1. Introduction
The gallbladder is an easily accessible organ at US examination, due to its anatomical location in the right upper quadrant of the abdomen. For this reason, the gallbladder can be thoroughly evaluated by conventional B-mode US, as well as by color Doppler (CD) and CEUS.
When compared to alternative imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), US has the advantage of a lower cost, easy accessibility and high diagnostic performance in evaluating the gallbladder. Furthermore, US avoids radiation exposure, and this is even more important in pediatric population and in pregnant women.
Gallbladder US can be performed directly at the patient bed-side, in the emergency department, in the hospital wards or at the doctor’s office. For this reason, US is time-saving, well-tolerated and extremely convenient for the patient and for the clinician. Therefore, in the suspect of biliary disease, US is considered the first imaging technique.
Even more advanced and detailed diagnostic imaging can be obtained by CEUS or by using new sonographic techniques, combined with conventional B-mode US.
Point-of-care ultrasound (POCUS) is an important application of US, that allows a ready imaging support during the clinical evaluation. Furthermore, US can be easily repeated in the case of gallbladder diseases that require monitoring over time.
2. Cholelithiasis
Gallstones are a common disease and US is the method of choice for diagnosis, with an accuracy as high as 95%. [
1,
2]
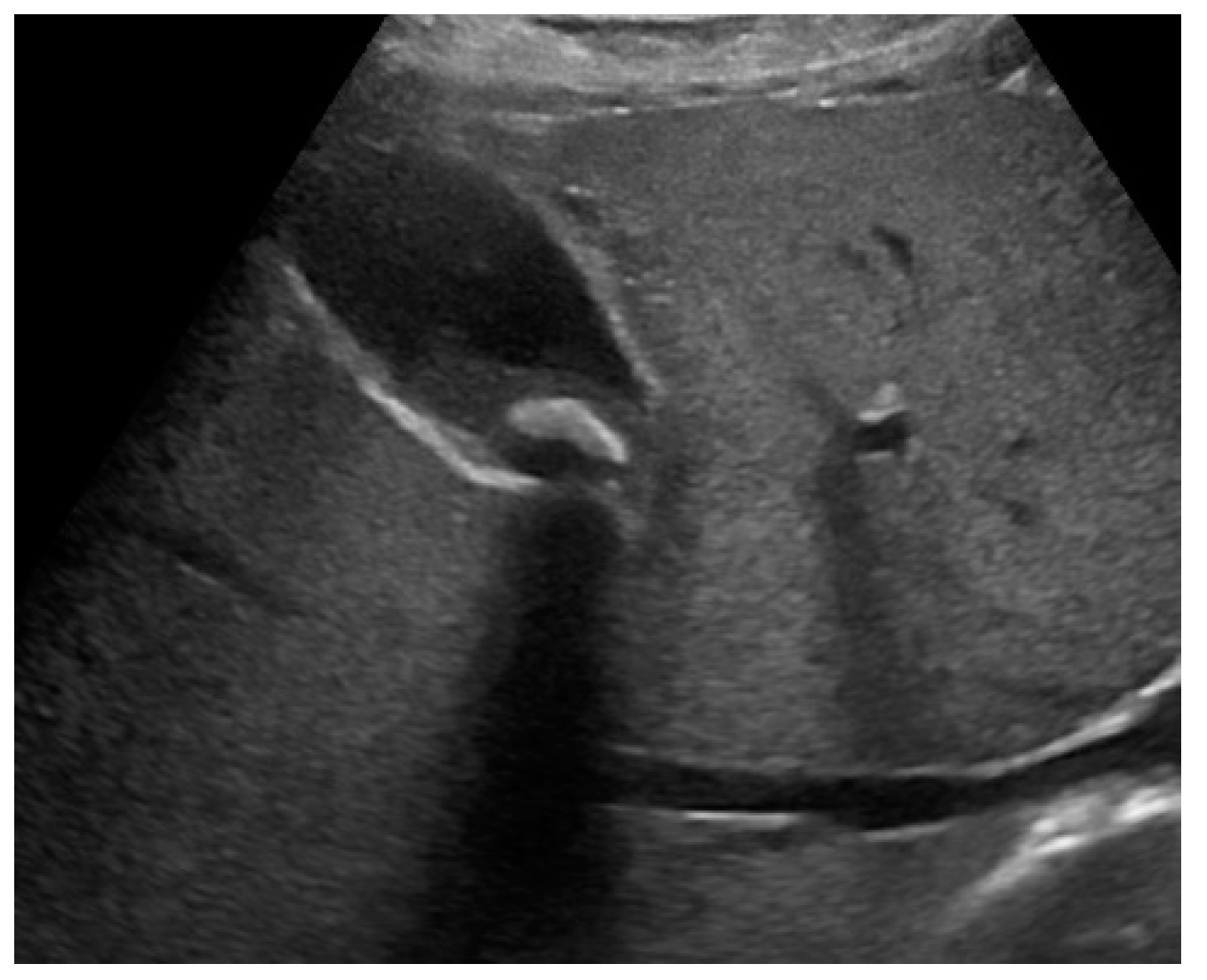
The US typical appearance of gallstones is an echogenic focus in the gallbladder lumen that casts a posterior acoustic shadow and changes position according to the variation of patient decubitus (
Figure 1).[
3] Stones smaller than 2 or 3 mm may be difficult to visualize, especially if isolated. Gallstones typically produce complete shadowing without reverberation because most of the ultrasound is absorbed by the stone. Rarely, reverberation artifacts may be seen posterior to calcified stones if they contain gas within fissures. [
1]
When the lumen of the gallbladder is completely filled with stones, the usual finding is a highly reflective gallbladder fossa, known as the “wall-echo-shadow” (WES) triad [
4] or “double-arc-shadow sign”. WES complex consists of two parallel arcuate hyperechoic lines separated by a thin hypoechoic space and distal acoustic shadowing. The most external hyperechoic arc represents the anterior side of the gallbladder wall. The hypoechoic space in between is a small film of bile separating the gallbladder wall from the stones. Alternatively, the hypoechoic layer could represent a portion of the gallbladder wall. Finally, the deeper hyperechoic line represents the gallstones with an acoustic shadow that masks the rest of intraluminal stones and the posterior gallbladder wall. [
5,
6]
On CD, the highly reflective surface of the stones, particularly in case of cholesterol stones, can produce the typical “twinkling artifact”, characterized by a mosaic of colored pixels posterior to the stone. [
7,
8]
Finally, US is very useful in the setting of biliary colic. In particular, POCUS is increasingly employed in the emergency room for the evaluation of patients with right upper quadrant pain,[
9] mainly to confirm the suspicion of gallstones or different gallbladder disease.
3. Gallbladder sludge
Gallbladder sludge is made of mucins, glycoproteins, calcium, and pigments. This environment facilitates cholesterol crystallization and calcium bilirubinate precipitation, predisposing to gallstones development.[
10]
Typically, biliary sludge is a slight sonographic finding,[
1] which appears as non-shadowing echoes with an indefinite shape that tend to layer in the most declivous portion of the gallbladder. Biliary sludge forms a horizontal level that moves slowly, according to changes in patient’s decubitus (
Figure 1). [
1,
2]
Rarely, aggregated sludge may appear as a static, variably echogenic, intraluminal mass, without acoustic shadows nor internal vascular signals, in close proximity with the gallbladder wall (“sludge ball”) or as a polypoid mass (“tumefactive sludge”). [
2,
3] A gallbladder completely filled with sludge may be isoechoic with the adjacent liver, leading to the so called “hepatization of the gallbladder”. [
3] In this setting, it can be difficult to distinguish biliary sludge from polypoid lesions, if not from gallbladder carcinoma (GBC). [
11,
12]
CD examination can be useful in differentiating biliary sludge from a solid mass. In particular, the presence of CD signals can be considered a reliable indicator of a malignant lesion, while the lack of signal does not exclude malignancy. [
13]
In case of tumefactive sludge, some authors suggest to repeat US examination after a short interval of time (from 1 day to 2 weeks), especially in patients on prolonged fasting, after resuming normal habits. [
11]
CEUS can greatly improve the diagnostic confidence in the differential diagnosis between sludge and mass-forming lesions, especially when CD signals are not detectable. Indeed, sludge typically does not show any kind of enhancement at any contrast phase, due to the absence of vascularization, with an accuracy of 100%.[
14,
15,
16,
17,
18,
19,
20]
The pseudo-enhancement of gallbladder sludge, caused by an artifact due to nonlinear propagation of US through microbubbles, has been reported in a single case report. [
21]
According to some authors, new techniques for the detection of small vessels flow (MVFI, Microvascular Flow Imaging, and SMI, Superb Microvascular Imaging) can help differentiate tumefactive sludge from solid lesions of the gallbladder.[
22]
4. Gallbladder hydrops
Gallbladder hydrops, sometimes indicated as mucocele, is generally defined as a distended gallbladder filled with mucoid fluid, due to prolonged impaction of a stone in the cystic duct or in the gallbladder neck. This condition is associated with the interruption of gallbladder filling and with reabsorption of the endoluminal bile.[
23] In turn, this can evolve into acute cholecystitis due to bacterial growth.[
24] Additional causes of gallbladder hydrops are obstructing polyps and tumors, congenital strictures, ascariasis infestation or external compression by enlarged lymph nodes.[
25] A specific form of gallbladder hydrops is observed in children during the acute phase of Kawasaki disease. [
25]
US shows a distended gallbladder with intraluminal clear fluid and possibly gallstones or biliary sludge in the gallbladder neck or in the cystic duct. [
23] A distended gallbladder is defined as an axial diameter >4-5 cm. [
23,
25] Some authors also take into account a longitudinal diameter > 10 cm. [
24]
5. Acute cholecystitis
In most patients the acute inflammation of the gallbladder wall is caused by gallstones, impacted in the cystic duct or in the gallbladder neck (calculous cholecystitis).
Recently, the Tokyo guidelines proposed specific criteria for the diagnosis of AC, providing a pivotal role for imaging studies.[
26] In particular, US is recommended as the imaging examination for the diagnosis of AC, due to its high sensitivity and specificity, (88% and 80%, respectively).[
26,
27]
In patients with suspected AC, POCUS can be readily performed at the admission in the Emergency Department. In particular, the presence of specific sonographic findings (gallstones, sludge, gallbladder wall thickening, pericholecystic fluid), especially if combined, has a good sensitivity and a high specificity for the diagnosis of AC. [
9,
28]
Besides the presence of gallstones or biliary sludge, the sonographic criteria for AC include gallbladder wall thickening (> 3 mm, in fasting condition) with layered appearance, gallbladder enlargement (longitudinal diameter > 8 cm, axial diameter > 4 cm), pericholecystic fluid, and a positive sonographic Murphy sign. [
2] The sonographic Murphy’s sign (tenderness elicited by the compression of the transducer over the gallbladder) is considered more accurate than a conventional physical examination. In particular, the presence of cholelithiasis combined with a positive sonographic Murphy’s sign seems to be the most specific diagnostic finding in AC. [
1,
29,
30,
31] Of note, it may not always be possible to evaluate for sonographic Murphy’s sign (
e.g., in unresponsive patients or after pain medication). [
1]
An isolated sonographic finding is not per se sufficient, while, in the proper clinical setting, concurrent multiple US features are highly accurate for the diagnosis of AC. [
3]
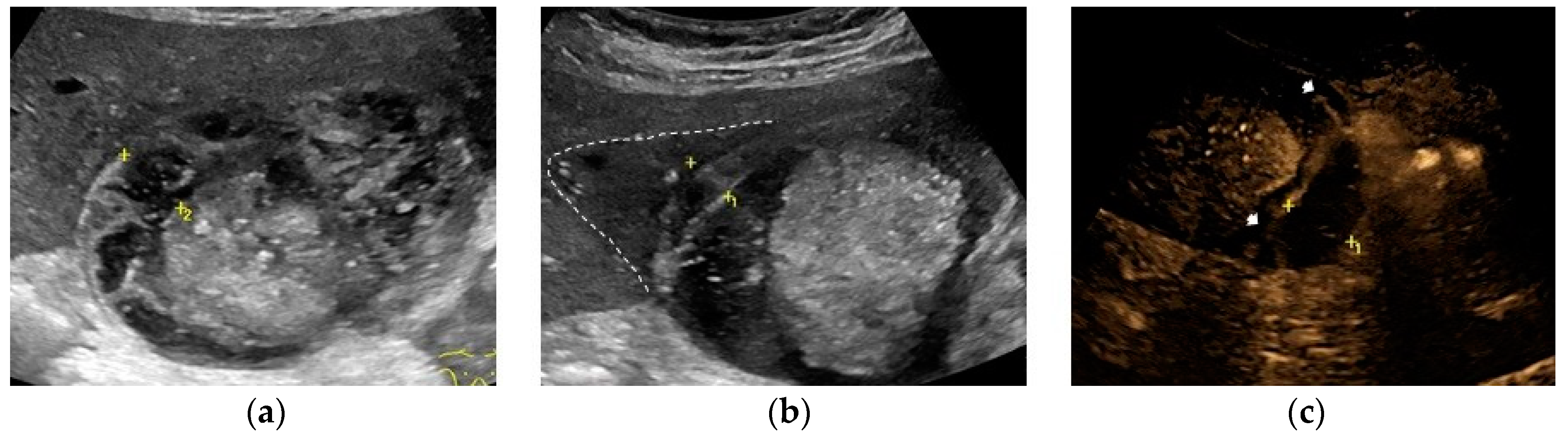
Sometimes, the adjacent liver parenchyma may show findings suggestive of diffuse edema, such as a hypoechoic aspect, possibly with prominent echogenic portal triads, known as “starry-sky” appearance (
Figure 2). [
29,
32]
Hyperemia of the gallbladder wall can be detected by CD evaluation in AC. [
12,
26] MVFI has also been proposed to improve the detection of parietal blood flow. [
22,
33]
In course of AC, CEUS shows fast, homogeneous and intense arterial uptake of the thickened gallbladder wall compared to the liver parenchyma [
14,
16], with a slight hypoenhancement in the late phase. [
20,
34] An indirect sign of AC is the hyperenhancement of the adjacent liver tissue (pericholecystic hepatitis) respect to the rest of the liver parenchyma. [
32,
35,
36,
37] For the same reason, shear wave elastography (SWE) and shear wave dispersion slope (SWD) of the pericholecystic liver tissue may be increased in course of AC. [
38]
6. Acute acalculous cholecystitis
Acute acalculous cholecystitis (AAC) is an acute inflammatory disease of the gallbladder in the absence of intraluminal gallstones. This clinical entity accounts for 5-10% of AC cases and it is associated with high morbidity and mortality. [
39]
Usually, it is found in special clinical settings, e.g., total parenteral nutrition, bone marrow transplant, severe trauma, burns, critical illness, cardiac surgery with cardiopulmonary bypass, immunodeficiency, immunosuppression, diabetes mellitus, systemic vasculitis, COVID-19. [
40] In these clinical contexts, gallbladder stasis and ischemia con occur, with subsequent development of AAC. Sometimes, AAC is due to a primary infection, especially by opportunistic pathogens in course of AIDS (
e.g., Cryptosporidium, Cytomegalovirus or Microsporidia). [
41] The obstruction of cystic duct by biliary cancer, extrinsic inflammation, lymphadenopathy, or metastasis can also lead to AAC. [
27,
34] Notably, AAC represents the most frequent form of acute cholecystitis in children. It usually develops in the setting of infectious or parasitic diseases (in particular Epstein-Barr virus and hepatitis A infection), systemic vasculitis (
e.g., Kawasaki disease and polyarteritis nodosa) or congenital malformations of gallbladder and biliary tract. [
40]
Sonographic features suggestive of AAC are nearly the same of AC, except for the absence of gallstones in the first condition. [
32,
34,
42]In AAC, the sensitivity and specificity of US reach 92% and 100%, respectively. [
39,
43,
44] In case of high clinical suspicion, but unspecific US picture, serial sonographic examination can be easily performed to monitor for the development of AAC. [
45,
46]
7. Acute complicated cholecystitis
The nosological pictures of acute complicated cholecystitis are typically represented by gangrenous cholecystitis (GC), gallbladder perforation, pericholecystic abscess, emphysematous cholecystitis (EC), gallbladder empyema (GE), and Mirizzi syndrome. These complications often coexist in the clinical practice and can be recognized at the US examination.
