Submitted:
23 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
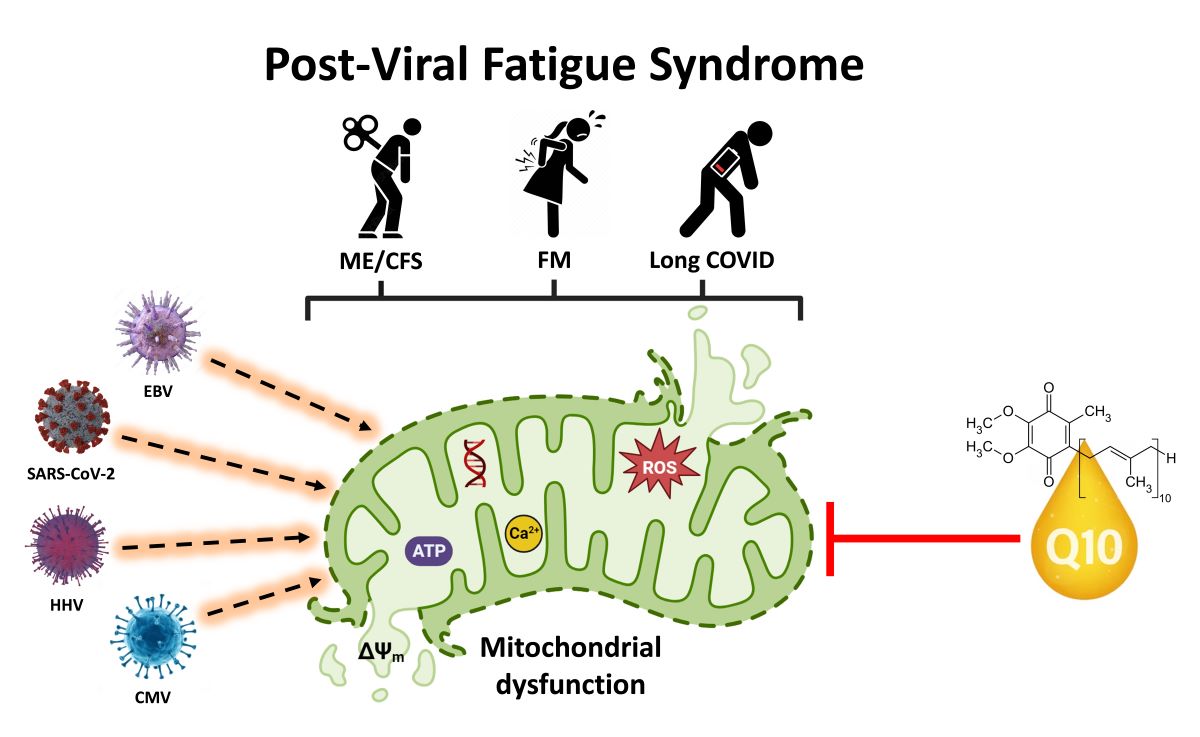
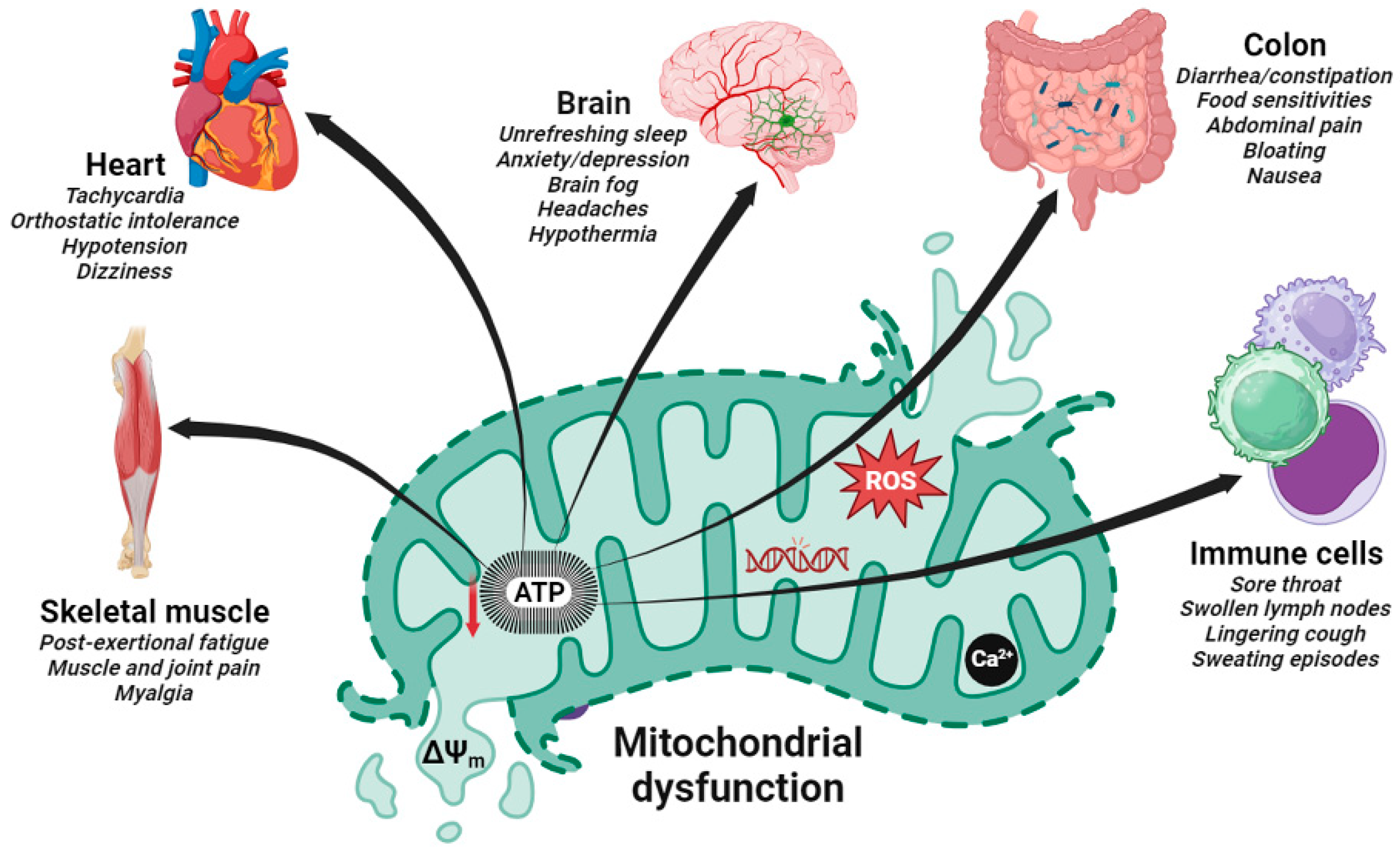
Keywords:
1. Introduction
2. Evidence of Mitochondrial Dysfunction in Post-Viral Fatigue Syndrome
3. Hijacking of Host Mitochondria in Post-Viral Fatigue Related Disorders
4. Effects of Coenzyme Q10 Supplementation in Post-Viral Fatigue Related Conditions
4.1. Coenzyme Q10 Supplementation in Fibromyalgia
4.2. Coenzyme Q10 Supplementation in Acute COVID-19 Infection
4.3. Coenzyme Q10 Supplementation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
5. Importance of CoQ10 Supplement Quality and Bioavailability
6. Conclusions, Unresolved Issues and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Komaroff, A.L.; Lipkin, W.I. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med (Lausanne) 2023, 10, 1187163. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L. Chronic "post-infectious" fatigue syndrome. Trans Am Acad Insur Med 1993, 76, 82–95. [Google Scholar] [PubMed]
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther Adv Infect Dis 2021, 8, 20499361211009385. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Shoenfeld, Y. Autoimmune Autonomic Dysfunction Syndromes: Potential Involvement and Pathophysiology Related to Complex Regional Pain Syndrome, Fibromyalgia, Chronic Fatigue Syndrome, Silicone Breast Implant-Related Symptoms and Post-COVID Syndrome. Pathophysiology 2022, 29(3), 414–425. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.T.; Gallagher, A.M.; Thomas, J.M.; White, P.D. The prognosis of different fatigue diagnostic labels: a longitudinal survey. Fam Pract 2005, 22(4), 383–8. [Google Scholar] [CrossRef] [PubMed]
- Behan, P.O.; Behan, W.M.; Gow, J.W.; Cavanagh, H.; Gillespie, S. Enteroviruses and postviral fatigue syndrome. Ciba Found Symp 1993, 173, 146-54, discussion 154-9. [Google Scholar] [CrossRef]
- Murga, I.; Aranburu, L.; Gargiulo, P.A.; Gomez Esteban, J.C.; Lafuente, J.V. Clinical Heterogeneity in ME/CFS. A Way to Understand Long-COVID19 Fatigue. Front Psychiatry, 2021; 12, 735784. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Saez-Francas, N.; Santillo, D.; Alegre, J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br J Pharmacol 2017, 174(5), 345–369. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Gradisnik, S.; Eaton-Fitch, N. Understanding myalgic encephalomyelitis. Science 2022, 377(6611), 1150–1151. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, I.; Chatys-Bogacka, Z.; Slowik, J.; Klich-Raczka, A.; Fedyk-Lukasik, M.; Slowik, A.; Wnuk, M.; Drabik, L. Fatigue after COVID-19 in non-hospitalized patients according to sex. Brain Behav 2023, 13(2), e2849. [Google Scholar] [CrossRef]
