1. Introduction
Many bacteria produce extracellular polysaccharides (EPS) that are involved in attachment. EPS enable cell-cell attachment, facilitating the formation of flocs (rafts of aggregated cells in aqueous environments) and biofilms through enhancing attachment to surfaces in soils or on plants and animals. In pathogenesis they may confer resistance to host defences [
1], thereby promoting intracellular survival [
2]. In non-pathogenic contexts, they may increase access to nutrients and protect against environmental stresses (e.g., desiccation and toxic agents) [
3,
4]. Some EPS are hydrophilic or gel forming polymers and are loosely adherent to the bacterial surface forming a slime layer (e.g., xanthan from
Xanthomonas campestris and succinoglycan from
Rhizobia and
Agrobacteria species). Others form a discrete capsule, which overlies the cell surface but is not attached to it (e.g., hyaluronan from
Streptococcus species and
Pasteurella multocida [
5,
6]). In
Azotobacter vinelandii a calcium alginate gel forms a coat around the resting cyst stage [
7]. Some bacteria are known to produce several different EPSs simultaneously, such as
Agrobacterium tumefaciens, which can produce at least five [
8].
A few EPS are water-insoluble, notably the (1-4)-β-glucan, cellulose, which is produced by some species recoverable from soil, e.g.,
Gluconacetobacter xylinus [
9],
Agrobacterium tumefaciens [
8], species of
Pseudomonas,
Achromobacter,
Alcaligenes,
Rhizobium,
Azotobacter [
10], members of the Enterobacteriaceae [
11,
12], and as a sheath around filamentous cyanobacteria [
13].
Curdlan, a linear (1→3)-β-D-glucan, is another example of a water insoluble EPS. This polysaccharide is produced by various
Agrobacterium species [
14], some Rhizobium isolates [
6], the Gram-positive
Cellulomonas flavigena [
15,
16] and an isolate of
Rahnella variigena, a member of the Enterobacteriaceae family [
17]. Interestingly, in all cases cited above, curdlan production in the laboratory requires N-limited culture medium with ample carbohydrate, which fits with the general observation that the production of EPS by bacteria is frequently induced by nutritional stress e.g., N, P or S limitation [
18,
19,
20,
21,
22]. It is nonetheless remarkable that the capacity for curdlan production is not restricted to a single Class or even a Phylum (e.g., Gram negative), but rather to a handful of bacteria that seem related only by their soil habitat. So far, curdlan production has only been detected in rhizosphere-dwelling bacteria, and is in every case regulated by N-limitation.
The biological role of curdlan has not been demonstrated, but it has been suggested to help form a biofilm that protects against predation, starvation and desiccation [
8]. Such a role is consistent with microscopic observations of
C. flavigena [
23], a soil-dwelling, curdlan-producing bacterium that colonizes and decomposes plant material using cellulose as a source of carbohydrate. However, little information is available on the form of nascent curdlan, its deposition, and the ongoing nature of its interaction with the cell surface. Curdlan is occasionally referred to in the literature as a capsule, although clear evidence for a capsular form has not been provided. Curdlan-producing Agrobacterium strains grown on glucose-rich yeast extract agar medium produce a coherent, aniline blue-staining, pellicle which may be stripped from areas of confluent bacterial growth [
14], giving the appearance of curdlan deposition as a disorganised mass at the colony surface. However, such an appearance does not rule out the possibility of a capsular form. One possibility is that curdlan is produced as a discrete capsule by some cells located strictly in the periphery of a biofilm. This scenario takes into account the strong dependency of curdlan production on oxygen availability [
24]. Peripheral cells would in this way have the role of providing curdlan as a protecting layer for the whole population. Alternatively, it is possible that upon production, curdlan helps to form a biofilm which engulfs the colony and forms a nebulous matrix of a biofilm without any discrete individual cell encapsulation. In my view, only the former scenario would justify the formal designation of curdlan as a capsule according to the literature [
25]. As one of the goals to this study, I wanted to investigate curdlan production by Agrobacterium species, in particular to see whether curdlan was also deposited as a discrete capsule on this bacterium.
A single molecule of curdlan can be quite large, consisting of up to 12 000 polymerized glucose residues [
26]. Its size, as well as the unique, unbranched (1→3)-β-D-glucan structure confers upon curdlan its unique gelling and high water-retention properties. There have been many reviews of the large body of literature covering the chemistry and physico-chemistry of curdlan, its pharmacological and immunomodulation activities and its commercial applications [
26,
27,
28,
29,
30,
31,
32]. Curdlan is widely used, for example, as an additive to cement (super-workable concrete) for the construction industry, an additive to tablet formulations in the pharmaceutical industry, as well as an antiviral agent in immunotherapy and numerous applications in tissue engineering, drug delivery, and other biomedical applications. Moreover, curdlan is one of the most desirable edible polysaccharides because it lacks any unpleasant taste and because of its versatile applications as a thickener and healthy fiber in the food industry. Unfortunately, the industrial use of curdlan is limited by its high cost of production. One way to reduce the cost of production is to develop higher curdlan-yielding strains through genetic engineering. Reducing the cost of production was another goal of this study.
The genetic basis of curdlan production has been investigated using Agrobacterium sp. strain ATCC31749. Earlier studies found that the essential genes include a biosynthetic gene cluster,
crdASC, and
crdR, which codes for a transcription regulator [
33,
34]. CrdS shows some similarity to beta-glycoxyl transferases with repetitive action patterns [
34]. The extremely hydrophobic CrdS protein is thought to form a multimeric (possibly an octamer) complex located in the inner membrane. This location is stabilized by seven transmembrane helices and a central large hydrophilic cytoplasmic region which contains the substrate-binding and catalytic motif.
The
crdASC genes form a gene cluster (i.e., all transcribed in the same direction) within 5 kb of the linear chromosome of Agrobacterium. Upstream of the first gene (
crdA) in the
crd cluster is a strong promoter, referred to as
crdP [
35] or Pcrd. It was not known whether Pcrd is the only promoter for the
crdASC cluster. Transposon insertions into any of the genes
crdA,
crdS, or
crdC result in the inability to produce curdlan. Loss of curdlan production by the insertion disruption of
crdA is interesting because of the implication that either
crdA is essential for curdlan production and/or because of the polar effect of disruption of transcription of the downstream gene
crdS. The functions of the protein products of the genes
crdA and
crdC are not known.
The gene
pssAG, coding for a phosphatidylserine synthase, also affects curdlan yield [
33]. It is likely that specific phospholipids are important for the activity of the synthase complex in the inner membrane of this Gram negative bacterium [
36]. In addition, curdlan production appears to be strictly governed by complex gene expression regulation [
26,
35,
37]. Upon encountering the environmental triggers for curdlan production (e.g., low nitrogen and abundant sugars) the transcription of the
crdASC genes increases by almost 100-fold, and this depended upon phosphate accumulation and the stringent response [
37]. Considering that curdlan is a polymer of glucose that is destined for the cell exterior, it seems reasonable that the expression of genes that incorporates this high-energy molecule into an exopolysaccharide should be highly regulated. So far, the only regulatory mechanisms governing curdlan production that have been reported operate at the level of transcription control. This is in contrast to another exopolysaccharide produced by Agrobacterium, succinoglycan, which does not appear to be regulated at the level of transcription [
8].
Curdlan has been intensively studied to increase production and lower costs during fermentation [
24,
35,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48]. Two of the major considerations for improving production and lowering the costs are the complex native regulation controlling production and the specific nutritional conditions required during fermentation. These two considerations are related, since the specific conditions required during fermentation drive up the cost of production. Generally, the native regulation serves to restrain curdlan production until certain specific conditions are met. These include low nitrogen, high carbon sources, generous phosphate levels, and high aeration [
26]. Some strains of Agrobacterium, e.g., the well-known plant pathogen
A. tumefaciens C58 that is used in plant genetic manipulations, do not produce detectable levels of curdlan [
8], even though they carry the necessary genes (
crdASC and
crdR). Other strains, such as Agrobacterium sp. strain ATCC31749, have ‘suffered’ from spontaneous mutations that appear to have partially relaxed the regulatory control over curdlan production, leading to high yields [
6,
19].
