in interconnected small reservoirs.
Figure 7.
Changes in transparency in reservoirs with a primary SOSF layer exceeding 1 meter.
Figure 7.
Changes in transparency in reservoirs with a primary SOSF layer exceeding 1 meter.
Figure 8.
Changes in transparency in reservoirs with a primary SOSF layer below 1 meter.
Figure 8.
Changes in transparency in reservoirs with a primary SOSF layer below 1 meter.
Figure 9.
Changes in transparency in interconnected small reservoirs (water ponds).
Figure 9.
Changes in transparency in interconnected small reservoirs (water ponds).
Figure 10.
Changes in turbidity in reservoirs with a primary SOSF layer exceeding 1 meter.
Figure 10.
Changes in turbidity in reservoirs with a primary SOSF layer exceeding 1 meter.
Figure 11.
Changes in turbidity in reservoirs with a primary SOSF layer below 1 meter.
Figure 11.
Changes in turbidity in reservoirs with a primary SOSF layer below 1 meter.
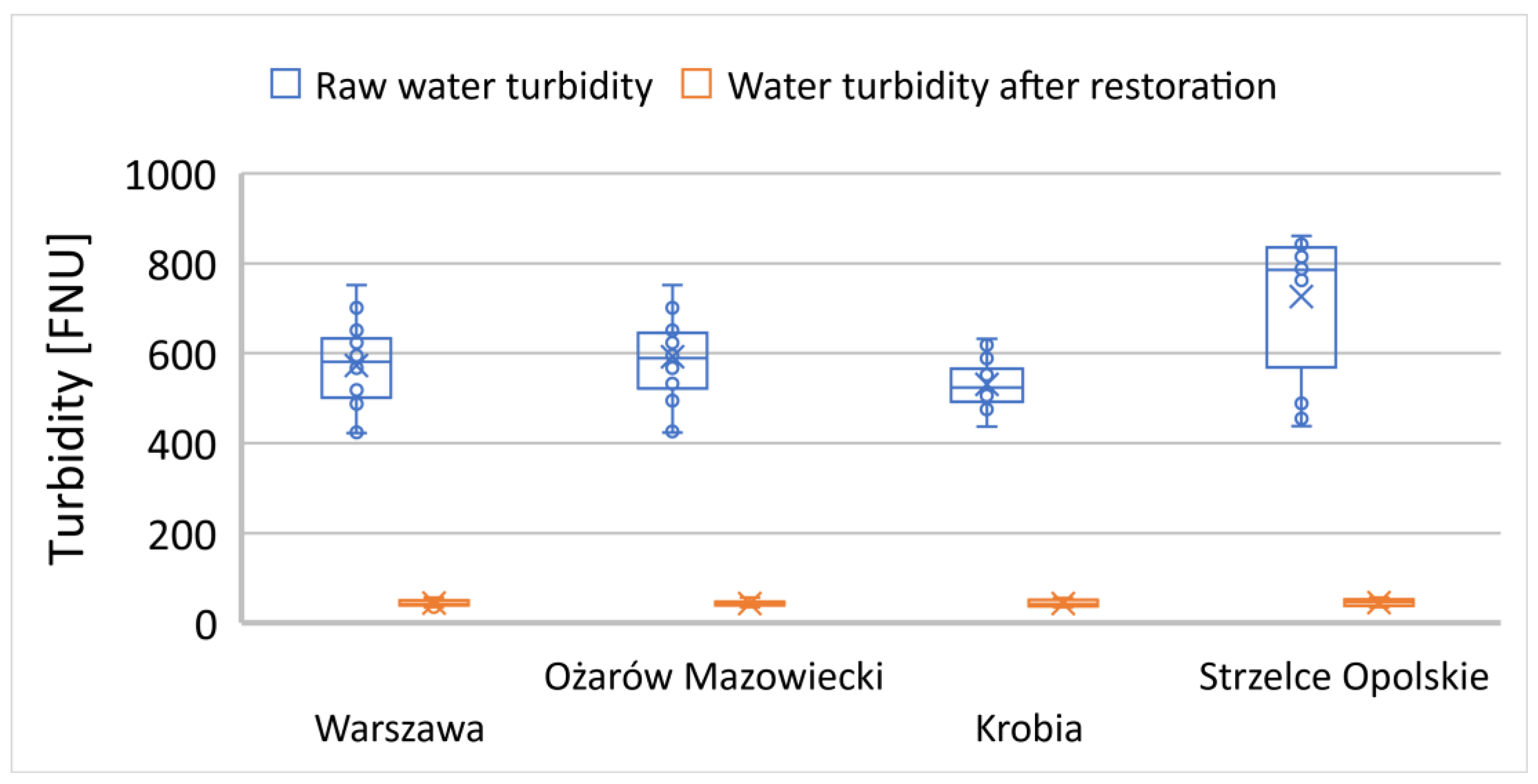
Figure 12.