- Faro, M.; Saez-Francas, N.; Castro-Marrero, J.; Aliste, L.; Fernandez de Sevilla, T.; Alegre, J. Gender differences in chronic fatigue syndrome. Reumatol Clin 2016, 12(2), 72–7. [Google Scholar] [CrossRef]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; Smirnoff, L.; Simpson, N.; Halawi, H.; Sum-Ping, O.; Kalinowski, A.; Patel, Z.M.; Shafer, R.W.; Geng, L.N. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front Neurol 2023, 14, 1090747. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Kirchner, E.; Calabrese, L.H. Long COVID and rheumatology: Clinical, diagnostic, and therapeutic implications. Best Pract Res Clin Rheumatol 2022, 36(4), 101794. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.B.; Javanmard, S.H. Post-COVID-19 Syndrome Mechanisms, Prevention and Management. Int J Prev Med 2023, 14, 59. [Google Scholar] [PubMed]
- Natarajan, A.; Shetty, A.; Delanerolle, G.; Zeng, Y.; Zhang, Y.; Raymont, V.; Rathod, S.; Halabi, S.; Elliot, K.; Shi, J.Q.; Phiri, P. A systematic review and meta-analysis of long COVID symptoms. Syst Rev 2023, 12(1), 88. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Olivier, T.; Prasad, V. The definition of long COVID used in interventional studies. Eur J Clin Invest 2023, e13989. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Al-Najjar, Y.; Sharma, O.; Kamal, I.; Javed, A.; Gohil, H.S.; Paul, P.; Al-Khalifa, A.M.; Laws, S.; Zakaria, D. Role of previous infection with SARS-CoV-2 in protecting against omicron reinfections and severe complications of COVID-19 compared to pre-omicron variants: a systematic review. BMC Infect Dis 2023, 23(1), 432. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Bluemke, D.A.; Luscher, T.F.; Neubauer, S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J 2022, 43(11), 1157–1172. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr 2021, 110(3), 914–921. [Google Scholar] [CrossRef]
- Tate, W.P.; Walker, M.O.M.; Peppercorn, K.; Blair, A.L.H.; Edgar, C.D. Towards a Better Understanding of the Complexities of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Long COVID. Int J Mol Sci 2023, 24(6). [Google Scholar] [CrossRef]
- Yong, S.J.; Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev Med Virol 2022, 32(4), e2315. [Google Scholar] [CrossRef]
- Barhorst, E.E.; Boruch, A.E.; Cook, D.B.; Lindheimer, J.B. Pain-Related Post-Exertional Malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Fibromyalgia: A Systematic Review and Three-Level Meta-Analysis. Pain Med 2022, 23(6), 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Possible Application of Melatonin in Long COVID. Biomolecules 2022, 12(11). [Google Scholar] [CrossRef] [PubMed]
- Porter, N.S.; Jason, L.A.; Boulton, A.; Bothne, N.; Coleman, B. Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med 2010, 16(3), 235–49. [Google Scholar] [CrossRef] [PubMed]
- Skilbeck, L. Patient-led integrated cognitive behavioural therapy for management of long COVID with comorbid depression and anxiety in primary care - A case study. Chronic Illn 2022, 18(3), 691–701. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Sawant, H.B.; Flannery, T.; Tarrant, R.; Shardha, J.; Bannister, R.; Ross, D.; Halpin, S.; Greenwood, D.C.; Sivan, M. Effect of using a structured pacing protocol on post-exertional symptom exacerbation and health status in a longitudinal cohort with the post-COVID-19 syndrome. J Med Virol 2023, 95(1), e28373. [Google Scholar] [CrossRef] [PubMed]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8(7). [Google Scholar] [CrossRef] [PubMed]