Various genetic modifications of strain ATCC31749 have generally resulted in not more than 10-50% improvement [
35,
44,
46,
49]. For example, Kim et al. [
49] reported a mutant strain that was able to produce 76 g/l curdlan in a stirred tank reactor after 120 hours of cultivation, while the parent strain produced 64 g/l under comparable conditions. One possible explanation was that a greater production rate of curdlan is not possible, i.e., that the production rate is limited by cellular metabolism capacities, uptake of carbon source, or lifetime of the bacterial cell. In that case, no amount of genetic engineering could greatly improve the yield. However, another possibility (tested in this study) was that a constitutive transcription of the
crdASC genes might yield higher levels of curdlan – an approach that to my knowledge has not been attempted. To state the hypothesis succinctly, if the native promoter Pcrd that controls the expression of the entire
crdASC gene cluster were to be replaced with a stronger, unregulated promoter, this might lead to a higher curdlan yield, given that curdlan production is highly dependent upon transcription control. Related to the question of whether increased transcription of the
crdASC genes would increase production is whether a stronger synthetic promoter would relax the requirement for specific conditions (e.g., high oxygen, low nitrogen) and support high curdlan production independently of the nutritional conditions.
The key to engineering a higher transcription rate for the
crdASC gene cluster is the knowledge of suitable promoters capable of strong transcription in Agrobacterium. During investigations on transcription regulation in the Alphaproteobacteria, I found a strong promoter named promoter PphaP (promoter of gene
phaP) from the Alphaproteobacterium
Rhodobacter sphaeroides [
50,
51]. I decided to test whether curdlan production could be improved using PphaP in the industrially used strain Agrobacterium sp. ATTC31749 and the ‘wild type’
A. tumefaciens strain C58. DNA sequence alignments using blastn from NCBI on the
crdASC gene clusters from each of the strains reveals 98.72% nucleotide identity. This analysis was based on 5.3 kb which includes the
crdASC genes and the 300 bp promoter region, using C58 (in NCBI:
Agrobacterium fabrum str. C58) and 31749 (in NCBI:
Agrobacterium sp. CGMCC 11546). The key conclusion of this comparison is that both 31749 and C58 carry a
crdASC gene cluster. (However, C58 does not produce quantifiable curdlan levels under standard laboratory conditions.) Therefore, I wanted to see if the insertion of PphaP into the chromosome of each Agrobacterium, replacing the native
crdASC promoter (Pcrd) and driving the expression of the
crdASC gene cluster, could increase curdlan production in each strain. A similar outcome in both strains could potentially lend greater weight to the conclusions.
2. Materials and Methods
Bacterial strains and plasmids. The strains used in this study were the curdlan producing Agrobacterium sp. strain ATCC 31749, whose genome has been sequenced [
52] and
Agrobacterium tumefaciens C58 [
53]. Mutants were generated using a chromosomally inserted suicide plasmid (pK18mob2) [
54]. For transferring plasmids into the Agrobacteria, the
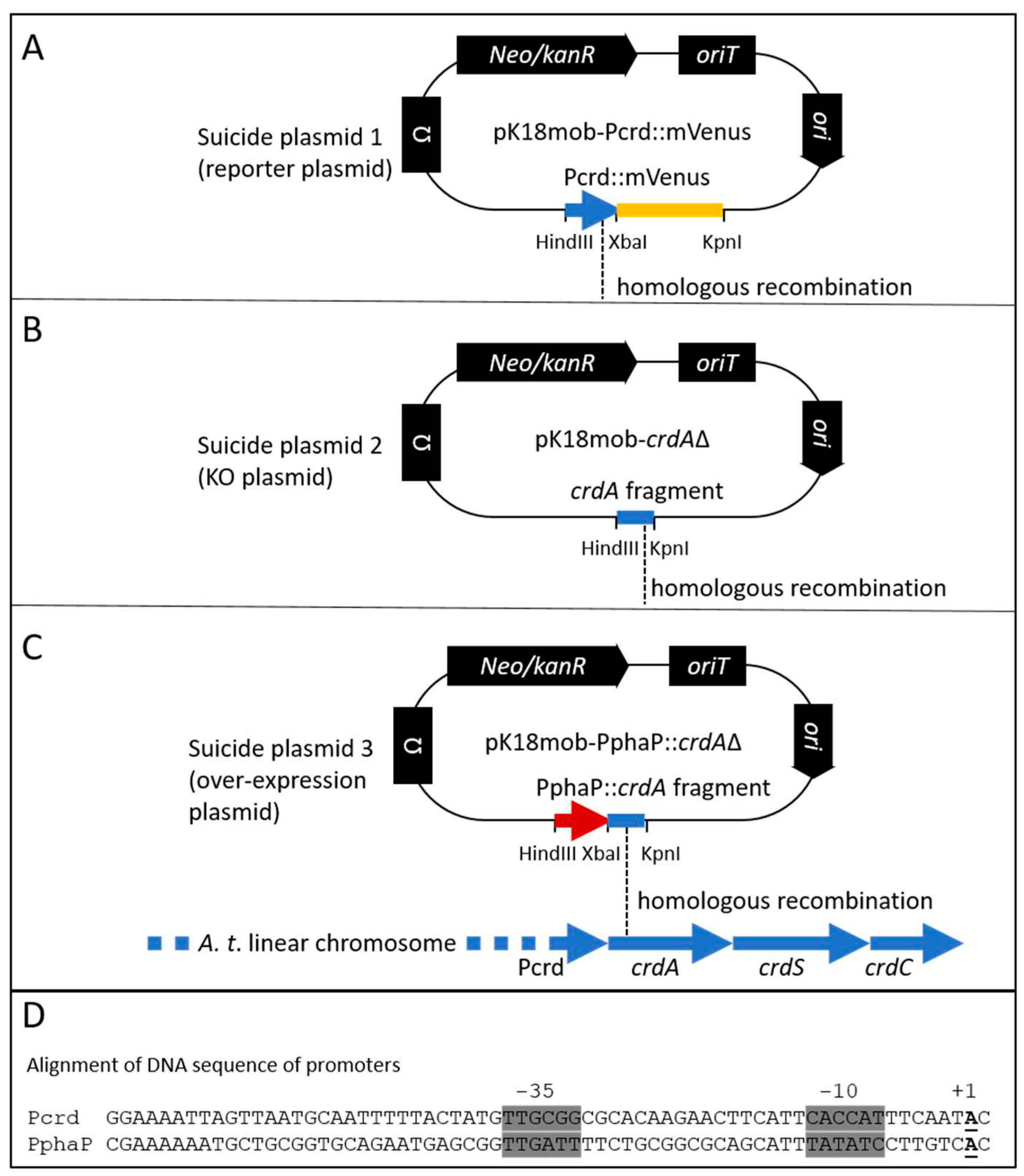
E. coli strain S17-1 was used for conjugation. Plasmid construction was performed using the restriction digest sites indicated in
Figure 1.
Upon homologous recombination with the chromosome, the suicide plasmid inserts an additional copy of the homologous fragment carried on the plasmid. For this approach, I used different fragments from the
crdASC gene cluster. Firstly, to learn about the expression of this cluster, a copy of the promoter region of
crdA (upstream 300 bp) plus the first 51 bp of the coding region of
crdA (351 bp total) was fused to the gene for the reporter protein mVenus. This translational fusion construct was inserted into the chromosome using the suicide vector pK18mob2 and homologous recombination (see
Figure 1A, suicide plasmid 1, reporter plasmid). Upon insertion, the CrdA::mVenus fusion provided a readout of
crdA expression. Importantly, the insertion created an additional copy of the
crd promoter (Pcrd) without interrupting the native P
crd-
crdASC expression and curdlan production.