7.1. Gangrenous cholecystitis
GC is defined as a form of AC with ischemia and secondary necrosis of the gallbladder wall. GC is the most common complication of AC, with a prevalence up to 20%, particularly in patients with risk factors such as older age, diabetes mellitus, cardiovascular disease. [
47] The early recognition of GC is important because it is associated with increased morbidity and mortality.[
48]
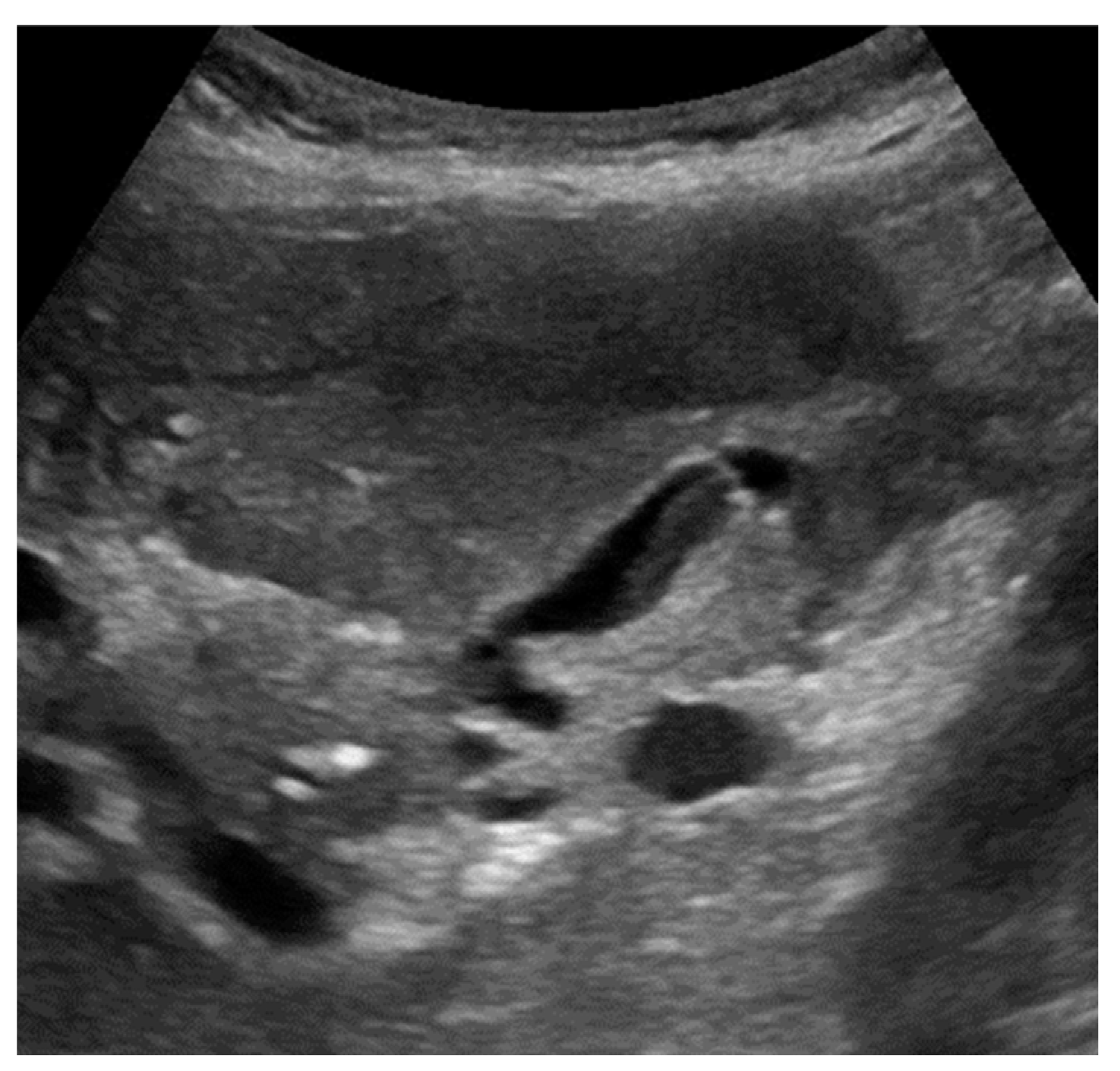
The most common sonographic finding in GC is an irregular gallbladder wall thickening,[
49,
50] characterized by multiple striations with alternating hypoechoic or hyperechoic bands (
Figure 2). [
27] This sonographic pattern is due to the presence of intramural hemorrhage or micro-abscesses. [
3,
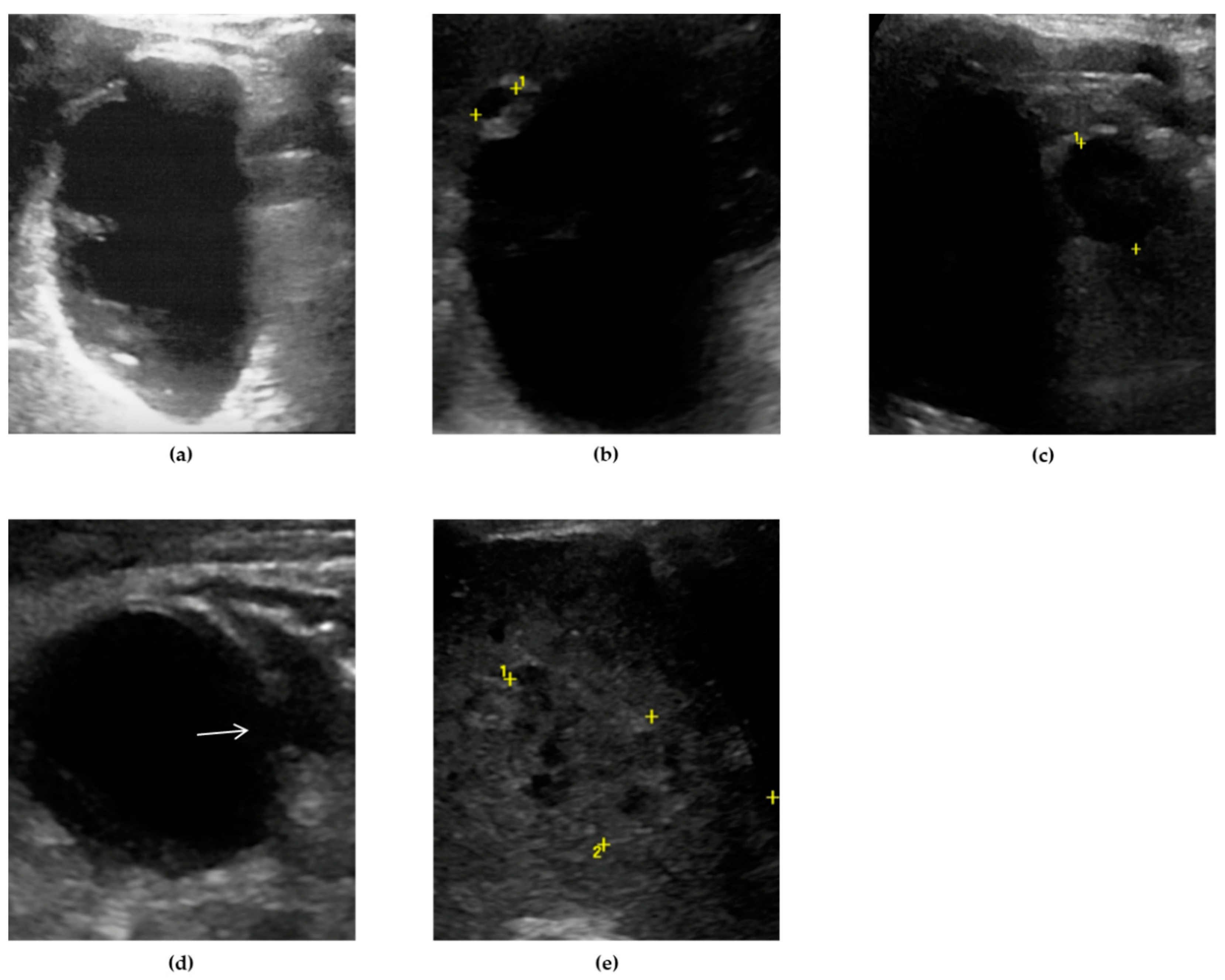
44] Intraluminal membranes, formed by strands of fibrinous exudate and desquamated or “denuded” mucosa, are considered a more specific finding in GC, although less common (
Figure 3).[
3,
44,
49,
50]
Of note, the sonographic Murphy sign is negative in about two thirds of patients, presumably due to ischemic denervation. [
29,
44,
49] According to some authors, the presence of hyperechoic pericholecystic fat is indicative of flogistic involvement and is a specific finding for early gangrenous evolution. [
51]
Some authors suggest that CD examination can help diagnose GC showing focal decrease in the wall perfusion.[
52]
At CEUS examination, the hallmark of GC consists in discontinuous or irregular enhancement of the gallbladder wall, due to perfusion defects in presence of gallbladder wall necrosis. [
35,
53,
54,
55] Similarly, intraluminal membranes don’t show vascular signal. [
16] In a comparative study, CEUS had a high sensitivity and specificity in detecting GC, referred to successive surgical and pathological findings. On the other hand, the same authors reported a few cases with a focal wall defect secondary to perforation in AC without concomitant gangrene. [
55]
In presence of edema and necrosis, MVFI can show the alterations of the gallbladder wall already described by CEUS (
Figure 2).
7.2. Gallbladder perforation
Gallbladder perforation is caused by transmural necrosis, usually in the setting of acute cholecystitis.[
32] In particular, perforation occurs in course of gangrenous cholecystitis or, rarely, in course of acute not gangrenous cholecystitis.[
55] Perforation occurs in about 10% of acute cholecystitis and is usually localized in the fundus, because of its relatively poor blood supply by the terminal vascular branches of the cystic artery.
According to Niemeier classification, US can describe the initial rupture of the gallbladder wall into the peritoneal cavity, the subsequent development of a pericholecystic or liver abscess and finally a bilio-enteric fistula. [
56]
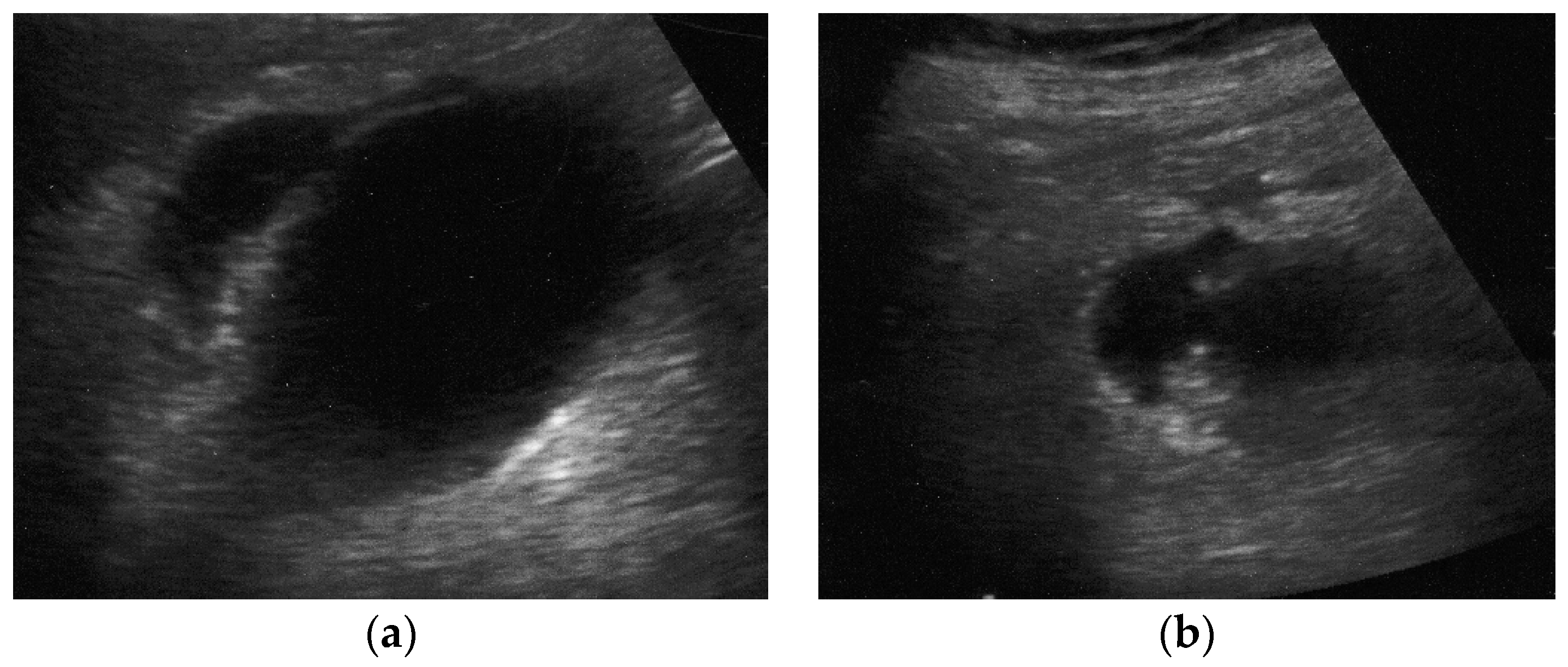
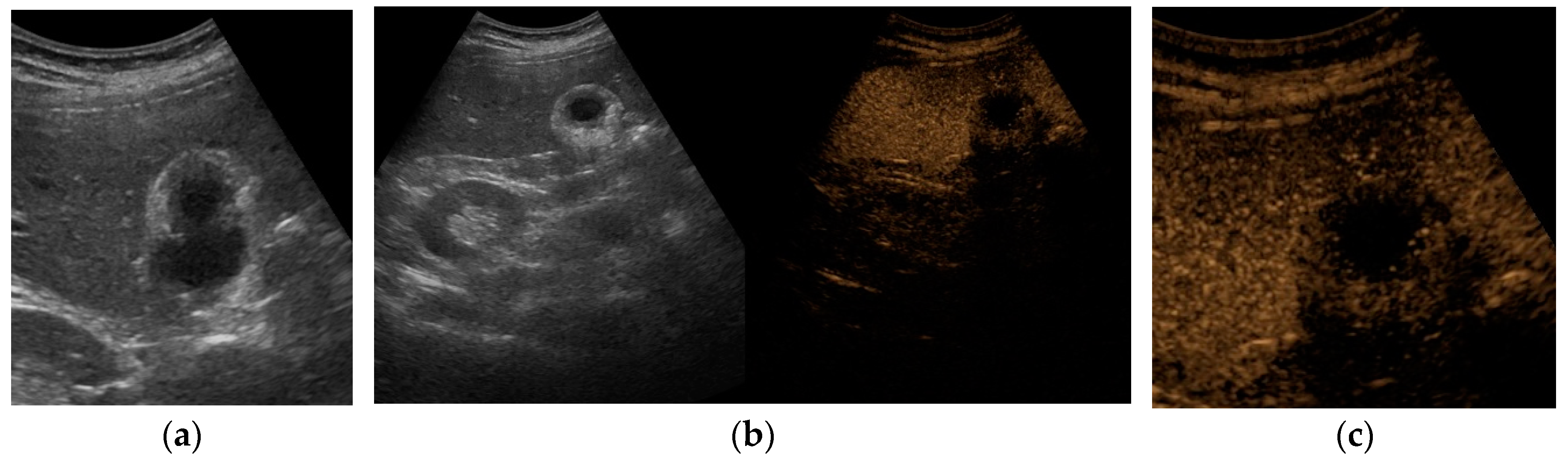
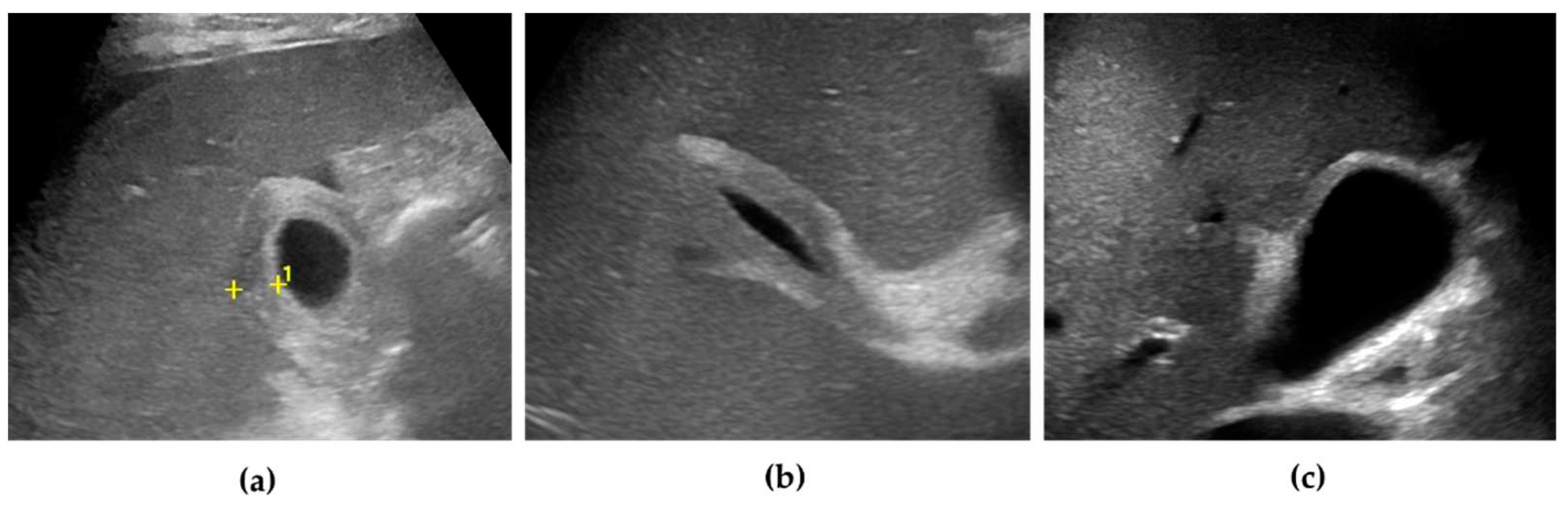
At US examination, the hallmark for the diagnosis of gallbladder perforation consists in the “hole sign”, present in 45-70% of cases [
27] and characterized by a full-thickness defect of the gallbladder wall (
Figure 3 and
Figure 4).[
44,
50,
57,
58] Defects in the gallbladder wall are usually focal and small. However, in case of perforation secondary to infectious necrosis, a large defect may be observed. [
59] The full-thickness disruption of the gallbladder wall can be further highlighted by CEUS examination. [
20,
37,
58,
60]
An indirect although specific sign of gallbladder perforation consists in the detection of gallstones outside the gallbladder lumen, typically in the peritoneal cavity. [
32,
44,
61] Besides the above-mentioned typical features, additional signs may be found. Some authors suggest that in case of thicker gallbladder wall perforation is more frequent. [
27] Pericholecystic fluid or increased fat echogenicity due to mesenteric reaction may be observed adjacent to the site of perforation. [
3,
32,
44,
62]
According to some authors, MVFI may help detect focal areas of decreased vascular perfusion in the gallbladder wall. [
22,
63]
A careful CD analysis can demonstrate to-and-fro signal of gallbladder content across the wall defect. [
64]
7.3. Pericholecystic abscess
In case of a subacute process, gallbladder perforation generally results in pericholecystic abscess or, rarely, in a liver abscess (
Figure 3).[
49]
CEUS is very useful in order to exactly define the presence of pericholecystic collections and abscesses. [
16,
20] In case of pericholecystic phlegmon CEUS findings are variable. Usually, the lesions may show hyperenhancement in the arterial phase, while in a later phase they may show non-enhancing foci, due to the liquefaction process.[
65] The typical CEUS appearance of a mature abscess consists of a hyperenhanced peripheral rim with a central area completely devoid of vascular signal.[
65] Sometimes, the abscess displays a honeycomb pattern with multiple septa and areas of nonenhancement,[
14,
60] based on the amount and distribution of the necrotic material. [
16] The hyper-enhanced portions correspond to the capsule or to the septa provided with vascularized tissue.[
65] In some cases, CEUS demonstrates a hypoenhancement in the peripheral rim or septa of hepatic abscess during the portal venous phase. At the same time, the surrounding liver parenchyma may show diffuse hyperenhancement and possibly a subsequent signal decrease in the late phase. [
65]
Recently, in cases of in acute complicated cholecystitis treated by percutaneous cholecystostomy, intracavitary CEUS has been described. The technique refers to the administration of ultrasound contrast agent inside a physiological or pathological body cavity through a drainage catheter or a puncture needle.[
35] In case of percutaneous cholecystostomy, ultrasound contrast agent can be injected directly in the gallbladder lumen, in order to verify the correct position of the drainage catheter, to detect any possible leak beyond the organ that could indicate a perforation. [
66] Similarly, direct injection of ultrasound contrast agent inside an abscess can be used to confirm the successful placement of the drainage catheter. Moreover, this technique can be useful to highlight the size of the abscess, the presence of septa, communicating compartments inside the abscess or the development of fistulas. [
67,
68] Intracavitary CEUS can be repeated over time to monitor the evolution of gallbladder wall defect and the pericholecystic abscess, in order to improve the therapeutic management.
