Submitted:
18 November 2023
Posted:
23 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. MDSC development and functions
3. MDSC identification and characterisation
4. Implications of MDSCs in COVID-19 pathogenesis
5. Role of inflammatory responses to MDSC development in severe COVID-19
6. Long-term changes in MDSCs after severe COVID-19 resolution
7. Potential therapeutic drugs for ameliorating MDSC levels in COVID-19 patients
8. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDSC | Myeloid derived suppressor cells |
| ACE2 | Angiotensin-converting enzyme 2 |
| Arg-1 | Arginase-1 |
| ASC | Alanine-serine-cysteine |
| CMP | Common myeloid progenitor |
| CSF | Colony-stimulating factor |
| DAMP | Damage associated molecular pattern |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| H2O2 | Hydrogen peroxide |
| HSC | Hematopoietic stem cells |
| LOX1 | Lectin-type oxidized LDL receptor 1 |
| M-CSF | Macrophage colony-stimulating factor |
| M-MDSC | Monocytic MDSC |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death ligand 1 |
| PMN-MDSC | Polymorphonuclear mdscs |
| ROS | Reactive oxygen species |
| SLC7A11 | Solute carrier family 7 member 11 |
| Syk | Spleen tyrosine kinase |
| Treg | Regulatory T cells |
References
- Johns Hopkins University COVID-19 Map - Johns Hopkins Coronavirus Resource Center Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 October 2023).
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nature Reviews Drug Discovery 2023, 22, 449–475. [CrossRef]
- Ioannidis, J.P.A. Global Perspective of COVID-19 Epidemiology for a Full-Cycle Pandemic. European Journal of Clinical Investigation 2020, 50, e13423. [CrossRef]
- Chan, K.R.; Koh, C.W.T.; Ng, D.H.L.; Qin, S.; Ooi, J.S.G.; Ong, E.Z.; Zhang, S.L.X.; Sam, H.; Kalimuddin, S.; Low, J.G.H.; et al. Early Peripheral Blood MCEMP1 and HLA-DRA Expression Predicts COVID-19 Prognosis. eBioMedicine 2023, 89. [CrossRef]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.-H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Research 2007, 67, 425–425. [CrossRef]
- Condamine, T.; Gabrilovich, D.I. Molecular Mechanisms Regulating Myeloid-Derived Suppressor Cell Differentiation and Function. Trends in Immunology 2011, 32, 19–25. [CrossRef]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, Development, States, and Unaddressed Complexity. Immunity 2021, 54, 875–884. [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nature Reviews Immunology 2021, 21, 485–498. [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nature Immunology 2018, 19, 108–119. [CrossRef]
- Ushach, I.; Zlotnik, A. Biological Role of Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF) and Macrophage Colony-Stimulating Factor (M-CSF) on Cells of the Myeloid Lineage. Journal of Leukocyte Biology 2016, 100, 481–489. [CrossRef]
- Ohl, K.; Tenbrock, K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Frontiers in Immunology 2018, 9.
- Bordoni, V.; Sacchi, A.; Cimini, E.; Notari, S.; Grassi, G.; Tartaglia, E.; Casetti, R.; Giancola, M.L.; Bevilacqua, N.; Maeurer, M.; et al. An Inflammatory Profile Correlates With Decreased Frequency of Cytotoxic Cells in Coronavirus Disease 2019. Clinical Infectious Diseases 2020, 71, 2272–2275. [CrossRef]
- Garg, A.; Spector, S.A. HIV Type 1 Gp120–Induced Expansion of Myeloid Derived Suppressor Cells Is Dependent on Interleukin 6 and Suppresses Immunity. The Journal of Infectious Diseases 2014, 209, 441–451. [CrossRef]
- Yang, F.; Yu, X.; Zhou, C.; Mao, R.; Zhu, M.; Zhu, H.; Ma, Z.; Mitra, B.; Zhao, G.; Huang, Y.; et al. Hepatitis B e Antigen Induces the Expansion of Monocytic Myeloid-Derived Suppressor Cells to Dampen T-Cell Function in Chronic Hepatitis B Virus Infection. PLOS Pathogens 2019, 15, e1007690. [CrossRef]
- Tcyganov, E.N.; Hanabuchi, S.; Hashimoto, A.; Campbell, D.; Kar, G.; Slidel, T.W.F.; Cayatte, C.; Landry, A.; Pilataxi, F.; Hayes, S.; et al. Distinct Mechanisms Govern Populations of Myeloid-Derived Suppressor Cells in Chronic Viral Infection and Cancer. J Clin Invest 2021, 131. [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunology Research 2017, 5, 3–8. [CrossRef]
- Ostrand-Rosenberg, S.; Lamb, T.J.; Pawelec, G. Here, There, and Everywhere: Myeloid-Derived Suppressor Cells in Immunology. The Journal of Immunology 2023, 210, 1183–1197. [CrossRef]
- Ballbach, M.; Dannert, A.; Singh, A.; Siegmund, D.M.; Handgretinger, R.; Piali, L.; Rieber, N.; Hartl, D. Expression of Checkpoint Molecules on Myeloid-Derived Suppressor Cells. Immunology Letters 2017, 192, 1–6. [CrossRef]
- Dean, M.J.; Ochoa, J.B.; Sanchez-Pino, M.D.; Zabaleta, J.; Garai, J.; Del Valle, L.; Wyczechowska, D.; Baiamonte, L.B.; Philbrook, P.; Majumder, R.; et al. Severe COVID-19 Is Characterized by an Impaired Type I Interferon Response and Elevated Levels of Arginase Producing Granulocytic Myeloid Derived Suppressor Cells. Frontiers in Immunology 2021, 12.