- San-Millan, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants (Basel) 2023, 12(4). [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial bioenergetic etiology of disease. J Clin Invest 2013, 123(4), 1405–12. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front Med (Lausanne) 2020, 7, 606824. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med 2021, 27(9), 895–906. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.; Maksoud, R.; Eaton-Fitch, N.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med 2020, 18(1), 290. [Google Scholar] [CrossRef] [PubMed]
- Myhill, S.; Booth, N.E.; McLaren-Howard, J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med 2009, 2(1), 1–16. [Google Scholar] [PubMed]
- Castro-Marrero, J.; Cordero, M.D.; Saez-Francas, N.; Jimenez-Gutierrez, C.; Aguilar-Montilla, F.J.; Aliste, L.; Alegre-Martin, J. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal 2013, 19(15), 1855–60. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.; Brown, A.E.; Newton, J.L.; Elson, J.L. Mitochondrial complex activity in permeabilised cells of chronic fatigue syndrome patients using two cell types. PeerJ 2019, 7, e6500. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.L.; Bore, P.J.; Radda, G.K.; Styles, P.; Taylor, D.J. Excessive intracellular acidosis of skeletal muscle on exercise in a patient with a post-viral exhaustion/fatigue syndrome. A 31P nuclear magnetic resonance study. Lancet 1984, 1(8391), 1367–9. [Google Scholar] [CrossRef] [PubMed]
- Plioplys, A.V.; Plioplys, S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology 1995, 32(4), 175–81. [Google Scholar] [CrossRef] [PubMed]
- Pintos-Pascual, I.; Moreno-Torres, V.; Ibanez-Estellez, F.; Corrales-Rodriguez, P.; Trevino, A.; Corpas, M.; Corral, O.; Soriano, V.; de Mendoza, C. Is SARS-CoV-2 the only cause of long-COVID? AIDS Rev 2022, 24(4), 183–196. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, K.; Marshall-Gradisnik, S.; Barth, M.; Eaton-Fitch, N.; Barnden, L. Brainstem volume changes in myalgic encephalomyelitis/chronic fatigue syndrome and long COVID patients. Front Neurosci 2023, 17, 1125208. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.; Brown, A.; Strassheim, V.; Elson, J.L.; Newton, J.; Manning, P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS One 2017, 12(10), e0186802. [Google Scholar] [CrossRef] [PubMed]