Another fragment of
crdA was used in the second and third suicide variants (see
Figure 2B,C, suicide plasmids 2 and 3). For suicide plasmid 3, this
crdA fragment (489 bp) was preceded by a fragment from the bacterium
R. sphaeroides encoding the
phaP promoter (PphaP, 127 bp). Upon homologous recombination with the chromosome, this suicide plasmid variant inserts PphaP upstream of the
crdASC gene cluster, meaning that expression of the cluster is now dependent upon PphaP. The DNA sequences of the suicide plasmids used in this study are included in a text file as Appendix B.
For the measurement of the activity of Pcrd and PphaP using the reporter gene for mVenus, the low copy plasmid pPHU231 was used in which either Pcrd or PphaP was fused to the gene for mVenus, as previously described [
50]. In the case of Pcrd::mVenus, the fusion was a translational fusion, and was the identical fragment which was used for the suicide vector pK18mob-Pcrd::mVenus (see
Figure 1A). This fragment included the ribosome-binding site of
crdA plus the first 27 codons of
crdA (including the translational start). In the case of PphaP::mVenus, the fusion included the native ribosome binding site of
phaP plus the first 13 codons of
phaP (including the translational start). A comparison of the two ribosome binding sites reveals some similarity:
CAGGAGACAAGGCAatg for
phaP and
GAGGAGTTGATTTCatg for
crdA (ribosome binding sites are underlined).
Media and culture conditions. Agrobacterium strains were routinely grown at 30°C for a minimum of 3 d on aniline blue agar (ABA) [
34]. For liquid cultures (shaken at 130 rpm), strains were grown in Luria broth (LB, 10% Oxoid bacto-tryptone, 5% Oxoid yeast extract, 10% NaCl) and Agrobacterium defined broth (ADB) containing 20 mM KNO
3, 2.8 mM K
2HPO
4, 12.8 mM KH
2PO
4, 11.5 mM Na
2SO
4, 1.25 mM MgCl
2, 0.1 CaCl
2, 0.09 mM FeCl
3, 0.05 mM MnCl
2, 1.0 mM citric acid and adjusted to pH 7.0 prior to autoclaving. Sterile D-glucose or D-mannitol (4% w/v final) was then added as a carbon source. The volume of the ADB cultures was 50 mL, grown on a rotary shaker at 350 rpm in 150 mL conical flasks plugged with cotton wool.
Microscopy. Fluorescence microscopy: An Olympus BX50 microscope with a xenon lamp and on a Zeiss Universal light microscope with ultraviolet light (365 nm from an OSRAM HBO-100 lamp) were used. Images were captured with an RT Monochrome SPOT camera (Diagnostic Instruments Inc.). Complexes of curdlan with the (1→3)-β-glucan specific, Aniline Blue Fluorochrome (ABF) (Biosupplies Australia Pty. Ltd.) were detected by mixing one drop of a 7 day culture from ADB with one drop of a 0.01% ABF solution on a glass slide, covering with a coverslip, which was then firmly pressed onto the slide. Fluorescence from the ABF-curdlan complex was observed with excitation at 330-385 nm and emission at 420+ nm. For vital staining: 10 ml of a 4d-ADB culture was incubated with 5 mM 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) [
56] for 1 h on a shaker (130 rpm) at room temperature. A drop of the culture was prepared as described above for fluorescence of ABF-curdlan complexes. Intracellular CTC fluorescence was observed with excitation at 530-550 nm and emission at 590+ nm using the same optics as described above.
Electron microscopy. Cells from ADB cultures were harvested and washed in water by centrifugation at forces not exceeding 500 g. Samples were mounted on formvar-coated, 300-mesh copper grids, and negatively stained with 2.5% uranyl acetate for observations using a Philips 420 transmission electron microscope (TEM) at 100 kV. Images were captured with a GATAN camera, with a 20x magnification factor. For cell-sections, colonies were harvested from ABA and fixed in a solution of 5% glutaraldehyde and 4% para-aldehyde in 0.2 M calcium cacodylate, 0. 1 M K2HP03 (pH 7.0), for one day, washed in 0.1 M cacodylate, and post-fixed in 1% osmium tetroxide in 0. 1 M cacodylate. The cells were dehydrated in a graded series of ethanol (50-100%) each for 15 min, followed by 100% acetone, infiltrated in a 25% solution of Spurr resin (Electron Microscopy Science) in acetone for 1 h, followed by 1 h incubations in 50% and 75% resin. Specimens were finally embedded in 100% resin overnight, and the resin polymerized at 60°C for 30 h. Specimen blocks were sectioned at 0.5 µm thickness, stained with lead citrate-uranyl acetate and viewed under a Philips 420 TEM at 100 kV.
Particle counting. ADB cultures grown for 2 to 7 d were diluted (1 in 10) with 50 mM Na2HP04/NaH2P04 (pH 7.0) before analysing with a particle counter/sizer (CDA-500, Model: F-520p, Sysmex) with a 100 gm aperture detector. Particles were measured at two ranges: 10-40 μm and 40-100 μm.
96-well plate assay. Sterile, transparent, flat-bottom 96-well microplates (Greiner Bio-One) were prepared by adding 100 µL of 1% agar (liquid, 60°C) containing aniline blue and nutrients from ADB or MOPS medium to each well. After the agar had cooled to room temperature, 20 µL of ADB or MOPS medium inoculated with Agrobacterium was pipetted onto the agar, and the plate was covered with a transparent plastic lid (with condensation rings, high profile, clear, sterile, Greiner Bio-One) and incubated in a Tecan reader (Infinite 200Pro (M Nano+)) at 30°C up to 72 hours. The Tecan reader was programmed to measure the cultures growing in the well automatically at 3 hour intervals.
Curdlan extraction. Curdlan was harvested from liquid cultures of Agrobacterium as follows: The liquid culture (1 L) was subjected to centrifugation at 4000 x g, 10 min. The supernatant was discarded and the pellet was resuspended in 1 L water. NaOH was added to the cell suspension a final concentration of 0.5 M, and stirred for 5 minutes. The cells were removed by centrifugation at 10000 x g for 10 minutes. The supernatant was removed to a clean container carefully without disturbing the cell pellet. HCl (32%) was added in slowly to the supernatant in a step-wise fashion, with good mixing, until pH 7. Then the neutralized suspension was centrifuged at 10000 x g for 10 min. The supernatant carefully removed and discarded. The almost-transparent curdlan pellet was washed by resuspending in water and centrifugation at 10000 x g. This step was repeated 3 times, before the curdlan was placed in a Petri-dish and incubated at 40-50°C for 2-3 days. The dried curdlan was then weighed.
Media for curdlan production. ADB (Agrobacterium defined broth) is medium that is weakly buffered with a phosphate solution, so that initial growth of Agrobacterium is at around pH 7, which then subsequently drops to around pH 5.5 at the onset of curdlan production [
19,
34]. ADB consists of 4% (w/v) glucose, 20 mM KNO
3, 2.8 mM K2HPO
4, 12.8 mM KH
2SO
4, 11.5 mM Na
2SO
4, 1.25 mM MgCl
2, 0.09 mM FeCl
3, 0.05 mM MnCl
2, 1.0 mM citric acid, adjusted to pH 7.0 with NaOH before autoclaving. (Sterile glucose was added to the salts solution after autoclaving.)
