Submitted:
23 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials AND METHODS
Hardware
Software
Protein Structure Preparation
Ligand Structures Preparation
Molecular Docking Simulation
Results AND DISCUSSION
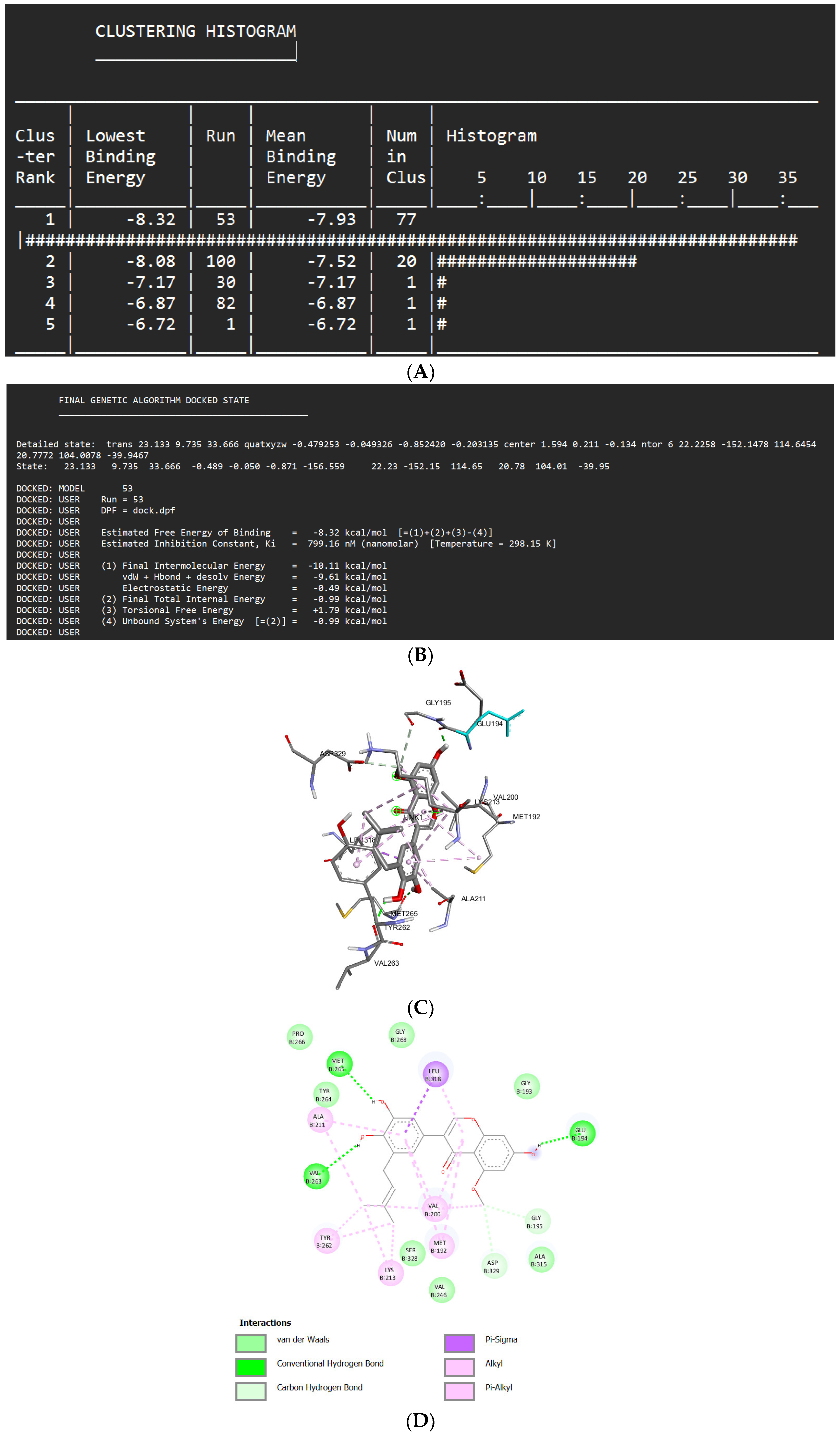
Docking Results of Isoliquiritigenin with IL-1 Receptor (IL-1R)
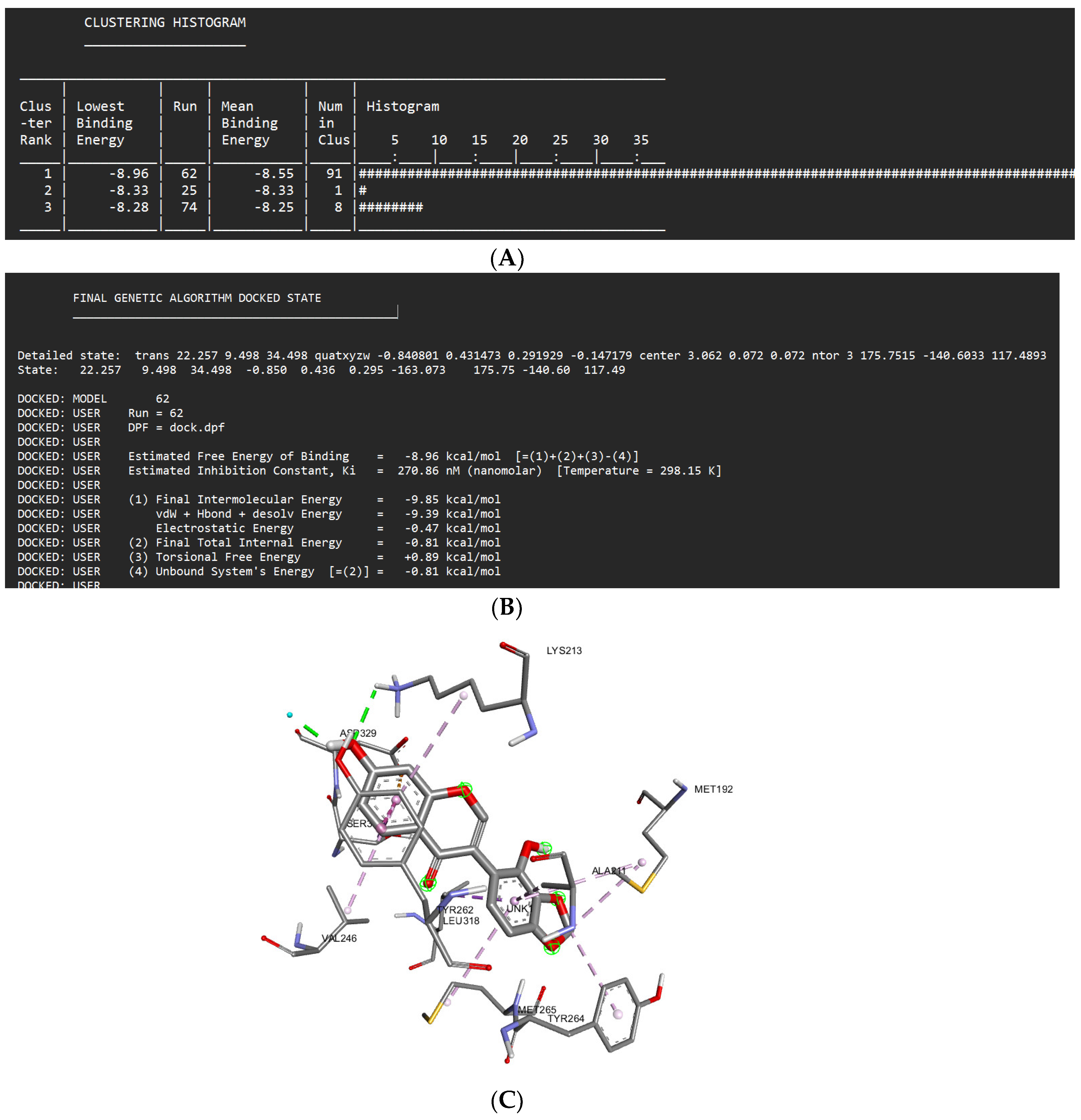
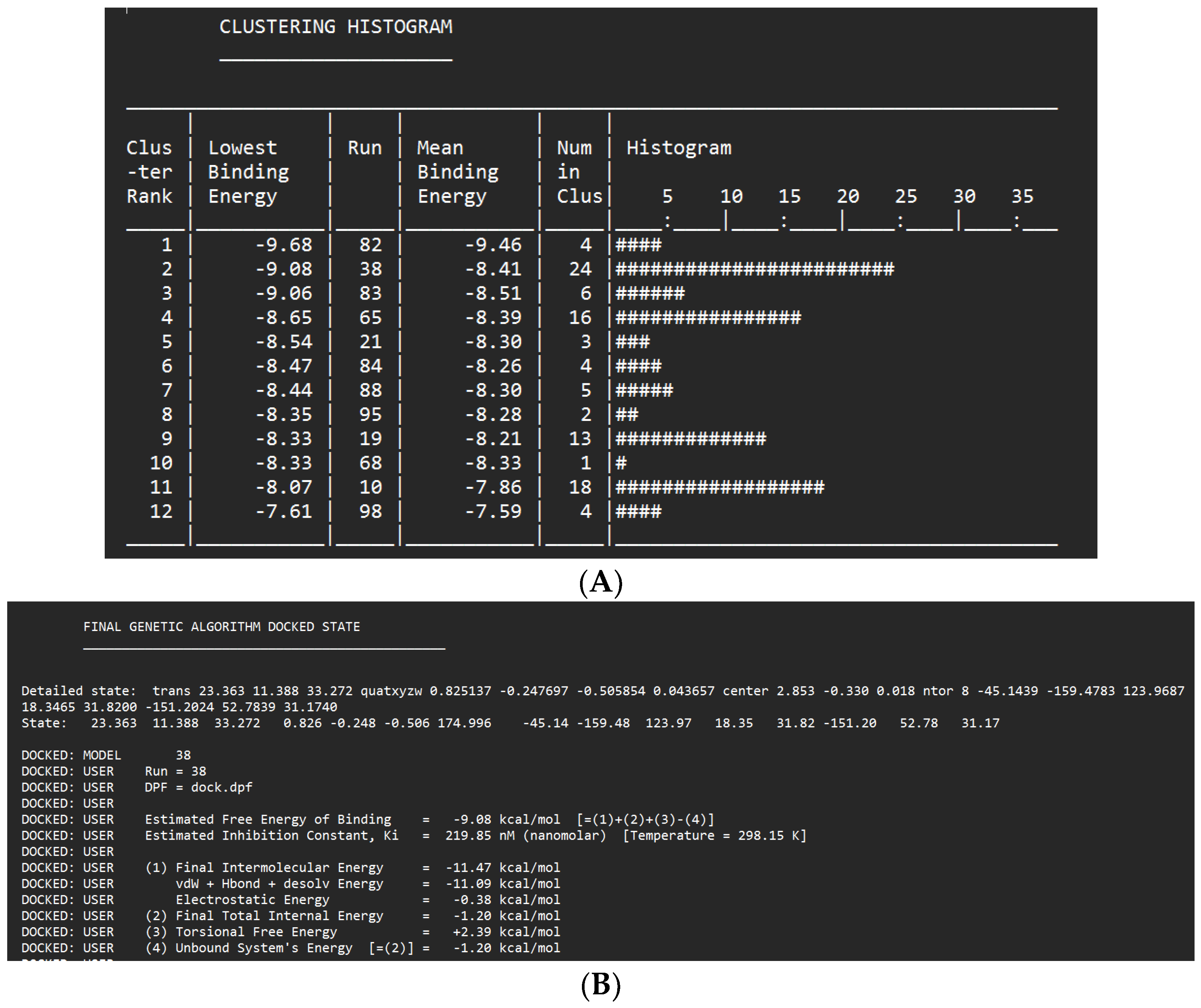
Docking Results of Glyzaglabrin with IL-1R
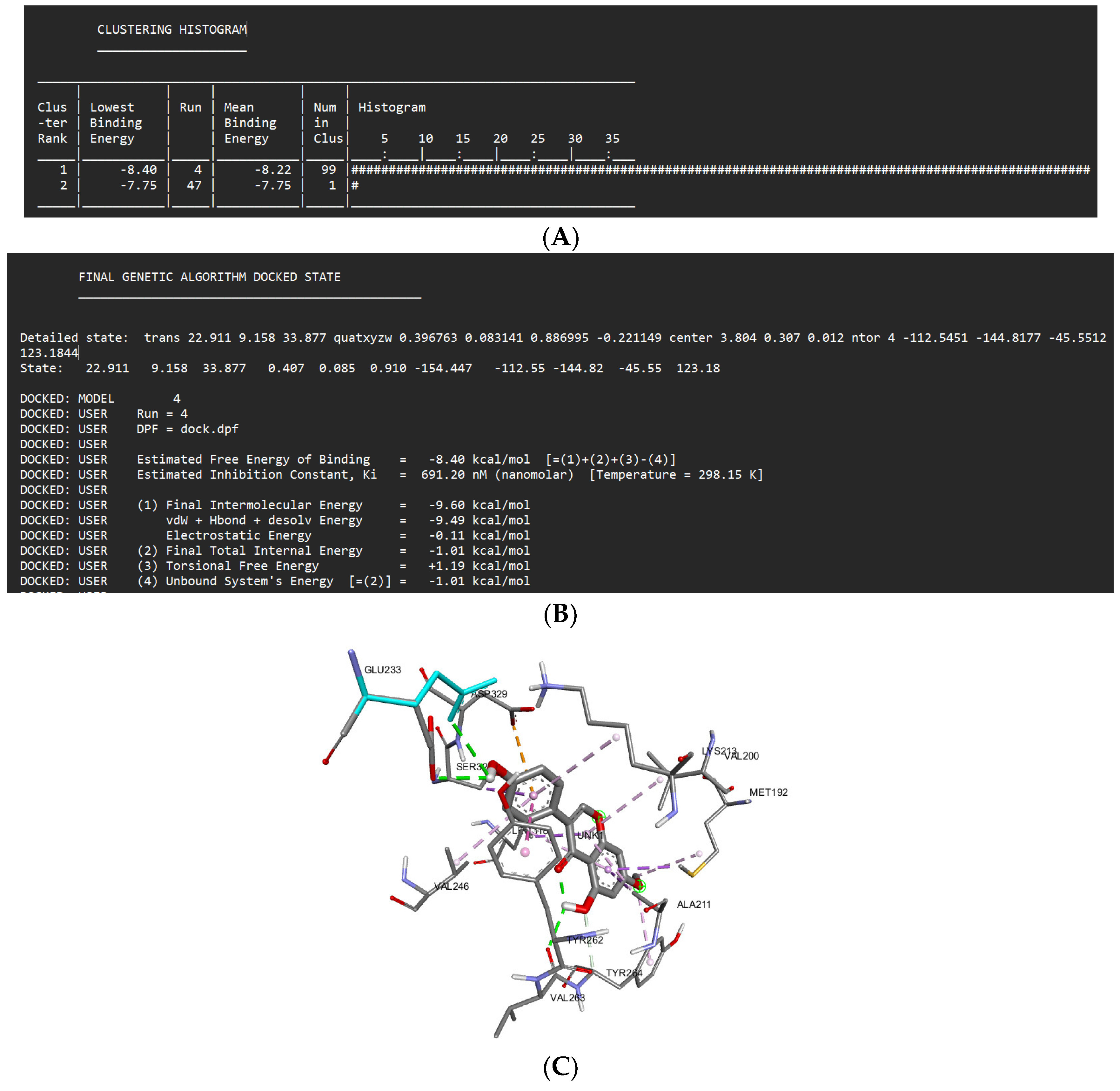