Changes in turbidity in interconnected small reservoirs (water ponds).
Figure 12.
Changes in turbidity in interconnected small reservoirs (water ponds).
Figure 13.
Changes in DO in reservoirs with a primary SOSF layer below 1 meter.
Figure 13.
Changes in DO in reservoirs with a primary SOSF layer below 1 meter.
Figure 14.
Changes in DO in reservoirs with a primary SOSF layer exceeding 1 meter.
Figure 14.
Changes in DO in reservoirs with a primary SOSF layer exceeding 1 meter.
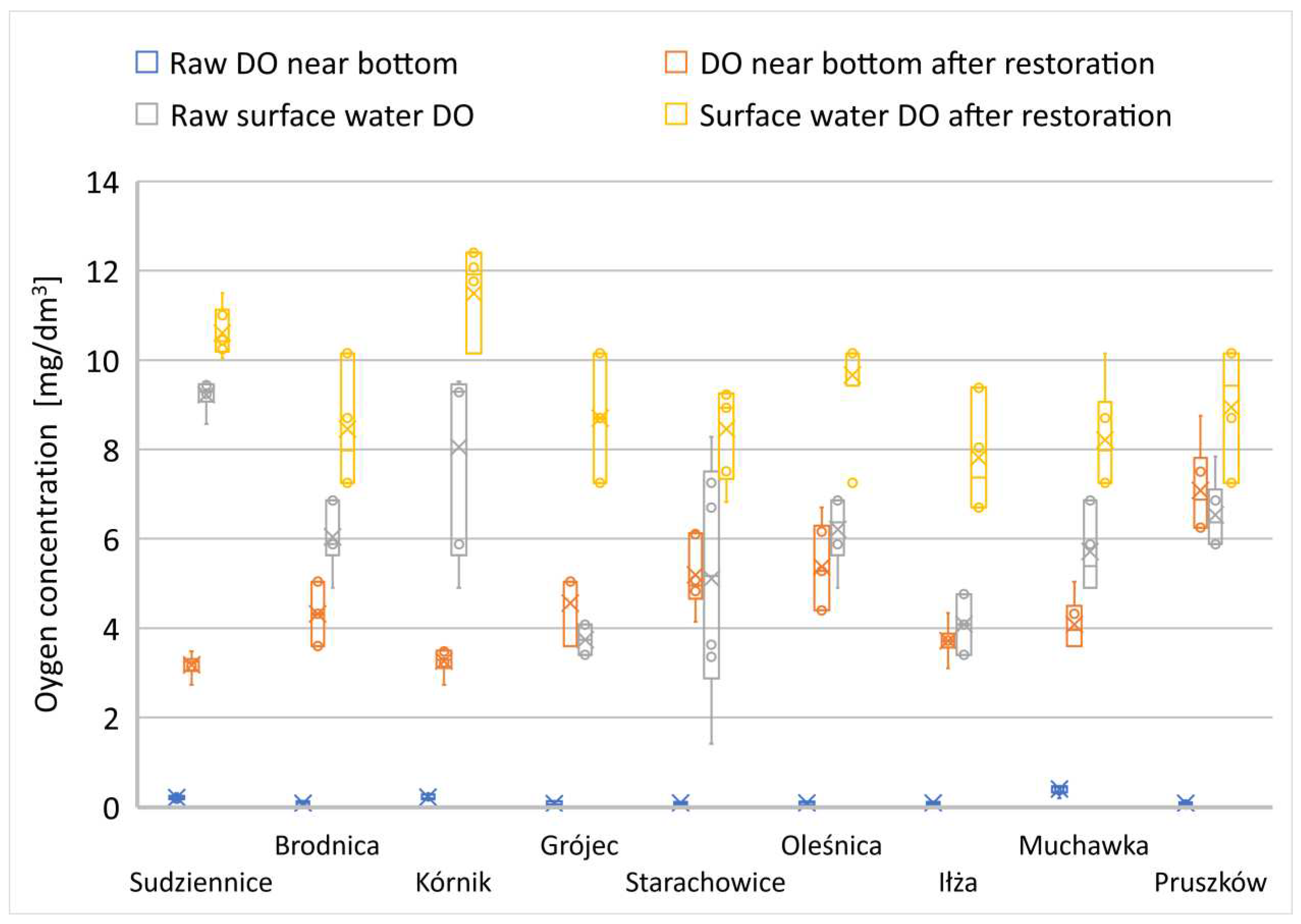
Figure 15.