MOPS-buffered medium was previously used for growth of
Sinorhizobium meliloti in a defined medium [
57] and also supported curdlan production by Agrobacterium. The MOPS medium consists of 50 mM MOPS (adjusted to pH 7 with KOH), 55 mM mannitol, 5 mM Na glutamate (or 20 mM for excess N), 250 µM CaCl
2.2H
2O, 37 µM FeCl
3.6H
2O, 48 µM H
3BO
3, 10 µM MnSO
4.H
2O, 1 µM NaMoO
4.2H
2O, 0.3 µM CoCl
2.6H
2O, 2 mM K
2HPO
4, and 4 µM biotin.
3. Results
Microscopical analysis of curdlan producing Agrobacterium cells reveals curdlan capsule. The strain ATCC31749 routinely produces curdlan when grown in liquid minimal salts with limiting nitrogen for longer than 3 days, whereas a knock-out (KO) strain of
crdA produced no detectable curdlan (<0.01 g/L). This 3-day delay in curdlan production fits with the knowledge that curdlan producing Agrobacterium cultures undergo a two stage development: an initial stage involving cell growth, and a later stage capable of producing curdlan provided that nitrogen is limiting and a suitable carbon source is present [
26]. The 3 day growth period was also necessary for the appearance of flocs of variable size which were visible to the naked eye in cultures of the curdlan producing strain, but not in those of the
crdA mutant. From this observation, I suspected that curdlan production was associated with floc formation.
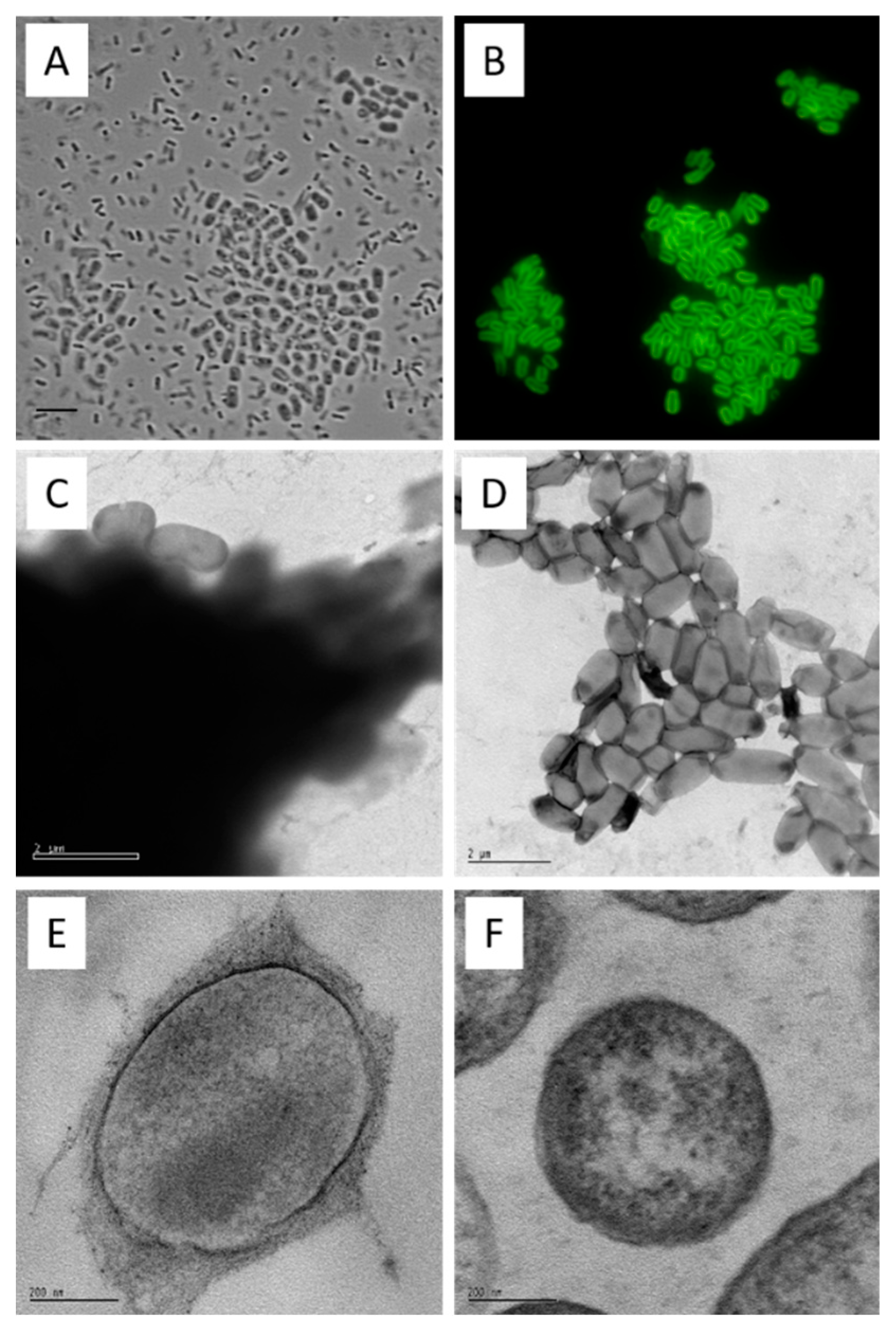
From a 7 day culture, I examined the cells using phase contrast microscopy. Two prominent morphological forms of cells were observable in the curdlan producing cultures. These were a thicker rod form averaging 1.5 x 3.0 μm, located in flocs (cell aggregates) and a slender rod form, averaging 0.5 x 2.0 μm that was planktonic (
Figure 2A). Only the slender rod form was observed in cultures of the
crdA mutant. When stained with the (1→3)-β-glucan-specific aniline blue fluorochrome (ABF) [
58,
59] only the larger form exhibited strong yellow/green fluorescence in a defined zone around the cell periphery (
Figure 2B). These observations suggest that curdlan is deposited as a capsule and that production of the capsule is restricted to cells located in flocs. Since curdlan production commences primarily in the post-growth stage of fermentation, it is likely that floc formation is mostly through the recruitment of capsulated cells, rather than through growth of new cells within a floc.
Electron microscopy of curdlan capsules. The physical form of native curdlan on the Agrobacterium cell surface was investigated by TEM of uranyl acetate stained preparations. At low magnification (9600 x), flocs from the curdlan producing culture were heavily stained, as might be expected for encapsulated cells. Occasional cells appeared to contain incomplete capsules (
Figure 2C). In contrast, the aggregates of cells from the
crdS mutant culture were quite difficult to find (being very rare) and not heavily stained (
Figure 2D). The fibrillary nature of the capsular material is evident in sections of embedded curdlan producing cells stained with lead citrate-uranyl acetate (
Figure 2E) when compared with the naked surface of the
crdS mutant cells (
Figure 2F). The use of electron microscopy (
Figure 2C–F) confirmed the deposition of curdlan as a capsule – defined as a polysaccharide layer that lies outside the cell envelope. In contrast, bacteria incapable of producing curdlan had a ‘clean’ surface. These analyses confirmed curdlan as a discreet capsule found only on the surface of curdlan producing cells.
Formation and organization of flocs. As noted above, the curdlan producing form was rarely planktonic. Rather, the curdlan capsule was usually confined to cell aggregates or flocs. This observation lead to the hypothesis that abundant flocs ought to appear in curdlan producing cultures, but not in cultures incapable of curdlan production. Floc formation was assessed using a particle counter capable of detecting flocs as particles. (The particle counter detects flocs as particles using a cut-off of 10 and 40 µm, meaning that all flocs outside of this range, both larger and smaller, are excluded.) When grown in liquid minimal salts for 3 d on a rotary shaker, ATCC31749 contained a proportion of particles larger than 20 µm in diameter, while the
crdS disruptant contained mostly particles with a diameter less than 20 µm (
Figure S1, Appendix A). At 6 days, the particles in the ATCC31749 culture were mostly larger than 20 µm in diameter. However, the particles from the
crdS disruptant did not increase in diameter. This result supported the hypothesis that curdlan production was indeed associated with floc formation, and that the loss of curdlan production is associated with failure to form high numbers of flocs in liquid cultures.