Docking Results of Prunetin with IL-1R
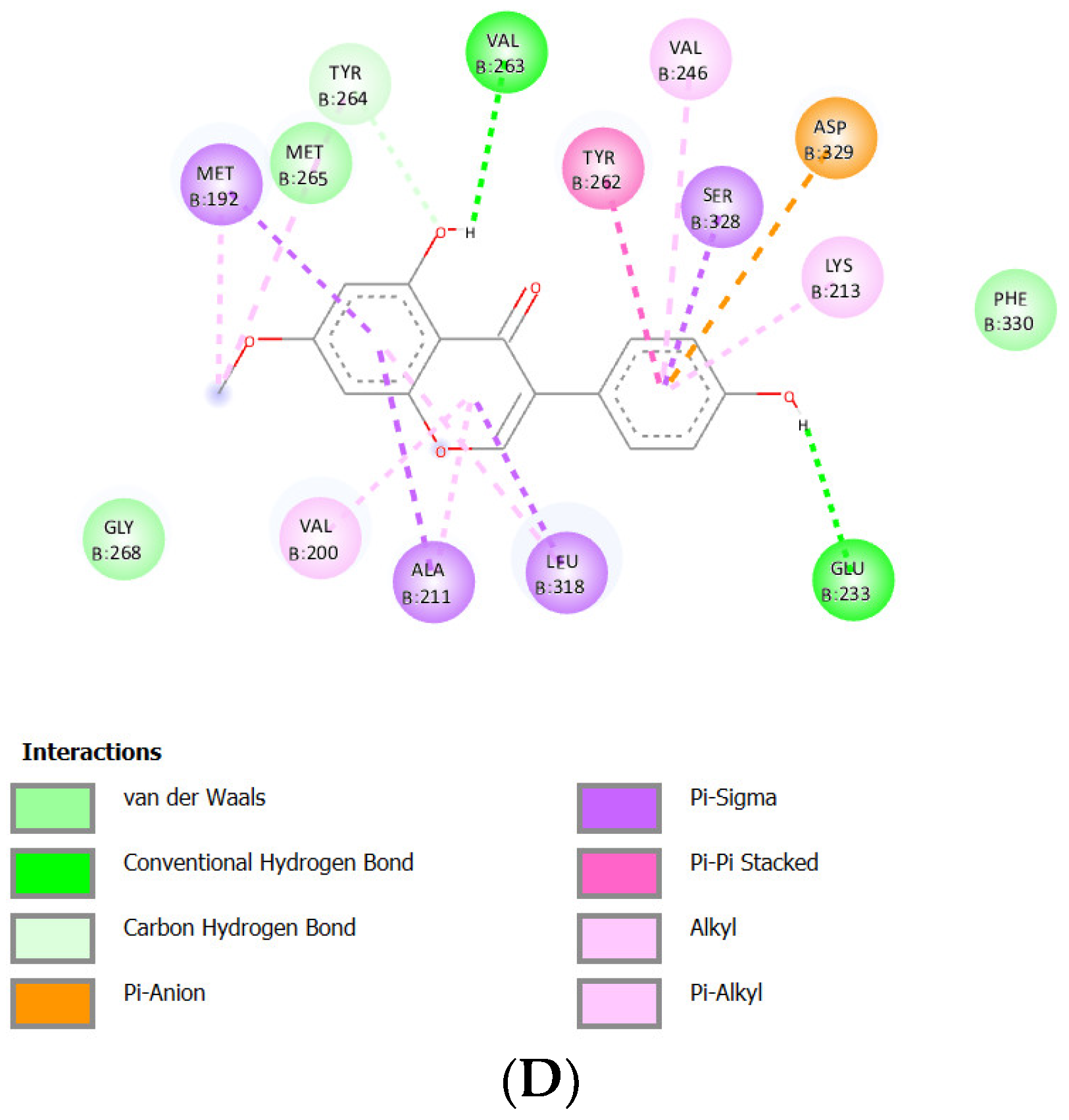
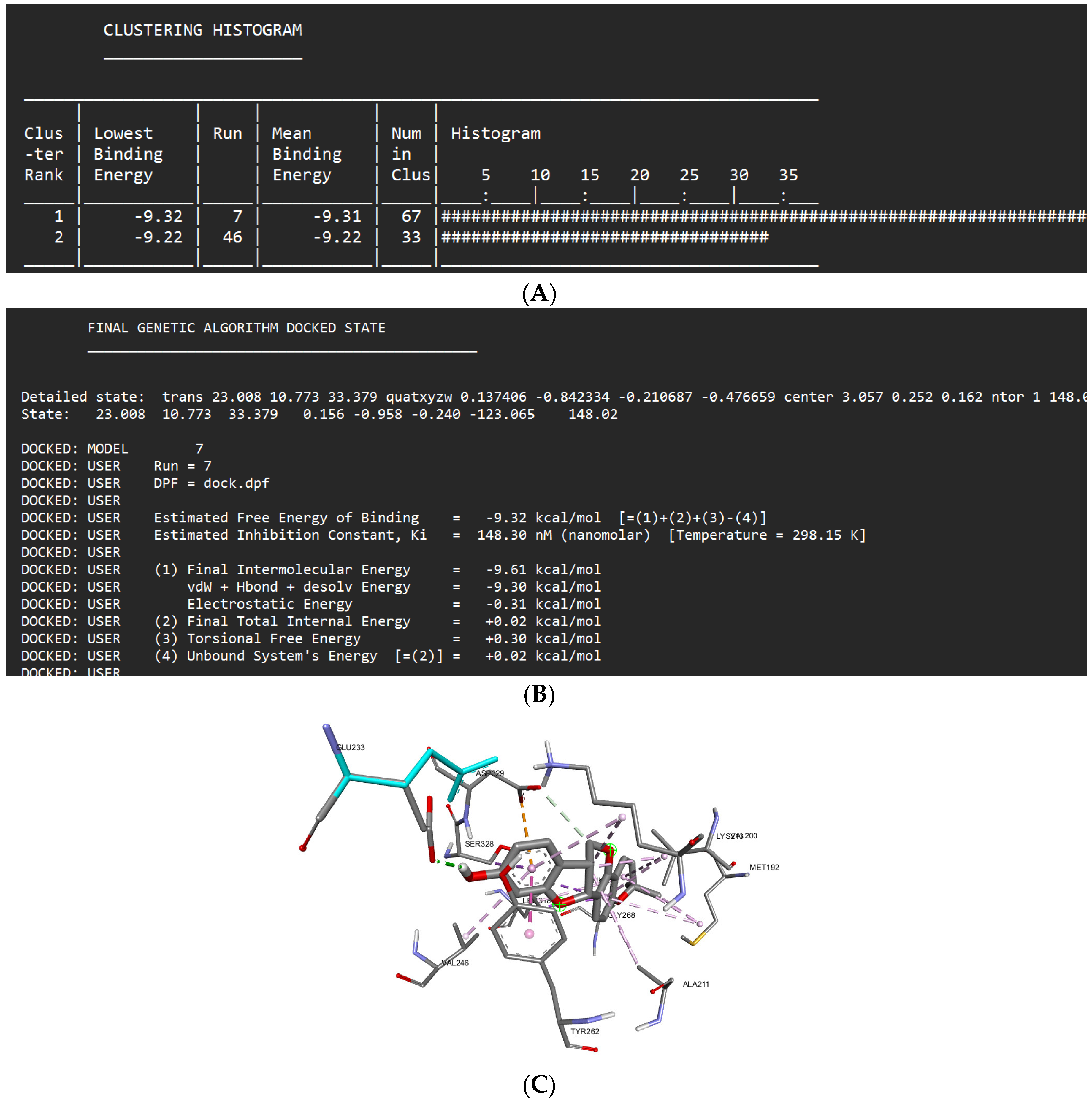
Docking Results of Shinpterocarpin with IL-1R
Docking Results of Licochalcone A with IL-1R
Docking Results of Glabridin Withil-1R
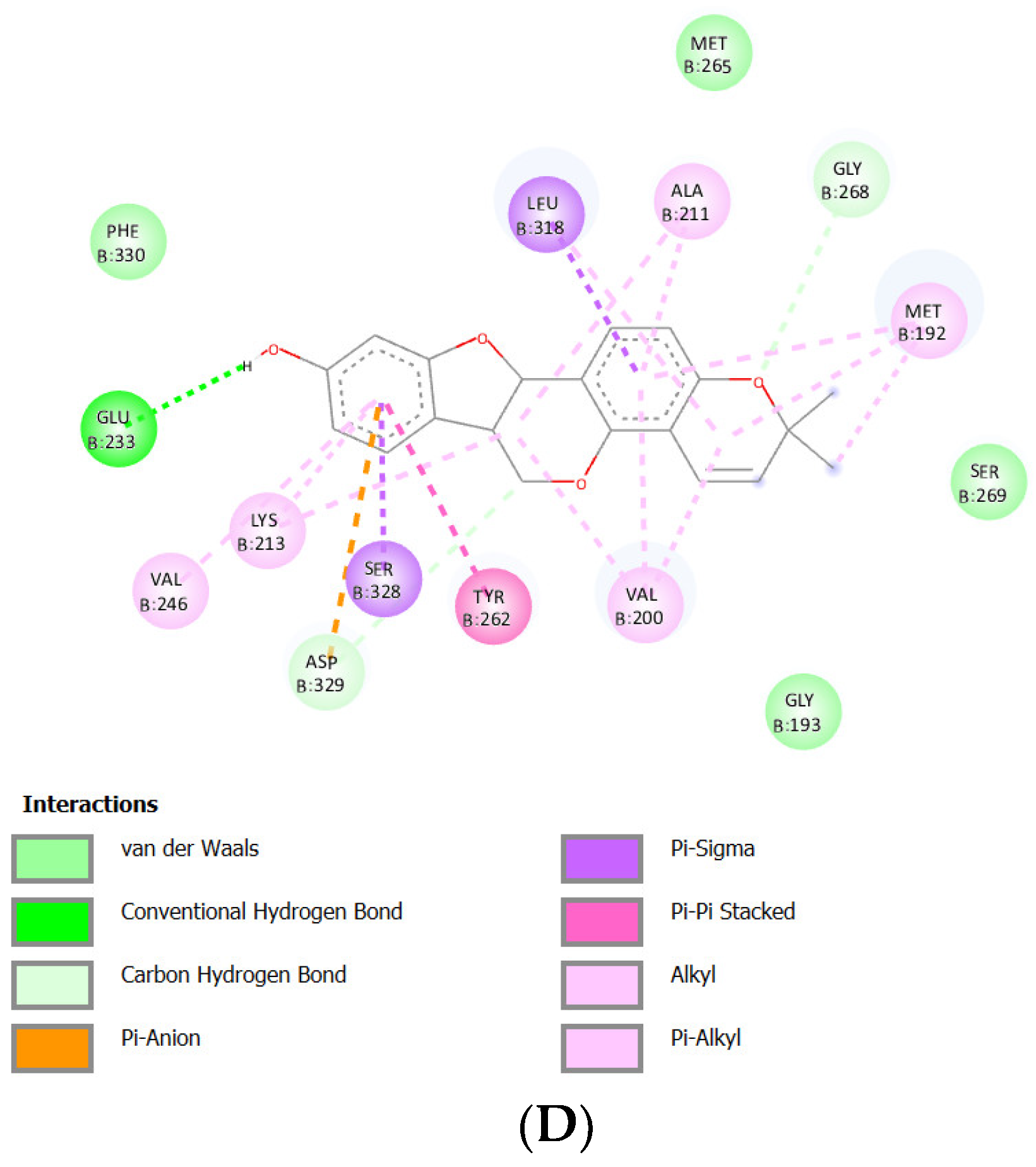
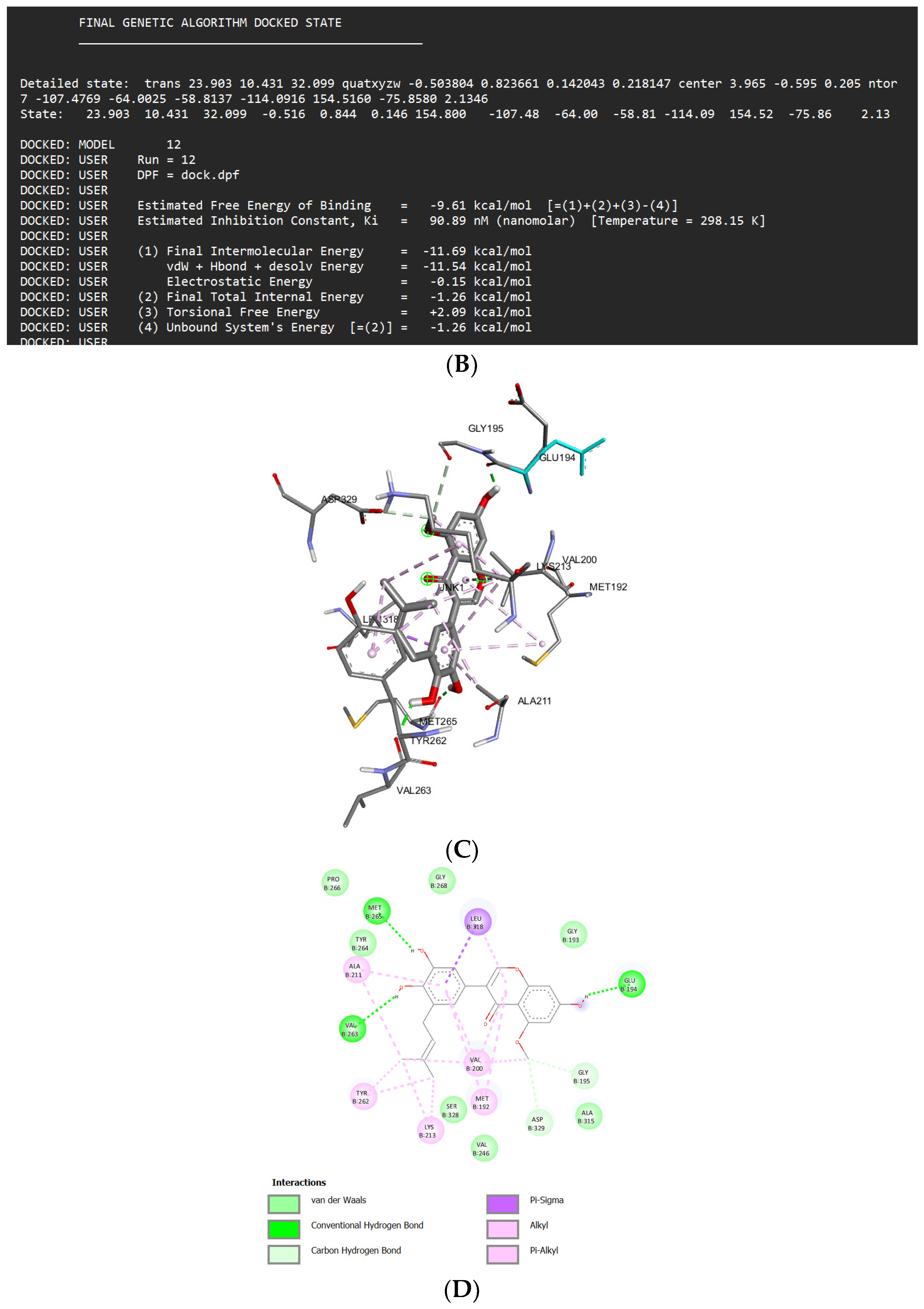
Docking Results of Glisoflavone with IL-1R
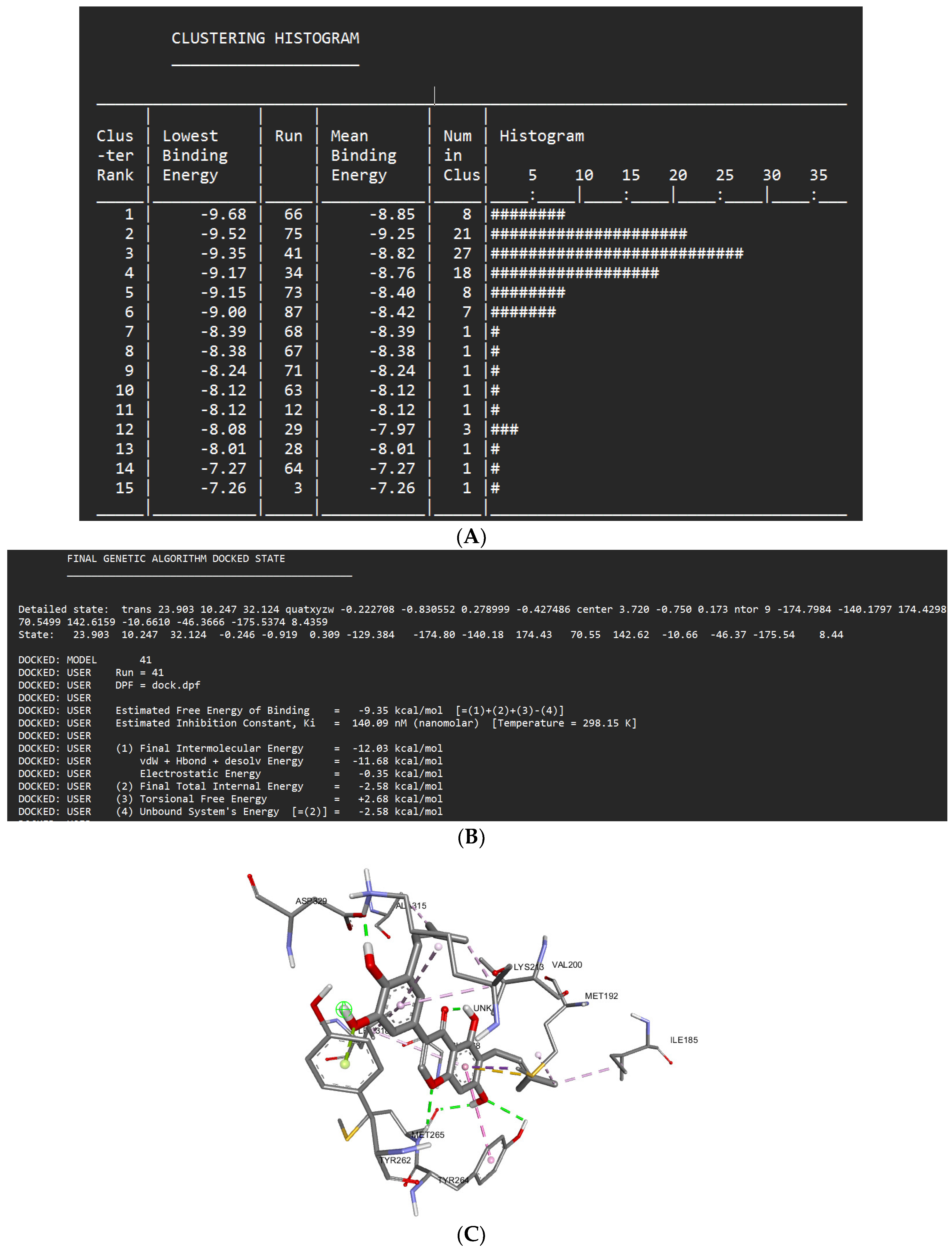
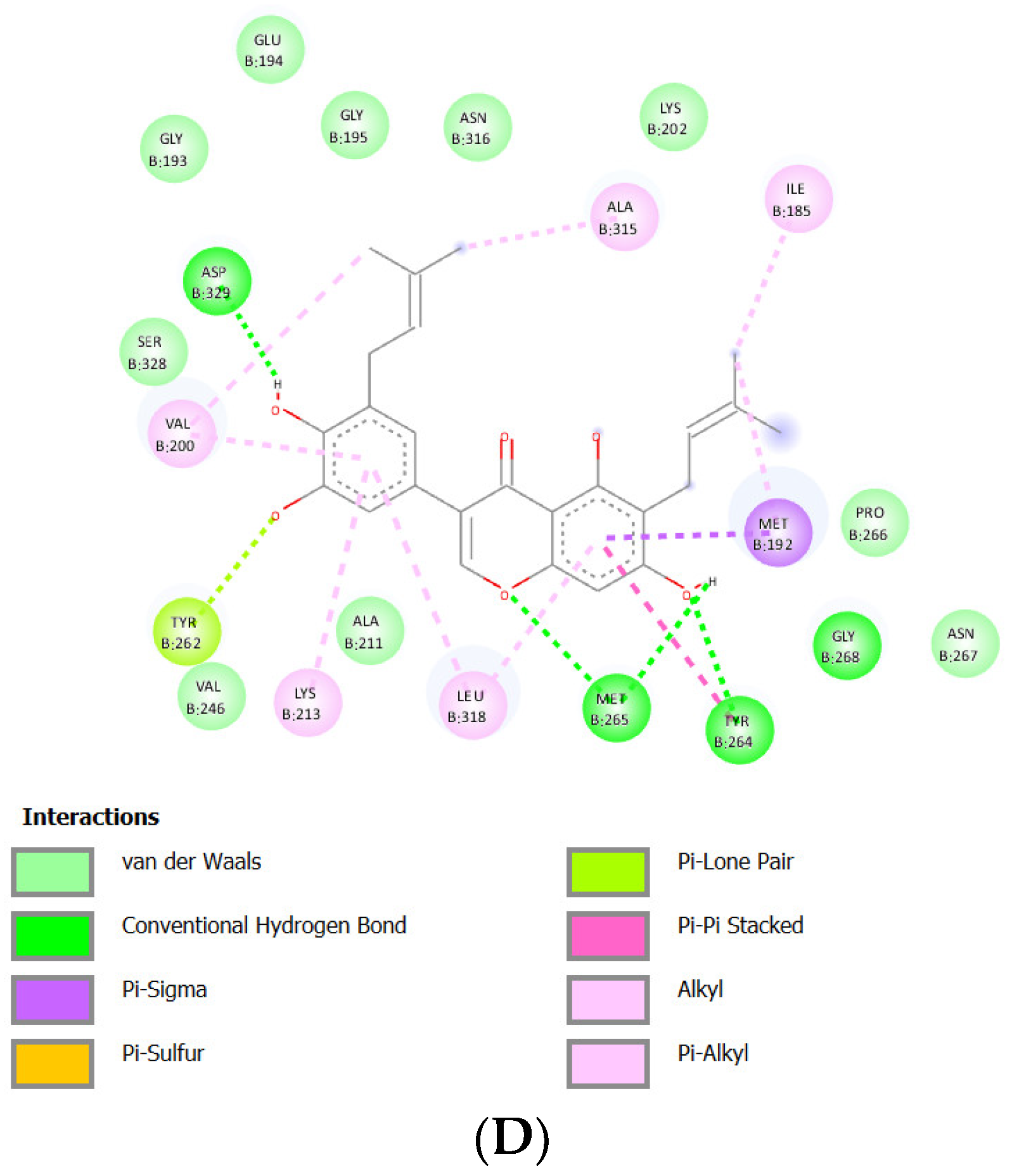