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Inhibit T-Cell Activation by Depleting Cystine and Cysteine. Cancer Research 2010, 70, 68–77. [CrossRef]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered Recognition of Antigen Is a Mechanism of CD8+ T Cell Tolerance in Cancer. Nature Medicine 2007, 13, 828–835. [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for Myeloid-Derived Suppressor Cell Nomenclature and Characterization Standards. Nature Communications 2016, 7, 12150. [CrossRef]
- Aarts, C.E.M.; Hiemstra, I.H.; Béguin, E.P.; Hoogendijk, A.J.; Bouchmal, S.; van Houdt, M.; Tool, A.T.J.; Mul, E.; Jansen, M.H.; Janssen, H.; et al. Activated Neutrophils Exert Myeloid-Derived Suppressor Cell Activity Damaging T Cells beyond Repair. Blood Advances 2019, 3, 3562–3574. [CrossRef]
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune Suppression by Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells: Similarities and Differences. Cellular and Molecular Life Sciences 2013, 70, 3813–3827. [CrossRef]
- Nefedova, Y.; Nagaraj, S.; Rosenbauer, A.; Muro-Cacho, C.; Sebti, S.M.; Gabrilovich, D.I. Regulation of Dendritic Cell Differentiation and Antitumor Immune Response in Cancer by Pharmacologic-Selective Inhibition of the Janus-Activated Kinase 2/Signal Transducers and Activators of Transcription 3 Pathway. Cancer Research 2005, 65, 9525–9535. [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-Derived Suppressor Cells as Immunosuppressive Regulators and Therapeutic Targets in Cancer. Signal Transduction and Targeted Therapy 2021, 6, 362. [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nature Reviews Immunology 2009, 9, 162–174. [CrossRef]
- Ong, E.Z.; Kalimuddin, S.; Chia, W.C.; Ooi, S.H.; Koh, C.W.; Tan, H.C.; Zhang, S.L.; Low, J.G.; Ooi, E.E.; Chan, K.R. Temporal Dynamics of the Host Molecular Responses Underlying Severe COVID-19 Progression and Disease Resolution. eBioMedicine 2021, 65. [CrossRef]
- Reyes, M.; Filbin, M.R.; Bhattacharyya, R.P.; Sonny, A.; Mehta, A.; Billman, K.; Kays, K.R.; Pinilla-Vera, M.; Benson, M.E.; Cosimi, L.A.; et al. Plasma from Patients with Bacterial Sepsis or Severe COVID-19 Induces Suppressive Myeloid Cell Production from Hematopoietic Progenitors in Vitro. Science Translational Medicine 2021, 13, eabe9599. [CrossRef]
- Wilk, A.J.; Lee, M.J.; Wei, B.; Parks, B.; Pi, R.; Martínez-Colón, G.J.; Ranganath, T.; Zhao, N.Q.; Taylor, S.; Becker, W.; et al. Multi-Omic Profiling Reveals Widespread Dysregulation of Innate Immunity and Hematopoiesis in COVID-19. Journal of Experimental Medicine 2021, 218, e20210582. [CrossRef]
- Kvedaraite, E.; Hertwig, L.; Sinha, I.; Ponzetta, A.; Hed Myrberg, I.; Lourda, M.; Dzidic, M.; Akber, M.; Klingström, J.; Folkesson, E.; et al. Major Alterations in the Mononuclear Phagocyte Landscape Associated with COVID-19 Severity. Proceedings of the National Academy of Sciences 2021, 118, e2018587118. [CrossRef]
- Shivram, H.; Hackney, J.A.; Rosenberger, C.M.; Teterina, A.; Qamra, A.; Onabajo, O.; McBride, J.; Cai, F.; Bao, M.; Tsai, L.; et al. Transcriptomic and Proteomic Assessment of Tocilizumab Response in a Randomized Controlled Trial of Patients Hospitalized with COVID-19. iScience 2023, 26. [CrossRef]
- LaSalle, T.J.; Gonye, A.L.K.; Freeman, S.S.; Kaplonek, P.; Gushterova, I.; Kays, K.R.; Manakongtreecheep, K.; Tantivit, J.; Rojas-Lopez, M.; Russo, B.C.; et al. Longitudinal Characterization of Circulating Neutrophils Uncovers Phenotypes Associated with Severity in Hospitalized COVID-19 Patients. Cell Reports Medicine 2022, 3, 100779. [CrossRef]
- Wang, X.; Wen, Y.; Xie, X.; Liu, Y.; Tan, X.; Cai, Q.; Zhang, Y.; Cheng, L.; Xu, G.; Zhang, S.; et al. Dysregulated Hematopoiesis in Bone Marrow Marks Severe COVID-19. Cell Discovery 2021, 7, 60. [CrossRef]
- Takano, T.; Matsumura, T.; Adachi, Y.; Terahara, K.; Moriyama, S.; Onodera, T.; Nishiyama, A.; Kawana-Tachikawa, A.; Miki, S.; Hosoya-Nakayama, K.; et al. Myeloid Cell Dynamics Correlating with Clinical Outcomes of Severe COVID-19 in Japan. International Immunology 2021, 33, 241–247. [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.-G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401-1418.e18. [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419-1440.e23. [CrossRef]