96-well plate assay reveals onset of curdlan production. How well does expression of the
crdASC genes correlate with curdlan production? A tight correlation would provide more support for the idea that curdlan production is controlled by Agrobacterium at the level of
crdASC gene expression. On the other hand, a poor correlation would indicate that curdlan production was controlled by additional unknown factors. A previous study using transcription analysis found a 100-fold upregulation of the
crdASC genes upon the transition to nitrogen starvation [
37], which lends weight to a tight correlation. To recapture this result, as well as to have a more sensitive, rapid and cheaper technique for the detection of both gene expression and curdlan production, I developed a novel assay capable of a high throughput. Until now, detection of curdlan production had been performed qualitatively via growth on agar containing aniline blue (where curdlan producing colonies turn intense blue while non-producing colonies remain white) or quantitatively following the extraction of curdlan via alkaline solution, neutralization, washing, drying and weighing. The extraction method is relatively laborious and impractical for analysing a high number of samples, while growth on standard aniline blue agar plates is impractical for obtaining temporal data. For developing this assay, I took advantage of the knowledge that curdlan accumulation in bacterial cultures is easily detectable using the (1→3)-β-glucan-specific dye aniline blue [
26]. For the setup, 100 µL of 1% agar containing aniline blue and nutrients from ADB (Agrobacterium define broth) was placed in each well in a 96-well plate. The rational for using agar in the 96-well plate was to minimize the volume of liquid cultures. This reduces the problem of measuring growth in liquid cultures with strong floc formation and the associated variation between measurements. Once the agar was set, 20 µL of ADB inoculated with Agrobacterium was pipetted onto the agar, and the plate was covered with a transparent plastic lid to prevent drying of the agar and incubated in a Tecan reader at 30°C up to 72 hours. The Tecan reader was set to measure the cultures growing on the surface of the agar in the well automatically at 3 hour intervals.
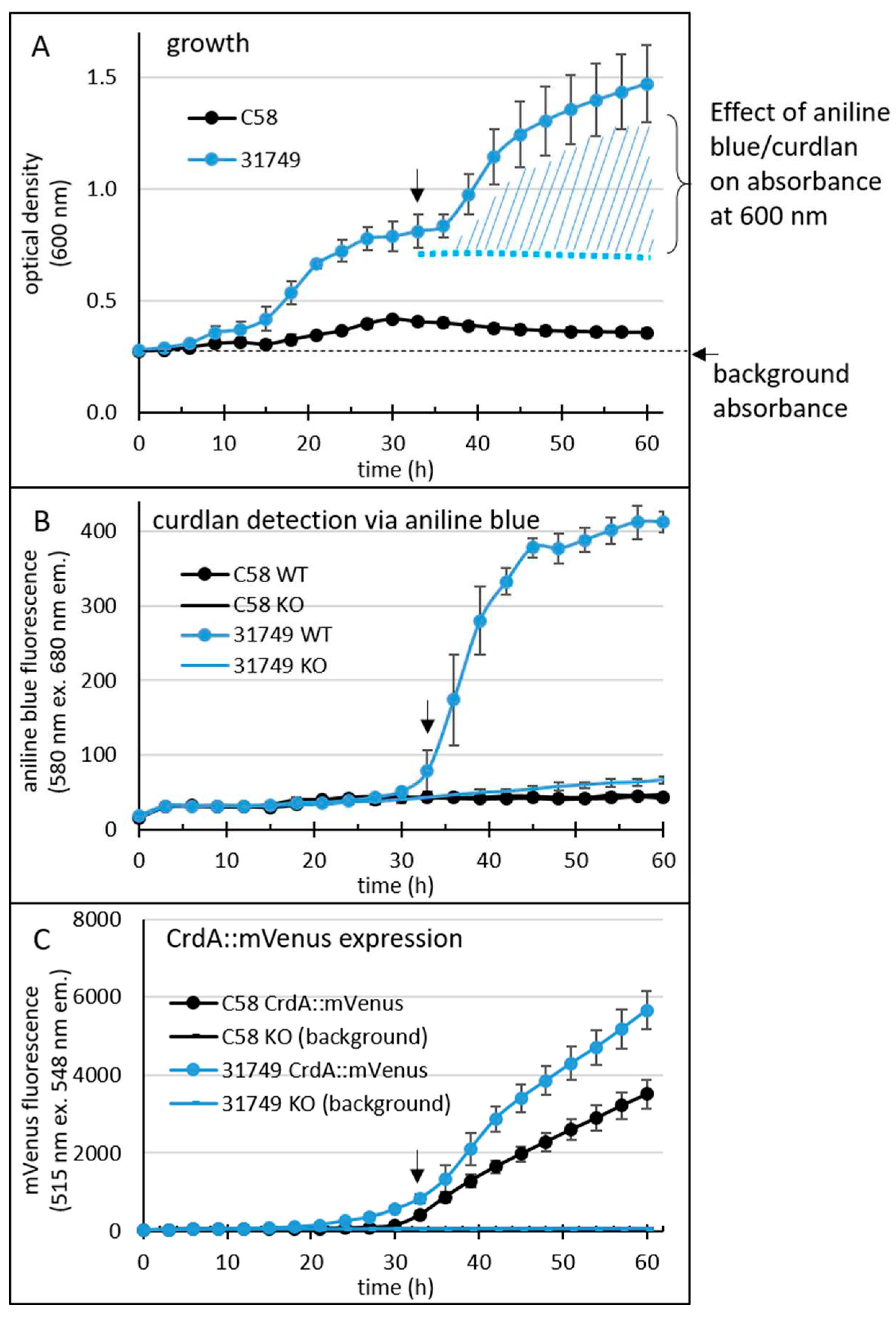
For the 96-well plate assay, reporter strains were prepared (using C58 and ATCC31749) in which the gene for mVenus was fused (translational fusion) to
crdA (see
Figure 1 for details of the genetic construct). Using these strains, I specifically looked for correlation between the onset of
crdA gene expression (detected as mVenus fluorescence) and the onset of curdlan production (as detected via aniline blue/curdlan fluorescence).