Docking Results of Isoangustone A with IL-1R
Binding Affinity and Molecular Interaction
Conclusions
- Protein Data Bank Website (http://www.rscb.org) with PDBID: 6MOM
-
PubChem Website (https://pubchem.ncbi.nlm.nih.gov/) with PubChem CID:
- ○
- Isoliquiritigenin : 638278
- ○
- Glyzaglabrin : 5317777
- ○
- Prunetin : 5281804
- ○
- Shinpterocarpin : 10336244
- ○
- Licochalcone A : 5318998
- ○
- Glabridin : 124052
- ○
- Glisoflavone : 5487298
- ○
- Isoangustone A : 21591148
References
- Petersen, P.E.; Baehni, P.C. Periodontal health and global public health. Periodontology 2000 2012, 60, 7–14. [CrossRef]
- World Health Organization. Oral Health: Periodontal (Gum) Disease. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 15 September 2023).
- Riskesdas. Laporan Nasional Riskesdas. 2018. Available online: https://dinkes.acehprov.go.id/l-content/uploads/riskesda_2018_nasional.pdf translate (accessed on 15 September 2023).
- Khan, S.F.; Shetty, B.; Fazal, I.; Khan, A.M.; Mir, F.M.; Moothedath, M.; et al. Licorice as A Herbal Extract in Periodontal Therapy. Drug Target Insights 2023, 17, 70–77. [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [CrossRef]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A Host-Mediated Disruption of Microbial Homeostasis. Unlearning Learned Concepts. Periodontol 2000, 2013, 62, 203–217. [CrossRef]
- Mehrotra, N.; Singh, S. Periodontitis 2023.