- Agrati, C.; Sacchi, A.; Bordoni, V.; Cimini, E.; Notari, S.; Grassi, G.; Casetti, R.; Tartaglia, E.; Lalle, E.; D’Abramo, A.; et al. Expansion of Myeloid-Derived Suppressor Cells in Patients with Severe Coronavirus Disease (COVID-19). Cell Death & Differentiation 2020, 27, 3196–3207. [CrossRef]
- Falck-Jones, S.; Vangeti, S.; Yu, M.; Falck-Jones, R.; Cagigi, A.; Badolati, I.; Österberg, B.; Lautenbach, M.J.; Åhlberg, E.; Lin, A.; et al. Functional Monocytic Myeloid-Derived Suppressor Cells Increase in Blood but Not Airways and Predict COVID-19 Severity. J Clin Invest 2021, 131. [CrossRef]
- Thompson, E.A.; Cascino, K.; Ordonez, A.A.; Zhou, W.; Vaghasia, A.; Hamacher-Brady, A.; Brady, N.R.; Sun, I.-H.; Wang, R.; Rosenberg, A.Z.; et al. Metabolic Programs Define Dysfunctional Immune Responses in Severe COVID-19 Patients. Cell Reports 2021, 34, 108863. [CrossRef]
- Xue, G.; Jiang, M.; Zhao, R.; Le, A.; Li, J. Elevated Frequencies of CD14+HLA-DRlo/neg MDSCs in COVID-19 Patients. Aging (Albany NY) 2021, 13, 6236–6246. [CrossRef]
- Marais, C.; Claude, C.; Semaan, N.; Charbel, R.; Barreault, S.; Travert, B.; Piloquet, J.-E.; Demailly, Z.; Morin, L.; Merchaoui, Z.; et al. Myeloid Phenotypes in Severe COVID-19 Predict Secondary Infection and Mortality: A Pilot Study. Annals of Intensive Care 2021, 11, 111. [CrossRef]
- Jiménez-Cortegana, C.; Sánchez-Jiménez, F.; Pérez-Pérez, A.; Álvarez, N.; Sousa, A.; Cantón-Bulnes, L.; Vilariño-García, T.; Fuentes, S.; Martín, S.; Jiménez, M.; et al. Low Levels of Granulocytic Myeloid-Derived Suppressor Cells May Be a Good Marker of Survival in the Follow-Up of Patients With Severe COVID-19. Frontiers in Immunology 2022, 12.
- Sacchi, A.; Grassi, G.; Bordoni, V.; Lorenzini, P.; Cimini, E.; Casetti, R.; Tartaglia, E.; Marchioni, L.; Petrosillo, N.; Palmieri, F.; et al. Early Expansion of Myeloid-Derived Suppressor Cells Inhibits SARS-CoV-2 Specific T-Cell Response and May Predict Fatal COVID-19 Outcome. Cell Death & Disease 2020, 11, 921. [CrossRef]
- Xu, G.; Qi, F.; Li, H.; Yang, Q.; Wang, H.; Wang, X.; Liu, X.; Zhao, J.; Liao, X.; Liu, Y.; et al. The Differential Immune Responses to COVID-19 in Peripheral and Lung Revealed by Single-Cell RNA Sequencing. Cell Discovery 2020, 6, 73. [CrossRef]
- Veenith, T.; Martin, H.; Le Breuilly, M.; Whitehouse, T.; Gao-Smith, F.; Duggal, N.; Lord, J.M.; Mian, R.; Sarphie, D.; Moss, P. High Generation of Reactive Oxygen Species from Neutrophils in Patients with Severe COVID-19. Scientific Reports 2022, 12, 10484. [CrossRef]
- Badawy, M.A.; Yasseen, B.A.; El-Messiery, R.M.; Abdel-Rahman, E.A.; Elkhodiry, A.A.; Kamel, A.G.; El-sayed, H.; Shedra, A.M.; Hamdy, R.; Zidan, M.; et al. Neutrophil-Mediated Oxidative Stress and Albumin Structural Damage Predict COVID-19-Associated Mortality. eLife 2021, 10, e69417. [CrossRef]
- Kiaee, F.; Jamaati, H.; Shahi, H.; Roofchayee, N.D.; Varahram, M.; Folkerts, G.; Garssen, J.; Adcock, I.M.; Mortaz, E. Immunophenotype and Function of Circulating Myeloid Derived Suppressor Cells in COVID-19 Patients. Scientific Reports 2022, 12, 22570. [CrossRef]
- Jiménez-Cortegana, C.; Liró, J.; Palazón-Carrión, N.; Salamanca, E.; Sojo-Dorado, J.; de la Cruz-Merino, L.; Pascual, Á.; Rodríguez-Baño, J.; Sánchez-Margalet, V. Increased Blood Monocytic Myeloid Derived Suppressor Cells but Low Regulatory T Lymphocytes in Patients with Mild COVID-19. Viral Immunology 2021, 34, 639–645. [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates with Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Reports 2021, 34, 108728. [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 Spike Protein Induces Inflammation via TLR2-Dependent Activation of the NF-κB Pathway. eLife 2021, 10, e68563. [CrossRef]
- DeDiego Marta L.; Nieto-Torres Jose L.; Regla-Nava Jose A.; Jimenez-Guardeño Jose M.; Fernandez-Delgado Raul; Fett Craig; Castaño-Rodriguez Carlos; Perlman Stanley; Enjuanes Luis Inhibition of NF-κB-Mediated Inflammation in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice Increases Survival. Journal of Virology 2014, 88, 913–924. [CrossRef]