Changes in DO in interconnected small reservoirs (water ponds).
Figure 15.
Changes in DO in interconnected small reservoirs (water ponds).
Table 1.
Applied measurement methods in the study of selected water quality parameters.
Table 1.
Applied measurement methods in the study of selected water quality parameters.
| The measured parameters |
Methods/tools |
| Dissolved oxygen 2014 - 2018 |
By portable Multi-Function Meter CX-401 with oxygen galvanic oxygen sensors COG-1 (made by Elmetron) |
| Dissolved oxygen 2014 - 2018 |
By portable Multi-Function Meter HQ 1130 with a 5-meter cable and oxygen sensor (probe). (made by Hach) |
| Transparency |
Measurement by Secchi disc |
| Sediment layer |
With the application of geodetic staff, an endoscopic camera with its light source, and a visual monitor connected to the camera via a USB cable – sediment thickness readings were displayed on a metric scale on the geodetic staff |
| Turbidity |
By portable Turbidity Meter 2100Q (made by Hach) |
| Chlorophyll-a |
spectrophotometric method (PN-86/C-05560/02) |
Table 2.
Characterization of water bodies subjected to microbiological bioremediation process in Poland.
Table 2.
Characterization of water bodies subjected to microbiological bioremediation process in Poland.
| No. |
Water reservoir |
Location |
Date of restoration |
Surface area [m2] |
Volume [m3] |
Maximum depth [m] |
Average depth [m] |
Water flow types |
Flow velocity |
River tributary |
| 1 |
Dam Reservoir Pniowiec |
Rybnik |
2018 - 2019 |
885000 |
4800000 |
12 |
5,4 |
exorheic reservoir |
slow |
Ruda |
| 2 |
Niskie Brodno Lake |
Brodnica |
2022 - 2023 |
823420 |
5681595 |
18,2 |
6,9 |
exorheic reservoir |
slow |
Brodniczka |
| 3 |
Kórnickie Lake |
Kórnik |
2022 - 2023 |
741669 |
2225007 |
6 |
3 |
exorheic reservoir |
slow |
Kórnicki canal |
| 4 |
Błędno Lake |
Grójec |
2022 - 2023 |
700000 |
1330000 |
5,6 |
1,9 |
exorheic reservoir |
slow |
Obra - Szarka |
| 5 |
Bugaj Lake |
Piotrków Trybunalski |
2017 - 2018 |
520000 |
936000 |
4 |
1,8 |
exorheic reservoir |
slow |
Wirzejka |
| 6 |
Pasternik Lake |
Starachowice |
2021 - 2022 |
420000 |
840000 |
4 |
2 |
exorheic reservoir |
slow |
Kamienna |
| 7 |
Dam Reservoir Muchawka |
Siedlce |
2014 - 2015 |
400000 |
600000 |
5 |
1,5 |
endorheic reservoir |
very slow / lack |
Muchawka |
| 8 |
Łokacz Lake in K.W. |
Krzyż Wielkopolski |
2022 - 2023 |
382241 |
1146722 |
3,4 |
3 |
exorheic reservoir |
slow |
Człopica |
| 9 |
Mrożyczka Dam Reservoir in Głowna |
Głowno |
2019 - 2020 |
380000 |
608000 |
3,3 |
1,6 |
exorheic reservoir |
slow |
Mroga |
| 10 |
Siemiatycze Dam Reservoir |
Siemiatycze |
2016-2017 |
274000 |
548000 |
5,1 |
2 |
exorheic reservoir |
slow |
Kamionka and Mahomet |
| 11 |
Tarpno Lake in Grudziądz |
Grudziądz |
2021 - 2022 |
270000 |
1512000 |
5,8 |
5,6 |
exorheic reservoir |
slow |
Trynkowa |
| 12 |
Studzienniczno Lake in Bytów |
Studziniczno, Bytów County |
2022 - 2023 |
210623 |
1474363 |
20 |
7 |
exorheic reservoir |
slow |
Studnica |
| 13 |