7.4. Emphysematous cholecystitis
EC is characterized by the presence of gas within the gallbladder wall or lumen in course of AC, in the absence of anomalous communication between the biliary system and the gastrointestinal tract (
e.g., previous sphincterotomy or biliary enteric anastomosis). [
32,
69] It occurs approximately in 1 to 3% of AC,[
50] particularly in the elderly male diabetic patients. [
32,
69] EC usually results from thrombosis or occlusion of the cystic artery leading to ischemic necrosis of the gallbladder wall and only two thirds of patients have gallstones. In turn, this leads to the proliferation of gas-forming organisms (
e.g., Klebsiella, Clostridium or
Escherichia coli). [
32,
69,
70,
71] Frequently, EC leads to gangrene, perforation and other complications. [
3,
50,
70]
Sonographic findings of EC are different according to the amount and the localization of gas. [
50,
71] Gas bubbles can be intraluminal, intramural or in the pericholecystic tissue. [
50,
69] In case of a small amount of gas, intraluminal bubbles appear as highly reflective punctate echoes, associated with distal dirty shadowing, known as ring-down or comet-tail artifact. [
32,
69] Sometimes, US examination displays intraluminal gas bubbles rising from the dependent up to the nondependent portions of the gallbladder cavity (“champagne sign” or “effervescent gallbladder”). [
72,
73] Of note, free bubble air in EC should be differentiated from the rare condition of gas-containing gallstones. [
74] In case of large amount of intraluminal bubbles, a wide linear or curvilinear hyperechoic band is shown at the top of the gallbladder lumen. In this case, a typical reverberation pattern known as powder snow–like posterior echo prevents the visualization of the underlying gallbladder wall. [
44,
50,
71] Notably, a gallbladder filled with gas can be confused with a gas-filled duodenum: US examination with intercostal scans helps make the correct diagnosis. [
71] Gallbladder filled with gas can resemble a porcelain gallbladder or a highly contracted gallbladder filled with gallstones. [
69,
71] However, in case of gallstones or porcelain gallbladder, the echogenic line is usually sharp and always associated with posterior acoustic shadow.[
71] In case of doubt, changing the patient’s position determines intraluminal gas movement, suggesting the diagnosis of EC. In case of intramural gas, US examination may show multiple areas of high reflectivity with distal reverberations or, alternatively, a single bright ring of hyper-reflective echoes within the thickened gallbladder wall. [
3,
69] The detection of pericholecystic gas bubbles suggests that EC has led to gallbladder perforation. [
69]
7.5. Gallbladder empyema
GE, also known as suppurative cholecystitis, occurs when purulent material accumulates within a distended gallbladder in the setting of AC, due to persistent obstruction of the cystic duct and stasis of contaminated bile. GE typically occurs in diabetic patients and can determine gallbladder perforation and sepsis. [
75]
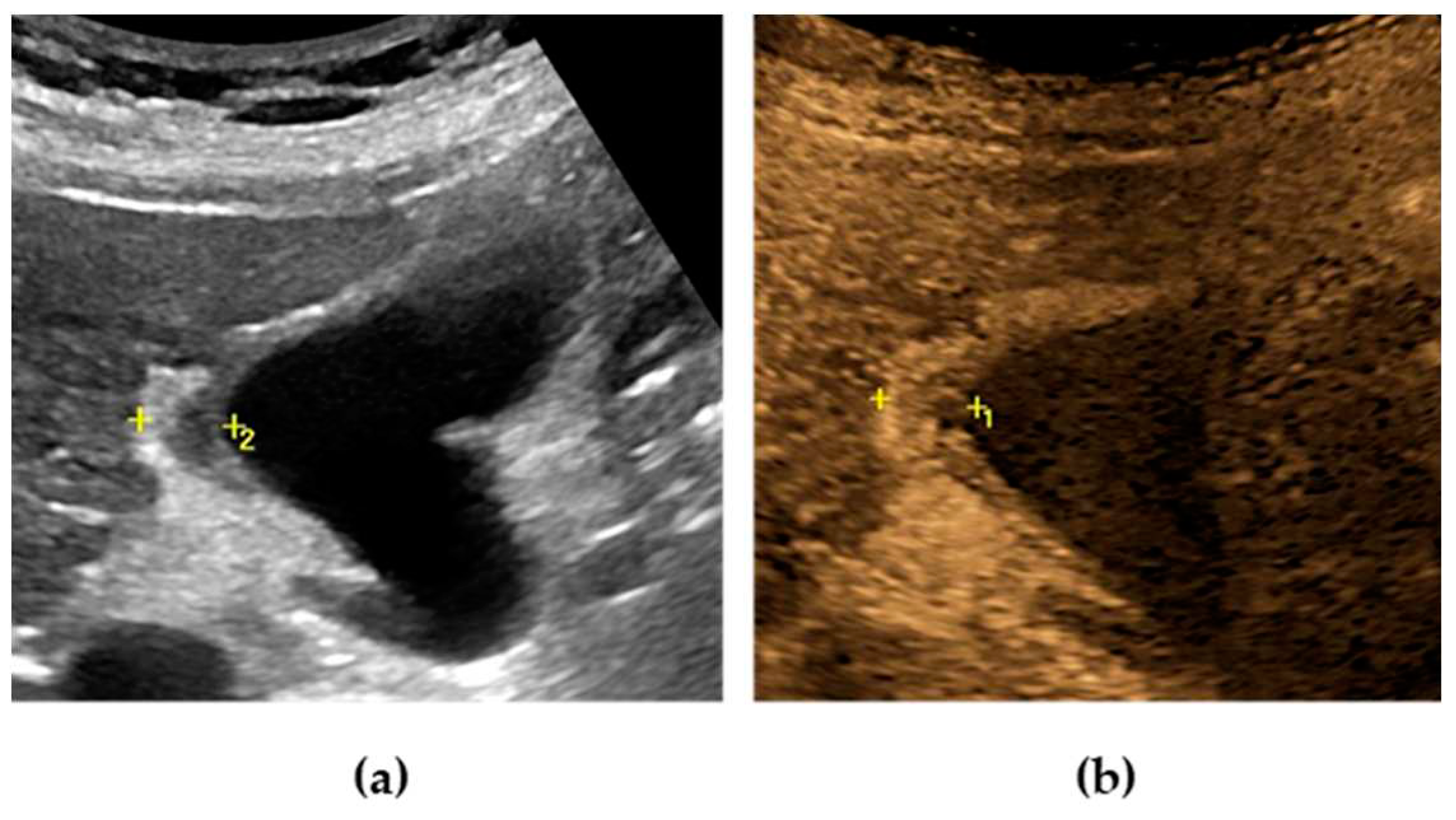
Typically, at US examination the gallbladder is distended and contains intraluminal echogenic material [
76] without posterior acoustic shadows, lying in the dependent portion of the lumen. The echogenic material moves as the patient’s position change, usually resembling biliary sludge (
Figure 2). [
70] Additional sonographic findings of GE are gallbladder wall thickening and pericholecystic fluid. [
76,
77] Rarely, the presence of intraluminal air been reported, suggesting infection by anaerobic pathogens such as
Clostridium or
Bacteroides. [
77]
7.6. Mirizzi syndrome
Mirizzi syndrome is a rare complication of cholelithiasis (up to 5.7% of patients undergoing surgery for cholelithiasis), [
78] occurring when an impacted gallstone in the gallbladder neck, infundibulum or cystic duct causes an extrinsic compression of the common hepatic duct. [
32,
44] A cholecysto-biliary fistula may eventually develop. Based on the presence and extent of cholecysto-biliary fistula, four types of Mirizzi syndrome have been described.[
32,
44,
79]
Mirizzi syndrome is generally suspected in course of abdominal US, which shows dilatation of the biliary system above the level of the gallbladder neck or cystic duct, in presence of one or more stones. Distally to the stenosis, the common bile duct maintains a normal caliber. [
32,
44,
80] Sonographic findings may include gallbladder hydrops, contracted atrophic gallbladder or, in case of concomitant AC, gallbladder wall thickening. US sensitivity is reported to be up to 50%, and in a single study even 77%. [
80,
81,
82] The presence of malignancy must be ruled out before therapeutic decision.
8. Chronic cholecystitis
Chronic cholecystitis is usually associated with gallstones and refers to chronic inflammatory cell infiltration and fibrosis of the gallbladder wall. It is the consequence of a mild, long-standing gallbladder inflammation. Around 5–10% of chronic cholecystitis develops in the absence of gallstones, although some authors have reported this condition in up to 25% of cases. [
2,
70] Most of patients are asymptomatic, although some patients report a history of recurrent acute cholecystitis or biliary colic. Chronic cholecystitis can evolve into AC and GBC. [
32]
At US examination, gallbladder is typically contracted, with uniform circumferential wall thickening, characterized by a preserved two-layer structure. [
2,
27] Rare forms of segmental chronic cholecystitis have been reported. [
83]
In most cases of chronic cholecystitis CEUS examination shows hyperenhancement of the gallbladder wall in the arterial phase, [
20] without the typical features of malignant lesions (see the specific section). [
19,
37,
84] In case of biliary symptoms, the management of chronic cholecystitis is cholecystectomy, possibly in a symptom free interval. [
32,
84]
Besides the typical presentation, different subtypes of chronic cholecystitis may be found, namely xanthogranulomatous cholecystitis (XGC), porcelain gallbladder, IgG4-associated cholecystitis (IgG4-CC), and some other rarer forms (hyalinazing cholecystitis, eosinophilic cholecystitis). [
27]
8.1. Xantogranulomatous cholecystitis
XGC is characterized by the accumulation of lipid-filled macrophages and mixed inflammatory cell infiltrates in the gallbladder wall.[
27,
85] It is a rare entity, with a prevalence ranging from 1-2% in cholecystectomized patients in Western countries and up to 9% in India. [
86] Xanthogranulomatous reaction is thought to originate from extravasation of bile into the wall and into the Rokitansky-Aschoff sinuses (RAS). Rarely, XGC has been found in association with gallbladder adenomyomatosis (GA). [
27]
At US examination, the presence of well-defined hypoechoic nodules or bands within the thickened gallbladder wall represents the xanthogranulomatous reaction documented at histopathologic analysis and it is considered highly suggestive of XGC. [
27,
85,
87] However, the hypoechoic nodules can be misdiagnosed with gallbladder intramural abscess [
85] or with RAS. [
3] The gallbladder wall is focally or diffusely thickened with preserved mucosal layer. [
27,
70] Intraluminal gallstones are present in about 85% of cases, suggesting a potential role in the pathogenesis of XGC. [
88] Additional sonographic findings may include hyperdense intramural nodules, pericholecystic fluid, sludge, and hyperechoic appearance of the adjacent liver parenchyma. [
70] In fact, in the most severe cases, xanthogranulomatous inflammation can extend from the gallbladder to the adjacent structures such as liver, bowel and stomach, resulting in adhesion, perforation, abscess and fistula, possibly detectable at US examination. [
85]
The diagnosis of XGC can be improved by mean of High-Resolution Ultrasound (HRUS), defined as the use of 7-10 MHz linear probe combined to the 3-5 MHz convex probe. The high-frequency linear transducer allows a higher imaging resolution of the gallbladder wall which can result in a clearer and more accurate visualization of XGC features. [
2,
89,
90,
91]
When XGC is suspected, an important issue is the differential diagnosis with GBC, both in the pre- and intra- operatory setting, especially in case of severe proliferative fibrosis of the gallbladder and surrounding tissues. [
92] Rarely, XGC has been found in association with GBC. [
93,
94] Therefore, the sonographic suspect of XGC warrants cholecystectomy. [
32]
According to some authors, CEUS may help diagnose XGC by detecting a continuous inner gallbladder wall enhancement in the arterial phase, with late hypoenhancement. Diffuse thickening of gallbladder wall and hypoechoic nodules are also highlighted by CEUS. [
95,
96]
8.2. Porcelain gallbladder
Porcelain gallbladder is a rare variant of chronic cholecystitis, diagnosed in less than 1% of cholecystectomy specimens, characterized by calcification of the thickened gallbladder wall, resulting from long-standing inflammation. [
1,
27] Its name derives from the brittle consistency of the gallbladder. [
1] The histological hallmark of porcelain gallbladder consists in mural calcification, ranging from focal plaques within the mucosal layer to a broad and continuous band that includes and replaces the muscular layer. [
1] [
2] These pathological changes can involve the entire gallbladder or may be confined to part of the organ.[
1] Of note, intraluminal gallstones are present in more than 95% of cases of porcelain gallbladder. [
97] Previous studies reported a cancer risk up to 60% in porcelain gallbladder, while recent data have found a much lower association (2-3%). [
2,
27] Interestingly, the increased risk may be confined to patients with selective mucosal calcification or incomplete mural calcification. [
98,
99]
At US examination porcelain gallbladder typically appears as a curvilinear or linear hyperechoic structure with a wide posterior acoustic shadow in the gallbladder fossa. This variant corresponds to complete intramural calcification and must be differentiated from a gallbladder completely filled with stones. [
27] In case of selective mucosal calcification, two different sonographic patterns are described: scattered punctuate echoes with acoustic shadow within the gallbladder wall or, alternatively, a biconvex, curvilinear hyperechoic structure with variable acoustic shadowing in the gallbladder fossa.[
1] The US appearance of porcelain gallbladder may be similar to EC, but the clinical setting is considerably different. [
2]
In the setting of porcelain gallbladder, CEUS can characterize a coexisting mass with signs of malignancy (see the specific section).
8.3. IgG4-related cholecystitis
IgG4-CC is an emergent organ manifestation of IgG4-related disease and only few cases are reported in literature. [
100]
At US examination, the main finding of IgG4-CC is the thickening of the gallbladder wall, which can be diffuse or localized [
27]. Recently, a further classification of IgG4-CC wall thickening has been proposed in Japan, due to the wider diffusion of IgG4-related diseases.
Sometimes, it can be challenging to differentiate IgG4-CC from GBC. [
100,
101] For this reason, the important role of CEUS in IgG4-CC is to exclude malignancy (see the specific section).[
100]
9. Hyperplastic cholecystoses
Hyperplastic cholecystoses refer to different conditions characterized by lipid accumulation (cholesterol and triglycerides) in the gallbladder wall. The two main variants of gallbladder cholecystoses are GA and gallbladder cholesterolosis.
9.1. Gallbladder adenomyomatosis
GA is relatively common, observed in 2% to 9% of cholecystectomy specimens. [
102,
103,
104] At the histological examination, GA is characterized by gallbladder wall thickening with epithelial proliferation, muscular hypertrophy and RAS (mucosal invaginations into the thickened muscularis propria). [
34] According to its distribution, GA can be classified in focal, segmental (or annular), and a diffuse type. [
2] Sometimes, these features may coexist (
Figure 5). The focal variant, also called adenomyoma, is the most frequent and the gallbladder fundus is most commonly involved. Rarely, a focal adenomyoma may appear as an intraluminal polypoid projection. The segmental type affects a ring of the gallbladder body with luminal narrowing, resembling an “hourglass” or a “waist-like” appearance.[
105] Some authors consider segmental GA as a precancerous condition. [
106,
107] The diffuse type is the less common form and involves the entire organ.