- Schmidlin, P.R.; Dehghannejad, M.; Fakheran, O. Interleukin-35 Pathobiology in Periodontal Disease: A Systematic Scoping Review. BMC Oral Health. 2021, 21, 139. [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β Is A Potential Therapeutic Target for Periodontitis: A Narrative Review. Int. J. Oral Sci. 2020, 12, 2. [CrossRef]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The Anti-Inflammatory Activity of Licorice, A Widely Used Chinese Herb. Pharm. Biol. 2017, 55, 5–18. [CrossRef]
- Ahn, S.J.; Cho, E.J.; Kim, H.J.; Park, S.N.; Lim, Y.K.; Kook, J.K. The Antimicrobial Effects of Deglycyrrhizinated Licorice Root Extract on Streptococcus Mutans UA159 in Both Planktonic and Biofilm Cultures. Anaerobe 2012, 18, 590–596. [CrossRef]
- Ladke, V.S.; Kumbhar, G.M.; Joshi, K.; Kheur, S. Systemic Explanation of Glycyrrhiza Glabra’s Analyzed Compounds and Anti-Cancer Mechanism Based on Network Pharmacology in Oral Cancer. J. Oral Biosci. 2022, 64, 452–460. [CrossRef]
- Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG; et al. Important Flavonoids and Their Role as A Therapeutic Agent. Molecules 2020, 25, 5243. [CrossRef]
- Leite CDos, S.; Bonafé, G.A.; Carvalho Santos, J.; Martinez, C.A.R.; Ortega, M.M.; Ribeiro, M.L. The Anti-Inflammatory Properties of Licorice (Glycyrrhiza Glabra)-Derived Compounds in Intestinal Disorders. Int. J. Mol. Sci. 2022, 23, 4121. [CrossRef]
- CarolineML; Muthukumar, R.S.; AHH; NN Anticancer Effect of Plectranthus Amboinicus and Glycyrrhiza Glabra on Oral Cancer Cell Line: An In vitro Experimental Study. Asian Pac. J. Cancer Prev. 2023, 24, 881–887. [CrossRef]
- Wahab S, Annadurai S, Abullais SS, Das G, Ahmad W, Ahmad MF; et al. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; M Abdel-Daim M, Prasad Devkota H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza Glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [CrossRef]
- Sharma, V.; Katiyar, A.; Agrawal, R.C. Glycyrrhiza Glabra: Chemistry and Pharmacological Activity; 2018.; pp. 87–100. [CrossRef]
- Tang ZH, Chen X, Wang ZY, Chai K, Wang YF, Xu XH; et al. Induction of C/EBP Homologous Protein-Mediated Apoptosis and Autophagy by Licochalcone A in Non-Small Cell Lung Cancer Cells. Sci. Rep. 2016, 6, 26241. [CrossRef]
- Wang J, Zhang YS, Thakur K, Hussain SS, Zhang JG, Xiao GR; et al. Licochalcone A From Licorice Root, An Inhibitor of Human Hepatoma Cell Growth Via Induction of Cell Apoptosis and Cell Cycle Arrest. Food Chem. Toxicol. 2018, 120, 407–417. [CrossRef]