- Wu, Y.; Ma, L.; Cai, S.; Zhuang, Z.; Zhao, Z.; Jin, S.; Xie, W.; Zhou, L.; Zhang, L.; Zhao, J.; et al. RNA-Induced Liquid Phase Separation of SARS-CoV-2 Nucleocapsid Protein Facilitates NF-κB Hyper-Activation and Inflammation. Signal Transduction and Targeted Therapy 2021, 6, 167. [CrossRef]
- Su, C.-M.; Wang, L.; Yoo, D. Activation of NF-κB and Induction of Proinflammatory Cytokine Expressions Mediated by ORF7a Protein of SARS-CoV-2. Scientific Reports 2021, 11, 13464. [CrossRef]
- Nilsson-Payant Benjamin E.; Uhl Skyler; Grimont Adrien; Doane Ashley S.; Cohen Phillip; Patel Roosheel S.; Higgins Christina A.; Acklin Joshua A.; Bram Yaron; Chandar Vasuretha; et al. The NF-κB Transcriptional Footprint Is Essential for SARS-CoV-2 Replication. Journal of Virology 2021, 95, 10.1128/jvi.01257-21. [CrossRef]
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; Martinez de la Escalera, G.; Clapp, C. The Spike Protein of SARS-CoV-2 Induces Endothelial Inflammation through Integrin A5β1 and NF-κB Signaling. Journal of Biological Chemistry 2022, 298. [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nature Medicine 2020, 26, 1636–1643. [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest 2020, 130, 2620–2629. [CrossRef]
- Hackney, J.A.; Shivram, H.; Vander Heiden, J.; Overall, C.; Orozco, L.; Gao, X.; Kim, E.; West, N.; Qamra, A.; Chang, D.; et al. A Myeloid Program Associated with COVID-19 Severity Is Decreased by Therapeutic Blockade of IL-6 Signaling. iScience 2023, 26. [CrossRef]
- Thwaites, R.S.; Sanchez Sevilla Uruchurtu, A.; Siggins, M.K.; Liew, F.; Russell, C.D.; Moore, S.C.; Fairfield, C.; Carter, E.; Abrams, S.; Short, C.-E.; et al. Inflammatory Profiles across the Spectrum of Disease Reveal a Distinct Role for GM-CSF in Severe COVID-19. Science Immunology 2021, 6, eabg9873. [CrossRef]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. The Journal of Infectious Diseases 2020, 222, 746–754. [CrossRef]
- Cabrera, L.E.; Pekkarinen, P.T.; Alander, M.; Nowlan, K.H.A.; Nguyen, N.A.; Jokiranta, S.; Kuivanen, S.; Patjas, A.; Mero, S.; Pakkanen, S.H.; et al. Characterization of Low-Density Granulocytes in COVID-19. PLOS Pathogens 2021, 17, e1009721. [CrossRef]
- Chakraborty, S.; Gonzalez, J.; Edwards, K.; Mallajosyula, V.; Buzzanco, A.S.; Sherwood, R.; Buffone, C.; Kathale, N.; Providenza, S.; Xie, M.M.; et al. Proinflammatory IgG Fc Structures in Patients with Severe COVID-19. Nature Immunology 2021, 22, 67–73. [CrossRef]
- Hoepel, W.; Chen, H.-J.; Geyer, C.E.; Allahverdiyeva, S.; Manz, X.D.; de Taeye, S.W.; Aman, J.; Mes, L.; Steenhuis, M.; Griffith, G.R.; et al. High Titers and Low Fucosylation of Early Human Anti–SARS-CoV-2 IgG Promote Inflammation by Alveolar Macrophages. Science Translational Medicine 2021, 13, eabf8654. [CrossRef]
- Wigerblad, G.; Warner, S.A.; Ramos-Benitez, M.J.; Kardava, L.; Tian, X.; Miao, R.; Reger, R.; Chakraborty, M.; Wong, S.; Kanthi, Y.; et al. Spleen Tyrosine Kinase Inhibition Restores Myeloid Homeostasis in COVID-19. Science Advances 2023, 9, eade8272. [CrossRef]
- Schrijver, I.T.; Théroude, C.; Antonakos, N.; Regina, J.; Le Roy, D.; Bart, P.-A.; Chiche, J.-D.; Perreau, M.; Pantaleo, G.; Calandra, T.; et al. COVID-19 Rapidly Increases MDSCs and Prolongs Innate Immune Dysfunctions. European Journal of Immunology 2022, 52, 1676–1679. [CrossRef]
- Cheong, J.-G.; Ravishankar, A.; Sharma, S.; Parkhurst, C.N.; Grassmann, S.A.; Wingert, C.K.; Laurent, P.; Ma, S.; Paddock, L.; Miranda, I.C.; et al. Epigenetic Memory of Coronavirus Infection in Innate Immune Cells and Their Progenitors. Cell 2023, 186, 3882-3902.e24. [CrossRef]
- Hopkins, F.R.; Govender, M.; Svanberg, C.; Nordgren, J.; Waller, H.; Nilsdotter-Augustinsson, Å.; Henningsson, A.J.; Hagbom, M.; Sjöwall, J.; Nyström, S.; et al. Major Alterations to Monocyte and Dendritic Cell Subsets Lasting More than 6 Months after Hospitalization for COVID-19. Frontiers in Immunology 2023, 13.