Iłżanka Reservoir |
Iłża |
2020 - 2021 |
185447 |
148358 |
3 |
0,8 |
exorheic reservoir |
slow |
Iłżanka |
| 14 |
Ożanna Lake in Kuryłówka |
Kuryłówka |
2020 - 2021 |
181245 |
253743 |
3,7 |
1,4 |
exorheic reservoir |
slow |
Złota |
| 15 |
Zimna Woda Lake in Łuków |
Łuków |
2020 - 2021 |
180000 |
270000 |
3 |
1,5 |
endorheic reservoir |
very slow / lack |
Krzna Południowa |
| 16 |
Pinczów Dam Reservoir |
Pińczów |
2022 - 2023 |
115000 |
218500 |
3,5 |
1,9 |
exorheic reservoir |
slow |
Nida canal |
| 17 |
Warszawa city ponds |
Warsaw |
2022 - 2023 |
108807 |
321091 |
5 |
2,5 |
exorheic multi reservoirs |
slow |
Wystawowy Canal - Vistula |
| 18 |
Śmieszek Lake in Żory |
Żory |
2019 - 2020 |
95000 |
190000 |
2,7 |
2 |
exorheic reservoir |
slow |
Ruda |
| 19 |
Bąk Lake in Okonek |
Okonek |
2018 - 2019 |
65000 |
97500 |
3 |
1,5 |
endorheic reservoir |
very slow / lack |
Czarna |
| 20 |
Leśny lake in Sosnowiec |
Sosnowiec |
2021 - 2022 |
50913 |
101826 |
2,5 |
2 |
endorheic reservoir |
lack |
noname water canal |
| 21 |
Pruszków city ponds |
Pruszków |
2020 - 2021 |
30998 |
46496 |
1,95 |
1,5 |
endorheic reservoir |
very slow / lack |
Utrata |
| 22 |
Oleśnica city ponds |
Oleśnica |
2021 - 2022 |
28408 |
42612 |
2 |
1,5 |
endorheic reservoir |
very slow / lack |
Oleśnica |
| 23 |
Ożarków Mazowiecki city ponds |
Ożarów Mazowiecki |
2020 - 2021 |
13314 |
19972 |
2 |
1,5 |
endorheic reservoir |
very slow / lack |
City canal |
| 24 |
Strzelce Opolskie city ponds |
Strzelce Opolskie |
2020 - 2021 |
12289 |
12289 |
1 |
1 |
endorheic reservoir |
lak |
groundwater-fed ponds |
| 25 |
Jędrzejów Dam Reservoir |
Jędrzejów, Świętokrzyskie Voivodeship |
2021 - 2022 |
11636 |
23272 |
3 |
2 |
endorheic reservoir |
very slow / lack |
Brzeźnica |
| 26 |
Kaczeńcowa city pond |
Kraków County |
2017 - 2018 |
8200 |
11480 |
2 |
1,4 |
endorheic reservoir |
very slow / lack |
groundwater-fed ponds - Młynówka |
| 27 |
Stawek pond in Gryfice |
Gryfice |
2021 - 2022 |
6866 |
27466 |
9 |
4 |
endorheic reservoir |
very slow / lack |
noname water canal |
| 28 |
Ostrów Wielkopolski city pond |
Ostrów Wielkopolski |
2022 - 2023 |
5900 |
8849 |
2 |
1,5 |
endorheic reservoir |
very slow / lack |
noname water canal |
| 29 |
Gedymin Pond in Szczawno Zdrój |
Szczawno-Zdrój |
2021 - 2022 |
5445 |
8167 |
2 |
1,5 |
exorheic reservoir |
very slow / lack |
Szczawnik |
| 30 |
Baranów Sandomierski Palast pond |
Baranów Sandomierski |
2022 - 2023 |
4718 |
9435 |
2 |
2 |
endorheic reservoir |
very slow / lack |
noname water canal |
| 31 |
Krobia community ponds |
Gmina Krobia |
2021 - 2022 |
4009 |
5124 |
1,5 |
1,5 |
endorheic multi reservoirs |
very slow / lack |
noname water canal |
| 32 |
Mysłakowice Palast pond |
Mysłakowice |
2021 - 2022 |
3640 |
5460 |
2 |
1,5 |
exorheic reservoir |
very slow / lack |
Łomnica |
| 33 |
Pond at Łańcut Castle Park |
Łańcut |
2022 - 2023 |
3121 |
4682 |
2 |
1,5 |
exorheic reservoir |
very slow / lack |
noname water canal |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).