- Bortolotto LFB, Barbosa FR, Silva G, Bitencourt TA, Beleboni RO, Baek SJ; et al. Cytotoxicity of Trans-Chalcone and Licochalcone A Against Breast Cancer Cells Is Due to Apoptosis Induction and Cell Cycle Arrest.Biomed. Pharmacother. 2017, 85, 425–433. [CrossRef]
- Qiu C, Zhang T, Zhang W, Zhou L, Yu B, Wang W; et al. Licochalcone A Inhibits the Proliferation of Human Lung Cancer Cell Lines A549 and H460 By Inducing G2/M Cell Cycle Arrest and ER Stress. Int. J. Mol. Sci. 2017, 18, 1761. [CrossRef]
- Lu WJ, Wu GJ, Chen RJ, Chang CC, Lien LM, Chiu CC; et al. Licochalcone A Attenuates Glioma Cell Growth In Vitro and In Vivo Through Cell Cycle Arrest. Food Funct. 2018, 9, 4500–4507. [CrossRef]
- Lin, X.; Tian, L.; Wang, L.; Li, W.; Xu, Q.; Xiao, X. Antitumor Effects and the Underlying Mechanism of Licochalcone A Combined with 5-Fluorouracil in Gastric Cancer Cells. Oncol. Lett. 2017, 13, 1695–1701. [CrossRef]
- Fu Y, Hsieh T Chen, Guo J, Kunicki J, Lee MYWT, Darzynkiewicz Z; et al. Licochalcone-A, A Novel Flavonoid Isolated from Licorice Root (Glycyrrhiza Glabra), Causes G2 and Late-G1 Arrests in Androgen-Independent PC-3 Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [CrossRef]
- Chen R, Wang M, Liu Q, Wu J, Huang W, Li X; et al. Sequential Treatment with At19 Cells Generates Memory CAR-T Cells and Prolongs the Lifespan of Raji-B-NDG Mice. Cancer Lett. 2020, 469, 162–172. [CrossRef]
- Dabke, A.; Ghosh, S.; Dabke, P.; Sawant, K.; Khopade, A. Revisiting the In-Vitro and In-Vivo Considerations for In-Silico Modelling of Complex Injectable Drug Products. J. Control. Release 2023, 360, 185–211. [CrossRef]
- Nesslany, F. The Current Limitations of in Vitro Genotoxicity Testing and Their Relevance to the in Vivo Situation. Food Chem. Toxicol. 2017, 106, 609–615. [CrossRef]
- Ekins S, Mestres J, Testa B.In SilicoPharmacology for Drug Discovery: Applications to Targets and Beyond. Br. J. Pharmacol. 2007, 152, 21–37. [CrossRef]
- Verma, S.K.; Deshmukh, R.; Joshi, N. Molecular Biology: Fundamentals and Applications. J. Cell Biochem. 2020, 122, 230–238.
- Guvench, O. Small-Molecule Docking as A Structural Biology Tool; 2020; pp. 57–86.
- Om Silakari, Pankaj Kumar Singh. Concepts and Experimental Protocols of Modelling and Informatics in Drug Design; Elsevier, 2021.