At US examination, the presence of RAS in a thickened gallbladder wall strongly suggests GA. However, RAS are not always detected, given their small size, ranging from 1 to 10 mm. Notably, the use of HRUS can highlight the presence of RAS, which appear as small anechoic spaces within the gallbladder wall. [
108] When RAS are filled with sludge, small stones, or cholesterol crystals, their internal structure become echoic. In particular, cholesterol crystals inside RAS appear as echogenic mural spots and act as highly reflective surfaces that generate posterior reverberation artifacts (vertical “V-shaped” echoes, known as “ring down” or “comet tail” artifact). [
109,
110] Similarly, CD examination can show a persistent signal composed of a rapidly changing mixture of red and blue color (twinkling artifact). [
111]
CEUS can be very useful in the diagnosis of GA by increasing the detection of RAS, that don’t show vascular enhancement. Typically, in the arterial phase the thickened gallbladder wall shows a “moth-eaten” pattern (
Figure 5). [
16] In the venous phase, the thickened wall can display a slight hypo-enhancement. [
14] Importantly, CEUS can help exclude malignant lesions (see the specific section). [
112,
113,
114,
115,
116]
Cholecystectomy can be considered in case of biliary symptoms or in the suspect of potential malignant evolution (
e.g., in case of segmental adenomyomatosis). [
117]
9.2. Gallbladder cholesterolosis
Gallbladder cholesterolosis is a form of hyperplastic cholecystosis characterized by lipid accumulation (cholesterol, cholesterol esters, and triglycerides) in the macrophages of the lamina propria. It may involve the gallbladder in a focal or diffuse form. The prevalence ranges from 9% up to 26% of cholecystectomy specimens. [
118]
At US examination, lipid accumulation appears as bright hyperechoic foci within the gallbladder wall, that may show a comet tail artifact. With time, they can be covered by normal gallbladder epithelium, with the development of cholesterol polyps. The diffuse form of gallbladder cholesterolosis corresponds to the so-called “strawberry gallbladder”, a pathologic finding characterized by a bright red mucosa with slightly raised interposed areas of yellow lipid aggregates at gross examination. [
102,
119]
10. Gallbladder polyps
Gallbladder polyps are mucosal projections of varying shape and size, rising from the gallbladder wall and protruding into the lumen. [
120,
121] Gallbladder polyps are a common finding at US examination (from 1.5% to 4.5%), with even higher prevalence at histological analysis (up to 13.8%). [
120] They are classified into benign (neoplastic or non-neoplastic) and malignant lesions. [
2] Cholesterol polyps are the most common type, followed by adenomyomas, inflammatory polyps and adenomas. Leiomyomas, fibromas, lipomas, and heterotopic mucosa have also been described. [
122] Adenocarcinoma is the most common type of malignant polyp. Less common forms are lymphoma, sarcoma, mucinous cystoadenoma, squamous cell carcinoma, adenoacanthoma, metastases from different malignant lesions. [
120,
121]
10.1. Cholesterol polyps
Cholesterol polyps are the most common form of benign gallbladder polyps and represent a morphologic variant of gallbladder cholesterolosis. [
3,
120] At US examination, they typically appear as multiple, small (usually 1-2 mm, rarely up to 10 mm), round shaped, intraluminal hyperechoic masses with smooth contours, fixed to the gallbladder wall regardless of positional change, without any acoustic shadow. Their stalks are rarely visible, giving a typical appearance known as the “ball on the wall” sign. [
102]
10.2. Inflammatory polyps
Inflammatory polyps result from granulation and fibrous tissue secondary to chronic inflammation. They are often associated with chronic cholecystitis and gallstones. At US examination they typically appear as small (5-10 mm), sessile or peduncolated polyps, without comet tail artifact, acoustic shadows or other specific sonographic features. [
122,
123,
124]
10.3. Adenomas
Adenomas are the most common benign neoplastic polypoid lesions of the gallbladder, accounting for a relatively low overall prevalence (4% of benign gallbladder polyps). US examination typically shows a single, sessile or pedunculated mass, variable in size (from 5 to 20 mm), with detectable vascular flow at CD and CEUS examination. In almost 50% of cases gallbladder adenomas are associated with intraluminal gallstones. [
120,
122]
Biliary papillomatosis is a rare condition characterized by the presence of multiple adenomatous foci, diffuse or confined to a specific segment of the biliary tract. When the gallbladder is involved, its internal surface is interested by multiple small polyps (multicentric papillomatosis). [
125,
126]
Although the progression from adenoma to carcinoma is not definitively proved, the polyp size is considered to be directly related to the risk of gallbladder malignancy. [
122] Some authors describe a cancerization risk of 128.2 per 100.000 person-years in case for adenomatous polyps more than 10 mm in size. [
127]
10.4. Sonographic differentiation and characterization of gallbladder polyps
US has the highest sensitivity (84%) and specificity (96%) in the detection of gallbladder polyps, and is the most used imaging technique in the diagnosis, characterization and follow up of these lesions (
Table 1). [
34,
128,
129,
130]
HRUS proved to be more accurate in the diagnosis and characterization of gallbladder polyps than conventional US. [
91,
131,
132]
CEUS can highlight some features of gallbladder polyps (
e.g., size, morphology, and stalk). Cholesterol polyps typically show iso-enhancement in the arterial phase while most adenomatous polyps show homogeneous hyperenhancement. Considering the microvascular pattern, most authors do not find substantial differences between cholesterol and adenomatous polyps. A single study described a different behavior in the arterial phase for cholesterol polyps (dotted pattern) and adenomatous polyps (linear pattern). [
133,
134] In the late phase, both cholesterol and adenomatous polyps tend to be isoechoic to the surrounding parenchyma. However, a minority of benign polyps show a slight and delayed hypoenhancement (differently from the more pronounced wash-out of malignant lesions). [
2,
15,
16,
113,
133,
134,
135,
136]
There is some evidence that high-frame rate CEUS may better differentiate between cholesterol and adenomatous polyps. [
137]
Recently, some studies have shown that MVFI can differentiate between benign, adenomatous and malignant polyps. [
22,
138]
Tridimensional ultrasound (3DUS) examination has also been proposed to better define gallbladder polyps. [
139]
Artificial intelligence, in particular radiomic analysis based on B-mode and SMI, has been applied in the sonographic differentiation between neoplastic and cholesterol polyps. [
140]
In recent years, some authors have developed scoring systems to predict the histologic type of gallbladder polyps, based on pre-operative sonographic findings and/or patients characteristics. [
141,
142] Polyps > 10 mm in size have been considered as preinvasive adenomas or papillary neoplasms, while polyps from 6 to 10 mm in size rarely progress to malignancy. [
3,
35] For this reason, cholecystectomy has been proposed for all the polypoid lesions larger than 10 mm, while for polyps smaller than 10 mm a simple follow up has been suggested. Recently, concomitant risk factors for gallbladder malignancy have been taken into account: elderly age (more than 60 years), Asian ethnicity (especially Indian), history of primary sclerosing cholangitis, concomitant focal gallbladder wall thickening (> 4 mm). [
143] According to recent guidelines, the sonographic follow up in patients without additional risk factors for malignancy should be related to the polyp size. [
144]
11. Gallbladder carcinoma
GBC is the most common malignant neoplasm of the biliary tract,[
145] especially in the Eastern countries. [
146] It is found incidentally at the histological examination in 1% of all cholecystectomy specimens. [
147] GBC usually develops from underlying chronic cholecystitis, especially porcelain gallbladder. Gallstones represent a risk factor according to some authors, especially if long-standing and large-sized (> 3 cm). [
148] Symptoms are often late and unspecific (
e.g., right upper quadrant pain, jaundice and weight loss).[
145] In fact, GBC is an incidental finding in almost 50% of cases, usually diagnosed in post-cholecystectomy histology.[
149] The most frequent site for GBC is the fundus of the gallbladder (about 60%), followed by the body (30%) and the neck (10%).[
150] Very rarely GBC arises from cystic duct. [
146]
US usually represents the first initial assessment in symptomatic patients, with a high accuracy (more than 80%), especially in the diagnosis of locally advanced GBC. However, US examination has a poor sensitivity in detecting early GBC. [
3,
151,
152] GBC can show different patterns: a mass obscuring or replacing the gallbladder, a polypoid mass, a focal or diffuse thickening or irregularity of the gallbladder wall. Intraluminal gallstones are usually found. [
1]
A mass obscuring or replacing the gallbladder is the most common presentation of GBC. At US examination, GBC appears as an ill-defined mass in the gallbladder fossa, mainly hypo- or iso- echoic to the liver. The echotexture is usually mixed and dishomogeneous, sometimes with anechoic regions (tissue necrosis, or, less frequently, residual bile) or intralesional gallstones (intralesional hyperechoic foci). Generally, the GBC shape is not well defined and the margins can be irregular, due to the invasion of the adjacent hepatic parenchyma. Notably, the gallbladder can be partially or completely masked or replaced by the malignant mass (
Figure 6).[
153] Small amounts of pericholecystic fluid can be detectable at US, suggesting a poor prognosis.[
1,
154]
The histological examination of a polypoid mass of the gallbladder wall can reveal an early-stage GBC, well-differentiated and confined to the mucosa or muscularis propria.[
154] At US examination, the polypoid mass can be hyper-, hypo- or isoechoic to the liver, usually with a homogeneous tissue texture and without acoustic shadow. A polypoid GBC generally has a large implant and smooth borders.[
144]
The rare GBC variant presenting as a focal or diffuse thickening or irregularity of the gallbladder wall is generally difficult to diagnose. [
1] GBC confined to gallbladder mucosa may present as flat or slightly raised lesion with luminal surface irregularity, sometimes without an appreciable wall thickening.[
1] At more advanced stages, GBC can show marked mural thickening, often with irregular and mixed echogenicity.[
1] Some authors described a thicker (> 10 mm) and less echoic gallbladder wall in GBC compared to chronic cholecystitis. [
1,
153]
11.1. Sonographic differentiation and characterization of GBC
Sonographic characterization and even differentiation between benign and malignant gallbladder lesions can be challenging.
At B-mode evaluation, a larger lesion and disrupted gallbladder wall suggest malignancy. [
15] However, only coexistent signs of pericholecystic invasion are reliable indicators for GBC, such as lymphoadenopathies, vascular infiltration of the hepatic pedicle, peritoneal involvement, liver metastases, and pericholecystic fluid. [
153]
HRUS may be useful in differentiating GBC from benign lesions, especially GA and XGC. In particular, some studies indicate that HRUS can differentiate GA from early-stage GBC, with a diagnostic performance comparable to MRI and magnetic resonance cholangiopancreatography. [
155,
156,
157]
Sometimes, CD analysis can help in the diagnosis of GBC. In case of detectable CD signal, a power Doppler blood flow higher than 20–30 cm/s suggests a malignant lesion.[
151,
158]
CEUS can be useful in differentiating GBC from benign lesions, mainly tumefactive sludge and chronic cholecystitis. [
35,
159,
160]Arterial hyperenhancement is not per se diagnostic, because it is also common in benign gallbladder lesions.[
35,
161] However, during the arterial phase, the detection of linear or irregular and branched vessels can suggest malignancy.[
15,
162,
163,
164] An early wash out (within 35 s) is strongly suggestive of malignancy.[
15,
35,
164] In fact, about 91% of GBC and only 17% of benign lesions display wash-out within 35 s. [
159] At CEUS examination, disruption of the gallbladder wall and infiltration of the adjacent liver tissue are considered accurate features for malignancy. [
35,
161,
163,
165,
166,
167] The CEUS sensitivity for GBC is significantly lower for lesions ≤ 1 cm,[
18,
168] while for larger lesions its performance seems to be comparable to CT and MRI. [
169] Recently, promising data have been proposed on the diagnostic accuracy of some parameters derived from the wash in / wash out curve in the automated CEUS quantitative analysis (
e.g., rise time, mean transit time, time to peak, fall time).[
15,
170] Of note, according to some authors, CEUS performed with high frequency linear transducers could be a useful tool in GBC diagnosis, especially in case of focal fundal gallbladder wall thickening. [
171]
At MVFI evaluation, malignant lesions can display tortuous micro-vessels or abrupt vascular caliber changes.[
22]
3DUS may be useful in the characterization of a gallbladder mass suspect for malignancy, to better evaluate its location and extension.[
139]
Elastography has also been proposed to the evaluation of GBC. [
139]
12. Gallbladder metastases from different organ neoplasia
Metastatic diffusion to the gallbladder wall is rare, accounting for less than 5% of all gallbladder malignancies. In more than 50%, the primitive tumor is melanoma, followed by breast cancer, hepatocellular carcinoma, renal cell carcinoma, gastrointestinal tract cancers. [
172,
173] Gallbladder metastases are usually asymptomatic but, in some cases, they can manifest as AC, especially in case of larger polypoid lesions or involvement of the neck or the infundibulum.[
174,
175]
At B-mode evaluation gallbladder metastases usually appear as single or multiple intraluminal nodules. [
35] Rarely, they present as focal or diffuse wall thickening. [
174,
176] Metastatic lesions from melanoma typically display a low-moderate echogenicity, probably due to the low reflectivity of melanin. [
176] Differently from primary gallbladder adenocarcinoma, secondary neoplastic lesions of the gallbladder are rarely associated with gallstones. [
177] CD evaluation may show intralesional signals, although the absence of vascular signals doesn’t exclude malignancy. [
177,
178,
179]
Secondary gallbladder localization of different neoplastic lesions may show vascular signal at CEUS examination, sometimes with arterial hyperenhancement and wash out in the late phase.[
35,
176,
177]
13. Rare gallbladder neoplasia
Rarely, gallbladder can be interested by lymphomas and neuroendocrine tumors, that generally show the same sonographic features of GBC (see the specific section). [
180,
181,
182]
Gallbladder cystoadenoma and mucinous cystic neoplasm appear at US evaluation as a cystic lesion, more often multiloculated. In most cases, the internal structure has a serous anechoic content, sometimes with the presence of echogenic material, suggestive of blood, mucin, or protein aggregates.[
183,
184] Walls and septa can be thickened, with calcifications, casting posterior acoustic shadows. Papillary projections and nodular solid components have also been described. [
185,
186]
14. Heterotopic tissue in the gallbladder
Heterotopia (normal tissue in an abnormal location) is rarely found in the gallbladder.[
187] Most frequently, it is represented by gastric mucosa, followed by pancreatic and, very rarely, thyroid, liver and adrenal gland tissue. [
187] The most frequently involved portions are the cystic duct and the gallbladder neck. [
188]
At US examination heterotopic tissue in the gallbladder appears mainly as a polypoid mass, either sessile or pedunculated, more often hyperechoic or sometimes isoechoic, of variable size (from 5 to 30 mm). Less frequently, it presents as focal gallbladder wall thickening, usually hyperechoic. [
188] Rarely, the mucus secretion of heterotopic gastric or pancreatic tissue can lead to the development of parietal cystic lesion. [
189,
190]
15. Gallbladder trauma
Gallbladder injury occurs in about 2% of blunt abdominal trauma and is usually associated with the involvement of the liver or different abdominal organs.[
191] Isolated gallbladder injury is even rarer, due to its size and its localization, protected by the liver and the ribs. Injury of the gallbladder includes contusion (intramural hematoma), perforation, and avulsion. [
192]
At US examination, the finding of discontinuous or irregular gallbladder wall is suggestive of perforation, especially in presence of a collapsed gallbladder. [
191,
193] The finding of echoic material within the gallbladder lumen should raise the suspicion of intraluminal bleeding. [
194,
195] Pericholecystic or perihepatic fluid and gallbladder wall thickening represent common although less specific findings. [
191,
194]
16. Gallbladder edema
Besides typical calculous and acalculous AC, different systemic and local diseases can determine gallbladder wall edema. Among these, the most frequent are hepatic cirrhosis (
Figure 7), acute hepatitis, acute pancreatitis, congestive heart failure, hypoalbuminemia, sepsis, acute renal failure, peritonitis and pyelonephritis. [
2,
196]
Notably, in bone marrow transplant patients, gallbladder edema is important for the suspicion of sinusoidal obstruction syndrome (SOS), also known as veno-occlusive disease (VOD), or hepato-biliary graft-versus-host-disease (GVHD) (
Figure 8).[
197,
198]
Gallbladder edema has been also described in course of salmonella enteric infection, molecular targeted therapies, hyperthyroidism, pre-eclampsia, Dengue fever, gold salt hypersensitivity, acute peri-myocarditis, scarlet fever, and COVID-19 infection. [
199,
200,
201,
202,
203,
204,
205,
206,
207]
In case of acute hepatitis, especially of viral etiology, gallbladder wall edema is often associated with biliary sludge, hepatomegaly and diffusely hypoechoic texture of the liver, sometimes with prominent portal triads (“starry sky” appearance). [
208,
209]
The sonographic hallmark of gallbladder edema is the presence of wall thickening (> 3 mm) with a multilayered and meshwork pattern. [
196] A double-wall appearance can be seen, characterized by the presence of hyperechoic outer and inner borders with a relatively less echogenic layer in between. [
208] Gallbladder edema can be misdiagnosed with the pseudo-thickening observed in the post prandial state, due to the gallbladder physiologic contraction. [
196]
CEUS could be useful to differentiate gallbladder wall edema from gallbladder wall thickening due to AC. In case of simple edema, the contrast enhancement is observed in the inner and outer layers of the gallbladder wall, but not in the hypoechogenic edematous area in between. [
16]
17. Gallbladder volvulus
Gallbladder volvulus (GV), also known as gallbladder torsion, is a rare condition, accounting less than 500 cases in literature. GV consists in the rotation of the gallbladder on its long axis, resulting in impaired vascular supply with subsequent gallbladder wall schemia. [
210] GV typically occurs in the elderly females or, less frequently, in pediatric patients and young adults. Patients with complete torsion (>180°) usually present a clinical picture resembling AC. Differently, in case of incomplete gallbladder torsion (<180°), patients may experience recurrent episodes of slowly progressive biliary pain. [
210,
211]
At US examination the most common finding of GV is the presence of a “floating gallbladder” with a thickened wall. [
210] US examination may show a hypoechoic edematous layer between the muscular wall and the mucosa, due to venous and lymphatic stasis. Some authors have reported the sonographic finding of stretched cystic duct and gallbladder neck, appearing as a conical-shaped structure composed of multiple linear echoes converging towards the tip (“cystic duct knot sign”). [
212,
213,
214] The sonographic finding of portal venous gas associated to gallbladder ischemia in GV has been described. [
215]
CD evaluation can detect blood flow interruption in the cystic pedicle, typically associated to the complete gallbladder torsion, and absence of vascular signals in the gallbladder wall. [
210,
211]
A sonographic picture suggestive of GV strongly indicates emergency cholecystectomy. [
210]
18. Hemobilia
Haemobilia is defined as the presence of macroscopic endoluminal blood in the biliary tree or in the gallbladder. [
216] Haemobilia is most frequently secondary to complications of invasive procedures on the hepatopancreatobiliary system.[
216] Additional causes of haemobilia include traumatic injury, biliary tumors, inflammatory disease, venous or arterial-biliary fistulae, rupture of aneurysm of the hepatic artery, hemorrhagic cholecystitis, and coagulation disorders. [
3] Concomitant right upper quadrant pain with jaundice and overt gastrointestinal bleeding (Quinckle’s triad) has been classically described. [
49]
At US examination, blood appears initially as echoic material in the gallbladder lumen, that tends to layer in the most declivous portion. [
3,
44,
49] Later in time, when the hematoma has developed, it looks like an echoic, usually dishomogeneous, nonmobile intraluminal mass, without acoustic posterior shadowing. [
3,
49] Rarely, intraluminal blood may develop a cystic appearance. [
44]
CEUS can be useful in the detection of active intraluminal gallbladder bleeding. [
16]
Sometimes, US evaluation can detect the underlying cause of hemobilia. In fact, some authors reported the rupture of artery pseudoaneurysm, appearing as a hypoechoic intraluminal mass of the gallbladder, with the typical “yin and yang” sign at CD examination. Power doppler analysis shows a pulsatile wave pattern. [
217,
218]
19. Gallbladder Ascariasis
Ascariasis is a human infestation caused by
Ascaris lumbricoides, diffused in tropical and subtropical areas.
Ascaris lumbricoides adult worms can migrate from the duodenum to the biliary tract and into the gallbladder. [
219]
At US examination, active live worms in the gallbladder appear as long, curved or coiled up echoic structures, with active movements and without acoustic posterior shadow. They can show a longitudinal central anechoic tube, representing the digestive tract of the worm, surrounded by a thick echoic stripe (“inner tube sign” or “triple line sign”). Alternatively, they can simply appear as a thin tubular echoic stripe (“stripe sign”). [
220] In the transversal scan, worms display round shaped structure, with a target-like appearance. [
221] Hook-shaped and coiled worms can lead to a septated appearance of the gallbladder or, sometimes, a “bull’s eye” sonographic picture. [
219,
222] In case of living worms, US is the first choice technique for the diagnosis of gallbladder ascariasis (84% sensitivity). [
223,
224] Notably, the sonographic finding of not movable worms is suggestive of their death or paralysis.
Some different worms (e.g., Clonorchis sinensis, Opisthorchis viverrini, Opisthorchis felineus, and Fasciola hepatica), can rarely lead to a similar sonographic picture. [
225]
20. Congenital variants of the gallbladder
Congenital variants of the gallbladder represent a rare entity and sometimes they are an incidental sonographic finding. Their early recognition avoids misdiagnosis and unnecessary diagnostic workup. Notably, 3DUS can be useful to highlight the anatomical features of gallbladder congenital variants.[
139]
Gallbladder is a pear-shaped organ, but different morphologies are described. The most frequent variants are the Phrygian cap gallbladder (the fundus is folded over the body of the organ) and the sigmoid gallbladder, appearing as a double cavity because of a septum or a folded morphology.[
226]
Further congenital gallbladder variants:
- -
Agenesis of the gallbladder. It’s defined as the absence of the gallbladder in patients without a history of cholecystectomy. This rare condition (10 to 65 per 100.000), is frequent in patients with biliary atresia (1 out of 6 patients). In almost half of patients, agenesis of the gallbladder is associated with the development of common duct stones. It is important to rule out gallbladder ectopia. [
227]
- -
Hypoplasia of the gallbladder. In adults, the minimal gallbladder length is generally 7 cm and the minimal width 2 cm. [
227,
228] It has been reported an association with cystic fibrosis, cholangitis, and biliary atresia. At US examination, the hypoplastic gallbladder seems to be contracted, collapsed or simply small in size. Microgallbladder, typically associated with cystic fibrosis, is defined as a gallbladder < 2-3 cm long and < 0.5-1.5 wide. [
229,
230]
- -
Septated and multiseptated gallbladder. Very rarely, US shows a multichambered or multiloculated gallbladder lumen with multiple thin intraluminal septa, sometimes with a “honeycomb” appearance. [
227]
- -
Duplicated gallbladder. Duplication of the gallbladder consists in the presence of two completely separated gallbladder cavities, that can present a common cystic duct (bilobed gallbladder) or two different cystic ducts. In the latter case, the two separated cystic ducts may have a common insertion in the main bile duct (Y-shaped gallbladder) or, alternatively, two distinct insertion points (V-shaped gallbladder). [
227] The presence of sludge, cholelithiasis or disease – for example cholecystitis - only in a single gallbladder cavity helps detect the presence of two different gallbladder lumens, suggesting the diagnosis of duplicated gallbladder. [
227]
- -
Intrahepatic gallbladder. US shows the gallbladder partially embedded or completely incorporated in the hepatic parenchyma. This anatomic variant is usually associated with biliary stasis, because of ineffective gallbladder emptying, and with an increased risk of torsion. [
227]
- -
Left-sided gallbladder. In this ectopic variant, the gallbladder is located on the left side of the
ligamentum teres, between the segments III and IV or on the segment III. It can be associated with
situs viscerum inversus, portal vein or biliary system anomalies, and segment IV atrophy. At US examination, left-sided gallbladder generally appears as a cystic mass near the left lobe of the liver, in front of the pancreas, with a narrow neck connecting to the bile duct. [
227,
231]
- -
Rarer variants of ectopic gallbladder. Rarer variants of ectopic gallbladder have been described in literature, namely retrohepatic, suprahepatic, supradiaphragmatic, retroperitoneal, intrathoracic, within the falciform ligament or within the abdominal wall musculature. The sonographic visualization of gallbladder may be particularly challenging for these rare variants. [
227]
21. Gallbladder dysmotility
Gallbladder dysmotility is a functional gallbladder disorder, characterized by biliary pain in the absence of gallstones, sludge or structural disease.[
232] Gallbladder dysmotility can be associated with different conditions, such as diabetes, obesity, myotonic dystrophy, cirrhosis, irritable bowel disease, slow transit constipation, medications, and celiac disease. [
233] Additionally, in patients with celiac disease an enlarged gallbladder, often containing sludge, can be found at US examination. [
234]
The finding of a low ejection fraction, evaluated by cholecystokinine – stimulated cholescintigraphy (HIDA scan), supports the diagnosis of gallbladder dysmotility.[
235] Similarly, US is used for the evaluation of gallbladder motility, both in research studies and in some clinical settings. [
236,
237] The gallbladder functional sonographic study is based on the calculation of its volume at the baseline and then at regular intervals after the ingestion of a standard fat meal, in order to evaluate gallbladder emptying and subsequent filling. A software has been developed to calculate the gallbladder volume after the acquisition of two-dimensional sonographic imaging. [
238,
239] Recently, 3DUS and 4DUS (
i.e., dynamic 3DUS) have improved the evaluation of gallbladder motility, with a diagnostic performance similar to cholescintigraphy. [
240]
22. Conclusion
US is a highly effective imaging tool for the diagnosis of gallbladder disease. Since its introduction in the medical practice, US has acquired much importance, not only in the elective setting, but also as a prompt confirmation of clinical suspicion in patients with right upper quadrant pain or possible gallbladder disease. In particular, gallbladder POCUS can be easily performed by the clinician in the medical office or in emergency room.