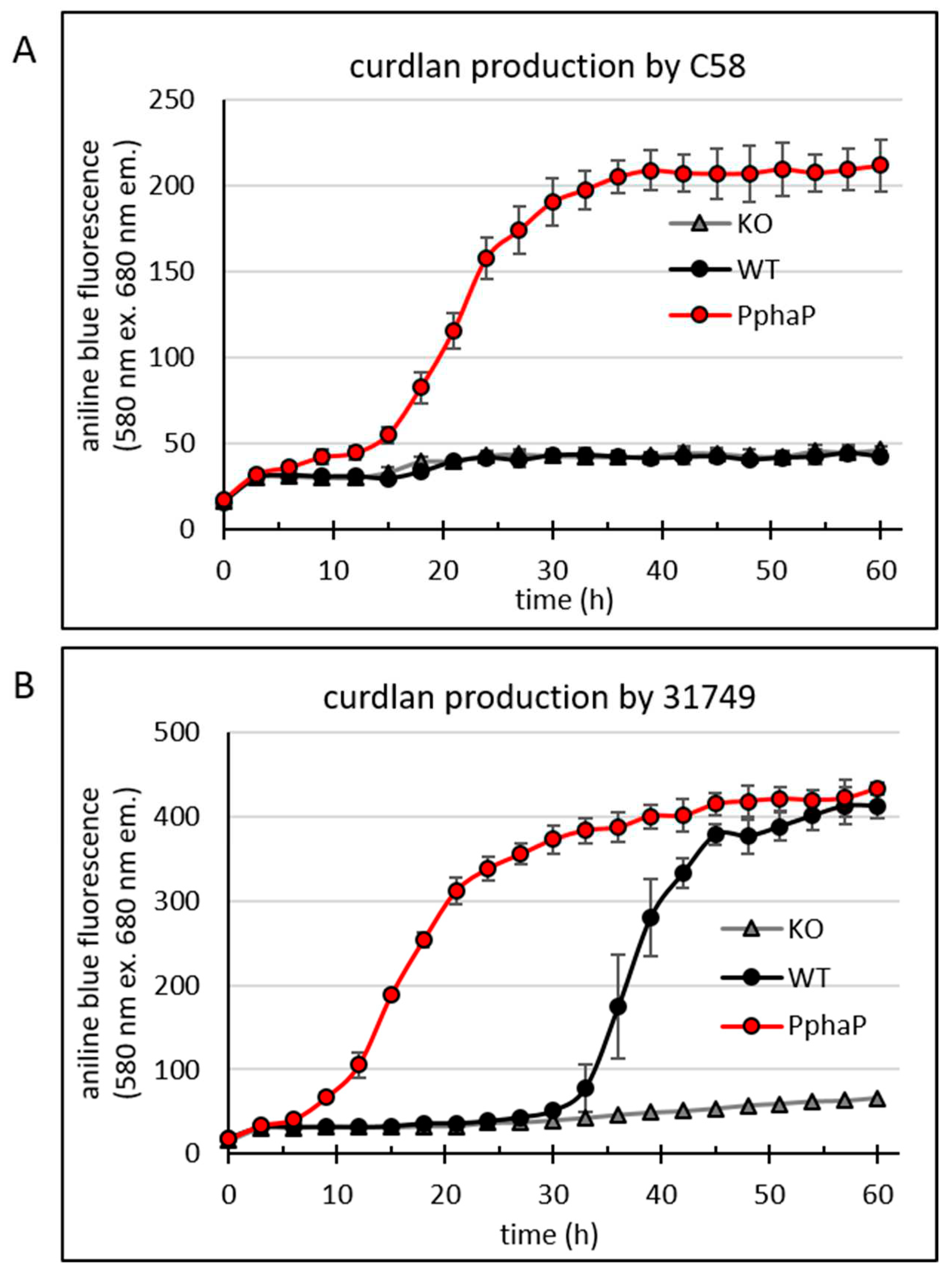
Absorbance at 600 nm (providing an indication of cell density,
Figure 3A) was strongly impacted by curdlan accumulation in strain ATCC31749 at around 30 hours, since binding between aniline blue and curdlan results in an intense blue colour that also absorbs light at 600 nm (
Figure 3A). The large error bars are likely because of the irregular colony surface that accompanies curdlan production. Growth of C58 was weaker than that of ATCC31749.
Curdlan binds to aniline blue, and the complex emits red fluorescence at 548 nm when excited by light at 515 nm. Fluorescence from the aniline blue-curdlan complex was apparent in cultures of ATCC31749 after 30 hours, but not in cultures of C58 (
Figure 3B). Interestingly, fluorescence from mVenus (
Figure 3C) was apparent in cultures of both ATCC31749 and C58 around 30 hours, although a weaker basal level of mVenus fluorescence was apparent slightly earlier, at 24 hrs for ATCC31749.
Since C58 did not produce curdlan under these conditions, the data suggests that the expression of the genes crdASC was too low to support curdlan production. Alternatively, the critical step controlling the onset of curdlan production lies elsewhere, so that the expression of these genes is not the deciding factor for curdlan production in this strain. This was not the case for ATCC31749, where the onset of strong crdASC gene expression (at about 30 h) correlated relatively well with the onset of curdlan accumulation (as would be expected if curdlan production was controlled by crdASC expression). Thus, curdlan accumulation correlated with crdA gene expression in ATCC31749, but not in C58. Altogether, the newly developed assay appeared to be reliable for sensitively detecting curdlan accumulation and gene expression at a high throughput level.
The R. sphaeroides promoter PphaP is much stronger than Pcrd in Agrobacterium. Since curdlan production correlates with
crdASC gene expression, one possibility for enhancing curdlan production is the enhanced expression of the
crdASC genes. For this goal, the replacement of the native
crdASC promoter (Pcrd) with a constitutively active promoter could suffice. I came across an obvious candidate promoter during investigations on transcription regulation in the Alphaproteobacteria. This candidate was PphaP from
R. sphaeroides [
50], which was very active in
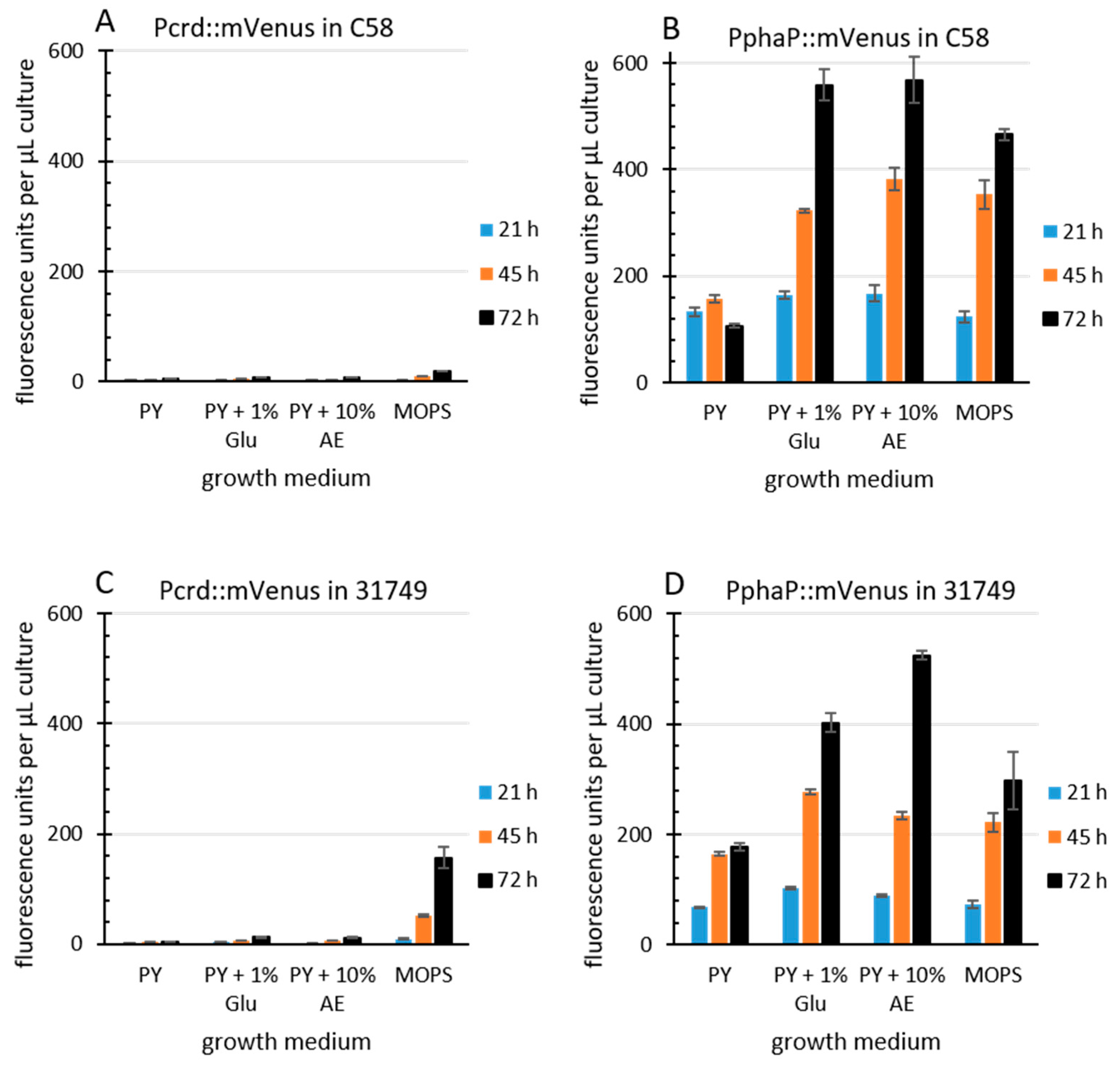
A. tumefaciens C58. To compare the activity of the two promoters, PphaP and Pcrd, in Agrobacterium, the reporter gene for mVenus was fused to each of the promoters on the low copy plasmid pPHU231 (see materials and methods for details). The Agrobacterium strains C58 and 31749 carrying the KO plasmid were used, since this avoided curdlan production and the resulting floc formation, which tends to interfere with fluorescence measurements and increase standard deviation. Furthermore, I used a variety of media. PY medium contains peptone and yeast extract (a rich source of nitrogen), while the MOPS medium contains a defined, growth limiting level of nitrogen. Apple extract (10%) represents a cheap and locally available source of nutrients. For details see materials and methods. In C58, the activity of Pcrd was low during growth in PY (
Figure 4A) and also in MOPS medium. In contrast, the PphaP promoter activity was much stronger in all media (
Figure 4B). A similar pattern was observed with the Pcrd and PphaP promoters in 31749 (
Figure 4C,D). I do not know why the presence of glucose or apple extract stimulated PphaP activity in PY medium. Also unexpected was the observation that Pcrd was rather weak in strain C58. This is probably related to the setup conditions, where 100 µL of liquid medium was used. The previous setup used in
Figure 3 involved only 20 µL of liquid culture overlaying 100 µL of agar, likely providing better access of the bacterial culture to oxygen, which is known to be important for curdlan production [
26], and is likely more critical for strain C58 than for the industrial strain 31749. Also in 31749, the Pcrd promoter was virtually inactive in PY medium, while in the MOPS medium, the Pcrd promoter was not active at 21 hours, but did show some activity at the 45 and 72-hour time points (
Figure 4C). This fits well with the data in
Figure 3C (the main difference being that the
crdA::mVenus fusion was carried on the chromosome in
Figure 3C, and on a low copy plasmid in
Figure 4). This also fits with the knowledge that the expression of the
crdASC genes is strongly activated upon N depletion [
37]. In summary, these data demonstrate that PphaP was far more active than Pcrd in all the growth media tested.
PphaP activates curdlan production. At this point, I was interested in seeing how the PphaP promoter affected curdlan accumulation. Specifically, would the use of PphaP to drive the expression of crdASC lead to a higher production of curdlan? To test this I used the 96-well plate assay.
The data shows that the C58 WT (
Figure 5A) did not produce curdlan (as was also shown in
Figure 3B), while the insertion of PphaP resulted in curdlan production. Also in 31749 the presence of PphaP clearly enhanced curdlan production so that curdlan accumulation was apparent much earlier, at around 10 hours of incubation, while in the 31749 WT, the onset of curdlan accumulation was apparent after 30 hours of incubation (
Figure 5C). Fluorescence reached maximum levels at around 400 fluorescence units in 31749, possibly because of exhaustion of the carbon source (mannitol).