- Beliakova-Bethell, N.; Maruthai, K.; Xu, R.; Salvador, L.C.M.; Garg, A. Monocytic-Myeloid Derived Suppressor Cells Suppress T-Cell Responses in Recovered SARS CoV2-Infected Individuals. Frontiers in Immunology 2022, 13.
- Ruenjaiman, V.; Sodsai, P.; Kueanjinda, P.; Bunrasmee, W.; Klinchanhom, S.; Reantragoon, R.; Tunvirachaisakul, C.; Manothummetha, K.; Mejun, N.; Liengswangwong, K.; et al. Impact of SARS-CoV-2 Infection on the Profiles and Responses of Innate Immune Cells after Recovery. Journal of Microbiology, Immunology and Infection 2022, 55, 993–1004. [CrossRef]
- André F Rendeiro; Joseph Casano; Charles Kyriakos Vorkas; Harjot Singh; Ayana Morales; Robert A DeSimone; Grant B Ellsworth; Rosemary Soave; Shashi N Kapadia; Kohta Saito; et al. Profiling of Immune Dysfunction in COVID-19 Patients Allows Early Prediction of Disease Progression. Life Sci. Alliance 2021, 4, e202000955. [CrossRef]
- Siemińska, I.; Węglarczyk, K.; Surmiak, M.; Kurowska-Baran, D.; Sanak, M.; Siedlar, M.; Baran, J. Mild and Asymptomatic COVID-19 Convalescents Present Long-Term Endotype of Immunosuppression Associated With Neutrophil Subsets Possessing Regulatory Functions. Frontiers in Immunology 2021, 12.
- Tomić, S.; Đokić, J.; Stevanović, D.; Ilić, N.; Gruden-Movsesijan, A.; Dinić, M.; Radojević, D.; Bekić, M.; Mitrović, N.; Tomašević, R.; et al. Reduced Expression of Autophagy Markers and Expansion of Myeloid-Derived Suppressor Cells Correlate With Poor T Cell Response in Severe COVID-19 Patients. Frontiers in Immunology 2021, 12.
- Taniguchi-Ponciano, K.; Vadillo, E.; Mayani, H.; Gonzalez-Bonilla, C.R.; Torres, J.; Majluf, A.; Flores-Padilla, G.; Wacher-Rodarte, N.; Galan, J.C.; Ferat-Osorio, E.; et al. Increased Expression of Hypoxia-Induced Factor 1α mRNA and Its Related Genes in Myeloid Blood Cells from Critically Ill COVID-19 Patients. Annals of Medicine 2021, 53, 197–207. [CrossRef]
- Ricke-Hoch, M.; Stelling, E.; Lasswitz, L.; Gunesch, A.P.; Kasten, M.; Zapatero-Belinchón, F.J.; Brogden, G.; Gerold, G.; Pietschmann, T.; Montiel, V.; et al. Impaired Immune Response Mediated by Prostaglandin E2 Promotes Severe COVID-19 Disease. PLOS ONE 2021, 16, e0255335. [CrossRef]
- Mao, Y.; Sarhan, D.; Steven, A.; Seliger, B.; Kiessling, R.; Lundqvist, A. Inhibition of Tumor-Derived Prostaglandin-E2 Blocks the Induction of Myeloid-Derived Suppressor Cells and Recovers Natural Killer Cell Activity. Clinical Cancer Research 2014, 20, 4096–4106. [CrossRef]
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The Immunology of Long COVID. Nature Reviews Immunology 2023, 23, 618–634. [CrossRef]
- Haunhorst, S.; Bloch, W.; Javelle, F.; Krüger, K.; Baumgart, S.; Drube, S.; Lemhöfer, C.; Reuken, P.; Stallmach, A.; Müller, M.; et al. A Scoping Review of Regulatory T Cell Dynamics in Convalescent COVID-19 Patients – Indications for Their Potential Involvement in the Development of Long COVID? Frontiers in Immunology 2022, 13.
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. Cell 2022, 185, 881-895.e20. [CrossRef]
- Park, S.-J.; Nam, D.; Seong, H.C.; Hahn, Y.S. New Discovery of Myeloid-Derived Suppressor Cell’s Tale on Viral Infection and COVID-19. Frontiers in Immunology 2022, 13.