Recently, new sonographic tools have been developed, such as HRUS, MVFI/SMI, CEUS, 3D-US, elastography, artificial intelligence-powered US. These new US-based techniques will need further evaluation to elucidate their individual diagnostic potential. Importantly, the combination of multiple sonographic tools (so-called multiparametric US) can improve the diagnostic yield of US examination.
Author Contributions
Conceptualization, L.M. and M.M.; methodology, L.M. and M.M.; software, L.M. and M.M.; validation, L.M., A.V. and M.M.; formal analysis, L.M. and M.M.; investigation, L.M. and M.M.; resources, L.M., A.V., R.M.Z. and M.M.; data curation, L.M. and M.M.; writing—original draft preparation, L.M. and M.M.; writing—review and editing, L.M., A.V., R.M.Z. and M.M.; visualization, L.M. and M.M.; supervision, R.M.Z.; project administration, R.M.Z.; funding acquisition, R.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gore RM, Thakrar KH, Newmark GM, Mehta UK, Berlin JW. Gallbladder imaging. Gastroenterol Clin North Am. 2010 Jun;39(2):265-87, ix. [CrossRef] [PubMed]
- Yu M. H. et al., Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases, World J Gastroenterol 2020 June 14; 26(22): 2967-2986. [CrossRef]
- Gore R. M. et al., Imaging benign and malignant disease of the gallbladder, Radiol Clin N Am 40 (2002) 1307 – 1323. [CrossRef]
- MacDonald FR, Cooperberg PL, Cohen MM. The WES triad -- a specific sonographic sign of gallstones in the contracted gallbladder. Gastrointest Radiol. 1981 Jan 15;6(1):39-41. [CrossRef] [PubMed]
- Rybicki FJ. The WES sign. Radiology. 2000 Mar;214(3):881-2. [CrossRef] [PubMed]
- George N, Dawkins A, DiSantis D. The wall-echo-shadow (WES) sign. Abdom Imaging. 2015 Oct;40(7):2903. [CrossRef] [PubMed]
- Kim HJ, Lee JY, Jang JY, Kim SJ, Kim SH, Han JK, Choi BI. Color Doppler twinkling artifacts from gallstones: in vitro analysis regarding their compositions and architectures. Ultrasound Med Biol. 2010 Dec;36(12):2117-22. Epub 2010 Oct 15. [CrossRef] [PubMed]
- Campbell SC, Cullinan JA, Rubens DJ. Slow flow or no flow? Color and power Doppler US pitfalls in the abdomen and pelvis. Radiographics. 2004 Mar-Apr;24(2):497-506. Erratum in: Radiographics. 2004 May-Jun;24(3):917. [CrossRef] [PubMed]
- Arnold MJ, Jonas CE, Carter RE. Point-of-Care Ultrasonography. Am Fam Physician. 2020 Mar 1;101(5):275-285. [PubMed]
- David Q.-H. Wang, Nezam H. Afdhal, Chapter 65 - Gallstone Disease, Editor(s): Mark Feldman, Lawrence S. Friedman, Lawrence J. Brandt, Sleisenger and Fordtran’s Gastrointestinal and Liver Disease (Ninth Edition), W.B. Saunders, 2010, Pages 1089-1120.e5, ISBN 9781416061892. [CrossRef]
- Lee C. C. et al., Tumefactive Sludge Mimicking Gallbladder Neoplasm: A Case Report and Review of the Literature, 2018 Journal of Medical Ultrasound. [CrossRef]
- Yarmenitis SD. Ultrasound of the gallbladder and the biliary tree. Eur Radiol. 2002 Feb;12(2):270-82. Epub 2001 Dec 20. [CrossRef] [PubMed]
- Hirooka Y, Naitoh Y, Goto H, Furukawa T, Ito A, Hayakawa T. Differential diagnosis of gall-bladder masses using colour Doppler ultrasonography. J Gastroenterol Hepatol. 1996 Sep;11(9):840-6. [CrossRef] [PubMed]
- Gerstenmaier, J.F., Hoang, K.N. & Gibson, R.N. Contrast-enhanced ultrasound in gallbladder disease: a pictorial review. Abdom Radiol 41, 1640–1652 (2016). [CrossRef]
- de Sio I, D’Onofrio M, Mirk P, Bertolotto M, Priadko K, Schiavone C, Cantisani V, Iannetti G, Vallone G, Vidili G; SIUMB experts committee. SIUMB recommendations on the use of ultrasound in neoplastic lesions of the gallbladder and extrahepatic biliary tract. J Ultrasound. 2023 May 5. Epub ahead of print. [CrossRef] [PubMed]
- Cokkinos D. D. et al., Contrast-enhanced ultrasound examination of the gallbladder and bile ducts: A pictorial essay, J Clin Ultrasound. 2017;1–14. [CrossRef]
- Kumar I. et al., Utility of Contrast-Enhanced Ultrasound in Differentiation between Benign Mural Lesions and Adenocarcinoma of Gallbladder, 2019 Journal of Medical Ultrasound. [CrossRef]
- Zhang H.-P. et al., Value of contrast-enhanced ultrasound in the differential diagnosis of gallbladder lesion, World J Gastroenterol 2018 February 14; 24(6): 744-751. [CrossRef]
- Serra C, Felicani C, Mazzotta E, Gabusi V, Grasso V, De Cinque A, Giannitrapani L, Soresi M. CEUS in the differential diagnosis between biliary sludge, benign lesions and malignant lesions. J Ultrasound. 2018 Jun;21(2):119-126. Epub 2018 Feb 23. [CrossRef] [PubMed] [PubMed Central]
- Sparchez et al., Role of CEUS in the diagnosis of gallbladder disease, Med Ultrason perf 2012, Vol. 14, no. 4, 326-330.
- SY, Huang P, Cosgrove D, Xu H, Xu LL, Liang X, Cai XJ. Pseudoenhancement of Gallbladder Sludge: A Confusing Artifact Caused by Nonlinear Propagation of Ultrasound Through Microbubbles. Ultraschall Med. 2016 Jun;37(3):307-9. English. Epub 2016 Feb 16. [CrossRef] [PubMed]
- Aziz M. U. et al., Microvascular Flow Imaging: A State-of-the-Art Review of Clinical Use and Promise, Radiology 2022; 305:250–264. [CrossRef]
- Lam R, Zakko A, Petrov JC, Kumar P, Duffy AJ, Muniraj T. Gallbladder Disorders: A Comprehensive Review. Dis Mon. 2021 Jul;67(7):101130. Epub 2021 Jan 18. [CrossRef] [PubMed]
- Amarnath S, Polavarapu AD, Gumaste V. Spontaneous Perforation of an Acalculous Hydropic Gallbladder in a Diabetic Patient With Neuropathy: An Underdiagnosed Entity. Gastroenterology Res. 2019 Dec;12(6):315-319. Epub 2019 Nov 21. [CrossRef] [PubMed] [PubMed Central]
- Shirah, B.H., Shirah, H.A. & Albeladi, K.B. The value of intraoperative percutaneous aspiration of the mucocele of the gallbladder for safe laparoscopic management. Updates Surg 70, 495–502 (2018). [CrossRef]
- Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, Kozaka K, Endo I, Deziel DJ, Miura F, Okamoto K, Hwang TL, Huang WS, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Noguchi Y, Shikata S, Ukai T, Higuchi R, Gabata T, Mori Y, Iwashita Y, Hibi T, Jagannath P, Jonas E, Liau KH, Dervenis C, Gouma DJ, Cherqui D, Belli G, Garden OJ, Giménez ME, de Santibañes E, Suzuki K, Umezawa A, Supe AN, Pitt HA, Singh H, Chan ACW, Lau WY, Teoh AYB, Honda G, Sugioka A, Asai K, Gomi H, Itoi T, Kiriyama S, Yoshida M, Mayumi T, Matsumura N, Tokumura H, Kitano S, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018 Jan;25(1):41-54. Epub 2018 Jan 9. [CrossRef] [PubMed]
- Miyoshi H, Inui K, Katano Y, Tachi Y, Yamamoto S. B-mode ultrasonographic diagnosis in gallbladder wall thickening. J Med Ultrason (2001). 2021 Apr;48(2):175-186. Epub 2020 Apr 24. [CrossRef] [PubMed]
- Jang TB, Ruggeri W, Kaji AH. The predictive value of specific emergency sonographic signs for cholecystitis. J Med Ultrasound. 2013;21(1):29-31. [CrossRef]
- Oppenheimer DC, Rubens DJ. Sonography of Acute Cholecystitis and Its Mimics. Radiol Clin North Am. 2019 May;57(3):535-548. Epub 2019 Feb 10. [CrossRef] [PubMed]
- Ralls PW, Colletti PM, Lapin SA, Chandrasoma P, Boswell WD Jr, Ngo C, Radin DR, Halls JM. Real-time sonography in suspected acute cholecystitis. Prospective evaluation of primary and secondary signs. Radiology. 1985 Jun;155(3):767-71. [CrossRef] [PubMed]
- Spence SC, Teichgraeber D, Chandrasekhar C. Emergent right upper quadrant sonography. J Ultrasound Med. 2009 Apr;28(4):479-96. [CrossRef] [PubMed]
- Smith EA, Dillman JR, Elsayes KM, Menias CO, Bude RO. Cross-sectional imaging of acute and chronic gallbladder inflammatory disease. AJR Am J Roentgenol. 2009 Jan;192(1):188-96. [CrossRef] [PubMed]
- Ra JC, Lee ES, Park HJ, Kim HS, Lee JB, Do JH, Park SB, Choi BI. Efficacy of Superb Microvascular Imaging for Diagnosing Acute Cholecystitis: Comparison with Conventional Ultrasonography. Ultrasound Med Biol. 2018 Sep;44(9):1968-1977. Epub 2018 Jun 21. [CrossRef] [PubMed]
- Meacock LM, Sellars ME, Sidhu PS. Evaluation of gallbladder and biliary duct disease using microbubble contrast-enhanced ultrasound. Br J Radiol. 2010 Jul;83(991):615-27. [CrossRef] [PubMed] [PubMed Central]
- Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D’Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018 Apr;39(2):e2-e44. English. Epub 2018 Mar 6. [CrossRef] [PubMed]
- Kawai R. et al., Increased enhancement of the liver adjacent to the gallbladder wall seen with contrast ultrasound: comparison between acute cholecystitis and non-cholecystitis, Kawai et al. BMC Medical Imaging (2016) 16:21. [CrossRef]
- Xu HX. Contrast-enhanced ultrasound in the biliary system: Potential uses and indications. World J Radiol. 2009 Dec 31;1(1):37-44. [CrossRef] [PubMed] [PubMed Central]
- Ko A, Lee ES, Park HJ, Park SB, Kim HS, Choi BI. Added value of 2D shear wave imaging of the gallbladder bed of the liver for acute cholecystitis. Ultrasonography. 2020 Oct;39(4):384-393. Epub 2020 Mar 23. [CrossRef] [PubMed] [PubMed Central]
- Nezam H., Afdhal M., Acalculous cholecystitis: Clinical manifestations, diagnosis, and management, In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on August 1, 2023.).
- Gallaher JR, Charles A. Acute Cholecystitis: A Review. JAMA. 2022 Mar 8;327(10):965-975. [CrossRef] [PubMed]
- Wind P, Chevallier JM, Jones D, Frileux P, Cugnenc PH. Cholecystectomy for cholecystitis in patients with acquired immune deficiency syndrome. Am J Surg. 1994 Sep;168(3):244-6. [CrossRef] [PubMed]
- Owen CC, Jain R. Acute Acalculous Cholecystitis. Curr Treat Options Gastroenterol. 2005 Apr;8(2):99-104. [CrossRef] [PubMed]
- Bennett GL, Balthazar EJ. Ultrasound and CT evaluation of emergent gallbladder pathology. Radiol Clin North Am. 2003 Nov;41(6):1203-16. [CrossRef] [PubMed]
- Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol. 2010 Jan;8(1):15-22. Epub 2009 Sep 10. [CrossRef] [PubMed]
- Lin Q, Shen L, Chen C, Yang Z, Que Y, Liu Y, Yin M, Xu G, Li J. Prognostic Significance of Ultrasound Findings of Acute Acalculous Cholecystitis for Elderly Long-Term Bedridden Patients. Front Med (Lausanne). 2021 Oct 8;8:743998. [CrossRef] [PubMed] [PubMed Central]
- Thampy R, Khan A, Zaki IH, Wei W, Korivi BR, Staerkel G, Bathala TK. Acute Acalculous Cholecystitis in Hospitalized Patients With Hematologic Malignancies and Prognostic Importance of Gallbladder Ultrasound Findings. J Ultrasound Med. 2019 Jan;38(1):51-61. Epub 2018 Apr 30. [CrossRef] [PubMed] [PubMed Central]
- Önder A, Kapan M, Ülger BV, Oğuz A, Türkoğlu A, Uslukaya Ö. Gangrenous cholecystitis: mortality and risk factors. Int Surg. 2015 Feb;100(2):254-60. [CrossRef] [PubMed] [PubMed Central]
- Katsumata R, Manabe N, Urano T, Tanikawa T, Ishii K, Ayaki M, Fujita M, Suehiro M, Fujiwara H, Monobe Y, Kamada T, Yamatsuji T, Naomoto Y, Haruma K, Kawamoto H. Asymptomatic gangrenous cholecystitis diagnosed using contrast-enhanced ultrasonography in a patient with pancreatic cancer. Radiol Case Rep. 2022 May 5;17(7):2309-2314. [CrossRef] [PubMed] [PubMed Central]
- Nugent JP, Li J, Pang E, Harris A. What’s new in the hot gallbladder: the evolving radiologic diagnosis and management of acute cholecystitis. Abdom Radiol (NY). 2023 Jan;48(1):31-46. Epub 2022 Mar 1. [CrossRef] [PubMed]
- Maddu K, Phadke S, Hoff C. Complications of cholecystitis: a comprehensive contemporary imaging review. Emerg Radiol. 2021 Oct;28(5):1011-1027. Epub 2021 Jun 10. [CrossRef] [PubMed]
- Tse JR, Gologorsky R, Shen L, Bingham DB, Jeffrey RB, Kamaya A. Evaluation of early sonographic predictors of gangrenous cholecystitis: mucosal discontinuity and echogenic pericholecystic fat. Abdom Radiol (NY). 2022 Mar;47(3):1061-1070. Epub 2022 Jan 5. [CrossRef] [PubMed]
- Corr P. Sonography of gangrenous cholecystitis. J Emerg Trauma Shock. 2012 Jan;5(1):82-3. [CrossRef] [PubMed] [PubMed Central]
- Revel L, Lubrano J, Badet N, Manzoni P, Degano SV, Delabrousse E. Preoperative diagnosis of gangrenous acute cholecystitis: usefulness of CEUS. Abdom Imaging. 2014 Dec;39(6):1175-81. [CrossRef] [PubMed]
- Kawai R, Hata J, Manabe N, Imamura H, Iida A, Nakatou R, Koyama N, Hirai T, Sadahira Y. Contrast-enhanced ultrasonography with Sonazoid for diagnosis of gangrenous cholecystitis. J Med Ultrason (2001). 2016 Apr;43(2):193-9. Epub 2015 Oct 27. [CrossRef] [PubMed]
- Ripollés T, Martínez-Pérez MJ, Martin G, Vizuete J, Martínez-García R, Diez J, Martí E. Usefulness of contrast-enhanced US in the diagnosis of acute gangrenous cholecystitis: A comparative study with surgical and pathological findings. Eur J Radiol. 2016 Jan;85(1):31-38. Epub 2015 Oct 26. [CrossRef] [PubMed]