Curdlan production requires both the expression of the crdASC genes and a suitable carbon source. Does the insertion of PphaP mean that curdlan production is independent upon N availability? When grown on PY agar with aniline blue but without any additional carbon source, the colonies did not stain blue, indicating the absence of curdlan production (
Figure 6A). However, when glucose was added (1% final concentration), both the strains carrying PphaP, but not the WT strains, stained blue during growth on agar, indicating curdlan production (
Figure 6A). This result implies that curdlan production requires at least two criteria: high expression of the
crdASC genes as well as the presence of a suitable carbon source. Only one of these two conditions does not lead to curdlan production. Importantly, when Pcrd was replaced with PphaP, the bacteria appeared capable of curdlan production regardless of whether nitrogen was limiting, provided that a suitable source of carbon was available.
Apple extract is a suitable carbon source for curdlan production. Given that one of the major costs associated with curdlan production is the carbon source, I looked for realistic alternatives to the the typical carbon sources. Apple juice (100%) was obtained from a local apple juice factory, sterilized via autoclaving and added to PY agar in increasing concentrations. At 1% (final concentration), a low level of curdlan production was apparent judging by the aniline blue stain (
Figure 6A). Production was improved with 3%, 10% and 30% apple extract, with 10% supporting the most intense blue colour formation.
To quantify curdlan, the bacteria were grown in liquid cultures for 3 days with ADB using glucose as a carbon source. The results show that curdlan production by C58 was not detected after growth in ADB, while C58 PphaP produced about 0.1-0.2 g/L curdlan. Curdlan production by 31749 in ADB was also about 0.2 g/L, while production by 31749 PphaP in ADB reached 1.6 g/L. Thus, under these conditions, PphaP improved curdlan production about 8-fold in strain 31749 and forced C58 to produce about 0.2 g/L curdlan under conditions where it would normally not produce any.
4. Discussion
Curdlan-producing Agrobacterium colonies grown on agar medium with a sufficient carbon source and aniline blue produce curdlan as a stable, intensely blue-staining layer which may be stripped, along with the cells, from the colony surface [
14]. This suggests close proximity between cells and curdlan, and that curdlan is somehow adhesive and involved in attachment between cells. However, the relationship between curdlan and the bacterial cell surface had not been clearly defined. Scanning electron microscopy of the Gram positive curdlan producing bacterium
Cellulomonas indicated that curdlan may be deposited as a capsule [
16]. However, staining with aniline blue could not support a capsular formation by
Cellulomonas since the native form of curdlan produced by this bacterium does not stain with aniline blue. It must first undergo alkaline extraction before staining, indicating a fundamentally different native form to curdlan produced by Agrobacterium [
15]. In this study, I showed that Agrobacterium deposits curdlan as a capsule, using the (1→3)-β-glucan-specific aniline blue fluorochrome (ABF). Additionally, curdlan was negatively-stained by uranyl acetate and thereby visible using TEM. Both ABF-induced fluorescence images and TEM showed that the curdlan capsule is tightly associated with the cell surface. Neither of these features was observed with a curdlan deficient mutant (
crdS-) or in cultures of strains grown in medium that does not elicit curdlan production, i.e., Luria broth.
Cell aggregates depend upon curdlan production, and these aggregates appear to be via interactions between curdlan-encapsulated cells. The data is consistent with a scenario where cells produce a capsule, whereupon these encapsulated cells are recruited to the aggregate on the basis of curdlan-curdlan interactions. In other words, after producing curdlan, the cell randomly encounters a neighbouring cell that has also produced curdlan. The two become attached and then encounter additional cells that have produced curdlan, and in this way the aggregates grow larger through cell recruitment over time (
Figure S1, Appendix A). Studies on the soil-dwelling Gram-positive
C. flavigena also found a correlation between aggregation and curdlan production. Furthermore, on subculturing
C. flavigena on a nitrogen-rich medium lacking a carbohydrate source, disaggregation occurs and the curdlan disappears, suggesting that the curdlan is mobilized as a carbohydrate source [
15,
16]. Consumption of curdlan by Agrobacterium has not been observed.
A. tumefaciens is considered one of the more important, well-studied plant pathogens, being a tumorigenic pathogen responsible for crown gall disease with a wide host range [
60]. So far, there is no indication that curdlan is necessary for pathogenesis. Agrobacteria strains are endemic in soils. Many are not necessarily pathogenic [
61], and may exhibit long term survival even in the absence of host colonization [
62]. Probably the ability to produce curdlan is related to long term survival, although this has never been demonstrated. Indeed, the
A. tumefaciens C58 strain used in this study did not produce curdlan under standard laboratory conditions. Interestingly, when C58 was grown on apple extract, some curdlan production was apparent. Importantly, when C58 carried PphaP, strong curdlan accumulation was apparent (
Figure 6A). It is likely that curdlan production is more highly regulated in C58 than in 31749 and that, in addition to nitrogen limitation, other environmental conditions (such as desiccation, or plant-derived molecules) are needed for the activation of curdlan production in C58.