- Guo, Q.; Zhao, Y.; Li, J.; Liu, J.; Yang, X.; Guo, X.; Kuang, M.; Xia, H.; Zhang, Z.; Cao, L.; et al. Induction of Alarmin S100A8/A9 Mediates Activation of Aberrant Neutrophils in the Pathogenesis of COVID-19. Cell Host & Microbe 2021, 29, 222-235.e4. [CrossRef]
- Fiorentino, G.; Coppola, A.; Izzo, R.; Annunziata, A.; Bernardo, M.; Lombardi, A.; Trimarco, V.; Santulli, G.; Trimarco, B. Effects of Adding L-Arginine Orally to Standard Therapy in Patients with COVID-19: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Results of the First Interim Analysis. eClinicalMedicine 2021, 40. [CrossRef]
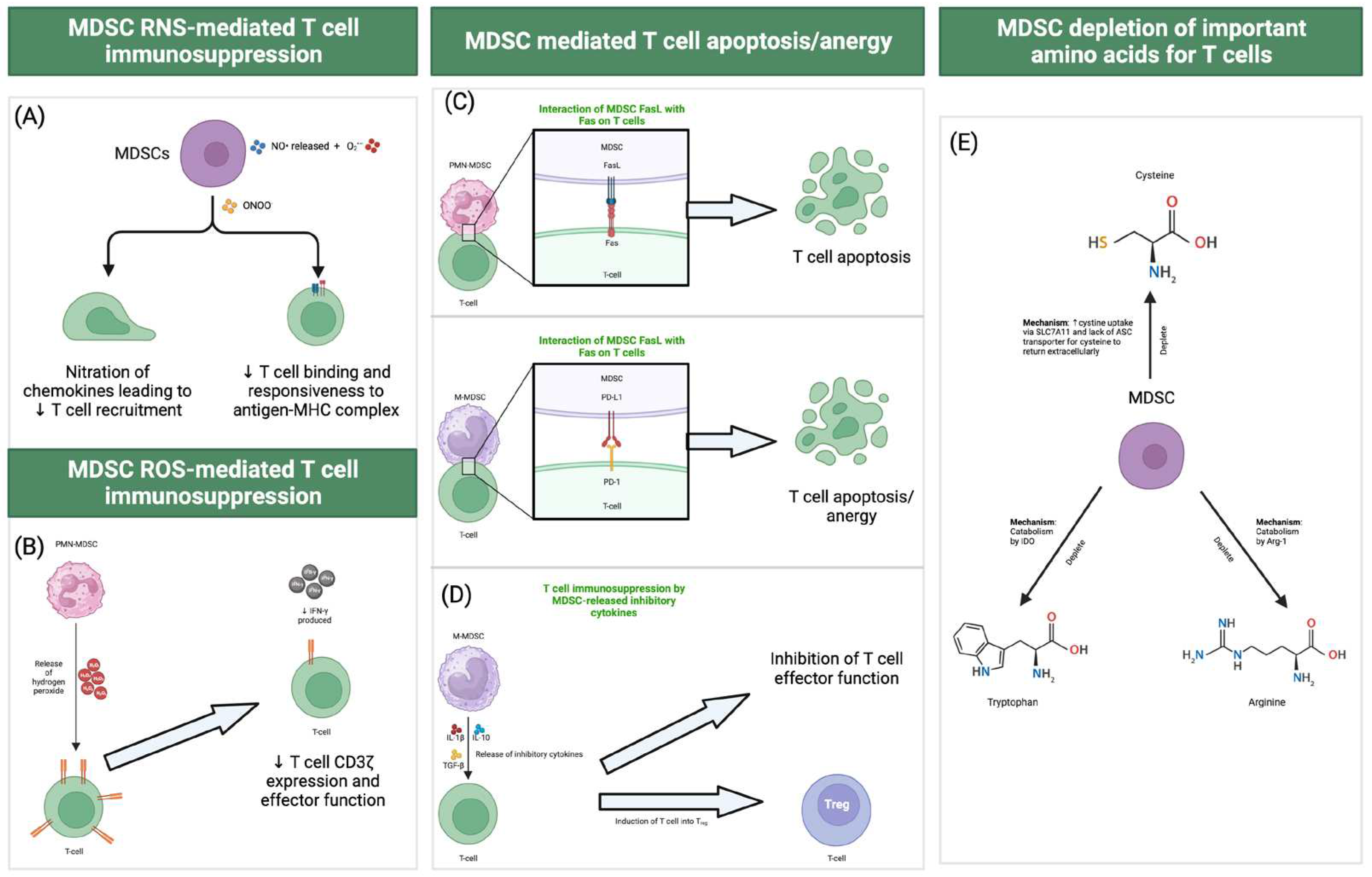
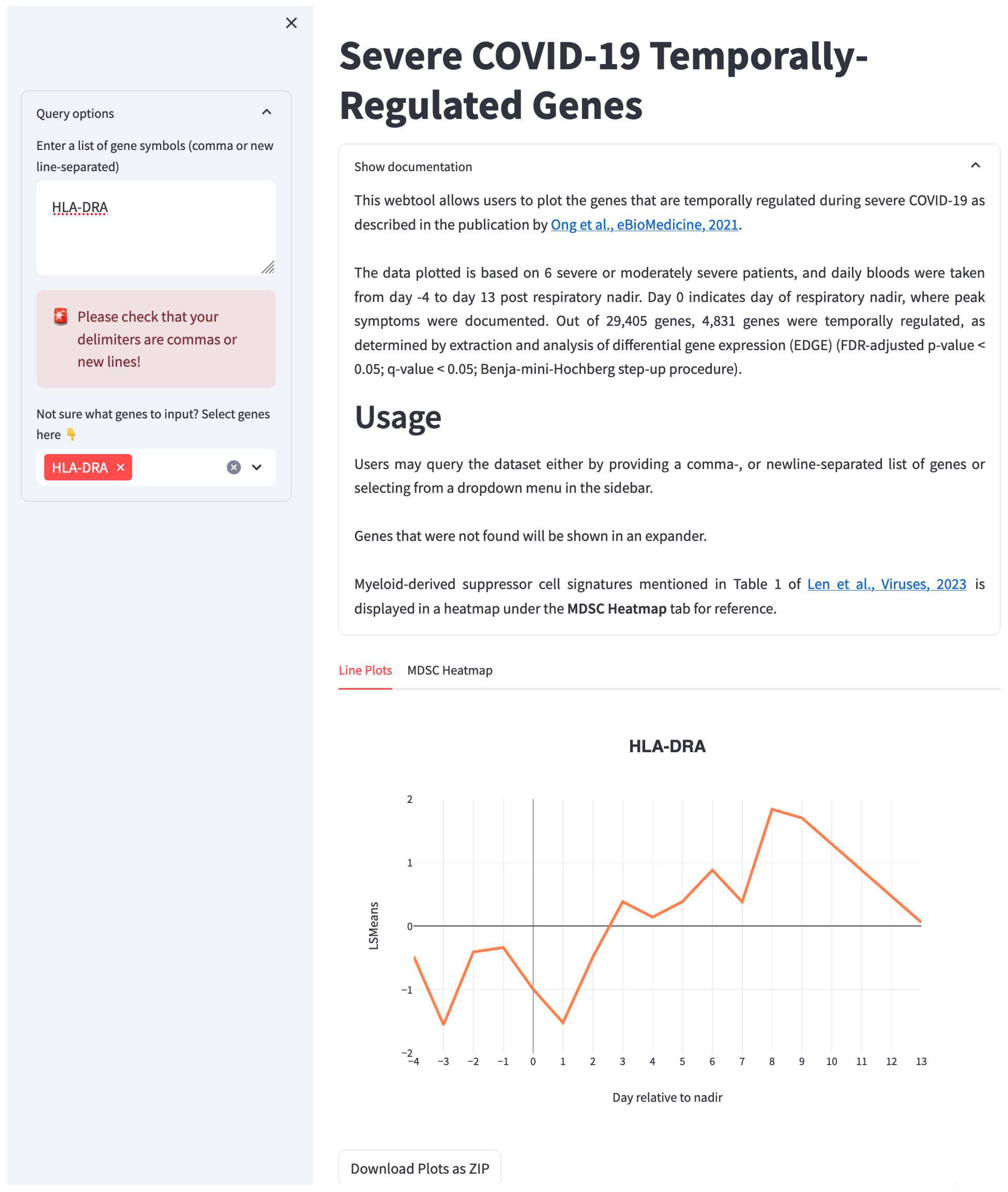
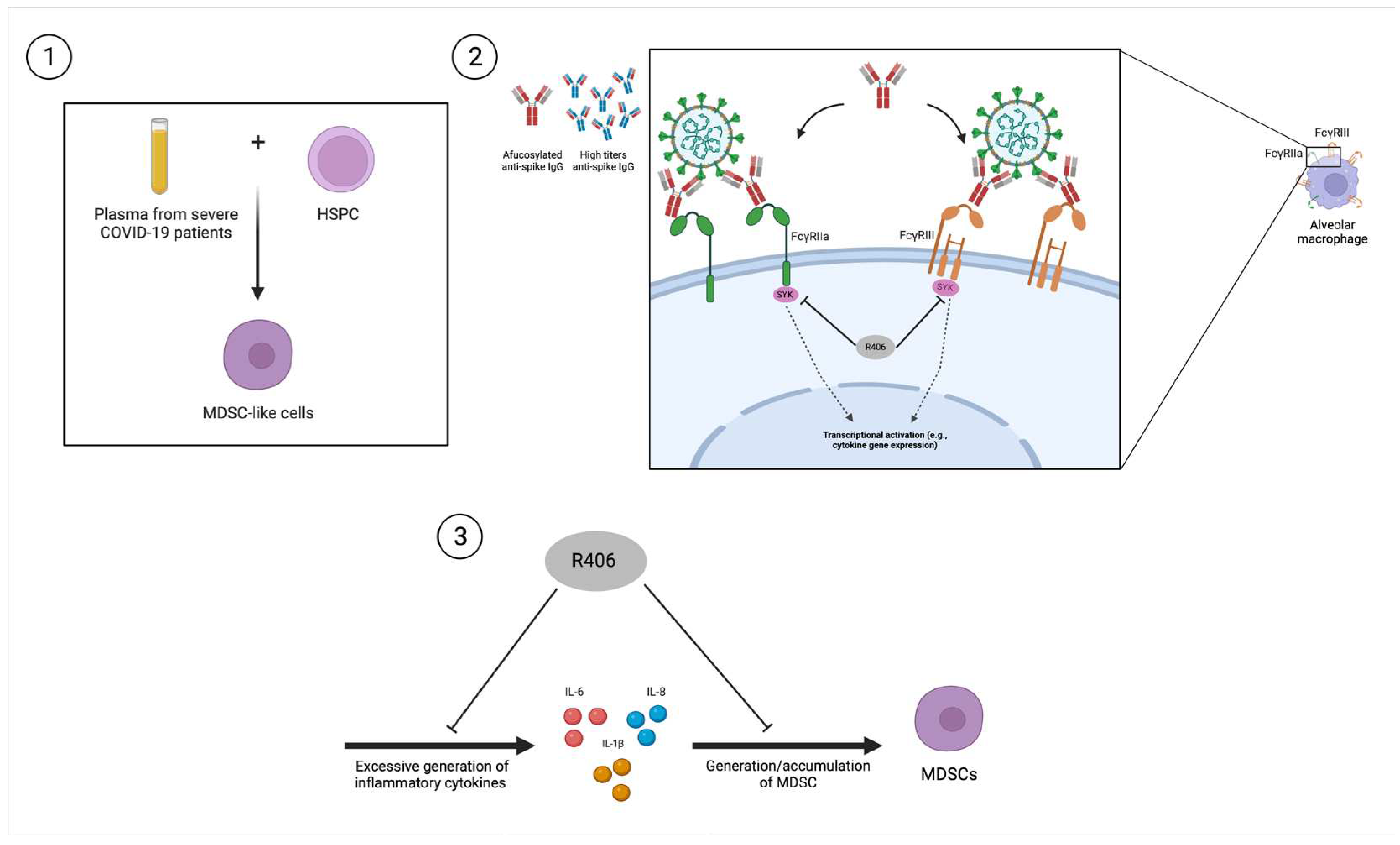
| M- or PMN-MDSC | Gene sets used/signature genes identified | Reference |
|---|---|---|
| M-MDSC | S100A8, S100A12, LGALS1, VCAN, RETN, LYZ, PLAC8, MNDA, CTSD, SELL, STXBP2, CYP1B1, VIM, CLU, NKG7, ALOX5AP, NCF1, MCEMP1, TIMP1, SOD2, CD163, NAMPT, FAM65B, ACSL1, VAMP5, LILRA5, VNN2, ANXA6, IL1R2, CALR | [29] |