- Niemeier OW. Acute Free Perforation of the Gall-Bladder. Ann Surg. 1934 Jun;99(6):922-4. [CrossRef] [PubMed] [PubMed Central]
- Indiran, V., Prabakaran, N. & Kannan, K. “Hole sign” of the gallbladder. Indian J Gastroenterol 36, 66–67 (2017). [CrossRef]
- Zechner PM, Rienmüller S, Dorr K, Genger C, Wurzer H. Contrast-enhanced ultrasound detects gallbladder perforation in a patient with acute abdominal pain. Am J Emerg Med. 2012 Mar;30(3):516.e5-6. Epub 2011 Mar 29. [CrossRef] [PubMed]
- Sood BP, Kalra N, Gupta S, Sidhu R, Gulati M, Khandelwal N, Suri S. Role of sonography in the diagnosis of gallbladder perforation. J Clin Ultrasound. 2002 Jun;30(5):270-4. [CrossRef] [PubMed]
- Tang S, Wang Y, Wang Y. Contrast-enhanced ultrasonography to diagnose gallbladder perforation. Am J Emerg Med. 2013 Aug;31(8):1240-3. Epub 2013 Jun 24. [CrossRef] [PubMed]
- Neimatullah MA, Rasuli P, Ashour M, Lewandowski BJ. Sonographic diagnosis of gallbladder perforation. J Ultrasound Med. 1998 Jun;17(6):389-91. [CrossRef] [PubMed]
- Shapira-Rootman M, Mahamid A, Reindorp N, Nachtigal A, Zeina AR. Diagnosis of gallbladder perforation by ultrasound. Clin Imaging. 2015 Sep-Oct;39(5):827-9. Epub 2015 May 27. [CrossRef] [PubMed]
- Aziz MU, Robbin ML. Improved Detection of Gallbladder Perforation Using Ultrasound Small Vessel Slow Flow “Perfusion” Imaging. J Ultrasound Med. 2022 Feb;41(2):511-518. Epub 2021 Apr 22. [CrossRef] [PubMed]
- Konno, K., Ishida, H., Sato, M. et al. Gallbladder perforation: color Doppler findings. Abdom Imaging 27, 47–50 (2002). [CrossRef]
- Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D’Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020 Oct;46(10):2579-2604. Epub 2020 Jul 24. [CrossRef] [PubMed]
- Francica G. Intracavitary contrast-enhanced ultrasound in ultrasound-guided percutaneous management of abdominal fluid collections/abscesses by a single clinician: an example of point-of-care ultrasound. J Ultrasound. 2020 Jun;23(2):175-181. Epub 2020 May 2. [CrossRef] [PubMed] [PubMed Central]
- Ignee A, Jenssen C, Cui XW, Schuessler G, Dietrich CF. Intracavitary contrast-enhanced ultrasound in abscess drainage--feasibility and clinical value. Scand J Gastroenterol. 2016 Jan;51(1):41-7. Epub 2015 Jul 11. [CrossRef] [PubMed]
- Sparchez Z, Mocan T, Craciun R, Sparchez M, Nolsøe C. Contrast enhancement for ultrasound-guided interventions: when to use it and what to expect? Ultrasonography. 2022 Apr;41(2):263-278. Epub 2021 Dec 9. [CrossRef] [PubMed] [PubMed Central]
- Charalel RA, Jeffrey RB, Shin LK. Complicated cholecystitis: the complementary roles of sonography and computed tomography. Ultrasound Q. 2011 Sep;27(3):161-70. [CrossRef] [PubMed]
- Runde R. et al., The gallbladder: what’s new in 2022?, Abdominal Radiology (2023) 48:2–28. [CrossRef]
- Konno K, Ishida H, Naganuma H, Sato M, Komatsuda T, Sato A, Ishida J, Sakai T, Watanabe S. Emphysematous cholecystitis: sonographic findings. Abdom Imaging. 2002 Mar-Apr;27(2):191-5. [CrossRef] [PubMed]
- Wu CS, Yao WJ, Hsiao CH. Effervescent gallbladder: sonographic findings in emphysematous cholecystitis. J Clin Ultrasound. 1998 Jun;26(5):272-5. [CrossRef] [PubMed]
- Nemcek AA Jr, Gore RM, Vogelzang RL, Grant M. The effervescent gallbladder: a sonographic sign of emphysematous cholecystitis. AJR Am J Roentgenol. 1988 Mar;150(3):575-7. [CrossRef] [PubMed]
- Huerta S, Kakati R, Lanier H. Gas-Containing Biliary Calculi: Case Series and a Systematic Review. Am Surg. 2023 Jun;89(6):2656-2664. Epub 2022 Aug 15. [CrossRef] [PubMed]
- Mehta V, Yarmish G, Greenstein J, Hahn B. Gallbladder Empyema. J Emerg Med. 2016 Jun;50(6):893-4. Epub 2016 May 21. [CrossRef] [PubMed]
- Zainal IA, Kew TY, Othman HA. Retrospective analysis of the sonographic and computed tomographic features of gallbladder empyema. Emerg Radiol. 2022 Apr;29(2):281-289. Epub 2021 Nov 23. [CrossRef] [PubMed]
- Elkbuli A, Sanchez C, Kinslow K, McKenney M, Boneva D. Uncommon Presentation of Severe Empyema of the Gallbladder: Case Report and Literature Review. Am J Case Rep. 2020 Jul 31;21:e923040. [CrossRef] [PubMed] [PubMed Central]
- Beltran MA, Csendes A, Cruces KS. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg. 2008 Oct;32(10):2237-43. [CrossRef] [PubMed]
- Csendes A, Díaz JC, Burdiles P, Maluenda F, Nava O. Mirizzi syndrome and cholecystobiliary fistula: a unifying classification. Br J Surg. 1989 Nov;76(11):1139-43. [CrossRef] [PubMed]
- Cui Y, Liu Y, Li Z, Zhao E, Zhang H, Cui N. Appraisal of diagnosis and surgical approach for Mirizzi syndrome. ANZ J Surg. 2012 Oct;82(10):708-13. Epub 2012 Aug 20. [CrossRef] [PubMed]
- Erben Y, Benavente-Chenhalls LA, Donohue JM, Que FG, Kendrick ML, Reid-Lombardo KM, Farnell MB, Nagorney DM. Diagnosis and treatment of Mirizzi syndrome: 23-year Mayo Clinic experience. J Am Coll Surg. 2011 Jul;213(1):114-9; discussion 120-1. Epub 2011 Apr 3. [CrossRef] [PubMed]
- Xu XQ, Hong T, Li BL, Liu W, He XD, Zheng CJ. Mirizzi syndrome: our experience with 27 cases in PUMC Hospital. Chin Med Sci J. 2013 Sep;28(3):172-7. [CrossRef] [PubMed]
- Sato M, Ishida H, Konno K, Naganuma H, Watanabe S, Hamashima Y, Komatsuda T, Sato A, Ishida J, Hirata M. Segmental chronic cholecystitis: sonographic findings and clinical manifestations. Abdom Imaging. 2002 Jan-Feb;27(1):43-6. [CrossRef] [PubMed]
- Adamietz B, Wenkel E, Uder M, Meyer T, Schneider I, Dimmler A, Bautz W, Janka R. Contrast enhanced sonography of the gallbladder: a tool in the diagnosis of cholecystitis? Eur J Radiol. 2007 Feb;61(2):262-6. Epub 2006 Oct 30. [CrossRef] [PubMed]
- Singh VP, Rajesh S, Bihari C, Desai SN, Pargewar SS, Arora A. Xanthogranulomatous cholecystitis: What every radiologist should know. World J Radiol. 2016 Feb 28;8(2):183-91. [CrossRef] [PubMed] [PubMed Central]
- Hale MD, Roberts KJ, Hodson J, Scott N, Sheridan M, Toogood GJ. Xanthogranulomatous cholecystitis: a European and global perspective. HPB (Oxford). 2014 May;16(5):448-58. Epub 2013 Aug 29. [CrossRef] [PubMed] [PubMed Central]
- Parra JA, Acinas O, Bueno J, Güezmes A, Fernández MA, Fariñas MC. Xanthogranulomatous cholecystitis: clinical, sonographic, and CT findings in 26 patients. AJR Am J Roentgenol. 2000 Apr;174(4):979-83. [CrossRef] [PubMed]
- Guzmán-Valdivia G. Xanthogranulomatous cholecystitis: 15 years’ experience. World J Surg. 2004 Mar;28(3):254-7. Epub 2004 Feb 17. [CrossRef] [PubMed]
- Joo et al., Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high-resolution ultrasound, Eur Radiol (2013) 23:730–738. [CrossRef]
- Kim JH, Lee JY, Baek JH, Eun HW, Kim YJ, Han JK, Choi BI. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am J Roentgenol. 2015 Feb;204(2):W150-9. [CrossRef] [PubMed]
- Jang JY, Kim SW, Lee SE, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg 2009; 250:943–949. [CrossRef]
- Suzuki H, Wada S, Araki K, Kubo N, Watanabe A, Tsukagoshi M, Kuwano H. Xanthogranulomatous cholecystitis: Difficulty in differentiating from gallbladder cancer. World J Gastroenterol. 2015 Sep 21;21(35):10166-73. [CrossRef] [PubMed] [PubMed Central]
- Giudicelli X, Rode A, Bancel B, Nguyen AT, Mabrut JY. Xanthogranulomatous cholecystitis: Diagnosis and management. J Visc Surg. 2021 Aug;158(4):326-336. Epub 2021 Mar 23. [CrossRef] [PubMed]
- Zhuang PY, Zhu MJ, Wang JD, Zhou XP, Quan ZW, Shen J. Xanthogranulomatous cholecystitis: a clinicopathological study of its association with gallbladder carcinoma. J Dig Dis. 2013 Jan;14(1):45-50. [CrossRef] [PubMed]
- Yuan HX, Wang WP, Wen JX, Ji ZB, Ding H, Huang BJ, Li CL. Xanthogranulomatous cholecystitis: contrast-enhanced ultrasound features and differential diagnosis from wall-thickening gallbladder carcinoma. Discov Med. 2016 Feb;21(114):89-98. [PubMed]
- Bo X, Chen E, Wang J, Nan L, Xin Y, Wang C, Lu Q, Rao S, Pang L, Li M, Lu P, Zhang D, Liu H, Wang Y. Diagnostic accuracy of imaging modalities in differentiating xanthogranulomatous cholecystitis from gallbladder cancer. Ann Transl Med. 2019 Nov;7(22):627. [CrossRef] [PubMed] [PubMed Central]
- Polk HC Jr. Carcinoma and the calcified gall bladder. Gastroenterology. 1966 Apr;50(4):582-5. [PubMed]
- Shimizu M, Miura J, Tanaka T, Itoh H, Saitoh Y. Porcelain gallbladder: relation between its type by ultrasound and incidence of cancer. J Clin Gastroenterol. 1989 Aug;11(4):471-6. [PubMed]
- DesJardins H, Duy L, Scheirey C, Schnelldorfer T. Porcelain Gallbladder: Is Observation a Safe Option in Select Populations? J Am Coll Surg. 2018 Jun;226(6):1064-1069. Epub 2018 Mar. [CrossRef] [PubMed]
- Kuwatani et al., Clinical and Image Characteristics of IgG4-Related Sclerosing Cholecystitis, Diagnostics 2021, 11, 1358. [CrossRef]
- Harada et al., Isolated IgG4-related cholecystitis with localized gallbladder wall thickening mimicking gallbladder cancer: a case report and literature review, BMC Gastroenterology (2022) 22:99. [CrossRef]
- Mellnick VM, Menias CO, Sandrasegaran K, Hara AK, Kielar AZ, Brunt EM, Doyle MB, Dahiya N, Elsayes KM. Polypoid lesions of the gallbladder: disease spectrum with pathologic correlation. Radiographics. 2015 Mar-Apr;35(2):387-99. Erratum in: Radiographics. 2015 May-Jun;35(3):973. Erratum in: Radiographics. 2015 Jul-Aug;35(4):1316. [CrossRef] [PubMed]
- Dursun N, Memis B, Pehlivanoglu B, Taskin OC, Okcu O, Akkas G, Bagci P, Balci S, Saka B, Araya JC, Bellolio E, Roa JC, Jang KT, Losada H, Maithel SK, Sarmiento J, Reid MD, Jang J, Cheng JD, Basturk O, Koshiol J, Adsay NV. Adenomyomas of the Gallbladder: An Analysis of Frequency, Clinicopathologic Associations, and Relationship to Carcinoma of a Malformative Lesion. Arch Pathol Lab Med. 2023 May 4. Epub ahead of print. [CrossRef] [PubMed]
- Riddell ZC, Corallo C, Albazaz R, Foley KG. Gallbladder polyps and adenomyomatosis. Br J Radiol. 2023 Feb;96(1142):20220115. Epub 2022 Jul 1. [CrossRef] [PubMed] [PubMed Central]
- Malik DG, Dahiya N, Lubner MG, Pickhardt PJ, Elsayes KM, Robinson KA, Menias CO. Spectrum of imaging findings in hyperplastic cholecystosis and potential mimics. Abdom Radiol (NY). 2023 Jan;48(1):47-62. Epub 2022 Oct 2. [CrossRef] [PubMed]
- Suzuki K. et al., A resected gallbladder carcinoma coexisting with adenomyomatosis involving varied degrees of intraepithelial dysplasia: a case report and literature review, Surg Laparosc Endosc Percutan Tech Volume 29, Number 4, August 2019. [CrossRef]
- Ootani T, Shirai Y, Tsukada K, Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992 Jun 1;69(11):2647-52. [CrossRef] [PubMed]
- Bonatti M, Vezzali N, Lombardo F, Ferro F, Zamboni G, Tauber M, Bonatti G. Gallbladder adenomyomatosis: imaging findings, tricks and pitfalls. Insights Imaging. 2017 Apr;8(2):243-253. Epub 2017 Jan 26. [CrossRef] [PubMed] [PubMed Central]
- Fonseca et al., Comet tail artifact in adenomyomatosis, Abdom Radiol (2018) 43:3516–3517. [CrossRef]
- Yu MH, Lee JY, Yoon JH, Baek JH, Han JK, Choi BI. Color Doppler twinkling artifacts from gallbladder adenomyomatosis with 1.8 MHz and 4.0 MHz color Doppler frequencies. Ultrasound Med Biol. 2012 Jul;38(7):1188-94. Epub 2012 May 12. [CrossRef] [PubMed]
- Ghersin E. et al., Twinkling artifact in Gallbladder Adenomyomatosis, J Ultrasound Med. 2003 Feb;22(2):229-31. [CrossRef]
- Liu, L. N., Xu, H. X., Lu, M. D., Xie, X. Y., Wang, W. P., Hu, B., Yan, K., Ding, H., Tang, S. S., Qian, L. X., Luo, B. M., & Wen, Y. L. (2012). Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PloSone, 7(10), e48371. [CrossRef]
- Xie XH, Xu HX, Xie XY, Lu MD, Kuang M, Xu ZF, Liu GJ, Wang Z, Liang JY, Chen LD, Lin MX. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol. 2010 Jan;20(1):239-48. Epub 2009 Aug 6. [CrossRef] [PubMed]
- de Sio I, D’Onofrio M, Mirk P, Bertolotto M, Priadko K, Schiavone C, Cantisani V, Iannetti G, Vallone G, Vidili G; SIUMB experts committee. SIUMB recommendations on the use of ultrasound in neoplastic lesions of the gallbladder and extrahepatic biliary tract. J Ultrasound. 2023 May 5. Epub ahead of print. [CrossRef] [PubMed]
- Tang et al., Contrast-enhanced ultrasonography diagnosis of fundal localized type of gallbladder adenomyomatosis, BMC Gastroenterology (2015) 15:99. [CrossRef]
- Yuan et al., Contrast-enhanced ultrasonography in differential diagnosis of focal gallbladder adenomyomatosis and gallbladder cancer, Clinical Hemorheology and Microcirculation xx (20xx) x–xx, DOI 10.3233/CH-180376.