- Smits, B.; van den Heuvel, L.; Knoop, H.; Kusters, B.; Janssen, A.; Borm, G.; Bleijenberg, G.; Rodenburg, R.; van Engelen, B. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion 2011, 11(5), 735–8. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; de Miguel, M.; Carmona-Lopez, I.; Bonal, P.; Campa, F.; Moreno-Fernandez, A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol Lett 2010, 31(2), 169–73. [Google Scholar] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; van der Voort, P.H.; Mulder, D.J.; van Goor, H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020, 251(3), 228–248. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol 2020, 319(2), C258–C267. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Tan, M.W.; Dikic, I. Mitochondrial Functions in Infection and Immunity. Trends Cell Biol 2020, 30(4), 263–275. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Munch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583(7816), 469–472. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Eaton, N.; Balinas, C.; Staines, D.; Marshall-Gradisnik, S. Loss of Transient Receptor Potential Melastatin 3 ion channel function in natural killer cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. Mol Med 2018, 24(1), 44. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Balinas, C.; Eaton-Fitch, N.; Staines, D.; Marshall-Gradisnik, S. Validation of impaired Transient Receptor Potential Melastatin 3 ion channel activity in natural killer cells from Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis patients. Mol Med 2019, 25(1), 14. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Eaton-Fitch, N.; Staines, D.R.; Marshall-Gradisnik, S. Potential Therapeutic Benefit of Low Dose Naltrexone in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Role of Transient Receptor Potential Melastatin 3 Ion Channels in Pathophysiology and Treatment. Front Immunol 2021, 12, 687806. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, H.; Muraki, K.; Staines, D.; Marshall-Gradisnik, S. Naltrexone Restores Impaired Transient Receptor Potential Melastatin 3 Ion Channel Function in Natural Killer Cells From Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Front Immunol 2019, 10, 2545. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Annesley, S.J.; Fisher, P.R. Pathological Mechanisms Underlying Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics (Basel) 2019, 9(3). [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol Neurobiol 2014, 49(2), 741–56. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, M.A.L.; Parisien, M.; Boles, R.G.; Drury, G.L.; Smith-Voudouris, J.; Verma, V.; Khoury, S.; Chabot-Dore, A.J.; Nackley, A.G.; Smith, S.B.; Whitehead, W.E.; Zolnoun, D.A.; Slade, G.D.; Tchivileva, I.; Maixner, W.; Diatchenko, L. A genetic polymorphism that is associated with mitochondrial energy metabolism increases risk of fibromyalgia. Pain 2020, 161(12), 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Ovrom, E.A.; Mostert, K.A.; Khakhkhar, S.; McKee, D.P.; Yang, P.; Her, Y.F. A Comprehensive Review of the Genetic and Epigenetic Contributions to the Development of Fibromyalgia. Biomedicines 2023, 11(4). [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Taylor, K.; Kozubek, J.; Sardell, J.; Gardner, S. Genetic risk factors for ME/CFS identified using combinatorial analysis. J Transl Med 2022, 20(1), 598. [Google Scholar] [CrossRef] [PubMed]
