Submitted:
23 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
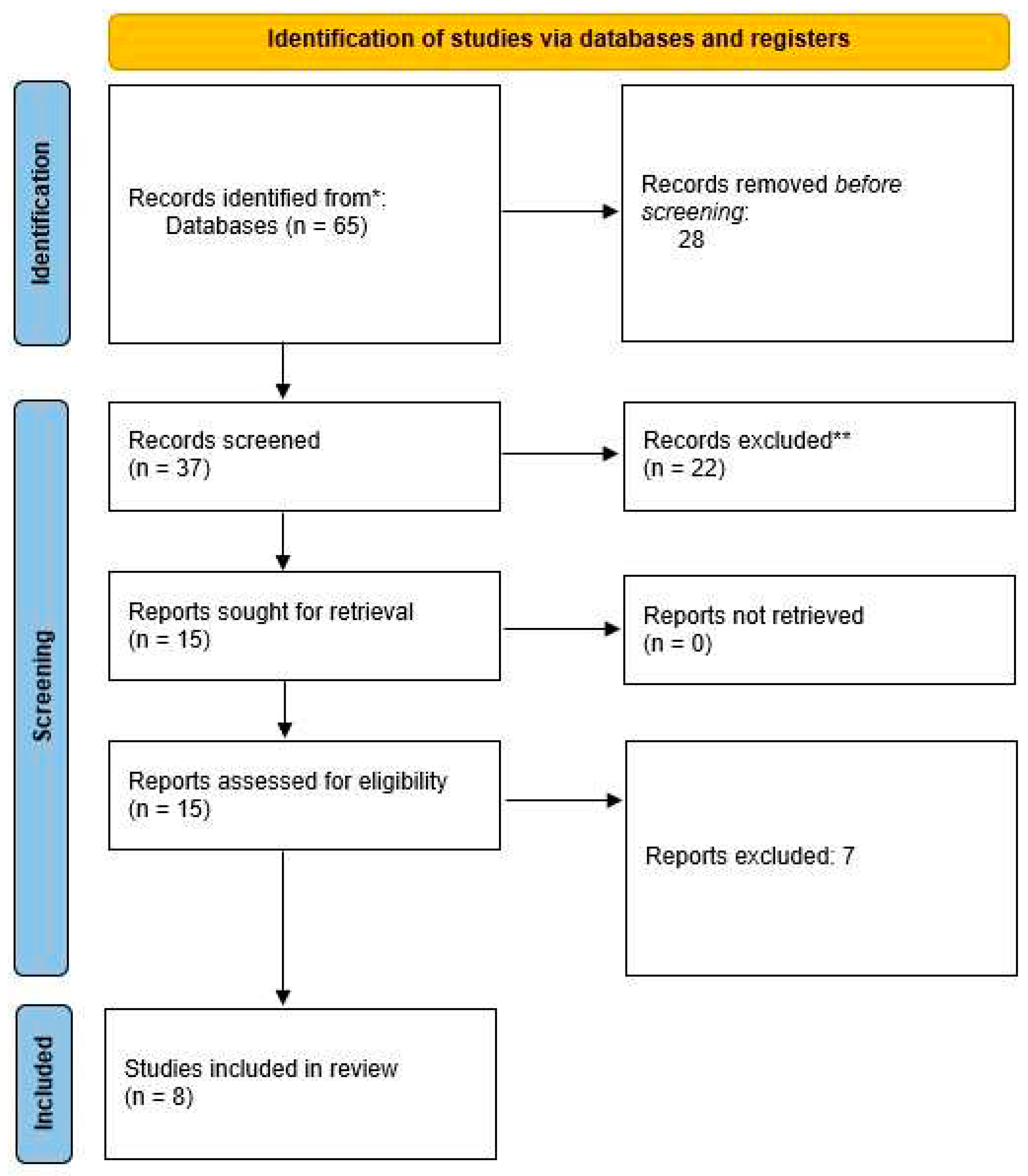
Literature search
Inclusion and exclusion criteria
Data extraction
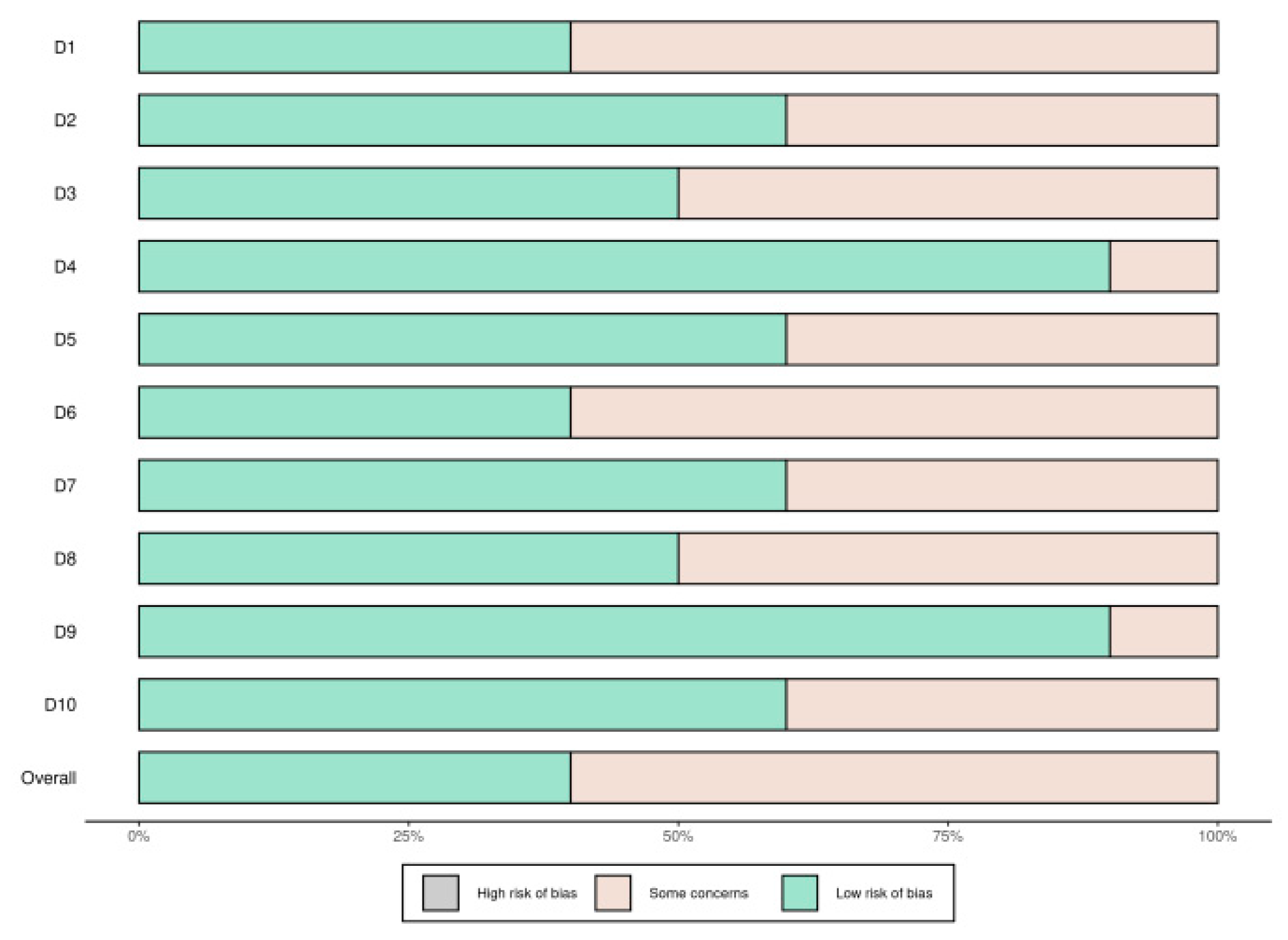
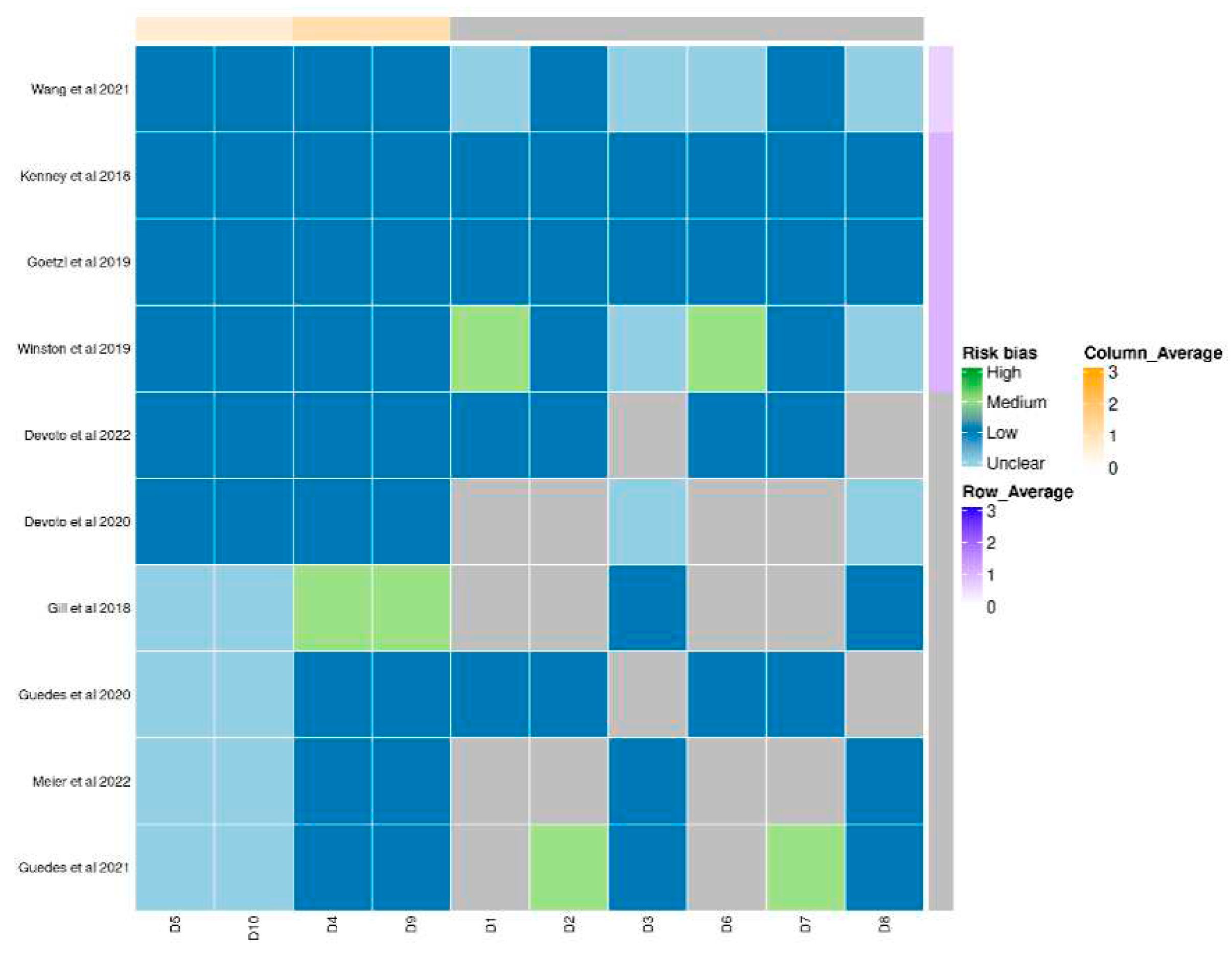
Quality assessment
Data synthesis
Results
Narrative summary of the studies
Tau protein
Exosomal miRNAs
Exosomal cytokines
Discussion
Conclusions
Conflicts of Interest
References
- Kazl C, Torres A. Definition, Classification, and Epidemiology of Concussion. Semin Pediatr Neurol. 2019 Jul;30:9-13. [CrossRef]
- Mavroudis I, Kazis D, Chowdhury R, Petridis F, Costa V, Balmus IM, Ciobica A, Luca AC, Radu I, Dobrin RP, Baloyannis S. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics (Basel). 2022 Mar 18;12(3):740. [CrossRef]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006;31:642–648. [CrossRef]
- Song Z, Xu Y, Deng W, Zhang L, Zhu H, Yu P, Qu Y, Zhao W, Han Y, Qin C. Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer's Disease. Front Mol Neurosci. 2020;13:79. [CrossRef]
- DeLeo AM, Ikezu T. Extracellular vesicle biology in Alzheimer’s disease and related tauopathy. J. Neuroimmune Pharmacol. 2018;13:292–308. [CrossRef]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420.
- Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. [CrossRef]
- Ratajczak MZ, Ratajczak J. Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia 2020;34:3126–3135. [CrossRef]
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles 2014;3:26913. [CrossRef]
- McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J. Circ. Biomark 2015;4:7. [CrossRef]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. [CrossRef]
- Manek R, Moghieb A, Yang Z, Kumar D, Kobeissy F, Sarkis GA et al. Correction to: protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol. Neurobiol. 2018;55:6129. [CrossRef]
- Peltz CB, Kenney K, Gill J, Diaz-Arrastia R, Gardner RC, Yaffe K. Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology 2020;95:e1126–e1133. [CrossRef]
- Goetzl EJ, Elahi FM, Mustapic M, Kapogiannis D, Pryhoda M, Gilmore A, et al. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019;33:5082–5088. [CrossRef]
- Guedes VA, Kenney K, Shahim P, Qu BX, Lai C, Devoto C et al. Exosomal neurofilament light: a prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology 2020;94: e2412–e2423. [CrossRef]
- Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193-203. [CrossRef]
- Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, Chen F, Wang H, Zhang J, Lei P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32(1):512-528. Erratum in: FASEB J. 2018;32(4):2315. [CrossRef]
- Li D, Huang S, Yin Z, Zhu J, Ge X, Han Z, Tan J, Zhang S, Zhao J, Chen F, Wang H, Lei P. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem Res. 2019;44(8):1903-1923. [CrossRef]
- Kenney K, Qu BX, Lai C, Devoto C, Motamedi V, Walker WC, Levin HS, Nolen T, Wilde EA, Diaz-Arrastia R, Gill J; CENC Multisite Observational Study Investigators. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 2018;32(10):1276-1284. [CrossRef]
- Wang Z, Wang H, Becker R, Rufo J, Yang S, Mace BE, Wu M, Zou J, Laskowitz DT, Huang TJ. Acoustofluidic separation enables early diagnosis of traumatic brain injury based on circulating exosomes. Microsyst Nanoeng. 2021;7:20. [CrossRef]
- Devoto C, Lai C, Qu BX, Guedes VA, Leete J, Wilde E, Walker WC, Diaz-Arrastia R, Kenney K, Gill J. Exosomal MicroRNAs in Military Personnel with Mild Traumatic Brain Injury: Preliminary Results from the Chronic Effects of Neurotrauma Consortium Biomarker Discovery Project. J Neurotrauma. 2020;37(23):2482-2492. [CrossRef]
- Devoto C, Guedes VA, Lai C, Leete JJ, Mithani S, Edwards K, Vorn R, Qu BX, Wilde EA, Walker WC, Diaz-Arrastia R, Werner JK, Kenney K, Gill JM. Remote blast-related mild traumatic brain injury is associated with differential expression of exosomal microRNAs identified in neurodegenerative and immunological processes. Brain Inj. 2022;36(5):652-661. [CrossRef]
- Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T, Motamedi V, Osier N, Stern RA, Kapogiannis D. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018;32(10):1277-1284. [CrossRef]
- Goetzl EJ, Ledreux A, Granholm AC, Elahi FM, Goetzl L, Hiramoto J, Kapogiannis D. Neuron-Derived Exosome Proteins May Contribute to Progression From Repetitive Mild Traumatic Brain Injuries to Chronic Traumatic Encephalopathy. Front Neurosci. 2019;13:452. [CrossRef]
- Winston CN, Romero HK, Ellisman M, Nauss S, Julovich DA, Conger T, Hall JR, Campana W, O'Bryant SE, Nievergelt CM, Baker DG, Risbrough VB, Rissman RA. Assessing Neuronal and Astrocyte Derived Exosomes From Individuals With Mild Traumatic Brain Injury for Markers of Neurodegeneration and Cytotoxic Activity. Front Neurosci. 2019;13:1005. [CrossRef]
- Guedes VA, Kenney K, Shahim P, Qu BX, Lai C, Devoto C, Walker WC, Nolen T, Diaz-Arrastia R, Gill JM; CENC Multisite Observational Study Investigators. Exosomal neurofilament light: A prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology. 2020;94(23):e2412-e2423. [CrossRef]
- Meier TB, Guedes VA, Smith EG, Sass D, Mithani S, Vorn R, Savitz J, Teague TK, McCrea MA, Gill JM. Extracellular vesicle-associated cytokines in sport-related concussion. Brain Behav Immun. 2022;100:83-87. [CrossRef]
- Guedes VA, Lai C, Devoto C, Edwards KA, Mithani S, Sass D, Vorn R, Qu BX, Rusch HL, Martin CA, Walker WC, Wilde EA, Diaz-Arrastia R, Gill JM, Kenney K. Extracellular Vesicle Proteins and MicroRNAs Are Linked to Chronic Post-Traumatic Stress Disorder Symptoms in Service Members and Veterans With Mild Traumatic Brain Injury. Front Pharmacol. 2021;12:745348. [CrossRef]