- Golse et al., Gallbladder adenomyomatosis: diagnosis and management; Journal of Visceral Surgery (2017) 154, 345—353. [CrossRef]
- SALMENKIVI K. CHOLESTEROSIS OF THE GALL-BLADDER. A CLINICAL STUDY BASED ON 269 CHOLECYSTECTOMIES. Acta Chir Scand Suppl. 1964;105:SUPPL 324:1-93. [PubMed]
- Boscak AR, Al-Hawary M, Ramsburgh SR. Best cases from the AFIP: Adenomyomatosis of the gallbladder. Radiographics. 2006 May-Jun;26(3):941-6. [CrossRef] [PubMed]
- Chatterjee A, Lopes Vendrami C, Nikolaidis P, Mittal PK, Bandy AJ, Menias CO, Hammond NA, Yaghmai V, Yang GY, Miller FH. Uncommon Intraluminal Tumors of the Gallbladder and Biliary Tract: Spectrum of Imaging Appearances. Radiographics. 2019 Mar-Apr;39(2):388-412. Epub 2019 Feb 1. [CrossRef] [PubMed]
- Karin L. Andersson, Lawrence S. Friedman, Chapter 67 - Acalculous Biliary Pain, Acalculous Cholecystitis, Cholesterolosis, Adenomyomatosis, and Polyps of the Gallbladder, Editor(s): Mark Feldman, Lawrence S. Friedman, Lawrence J. Brandt, Sleisenger and Fordtran’s Gastrointestinal and Liver Disease (Ninth Edition), W.B. Saunders, 2010, Pages 1139-1152.e3, ISBN 9781416061892,. [CrossRef]
- Gallahan WC, Conway JD. Diagnosis and management of gallbladder polyps. Gastroenterol Clin North Am. 2010 Jun;39(2):359-67, x. [CrossRef] [PubMed]
- Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N Am J Med Sci. 2012 May;4(5):203-11. [CrossRef] [PubMed] [PubMed Central]
- Cocco G, Basilico R, Delli Pizzi A, Cocco N, Boccatonda A, D’Ardes D, Fabiani S, Anzoletti N, D’Alessandro P, Vallone G, Cipollone F, Schiavone C. Gallbladder polyps ultrasound: what the sonographer needs to know. J Ultrasound. 2021 Jun;24(2):131-142. Epub 2021 Feb 6. [CrossRef] [PubMed] [PubMed Central]
- Khan M, Singh I. Multicentric biliary papillomatosis with synchronous gallbladder malignancy. Trop Gastroenterol. 2012 Jan-Mar;33(1):86-7. [CrossRef] [PubMed]
- Cox H, Ma M, Bridges R, Debru E, Bathe O, Sutherland F, Dixon E. Well differentiated intrahepatic cholangiocarcinoma in the setting of biliary papillomatosis: a case report and review of the literature. Can J Gastroenterol. 2005 Dec;19(12):731-3. [CrossRef] [PubMed]
- Szpakowski J-L, Tucker L-Y. Outcomes of gallbladder polyps and their association with gallbladder cancer in a 20-year cohort. JAMA Netw Open 2020; 3: e205143. [CrossRef]
- Wu CH, Luo Y, Fei X, Chou YH, Chiou HJ, Wang HK, Lai YC, Lin YH, Tiu CM, Wang J. Algorithmic approaches to the diagnosis of gallbladder intraluminal lesions on ultrasonography. J Chin Med Assoc. 2018 Apr;81(4):297-304. Epub 2018 Feb 21. [CrossRef] [PubMed]
- Wennmacker SZ, Lamberts MP, Di Martino M, et al. Transabdominal ultrasound and endoscopic ultrasound for diagnosis of gallbladder polyps. Cochrane Database Syst Rev 2018; 8:CD012233. [CrossRef]
- Martin E, Gill R, Debru E. Diagnostic accuracy of transabdominal ultrasonography for gallbladder polyps: systematic review. Can J Surg. 2018 Jun;61(3):200-207. [CrossRef] [PubMed] [PubMed Central]
- Jang JY, Kim SW, Lee SE, Hwang DW, Kim EJ, Lee JY, Kim SJ, Ryu JK, Kim YT. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009 Dec;250(6):943-9. [CrossRef] [PubMed]
- Liu J, Qian Y, Yang F, Huang S, Chen G, Yu J, Jiang S, Huang G. Value of prediction model in distinguishing gallbladder adenoma from cholesterol polyp. J Gastroenterol Hepatol. 2022 Oct;37(10):1893-1900. Epub 2022 Jul 22. [CrossRef] [PubMed]
- Fei X, Lu WP, Luo YK, Xu JH, Li YM, Shi HY, Jiao ZY, Li HT. Contrast-enhanced ultrasound may distinguish gallbladder adenoma from cholesterol polyps: a prospective case-control study. Abdom Imaging. 2015 Oct;40(7):2355-63. [CrossRef] [PubMed]
- Behzadmehr R, Salarzaei M. Is contrast enhanced ultrasonography an accurate way to diagnose gallbladder adenoma? A systematic review and meta-analysis. J Med Imaging Radiat Sci. 2021 Mar;52(1):127-136. Epub 2020 Oct 29. [CrossRef] [PubMed]
- Wang X, Zhu JA, Liu YJ, Liu YQ, Che DD, Niu SH, Gao S, Chen DB. Conventional Ultrasound Combined With Contrast-Enhanced Ultrasound in Differential Diagnosis of Gallbladder Cholesterol and Adenomatous Polyps (1-2 cm). J Ultrasound Med. 2022 Mar;41(3):617-626. Epub 2021 May 3. [CrossRef] [PubMed]
- Liu XS, Gu LH, Du J, Li FH, Wang J, Chen T, Zhang YH. Differential diagnosis of polypoid lesions of the gallbladder using contrast-enhanced sonography. J Ultrasound Med. 2015 Jun;34(6):1061-9. [CrossRef] [PubMed]
- Fei X, Li N, Zhu L, Han P, Jiang B, Tang W, Sang M, Zhang X, Luo Y. Value of high frame rate contrast-enhanced ultrasound in distinguishing gallbladder adenoma from cholesterol polyp lesion. Eur Radiol. 2021 Sep;31(9):6717-6725. Epub 2021 Feb 10. [CrossRef] [PubMed]
- Zhu L, Han P, Jiang B, Zhu Y, Li N, Fei X. Value of Micro Flow Imaging in the Prediction of Adenomatous Polyps. Ultrasound Med Biol. 2023 Jul;49(7):1586-1594. Epub 2023 Apr 2. [CrossRef] [PubMed]
- Badea R, Zaro R, Opincariu I, Chiorean L. Ultrasound in the examination of the gallbladder - a holistic approach: grey scale, Doppler, CEUS, elastography, and 3D. Med Ultrason. 2014 Dec;16(4):345-55. [CrossRef] [PubMed]
- Yuan HX, Wang C, Tang CY, You QQ, Zhang Q, Wang WP. Differential diagnosis of gallbladder neoplastic polyps and cholesterol polyps with radiomics of dual modal ultrasound: a pilot study. BMC Med Imaging. 2023 Feb 6;23(1):26. [CrossRef] [PubMed] [PubMed Central]
- Ma NQ, Lv HY, Bi J, Yu FX, Huang XM. A scoring system for gallbladder polyps based on the cross-sectional area and patient characteristics. Asian J Surg. 2022 Jan;45(1):332-338. Epub 2021 Jun 16. [CrossRef] [PubMed]
- Liu J, Qian Y, Yang F, Huang S, Chen G, Yu J, Jiang S, Huang G. Value of prediction model in distinguishing gallbladder adenoma from cholesterol polyp. J Gastroenterol Hepatol. 2022 Oct;37(10):1893-1900. Epub 2022 Jul 22. [CrossRef] [PubMed]
- Kamaya A, Fung C, Szpakowski JL, Fetzer DT, Walsh AJ, Alimi Y, Bingham DB, Corwin MT, Dahiya N, Gabriel H, Park WG, Porembka MR, Rodgers SK, Tublin ME, Yuan X, Zhang Y, Middleton WD. Management of Incidentally Detected Gallbladder Polyps: Society of Radiologists in Ultrasound Consensus Conference Recommendations. Radiology. 2022 Nov;305(2):277-289. Epub 2022 Jul 5. [CrossRef] [PubMed]
- Foley KG, Lahaye MJ, Thoeni RF, Soltes M, Dewhurst C, Barbu ST, Vashist YK, Rafaelsen SR, Arvanitakis M, Perinel J, Wiles R, Roberts SA. Management and follow-up of gallbladder polyps: updated joint guidelines between the ESGAR, EAES, EFISDS and ESGE. Eur Radiol. 2022 May;32(5):3358-3368. Epub 2021 Dec 17. [CrossRef] [PubMed] [PubMed Central]
- Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol. 2019 Apr 20;37(12):1015-1027. Epub 2019 Mar 11. [CrossRef] [PubMed]
- Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Feb;34(2):127-140. Epub 2022 Nov 10. [CrossRef] [PubMed]
- Onoyama H, Yamamoto M, Takada M, Urakawa T, Ajiki T, Yamada I, Fujita T, Saitoh Y. Diagnostic imaging of early gallbladder cancer: retrospective study of 53 cases. World J Surg. 1999 Jul;23(7):708-12. [CrossRef] [PubMed]
- Halaseh SA, Halaseh S, Shakman R. A Review of the Etiology and Epidemiology of Gallbladder Cancer: What You Need to Know. Cureus. 2022 Aug 22;14(8):e28260. [CrossRef] [PubMed] [PubMed Central]
- Malik H, Izwan S, Ng J, Teng R, Chan E, Damodaran Prabha R, Puhalla H. Incidence and management of gallbladder cancer in cholecystectomy specimens: a 5-year tertiary centre experience. ANZ J Surg. 2023 Jun 20. Epub ahead of print. [CrossRef] [PubMed]
- Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007 May;11(5):671-81. [CrossRef] [PubMed]
- Ramachandran A, Srivastava DN, Madhusudhan KS. Gallbladder cancer revisited: the evolving role of a radiologist. Br J Radiol. 2021 Jan 1;94(1117):20200726. Epub 2020 Oct 23. [CrossRef] [PubMed] [PubMed Central]
- Lee TY, Ko SF, Huang CC, Ng SH, Liang JL, Huang HY, Chen MC, Sheen-Chen SM. Intraluminal versus infiltrating gallbladder carcinoma: clinical presentation, ultrasound and computed tomography. World J Gastroenterol. 2009 Dec 7;15(45):5662-8. [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Fernández A, Gómez-Río M, Medina-Benítez A, Moral JV, Ramos-Font C, Ramia-Angel JM, Llamas-Elvira JM, Ferrón-Orihuela JA, Lardelli-Claret P. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol. 2006 Jun 15;93(8):650-64. [CrossRef] [PubMed]
- Gore RM, Shelhamer RP. Biliary tract neoplasms: diagnosis and staging. Cancer Imaging. 2007 Oct 1;7 Spec No A(Special issue A):S15-23. [CrossRef] [PubMed] [PubMed Central]
- Joo et al., Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high-resolution ultrasound, Eur Radiol (2013) 23:730–738. [CrossRef]
- Bang et al., Differentiating between Adenomyomatosis and Gallbladder Cancer: Revisiting a Comparative Study of High-Resolution Ultrasound, Multidetector CT, and MR Imaging, Korean J Radiol 2014;15(2):226-234. [CrossRef]
- Lee JS, Kim JH, Kim YJ, Ryu JK, Kim YT, Lee JY, Han JK. Diagnostic accuracy of transabdominal high-resolution US for staging gallbladder cancer and differential diagnosis of neoplastic polyps compared with EUS. Eur Radiol. 2017 Jul;27(7):3097-3103. Epub 2016 Nov 10. [CrossRef] [PubMed]
- Komatsuda T, Ishida H, Konno K, Hamashima Y, Naganuma H, Sato M, et al. Gallbladder carcinoma: color Doppler sonography. Abdom Imaging 2000; 25: 194–7. [CrossRef]
- Xie XH, Xu HX, Xie XY et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol 2010; 20: 239–248. [CrossRef]
- Kumar I, Yadav YK, Kumar S, Puneet, Shukla RC, Verma A. Utility of Contrast-Enhanced Ultrasound in Differentiation between Benign Mural Lesions and Adenocarcinoma of Gallbladder. J Med Ultrasound. 2019 Dec 16;28(3):143-150. [CrossRef] [PubMed] [PubMed Central]
- Xu JM, Guo LH, Xu HX et al. Differential Diagnosis of Gallbladder Wall Thickening: The-Usefulness of Contrast-Enhanced Ultrasound. Ultrasound Med Biol 2014; 40: 2794–2804. [CrossRef]
- Zhuang B, Li W, Wang W, Lin M, Xu M, Xie X, Lu M, Xie X. Contrast-enhanced ultrasonography improves the diagnostic specificity for gallbladder-confined focal tumors. Abdom Radiol (NY). 2018 May;43(5):1134-1142. [CrossRef] [PubMed]
- Liu LN, Xu HX, Lu MD, Xie XY, Wang WP, Hu B, Yan K, Ding H, Tang SS, Qian LX, Luo BM, Wen YL. Contrast-enhanced ultrasound in the diagnosis of gallbladder diseases: a multi-center experience. PLoS One. 2012;7(10):e48371. Epub 2012 Oct 31. [CrossRef] [PubMed] [PubMed Central]
- Boddapati SB, Lal A, Gupta P, Kalra N, Yadav TD, Gupta V, Dass A, Srinivasan R, Singhal M. Contrast enhanced ultrasound versus multiphasic contrast enhanced computed tomography in evaluation of gallbladder lesions. Abdom Radiol (NY). 2022 Feb;47(2):566-575. Epub 2021 Dec 7. [CrossRef] [PubMed]
- Sun LP, Guo LH, Xu HX, et al. Value of contrast-enhanced ultrasound in the differential diagnosis between gallbladder adenoma and gallbladder adenoma canceration. Int J Clin Exp Med 2015;8:1115–21. [PubMed]
- Yuan HX, Cao JY, Kong WT et al. Contrast-enhanced ultrasound in diagnosis of gallbladder adenoma. Hepatobiliary Pancreat Dis Int 2015; 14: 201–207. [CrossRef]
- Cheng Y, Wang M, Ma B, Ma X (2018) Potential role of contrast-enhanced ultrasound for the diferentiation of malignant and benign gallbladder lesions in East Asia: a meta-analysis and systematic review. Medicine (Baltimore) 97:e11808. [CrossRef]
- Zheng SG, Xu HX, Liu LN, Lu MD, Xie XY, Wang WP, Hu B, Yan K, Ding H, Tang SS, Qian LX, Luo BM. Contrast-enhanced ultrasound versus conventional ultrasound in the diagnosis of polypoid lesion of gallbladder: a multi-center study of dynamic microvascularization. Clin Hemorheol Microcirc. 2013;55(3):359-74. [CrossRef] [PubMed]
- Wang W, Fei Y, Wang F (2016) Meta-analysis of contrast-enhanced ultrasonography for the detection of gallbladder carcinoma. Med Ultrason 18:281–287. [CrossRef]
- Bae JS, Kim SJ, Kang HJ et al. (2019) Quantitative contrastenhanced US helps differentiating neoplastic vs. non-neoplastic gallbladder polyps. Eur Radiol 29:3772–3781. [CrossRef]
- Dong Y, Xu B, Cao Q, Zhang Q, Qiu Y, Yang D, Yu L, Wang WP. Incidentally detected focal fundal gallbladder wall thickening: Differentiation contrast enhanced ultrasound features with high-resolution linear transducers. Clin Hemorheol Microcirc. 2020;74(3):315-325. [CrossRef] [PubMed]
- Yoon WJ, Yoon YB, Kim YJ, Ryu JK, Kim YT. Metastasis to the gallbladder: a single-center experience of 20 cases in South Korea. World J Gastroenterol. 2009 Oct 14;15(38):4806-9. [CrossRef] [PubMed] [PubMed Central]
- de Bitter TJJ, Trapman DM, Simmer F, Hugen N, de Savornin Lohman EAJ, de Reuver PR, Verheij J, Nagtegaal ID, van der Post RS. Metastasis in the gallbladder: does literature reflect reality? Virchows Arch. 2022 Jun;480(6):1201-1209. Epub 2022 Mar 31. [CrossRef] [PubMed] [PubMed Central]
- Imaoka K, Satoh D, Oshita K, Yano T, Kubota T, Ishida M, Choda Y, Yoshimitsu M, Nakano K, Harano M, Matsukawa H, Idani H, Shiozaki S, Okajima M. Acute cholecystitis caused by gallbladder metastasis from non-small cell lung cancer: a case report. Clin J Gastroenterol. 2021 Feb;14(1):351-357. Epub 2021 Jan 25. [CrossRef] [PubMed]
- Urade T, Oka S, Iimori S, Man-I M, Abe T, Sawa H, Iwatani Y, Morinaga Y, Kuroda D. A resected case of gallbladder metastasis with symptoms of acute cholecystitis in multiple metastatic ductal carcinoma of the breast. Clin J Gastroenterol. 2019 Feb;12(1):52-56. Epub 2018 Aug 14. [CrossRef] [PubMed]
- Cocco G, Delli Pizzi A, Basilico R, Fabiani S, Taraschi AL, Pascucci L, Boccatonda A, Catalano O, Schiavone C. Imaging of gallbladder metastasis. Insights Imaging. 2021 Jul 14;12(1):100. [CrossRef] [PubMed] [PubMed Central]
- Barretta ML, Catalano O, Setola SV, Granata V, Marone U, D’Errico Gallipoli A. Gallbladder metastasis: spectrum of imaging findings. Abdom Imaging. 2011 Dec;36(6):729-34. [CrossRef] [PubMed]
- Shyr, BU., Chen, SC., Shyr, YM. et al. Metastatic polyp of the gallbladder from renal cell carcinoma. BMC Cancer 17, 244 (2017). [CrossRef]
- Ueda I, Aoki T, Oki H, Takahashi H, Hayashida Y, Minagawa N, Yamaguchi K, Fujimoto N, Matsumoto T, Yamada S, Korogi Y. Gallbladder metastasis from renal cell carcinoma: a case report with review of the literature. Magn Reson Med Sci. 2015;14(2):133-8. Epub 2014 Oct 27. [CrossRef] [PubMed]
- Honda M M D, Furuta Y M D Ph D, Naoe H M D Ph D, Sasaki Y M D Ph D. Primary mucosa-associated lymphoid tissue (MALT) lymphoma of the gallbladder and review of the literature. BMJ Case Rep. 2017 May 27;2017:bcr2017220161. [CrossRef] [PubMed] [PubMed Central]
- Niu C, Wang S, Guan Q, Ren X, Ji B, Liu Y. Neuroendocrine tumors of the gallbladder. Oncol Lett. 2020 May;19(5):3381-3388. Epub 2020 Mar 12. [CrossRef] [PubMed] [PubMed Central]
- Siddamreddy S, Meegada S, Syed A, Sarwar M, Muppidi V. Gallbladder Neuroendocrine Carcinoma: A Rare Endocrine Tumor. Cureus. 2020 Mar 31;12(3):e7487. [CrossRef] [PubMed] [PubMed Central]
- Rivero-Soto RJ, Hossein-Zadeh Z, Coleman J, Ahuja V. A Mucinous Cystic Neoplasm Originating from the Gallbladder: A Case Report and Literature Review. Perm J. 2019;23:18-077. [CrossRef] [PubMed] [PubMed Central]
- Sugawara S, Hirai I, Watanabe T, Tezuka K, Kimura W. A case of mucinous cystic neoplasm of the gallbladder. Clin J Gastroenterol. 2018 Oct;11(5):428-432. Epub 2018 Mar 13. [CrossRef] [PubMed]
- Rooney TB, Schofer JM, Stanley MD, Banks SL. Biliary cystadenoma of the gallbladder. AJR Am J Roentgenol. 2005 Dec;185(6):1571-2. [CrossRef] [PubMed]
- Gokalp G, Dusak A, Topal NB, Aker S. Cystadenoma originating from the gallbladder. J Ultrasound Med. 2010 Apr;29(4):663-6. [CrossRef] [PubMed]
- Pendharkar D, Khetrapal S, Jairajpuri ZS, Rana S, Jetley S. Pancreatic and Gastric Heterotopia in the Gallbladder: A Rare Incidental Finding. Int J Appl Basic Med Res. 2019 Apr-Jun;9(2):115-117. [CrossRef] [PubMed] [PubMed Central]
- Beeskow AB, Meyer HJ, Schierle K, Surov A. Heterotopic gastric mucosa in gallbladder-A rare differential diagnosis to gallbladder masses: A systematic review. Medicine (Baltimore). 2018 Mar;97(10):e0058. [CrossRef] [PubMed] [PubMed Central]
- Juillerat A, Rougemont AL, Wildhaber BE. Duplication de la vésicule biliaire avec hétérotopie: cas clinique et proposition de classification des duplications gastro-intestinales [Duplication of the gallbladder with heterotopic mucosa: A case report and proposal for a classification for gastrointestinal duplications]. Arch Pediatr. 2016 Jun;23(6):607-11. French. Epub 2016 Mar 25. [CrossRef] [PubMed]
- Kim J, Jarboe MD, Arnold MA, DiPietro MA, Bloom DA, Teitelbaum DH. Biliary duplication cyst with heterotopic gastric mucosa resulting in obstruction of the biliary system: a case report. J Pediatr Surg. 2012 Jun;47(6):e5-8. [CrossRef] [PubMed]
- Birn J, Jung M, Dearing M. Isolated gallbladder injury in a case of blunt abdominal trauma. J Radiol Case Rep. 2012 Apr;6(4):25-30. Epub 2012 Apr 1. [CrossRef] [PubMed] [PubMed Central]
- Gupta A, Stuhlfaut JW, Fleming KW, Lucey BC, Soto JA. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics. 2004 Sep-Oct;24(5):1381-95. [CrossRef] [PubMed]
- Le MTP, Herrmann J, Groth M, Reinshagen K, Boettcher M. Traumatic Gallbladder Perforation in Children - Case Report and Review. Rofo. 2021 Aug;193(8):889-897. English. Epub 2021 Feb 3. [CrossRef] [PubMed]
- Wiebe I, Baig Z, Sothilingam N. Hemorrhagic cholecystitis from isolated gallbladder injury following blunt abdominal trauma: An unusual case report. Int J Surg Case Rep. 2022 Jan;90:106680. Epub 2021 Dec 11. [CrossRef] [PubMed] [PubMed Central]
- Nishiwaki M, Ashida H, Nishimura T, Kimura M, Yagyu R, Nishioka A, Utsunomiya J, Yamamura T. Posttraumatic intra-gallbladder hemorrhage in a patient with liver cirrhosis. J Gastroenterol. 1999 Apr;34(2):282-5. [CrossRef] [PubMed]