The curdlan dependent aggregation phenomenon described here appear to enable the formation of microcolonies or flocs and biofilms in these nutritionally stressful situations. The curdlan capsule formed under these conditions may play a role in protection from desiccation by virtue of the impressive water-holding capacity of curdlan capsule, i.e., the encapsulated cell may act as a ‘spore’, as suggested in the case of cellulose-producing bacteria [
11]. Moreover, curdlan could protect not only the producing cells, but also the entire community of cells from desiccation as well as soil predators such as phagocytic protozoans [
63] and parasitic Bdellovibrio species [
64].
Interestingly, not all cells in the curdlan producing cultures were encapsulated. This heterogeneity within the population is somewhat similar for the production of another exopolysaccharide, galactoglucan, by another member of the family Rhizobiacea,
Sinorhizobium meliloti [
65]. The ability of clonal populations to display multiple phenotypes has been noted [
66], and provides a number of advantages for survival such as bet-hedging and division of labor. Without knowing the role of curdlan in survival, it is difficult to say which of these options applies to curdlan. For example, curdlan forms a capsule, which may provide an advantage for the producing cell (e.g., protection against predation or dehydration). This could be a bet-hedging strategy, which can be viewed as a preadaptation of a subpopulation to a danger before it is encountered. However, curdlan also promotes cell-cell aggregation and formation of flocs, and could therefore be important for the formation of a biofilm (which benefits the whole population). In such a scenario, curdlan production by a subpopulation could be a form of division of labor. Both scenarios are not mutually exclusive, and more experiments are needed to clarify the role (if any) of heterogeneity in curdlan producing cultures.
Relevant to the goal of maximal curdlan production, such heterogeneity likely has the undesired effect of decreasing curdlan production, since not all individuals contribute. A more desirable outcome would be that all cells contribute to curdlan production. The use here of a strong, constitutively active promoter such as PphaP to drive the crdASC gene expression may well have contributed to the increase in curdlan production by removing the heterogeneic pattern of production, i.e., by forcing all cells to contribute.
The
phaP promoter (PphaP) from
Rhodobacter sphaeroides controls the expression of the phasin gene
phaP. Previous work on this gene found that it was strongly active in the exponential growth phase of
R. sphaeroides [
50]. Interestingly, upon heat shock, transcription of the
phaP gene increased by over 20-fold [
51], making the promoter of
phaP one of the most active promoters that I have ever observed. Indeed, PphaP showed very high activity in Agrobacterium strains (
Figure 4). In comparison, the native
crd promoter (Pcrd) was not only much weaker than PphaP, it was also highly dependent upon the growth medium (e.g., nitrogen content). For example, PY medium (containing peptone and yeast extract) did not support Pcrd activity, while PphaP was relatively strong. This made PphaP the ideal candidate for strong, unregulated expression of the
crdASC genes. The outcome of this approach was a clear improvement in curdlan production (
Figure 5 and
Figure 6). However, curdlan production by strains carrying PphaP remained dependent upon the presence of a suitable carbon source (
Figure 6A). This outcome implies that curdlan production is not only dependent upon expression of the
crdASC genes, but also upon the presence of a suitable carbon source, which likely determines the metabolic status of the cell.
It is important that the KO strains did not produce curdlan (
Figure 5 and
Figure 6A). The significance here is that the suicide plasmid 2 (see
Figure 1B) does not disrupt the
crdASC genes upon insertion into the Agrobacterium chromosome. Rather, the insertion causes transcription from Pcrd to be terminated by a transcription terminator, and thus the
crdASC genes in the KO strains are not disrupted but rather transcriptionally inactive (i.e., isolated from Pcrd via the terminator). The outcome is that the KO strains do not produce curdlan, even in the presence of a suitable carbon source (
Figure 6A). This fits with the idea that the
crdASC gene cluster is indeed an operon with Pcrd as the promoter of the operon. Moreover, curdlan production requires both criteria:
crdASC expression and a suitable carbon source. Nitrogen limitation is not necessary, so long as these two requirements are satisfied. This scenario is therefore ideal for the engineering approach used in this study. Despite genetically fixing the high
crdASC gene expression, curdlan production will only follow once the bacterium encounters an adequate carbon source. This is useful for preparing starter cultures that are not encumbered with excessive curdlan production and cell aggregation.
How stable are the strains modified with a suicide vector? While such experiments are possible, I have not generated quantitative data to answer this question. However,
Figure 6A provides qualitative data. In this study, the insertion of pK18mob2 into the chromosome was used for genetic modification following homologous recombination. pK18mob2 contains a kanamycin selection marker, meaning that the loss of this plasmid would mean the loss of kanamycin resistance. Furthermore, such reverting mutants would accompany a phenotype. For example, the KO strains lost their ability to produce curdlan and therefore appeared as white colonies on agar with aniline blue. When grown on agar lacking the antibiotic kanamycin, any appearance of blue colonies would indicate the spontaneous loss of this suicide plasmid as cells recovered the ability to produce curdlan. Thus, the presence of blue colonies mixed in with the white colonies would provide a qualitative measurement of the stability of the suicide vectors. That no blue colonies appeared among the KO strains is an indication that the suicide plasmid is highly stable. It is also worth noting that if, for some reason, the presence of the suicide vector in the chromosome of the curdlan producing strain is undesirable, its removal is possible (while maintaining the presence of PphaP) through a well-established method that uses the
sacB gene [
54].
The work here describes a genetic engineering approach that enables higher curdlan yields. However, there is much work still needed to establish optimal conditions. Now that the high expression of the
crdASC genes is fixed, which sources of N and C are most suitable for high curdlan yields? What concentrations of P and dissolved oxygen are optimal, and at what pH? Such questions have been intensively addressed for
Agrobacterium sp. 31749 [
26,
31,
47], but these should be re-addressed for the genetically modified strains. I anticipate that the 96-well plate assay first described in this study could be a useful tool for answering these questions. Furthermore, which strains are most useful for high quality, high purity curdlan production? My approach using PphaP showed that curdlan production can be enhanced in both 31749 and C58 strains, despite the 1% genetic differences between the strains. Note that this difference likely accounts for different growth behaviour (31749 reaches a much higher cell density in liquid cultures than C58, see
Figure 3A) which could also account for the higher yield per liter of culture by strain 31749. However, strain C58 might be better at growth on various fermentation substrates, e.g., plant biomass.
Various plant biomasses have been tested as sources of carbon for curdlan production, such as sugar cane, cassava starch, wheat bran, orange peels and prairie cordgrass [
40,
44,
48,
67,
68,
69]. To my knowledge, however, apple extract has not been tested for curdlan production. Apples made up about 80-90% of all tree fruits harvested in Germany (
https://www.destatis.de/EN/Themes/Economic-Sectors-Enterprises/Agriculture-Forestry-Fisheries/Fruit-Vegetables-Horticulture/_node.html) and is therefore a local, cheap carbon source for curdlan production. In particular, the waste associated with apple storage, transport and pulp from juicing might be used for curdlan production. I found that apple extract added to agar at 10% supported what appeared to be a high yield of curdlan. It is even possible that apple extract might be superior to glucose as a carbon source, although this needs further research. Thus, apples could be cheap source of carbon for curdlan production. Combined with the new strains developed in this study that are not sensitive to nitrogen levels, new fermentation processes based on a variety of cheap plant biomass sources could dramatically decrease the cost of curdlan production, extending the use of curdlan in industry.