- Mavroudis, I.; Chatzikonstantinou, S.; Petridis, F.; Palade, O.D.; Ciobica, A.; Balmus, I.-M. Functional Overlay Model of Persistent Post-Concussion Syndrome. Brain Sci. 2023, 13, 1028. [CrossRef]
- Mavroudis, I.; Chatzikonstantinou, S.; Ciobica, A.; Balmus, I.-M.; Iordache, A.; Kazis, D.; Chowdhury, R.; Luca, A.-C. A Systematic Review and Meta-Analysis of the Grey Matter Volumetric Changes in Mild Traumatic Brain Injuries. Appl. Sci. 2022, 12, 9954. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petridis, F.; Balmus, I.-M.; Ciobica, A.; Gorgan, D.L.; Luca, A.C. Review on the Role of Salivary Biomarkers in the Diagnosis of Mild Traumatic Brain Injury and Post-Concussion Syndrome. Diagnostics 2023, 13, 1367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020; 15, 6917-6934. [CrossRef]
- Hofmann, L.; Medyany, V.; Ezić, J.; Lotfi, R.; Niesler, B.; Röth, R.; Engelhardt, D.; Laban, S.; Schuler, P.J.; Hoffmann, T.K.; Brunner, C.; Jackson, E.K.; Theodoraki, M.N. Cargo and Functional Profile of Saliva-Derived Exosomes Reveal Biomarkers Specific for Head and Neck Cancer. Front Med (Lausanne). 2022, 9, 904295. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Balmus, I.-M.; Ciobica, A.; Nicoara, M.N.; Luca, A.C.; Palade, D.O. The Role of Microglial Exosomes and miR-124-3p in Neuroinflammation and Neuronal Repair after Traumatic Brain Injury. Life 2023, 13, 1924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; He, F.; Li, T.; Chen, J.; Jiang, L.; Ouyang, X-P.; Zuo, L. Role of Exosomes in Brain Diseases. Front. Cell. Neurosci. 2021, 15, 743353. [CrossRef]
- Saeedi, S.; Israel, S.; Nagy, C.; Turecki, G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med, 2022, 20, 291. [CrossRef]
- Xu, X.; Xu, L.; Wen, C.; Xia, J.; Zhang, Y.; Liang, Y. Programming assembly of biomimetic exosomes: An emerging theranostic nanomedicine platform. Mater Today Bio. 2023, 22, 100760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. 2023, 1, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2004, 6 (1), 204. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Zhao, J.; Dash, P.K.; Soto, C.; Moreno-Gonzalez, I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J Neurotrauma. 2020, 37(1) ,80-92. [CrossRef]
- Hossain, I.; Blennow, K.; Posti, J.P.; Zetterberg, H. Tau as a fluid biomarker of concussion and neurodegeneration. Concussion. 2022, 7(2), CNC98. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, R.; Chang, B.; Yue, J.K.; Chiu, A.; Winkler, E.A.; Puccio, A.M.; Diaz-Arrastia, R.; Yuh, E.L.; et al. Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau-Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers. JAMA Neurol. 2017, 74(9), 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Bourgognon, J-M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain and Neuroscience Advances. 2020, 4. [CrossRef]
- Alam, A.; Thelin, E.P.; Tajsic, T.; et al. Cellular infiltration in traumatic brain injury. J Neuroinflammation, 2020, 17, 328. [CrossRef]
- Bao, W.; Lin, Y.; Chen, Z. The Peripheral Immune System and Traumatic Brain Injury: Insight into the role of T-helper cells. Int J Med Sci 2021, 18(16), 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, J.; Xu, X.; Li, W.; Zhong, R.; Qi, L.; Chen, J.; Cui, G.; et al. M-CSF, IL-6, and TGF-β promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci Adv. 2021, 7(11), eabb6260. [Google Scholar] [CrossRef] [PubMed]
| Exosomal biomarkers | Recent findings | References |
|---|---|---|
| tau and p-tau | Both exosomal tau and p-tau level changes were reported in mTBI and repetitive mTBI; exosomal p-tau levels were correlated with chronic neurophychological symptoms of PCS | [19,25,27,28] |
| micro-RNAs | The miRNAs for which changes in expression were reported in mTBI and PCS are involved in cell proliferation, cerebral microcirculation, apoptosis, tau accumulation, and neuronal differentiation | [22,28] |
| cytokines | IL-6 and IL-10 could be correlated with acute phase response in mTBI; IL-6 was associated with chronic post-concussion symptoms and the number of days of their manifestation | [23,24,27] |
| neuron-derived exosomes | Potential in transporting signal molecules, proteins (inflammatory factors), and neurotoxic peptides | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