- Runner GJ, Corwin MT, Siewert B, Eisenberg RL. Gallbladder wall thickening. AJR Am J Roentgenol. 2014 Jan;202(1):W1-W12. [CrossRef] [PubMed]
- Ravaioli F, Colecchia A, Alemanni LV, Vestito A, Dajti E, Marasco G, Sessa M, Pession A, Bonifazi F, Festi D. Role of imaging techniques in liver veno-occlusive disease diagnosis: recent advances and literature review. Expert Rev Gastroenterol Hepatol. 2019 May;13(5):463-484. Epub 2019 Mar 21. [CrossRef] [PubMed]
- Lubner MG, Menias CO, Agrons M, Alhalabi K, Katabathina VS, Elsayes KM, Pickhardt PJ. Imaging of Abdominal and Pelvic Manifestations of Graft-Versus-Host Disease After Hematopoietic Stem Cell Transplant. AJR Am J Roentgenol. 2017 Jul;209(1):33-45. Epub 2017 May 2. [CrossRef] [PubMed]
- Shetty PB, Broome DR. Sonographic analysis of gallbladder findings in Salmonella enteric fever. J Ultrasound Med. 1998 Apr;17(4):231-7. [CrossRef] [PubMed]
- Tirumani SH, Krajewski KM, Shinagare AB, Jagannathan JP, Ramaiya NH. Gallbladder complications associated with molecular targeted therapies: clinical and imaging features. Clin Imaging. 2014 Jan-Feb;38(1):50-5. Epub 2013 Oct 14. [CrossRef] [PubMed]
- Koyama K, Anno T, Kawasaki F, Nishino K, Kawamoto H, Kaneto H, Tomoda K. Edematous wall thickening of the gallbladder induced by hyperthyroidism: A case report. Medicine (Baltimore). 2022 Jan 28;101(4):e28720. [CrossRef] [PubMed] [PubMed Central]
- Murata T, Yoshimoto Y, Shibano Y, Nakamura S, Yamauchi R. Gallbladder wall thickening in a woman with postpartum preeclampsia: A case report. Case Rep Womens Health. 2021 Nov 15;33:e00370. [CrossRef] [PubMed] [PubMed Central]
- Motla M, Manaktala S, Gupta V, Aggarwal M, Bhoi SK, Aggarwal P, Goel A. Sonographic evidence of ascites, pleura-pericardial effusion and gallbladder wall edema for dengue fever. Prehosp Disaster Med. 2011 Oct;26(5):335-41. Epub 2011 Oct 26. [CrossRef] [PubMed]
- Rodríguez-Muguruza S, Mateo L, Sanchez MDC. Gold salt hypersensitivity associated with gallbladder edema. Int J Rheum Dis. 2017 Nov;20(11):1783-1784. Epub 2013 Dec 11. [CrossRef] [PubMed]
- Zenda T, Araki I, Nakamiya O, Ogawa M, Higashi K, Ueno T. Acute peri-myocarditis with an unusual initial manifestation of gallbladder edema and a profound eosinophilic surge during convalescence. Fukushima J Med Sci. 2018;64(2):95-102. [CrossRef]
- Wang LY, Young TH. Hepatitis, gallbladder hydrops, splenomegaly, and ascites in a child with scarlet fever. Pediatr Emerg Care. 2012 Nov;28(11):1215-7. [CrossRef] [PubMed]
- Palabiyik F, Akcay N, Sevketoglu E, Hatipoglu N, Sari EE, Inci E. Imaging of Multisystem Inflammatory Disease in Children (MIS-C) Associated With COVID-19. Acad Radiol. 2021 Sep;28(9):1200-1208. Epub 2021 Jun 5. [CrossRef] [PubMed] [PubMed Central]
- Koehler J, Stolz L, Minges P. Sonographic findings of acute hepatitis in the emergency department. Clin Case Rep. 2022 Aug 24;10(8):e6046. [CrossRef] [PubMed] [PubMed Central]
- Suk KT, Kim CH, Baik SK, Kim MY, Park DH, Kim KH, Kim JW, Kim HS, Kwon SO, Lee DK, Han KH, Um SH. Gallbladder wall thickening in patients with acute hepatitis. J Clin Ultrasound. 2009 Mar-Apr;37(3):144-8. [CrossRef] [PubMed]
- Keeratibharat N, Chansangrat J. Gallbladder Volvulus: A Review. Cureus. 2022 Mar 21;14(3):e23362. [CrossRef] [PubMed] [PubMed Central]
- Ohkura Y, Hashimoto M, Sasaki K, Watanabe G. Complete acute gallbladder torsion diagnosed with abdominal ultrasonography and colour Doppler imaging. BMJ Case Rep. 2013 Mar 14;2013:bcr2012008460. [CrossRef] [PubMed] [PubMed Central]
- Lim LX, Mahin HH, Burnett D. Gallbladder volvulus: An unexpected “twist”. Radiol Case Rep. 2022 Mar 25;17(5):1755-1759. [CrossRef] [PubMed] [PubMed Central]
- Moriwaki Y, Otani J, Okuda J, Niwano T. Gallbladder Torsion: US, CT, and MRI Findings. J Gastrointest Surg. 2019 May;23(5):1077-1079. Epub 2018 Sep 5. [CrossRef] [PubMed]
- Dasyam AK, Koo J, Stahlfeld Miller M, Sell HW Jr, Tublin ME. The cystic duct knot sign: case report with description of a new ultrasound sign of gallbladder torsion. Emerg Radiol. 2015 Aug;22(4):445-7. Epub 2015 Jul 2. [CrossRef] [PubMed]
- Tohma T, Kobe Y, Yoshida M, Ushio M. Portal venous gas accompanied by gallbladder torsion: a case report. J Surg Case Rep. 2022 Oct 31;2022(10):rjac491. [CrossRef] [PubMed] [PubMed Central]
- Zhornitskiy A. et al., Hemobilia: historical overview, clinical update, and current practices, Liver International. 2019;39:1378–1388. [CrossRef]
- Akatsu T, Hayashi S, Egawa T, Doi M, Nagashima A, Kitano M, Yamane T, Yoshii H, Kitajima M. Hepatic artery pseudoaneurysm associated with cholecystitis that ruptured into the gallbladder. J Gastroenterol. 2004 Sep;39(9):900-3. [CrossRef] [PubMed]
- Hangai S. et al., Successful transarterial embolization for recurrent pseudoaneurysm of the right hepatic artery with acute cholecystitis, Clin J Gastroenterol (2014) 7:164–169. [CrossRef]
- Khuroo MS, Rather AA, Khuroo NS, Khuroo MS. Hepatobiliary and pancreatic ascariasis. World J Gastroenterol. 2016 Sep 7;22(33):7507-17. [CrossRef] [PubMed] [PubMed Central]
- Silva C, Gonçalves IC, Neves S, Ferreira DC, Valdoleiros SR. Diagnosis of Asymptomatic Biliary Ascariasis by Abdominal Ultrasound in a Non-Endemic Area. Cureus. 2023 Jan 10;15(1):e33599. [CrossRef] [PubMed] [PubMed Central]
- Koumanidou C, Manoli E, Anagnostara A, Polyviou P, Vakaki M. Sonographic features of intestinal and biliary ascariasis in childhood: case report and review of the literature. Ann Trop Paediatr. 2004 Dec;24(4):329-35. [CrossRef] [PubMed]
- Keshvala C, Naidu L. Not everything in the gallbladder is gallstones: an unusual case of biliary ascariasis. BJR Case Rep. 2019 Nov 15;5(4):20180123. [CrossRef] [PubMed] [PubMed Central]
- Javid G, Wani N, Gulzar GM, Javid O, Khan B, Shah A. Gallbladder ascariasis: presentation and management. Br J Surg. 1999 Dec;86(12):1526-7. [CrossRef] [PubMed]
- Miller G, Schecter WP, Harris HW. Gallbladder ascariasis in a patient with severe pancreatitis. Surgery. 2003 Apr;133(4):445-6. [CrossRef] [PubMed]
- Lim JH, Kim SY, Park CM. Parasitic diseases of the biliary tract. AJR Am J Roentgenol. 2007 Jun;188(6):1596-603. [CrossRef] [PubMed]
- van Kamp MJ, Bouman DE, Steenvoorde P, Klaase JM. A phrygian cap. Case Rep Gastroenterol. 2013 Aug 17;7(2):347-51. [CrossRef] [PubMed] [PubMed Central]
- Whittle C, Skoknic V, Maldonado I, Schiappacasse G, Pose G. Multimodality Imaging of Congenital Variants in the Gallbladder: Pictorial Essay. Ultrasound Q. 2019 Jun;35(2):195-199. [CrossRef] [PubMed]
- Revzin MV, Scoutt L, Smitaman E, Israel GM. The gallbladder: uncommon gallbladder conditions and unusual presentations of the common gallbladder pathological processes. Abdom Imaging. 2015 Feb;40(2):385-99. [CrossRef] [PubMed]
- Dietrich CF, Chichakli M, Hirche TO, Bargon J, Leitzmann P, Wagner TO, Lembcke B. Sonographic findings of the hepatobiliary-pancreatic system in adult patients with cystic fibrosis. J Ultrasound Med. 2002 Apr;21(4):409-16; quiz 417. [CrossRef] [PubMed]
- Mousa MS, Feldman JC, Mahajan P. Microgallbladder: Self-Remitting Acute Cholecystitis-Like Condition Unique to Patients with Cystic Fibrosis. Case Rep Radiol. 2019 Jun 19;2019:6737428. [CrossRef] [PubMed] [PubMed Central]
- Hsu SL, Chen TY, Huang TL, Sun CK, Concejero AM, Tsang LL, Cheng YF. Left-sided gallbladder: its clinical significance and imaging presentations. World J Gastroenterol. 2007 Dec 21;13(47):6404-9. [CrossRef] [PubMed] [PubMed Central]
- Cotton PB, Elta GH, Carter CR, Pasricha PJ, Corazziari ES. Rome IV. Gallbladder and Sphincter of Oddi Disorders. Gastroenterology. 2016 Feb 19:S0016-5085(16)00224-9. Epub ahead of print. [CrossRef] [PubMed]
- Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010 Jun;39(2):369-79, x. [CrossRef] [PubMed]
- Dietrich CF, Hollerweger A, Dirks K, Higginson A, Serra C, Calabrese E, Dong Y, Hausken T, Maconi G, Mihmanli I, Nürnberg D, Nylund K, Pallotta N, Ripollés T, Romanini L, Săftoiu A, Sporea I, Wüstner M, Maaser C, Gilja OH. EFSUMB Gastrointestinal Ultrasound (GIUS) Task Force Group: Celiac sprue and other rare gastrointestinal diseases ultrasound features. Med Ultrason. 2019 Aug 31;21(3):299-315. [CrossRef] [PubMed]
- Yoon HJ, Kim PN, Kim AY, Lee MG. Three-dimensional sonographic evaluation of gallbladder contractility: comparison with cholescintigraphy. J Clin Ultrasound. 2006 Mar-Apr;34(3):123-7. [CrossRef] [PubMed]
- Lanzini A, Lanzarotto F, Baisini O, Amato M, Benini F. Value of measuring gallbladder motility in clinical practice. Dig Liver Dis. 2003 Jul;35 Suppl 3:S46-50. [CrossRef] [PubMed]
- Azzaroli F, Mazzella G, Mazzeo C, Simoni P, Festi D, Colecchia A, Montagnani M, Martino C, Villanova N, Roda A, Roda E. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am J Gastroenterol. 1999 Sep;94(9):2453-9. [CrossRef] [PubMed]
- Buchpiguel CA, Sapienza MT, Vezzozzo DP, Rockman R, Cerri GG, Magalhães AE. Gallbladder emptying in normal volunteers. Comparative study between cholescintigraphy and ultrasonography. Clin Nucl Med. 1996 Mar;21(3):208-12. [CrossRef] [PubMed]
- Stads S, Venneman NG, Scheffer RC, Samsom M, van Erpecum KJ. Evaluation of gallbladder motility: comparison of two-dimensional and three-dimensional ultrasonography. Ann Hepatol. 2007 Jul-Sep;6(3):164-9. [PubMed]
- Irshad A, Ackerman SJ, Spicer K, Baker NL, Campbell A, Anis M, Shazly M. Ultrasound evaluation of gallbladder dyskinesia: comparison of scintigraphy and dynamic 3D and 4D ultrasound techniques. AJR Am J Roentgenol. 2011 Nov;197(5):1103-10. Erratum in: AJR Am J Roentgenol. 2012 Jan;198(1):2. Baker, Nathanial [corrected to Baker, Nathaniel L]. [CrossRef] [PubMed]
Figure 1.
Gallbladder lithiasis and sludge. A gallstone can be seen in the bottom of the gallbladder lumen, with the typical sonographic appearance of an echogenic focus casting an acoustic shadow. Biliary sludge (echoic non-shadowing material with indefinite morphology) surrounds the gallstone, forming a horizontal fluid-fluid level.
Figure 1.
Gallbladder lithiasis and sludge. A gallstone can be seen in the bottom of the gallbladder lumen, with the typical sonographic appearance of an echogenic focus casting an acoustic shadow. Biliary sludge (echoic non-shadowing material with indefinite morphology) surrounds the gallstone, forming a horizontal fluid-fluid level.
Figure 2.
Complicated acute cholecystitis, leading to gangrenous cholecystitis (GC), with irregular gallbladder wall thickening with multiple striations and alternating hypo/hyperechoic bands, (a, b) and coexisting empyema (abundant dishomogeneous echogenic material occupying most of the gallbladder lumen, without posterior acoustic shadows) (a, b). Slightly hypoechoic pericholecystic liver parenchyma (delimited by dotted line) corresponds to concurrent hepatic edema (b). MVFI shows perfusion defects (arrows) of the inner layer with irregular and thickened gallbladder wall, without vascular signal in the underlying middle portion, due to edema and tissue necrosis. (c).
Figure 2.
Complicated acute cholecystitis, leading to gangrenous cholecystitis (GC), with irregular gallbladder wall thickening with multiple striations and alternating hypo/hyperechoic bands, (a, b) and coexisting empyema (abundant dishomogeneous echogenic material occupying most of the gallbladder lumen, without posterior acoustic shadows) (a, b). Slightly hypoechoic pericholecystic liver parenchyma (delimited by dotted line) corresponds to concurrent hepatic edema (b). MVFI shows perfusion defects (arrows) of the inner layer with irregular and thickened gallbladder wall, without vascular signal in the underlying middle portion, due to edema and tissue necrosis. (c).
Figure 3.
Complicated acute cholecystitis, leading to GC and gallbladder perforation. Gallbladder walls are irregularly thickened with an echogenic strand departing from the gallbladder wall and projecting into the lumen (intraluminal membrane: fibrinous exudate and desquamated mucosa). Microlithiasis and biliary sludge can be seen at the bottom of the gallbladder lumen. (a). At the level of the body, an intramural anechoic collection of the gallbladder wall represents a microabscess (calipers) (b). In the left side of the gallbladder, a pericholecystic collection (calipers) (c) communicates with the gallbladder lumen through a full thickness parietal defect (hole sign) (arrow) (d). Dishomogeneous pericholecystic liver parenchyma (delimited by calipers) corresponds to concurrent phlegmonous reaction / microabscesses of the V hepatic segment (e).
Figure 3.
Complicated acute cholecystitis, leading to GC and gallbladder perforation. Gallbladder walls are irregularly thickened with an echogenic strand departing from the gallbladder wall and projecting into the lumen (intraluminal membrane: fibrinous exudate and desquamated mucosa). Microlithiasis and biliary sludge can be seen at the bottom of the gallbladder lumen. (a). At the level of the body, an intramural anechoic collection of the gallbladder wall represents a microabscess (calipers) (b). In the left side of the gallbladder, a pericholecystic collection (calipers) (c) communicates with the gallbladder lumen through a full thickness parietal defect (hole sign) (arrow) (d). Dishomogeneous pericholecystic liver parenchyma (delimited by calipers) corresponds to concurrent phlegmonous reaction / microabscesses of the V hepatic segment (e).
Figure 4.
Gallbladder perforation. US B-mode evaluation shows a pericholecystic collection in the setting of acute cholecystitis (a). A different scan shows a full thickness defect in the gallbladder wall (hole sign) (b).
Figure 4.
Gallbladder perforation. US B-mode evaluation shows a pericholecystic collection in the setting of acute cholecystitis (a). A different scan shows a full thickness defect in the gallbladder wall (hole sign) (b).
Figure 5.
Mixed type gallbladder adenomyomatosis, showing body and fundic wall thickening coexisting with “hourglass” or “waist-like” appearance (a). CEUS examination shows a “mouth-eaten” enhancement in the arterial phase without wash-out in the late phase, where RAS appears as avascular spaces (arrows) (b, c).
Figure 5.
Mixed type gallbladder adenomyomatosis, showing body and fundic wall thickening coexisting with “hourglass” or “waist-like” appearance (a). CEUS examination shows a “mouth-eaten” enhancement in the arterial phase without wash-out in the late phase, where RAS appears as avascular spaces (arrows) (b, c).
Figure 6.
Gallbladder carcinoma. The gallbladder fossa is replaced by an ill-defined and heterogenous mass, infiltrating the liver parenchyma. The gallbladder is partially masked by the malignant mass and the residual lumen, containing a small amount of biliary sludge, can be recognized.
Figure 6.
Gallbladder carcinoma. The gallbladder fossa is replaced by an ill-defined and heterogenous mass, infiltrating the liver parenchyma. The gallbladder is partially masked by the malignant mass and the residual lumen, containing a small amount of biliary sludge, can be recognized.
Figure 7.
Gallbladder edema due to hypertensive cholecystopathy in a patient with hepatic cirrhosis. At B-mode evaluation, gallbladder walls are thickened with a double-wall appearance (calipers) (a). The same case evaluated by MVFI, that depicts the vascular pattern of the thickened gallbladder wall (calipers) with pronounced higher vascular signals in the outer layer and an edematous hypovascular inner layer (b).
Figure 7.
Gallbladder edema due to hypertensive cholecystopathy in a patient with hepatic cirrhosis. At B-mode evaluation, gallbladder walls are thickened with a double-wall appearance (calipers) (a). The same case evaluated by MVFI, that depicts the vascular pattern of the thickened gallbladder wall (calipers) with pronounced higher vascular signals in the outer layer and an edematous hypovascular inner layer (b).
Figure 8.
Gallbladder edema evolution in a young patient with VOD after bone marrow transplant, before and after treatment. At diagnosis, gallbladder walls appear markedly thickened. A small amount of perihepatic and pericholecystic fluid can be seen (a). After few days of treatment with defibrotide, a progressive decrease of the gallbladder wall was observed, with disappearance of pericholecystic fluid (b, c).
Figure 8.
Gallbladder edema evolution in a young patient with VOD after bone marrow transplant, before and after treatment. At diagnosis, gallbladder walls appear markedly thickened. A small amount of perihepatic and pericholecystic fluid can be seen (a). After few days of treatment with defibrotide, a progressive decrease of the gallbladder wall was observed, with disappearance of pericholecystic fluid (b, c).
Table 1.
Differential diagnosis between cholesterol and adenomatous gallbladder polyps, based on sonographic features.
Table 1.
Differential diagnosis between cholesterol and adenomatous gallbladder polyps, based on sonographic features.
| Differential diagnosis |
Cholesterol Polyps |
Adenoma |
| Number |
Multiple |
Single |
| Size |
< 10 mm (usually 2-4 mm) |
Usually > 10 mm (range 5-20 mm) |
| Echogenicity |
Iso-/hyperechoic, with internal hyperechoic foci |
Hypoechoic, sometimes with internal hypoechoic foci |
| Surface |
Smooth |
Sessile or lobulated contours |
| Stalk |
Thin, usually absent |
Wide |
| Vascular flow at CD evaluation |
Absent |
Present (not always detectable) |
| Arterial flow (CEUS) |
Iso-enhancement |
Hyper-enhancement |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).