| CSTA, IL1B, HBB, LRG1, CXCR2, PROK2, IGFBP6, HBA2, SOCS3, ASPRV1, C19orf12, GRINA, CSF3R, CCR1, IFITM2, HP, CTSD, TSPO, S100A11, JUNB, CYP4F2, CD84, CLEC4E, MAP1LC3B, GCNT2, GDA, DUSP1, HDC, C5AR1, MSRB1, SRGN, SELPLG, BTG1, MXD1, HIST1H2BI, STEAP4, SLC40A1, ARG2, SLPI, CLEC4D, TXN, FABP5, CDKN2D, UPP1, PRTN3, CHI3L1, TPD52, CXCL3, RNF149, GPCPD1, S100A6, GSR, LMNB1, TACSTD2, MTUS1, LITAF, NPL, C19orf38, SEPHS2, IL36G, GLIPR2, EIF4EBP1, F10, VCAN, PI16, SMPDL3A, GAPDH, ATP6V1G1, FCGR2A, PLA2G7, CD300LF, YPEL3, KIAA1551, ADIPOR1, UBA52, GLRX, SELL, ATG3, ABTB1, FGL2, ALOX5AP, GADD45A, CD14, SIGLEC9, FBXL5, MYD88, TGFBI, SNAP23, PILRA, FXYD5, BST1, FLOT1, LILRB4, PPT1, MRGPRX3, R3HDM4, PKM, PICALM, SORL1, ZYX, ATP11B, RGS3, CTSC, PTPN1, TALDO1, STK17B, ANKRD33B, GYG1, IL4R, IER3, NUDT4, SKAP2, IL13RA1, LILRB3, GATM, NCF4, F13A1, CDK2AP2, EMB, GCLM, RAB24, CD300LD, SERP1, CEBPB, ALDOA, UBE2H, TARM1, TSC22D3, CARHSP1, HEBP1, KLF13, RBM3, ELP1, PGD, ENO1, DHRS7, OGFRL1, GDPD3, SFXN5, PYGL, ANXA11, DCK, OSM, SDCBP, UBB, EDEM2, LDHA, UBALD2, CCRL2, FTH1, PKIB, PNPLA2, CAP1, KLF2, RND1, LCP1, ANXA2, HOPX, CAMK2D, SUPT4H1, GNG12, ARID5A, CD302, CSF2RB, PPP1R2, RAC2, SLC28A2, PIM1, MPP1, RNASEL, TONSL, SSU72, TBC1D14, ID2, SPI1, HIST1H1C, IER2, MMP8, RTP4, TMEM14C, UBE2B, GPR146, PGLYRP1, MBOAT7, LAMP2, BIN3, PLSCR1, EGR1, EMILIN2, C5orf30, TREM1, BIN2, CALM3, CBR3, SLFN12L | [30] | |
| IFITM1, IFITM2, IFITM3, GRINA, HLA-DRA, HLA-DQB1, HLA-DPA1, CD74 | [31] | |
| PMN-MDSC | SAMD9L, IL1RN, CSF3R, CSF1, GDA, HP, IFIT1, IFIT3, NUDT4, IFIT2, NDST1, IL18RAP, PGLYRP1, CD177, ZBP1, RSAD2, IL1R2, MCEMP1, TYROBP, SLPI, CRISPLD2, S100A6, CMPK2, PADI4, RAF1, S100A9, S100A8, SLC27A4, PFKFB4, C5AR1, FPR1, FPR2, ADIPOR1, RASGRP4, HCAR2, CD300LF, IP6K1, PAG1, CCR1, NBEAL2, ISG15, BST1, ABTB1, SELL, TREML2, TARM1, DMXL2, LCN2, FBXL5, MXD1, RTP4, TINAGL1, SLC2A3, PYGL, LYST, SLC2A6, TIMP2, FFAR2, UPP1, CD33, CERS6, SH2D3C, MMP8, MMP9, DUSP6, RHOB, IL17RA, FGR, MMP13, OAS2, IL1B, PECAM1, IRF7, CD44, TLR2, LILRA6, SLFN5, PTAFR, CXCR4, SAMHD1, THBS1, STX11, KCTD20, CXCR2, CCL3, EGLN3, GABBR1, SEMA4D, GSR, OSM, ASPRV1, KLF2, LRG1, PI16, SMAP2, AGRN, YPEL3, MSRB1, GRINA | [32] |
| S100A12, ARG1, CD177, MCEMP1, and GYG1 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