| Test ligand | Binding affinity (ΔG) |
Inhibition constant (Ki) |
Ligand – receptor bond | Run | |||
| Hydrogen bond | Van der Waals | Hydrophobic bond (Pi) | Others | ||||
| Isoliquiritigenin | -8.32 kcal/mol | 799.16 nM |
Met265 Val263 Glu194 Asp329 Gly195 |
Pro266 Gly268 Gly193 Tyr264 Ser328 Val246 Ala315 |
Leu318 (sigma) Ala211 (alkyl) Tyr263 (alkyl) Lys213 (alkyl) Val200 (alkyl) Met192 (alkyl) |
- | 53 |
| Glyzaglabrin | -8.96 kcal/mol | 270.86 nM |
Glu233 Lys213 |
Gly268 Val263 Val200 Phe330 Leu260 |
Asp329 (anion) Leu318 (sigma) Ser328 (sigma) Tyr262 (Pi stacked) Met192 (alkyl) Met256 (alkyl) Tyr264 (alkyl) Ala211 (alkyl) Val246 (alkyl) |
- | 62 |
| Prunetin | -8.40 kcal/mol | 691.20 nM |
Val263 Glu233 Tyr264 |
Met265 Gly268 Phe330 |
Asp329 (anion) Met192 (sigma) Ala211 (sigma) Leu318 (sigma) Ser328 (sigma) Tyr262 (Pi stacked) Val200 (alkyl) Val246 (alkyl) Lys213 (alkyl) |
- | 4 |
| Shinpterocarpin | -9.32 kcal/mol | 148.30 nM |
Glu233 Asp329 Gly268 |
Phe330 Met265 Gly193 Ser269 |
Asp329 (anion) Ser328 (sigma) Leu318 (sigma) Tyr262 (Pi stacked) Val246 (alkyl) Lys213 (alkyl) Val200 (alkyl) Ala211 (alkyl) Met192 (alkyl) |
- | 7 |
| Licochalcone A | -9.08 kcal/mol | 219.85 nM |
Tyr262 Glu233 |
Glu194 Gly193 Ser269 Leu319 Phe330 |
Asp329 (anion) Ser328 (sigma) Leu318 (sigma) Tyr262 (Pi stacked) Val200 (alkyl) Met265 (alkyl) Ala211 (alkyl) Met192 (alkyl) Tyr264 (alkyl) Val246 (alkyl) Lys213 (alkyl) |
- | 38 |
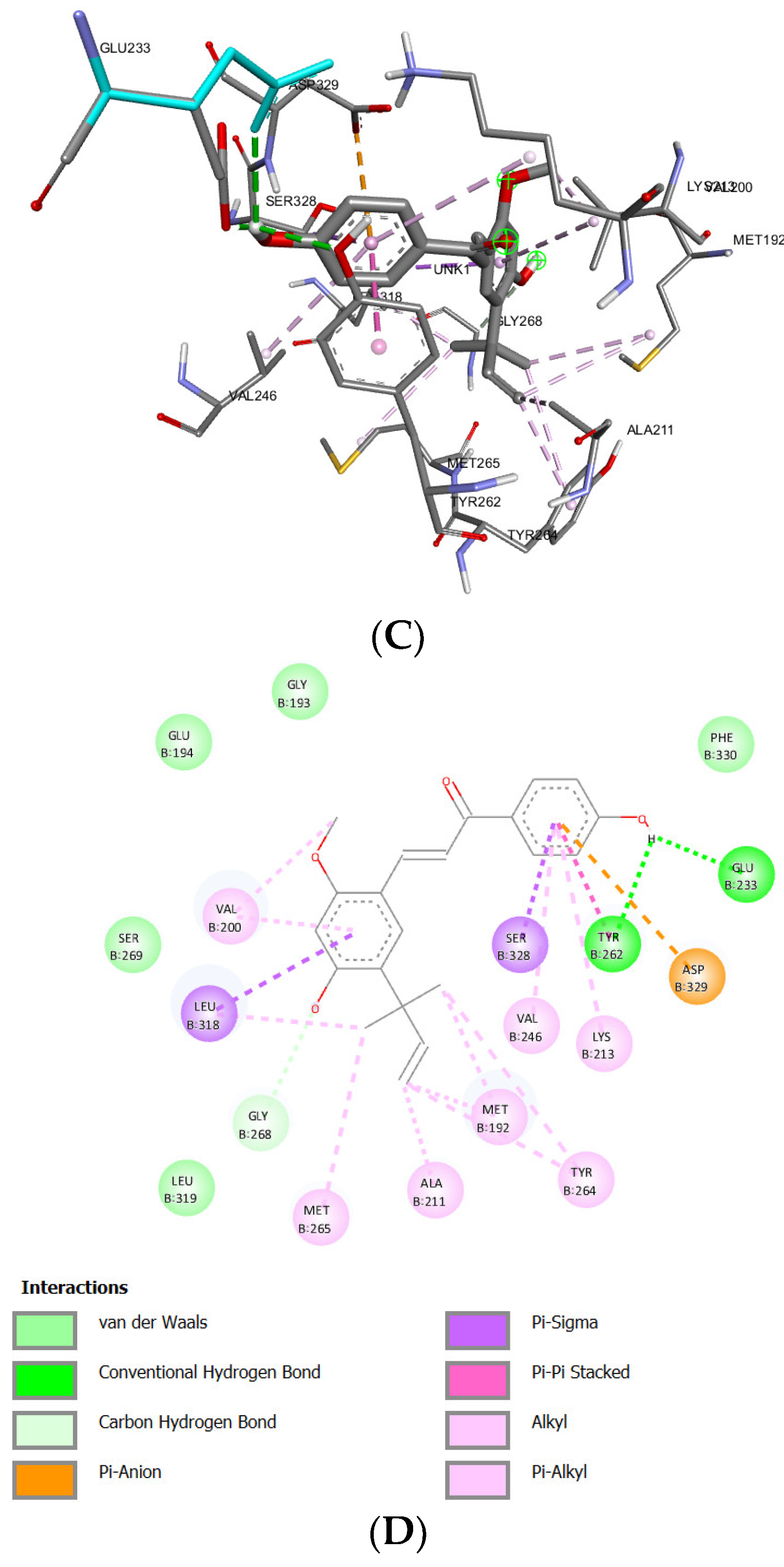
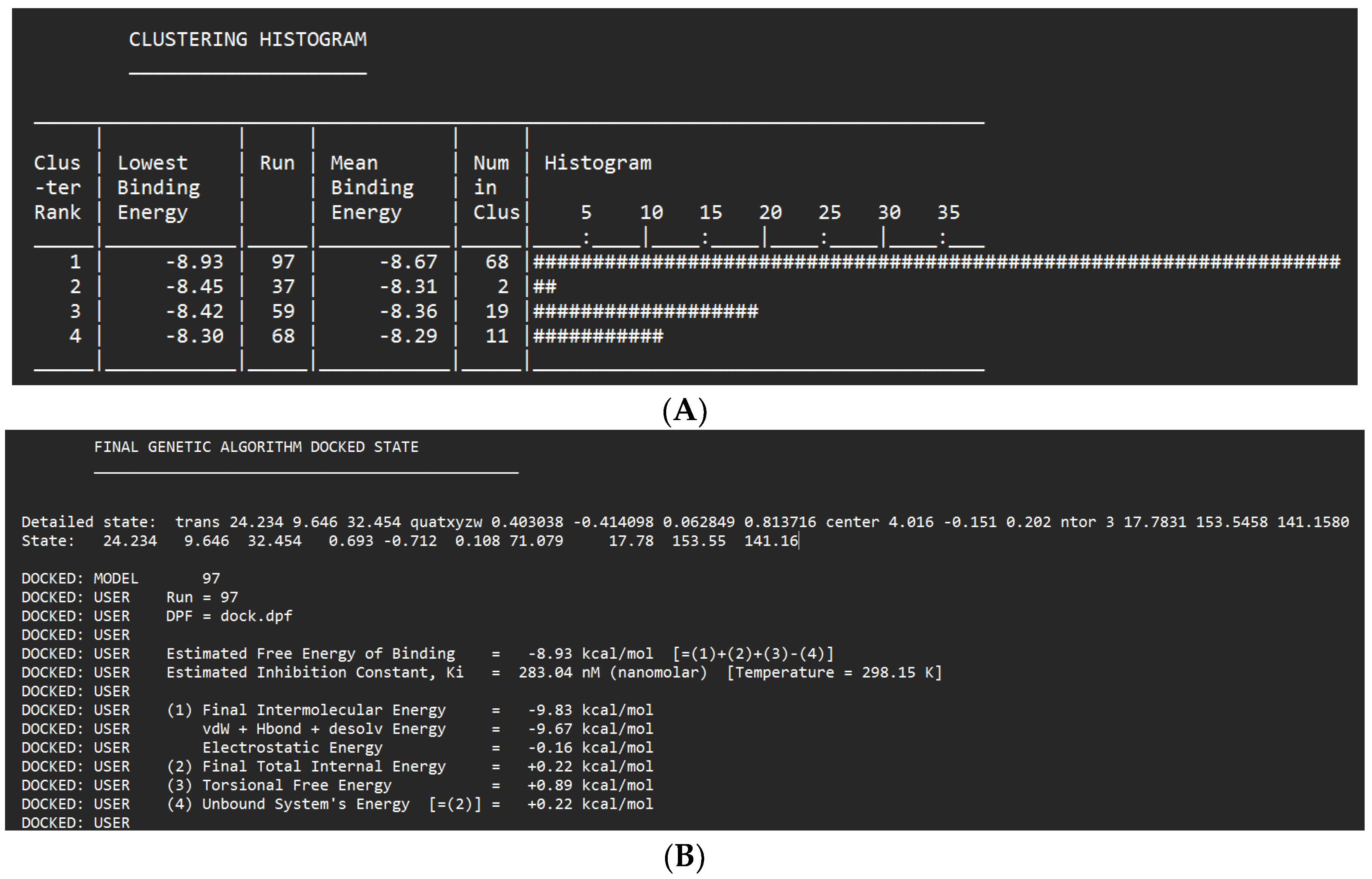
| Glabridin | -8.93 kcal/mol | 283.04 nM | Asp329 |
Phe330 Met265 Ser328 Asn316 Ala315 Gly268 Pro266 Ser269 |
Met192 (sigma) Tyr262 (t-shaped) Lys213 (alkyl) Val200 (alkyl) Leu318 (alkyl) Ala211 (alkyl) Tyr264 (alkyl) |
- | 97 |
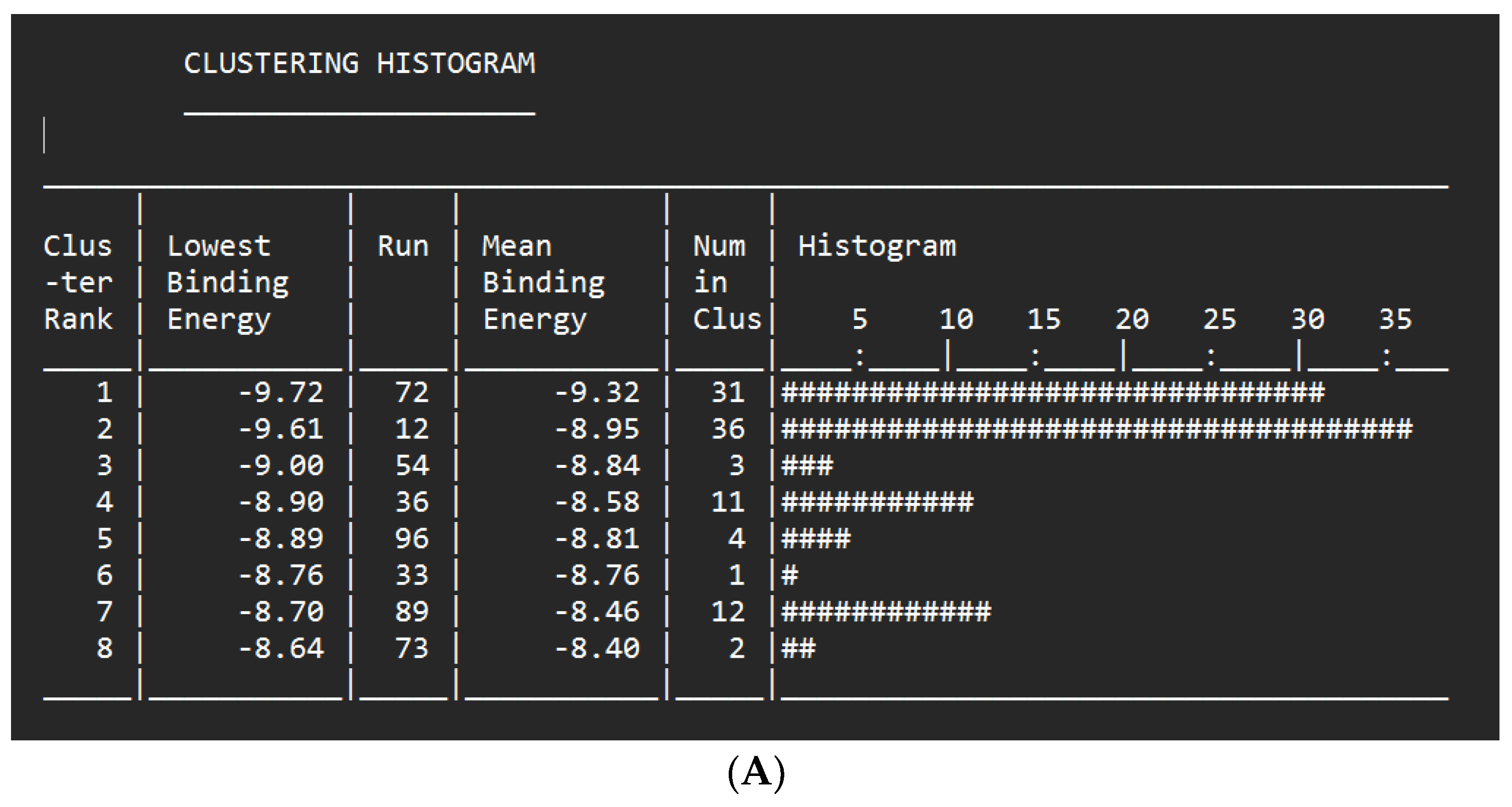
| Glisoflavone | -9.61 kcal/mol | 90.89 nM |
Met265 Val263 Glu194 Asp329 Gly195 |
Pro266 Gly268 Ser328 Val246 Gly193 Ala315 Tyr264 |
Leu318 (sigma) Ala211 (alkyl) Tyr262 (alkyl) Lys213 (alkyl) Val200 (alkyl) Met192 (alkyl) |
- | 41 |
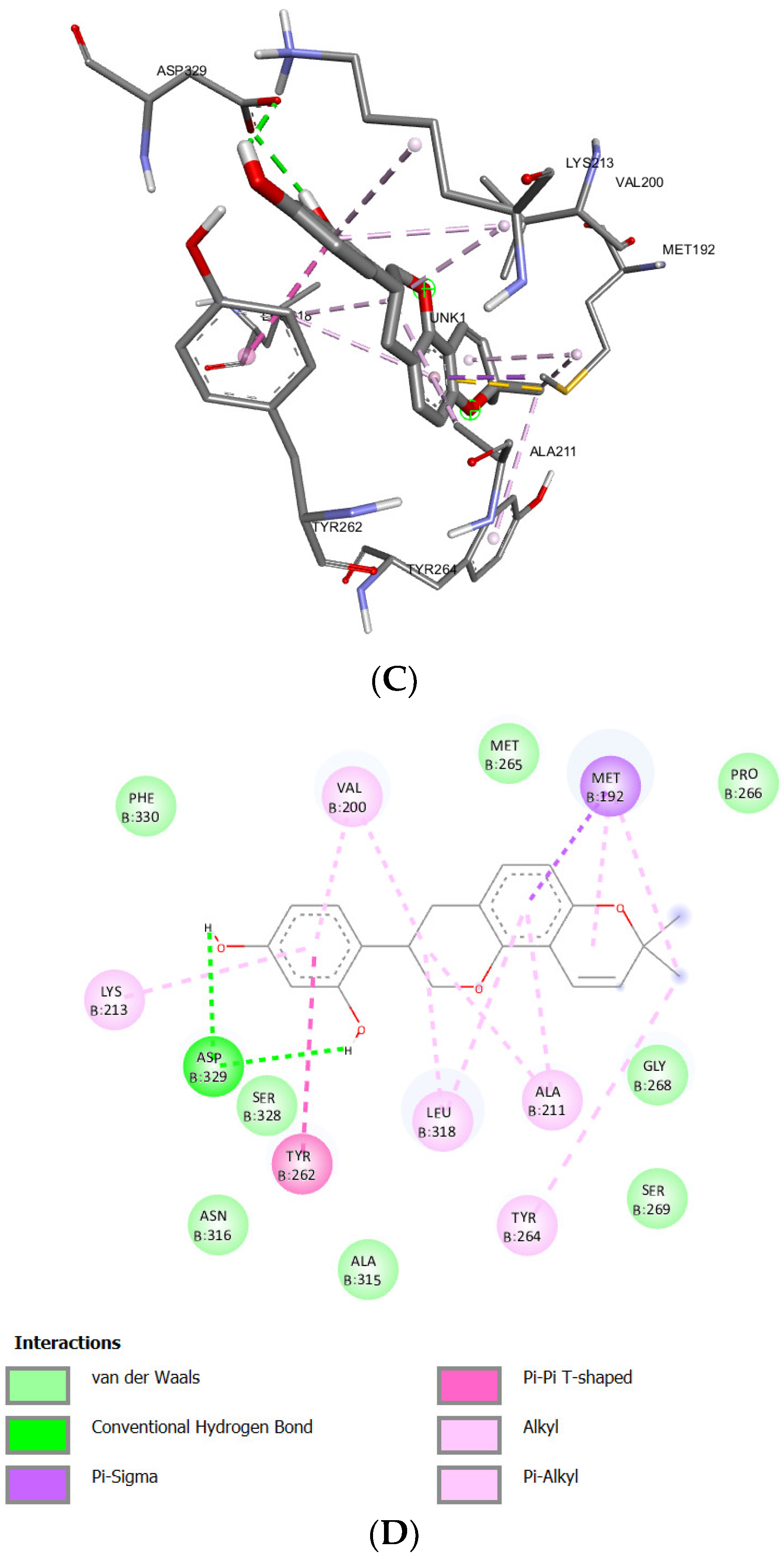
| Isoangustone A | -9.35 kcal/mol | 140.09 nM |
Asp329 Met265 Tyr264 Gly268 |
Glu194 Gly193 Gly195 Asn316 Val246 Ala211 Ser328 Lys202 Pro266 Asn267 |
Met192 (sigma) Tyr262 (lone pair) Tyr264 (Pi stacked) Val200 (alkyl) Lys213 (alkyl) Leu318 (alkyl) Ala315 (alkyl) Ile185 (alkyl) Met192 (alkyl) |
- | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