- Campisi, L.; La Motta, C. The Use of the Coenzyme Q10 as a Food Supplement in the Management of Fibromyalgia: A Critical Review. Antioxidants (Basel) 2022, 11(10). [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Araque, A.; Verde, Z.; Torres-Ortega, C.; Sainz-Gil, M.; Velasco-Gonzalez, V.; Gonzalez-Bernal, J.J.; Mielgo-Ayuso, J. Effects of Antioxidants on Pain Perception in Patients with Fibromyalgia-A Systematic Review. J Clin Med 2022, 11(9). [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.P.; Mantle, D. Targeted Treatment of Age-Related Fibromyalgia with Supplemental Coenzyme Q10. Adv Exp Med Biol 2021, 1286, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lowry, E.; Marley, J.; McVeigh, J.G.; McSorley, E.; Allsopp, P.; Kerr, D. Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients 2020, 12(9). [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, S.; Askari, G.; Miraghajani, M.; Tavakoly, R.; Arab, A. Effect of coenzyme Q10 supplementation on fatigue: A systematic review of interventional studies. Complement Ther Med 2019, 43, 181–187. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Cordero, M.D.; Segundo, M.J.; Saez-Francas, N.; Calvo, N.; Roman-Malo, L.; Aliste, L.; Fernandez de Sevilla, T.; Alegre, J. Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid Redox Signal 2015, 22(8), 679–85. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Saez-Francas, N.; Segundo, M.J.; Calvo, N.; Faro, M.; Aliste, L.; Fernandez de Sevilla, T.; Alegre, J. Effect of coenzyme Q10 plus nicotinamide adenine dinucleotide supplementation on maximum heart rate after exercise testing in chronic fatigue syndrome: a randomized, controlled, double-blind trial. Clin Nutr 2016, 35(4), 826–34. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Segundo, M.J.; Lacasa, M.; Martinez-Martinez, A.; Sentanes, R.S.; Alegre-Martin, J. Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13(8). [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Domingo, J.C.; Cordobilla, B.; Ferrer, R.; Giralt, M.; Sanmartin-Sentanes, R.; Alegre-Martin, J. Does Coenzyme Q10 Plus Selenium Supplementation Ameliorate Clinical Outcomes by Modulating Oxidative Stress and Inflammation in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Antioxid Redox Signal 2022, 36(10-12), 729–739. [Google Scholar] [CrossRef] [PubMed]
- Banoth, B.; Cassel, S.L. Mitochondria in innate immune signaling. Transl Res 2018, 202, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Horner, S.M. MAVS Coordination of Antiviral Innate Immunity. J Virol 2015, 89(14), 6974–7. [Google Scholar] [CrossRef] [PubMed]
- Roingeard, P.; Eymieux, S.; Burlaud-Gaillard, J.; Hourioux, C.; Patient, R.; Blanchard, E. The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell Mol Life Sci 2022, 79(8), 425. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Melia, C.E.; Snijder, E.J.; Barcena, M. Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol 2020, 28(12), 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Amar, S.; Gehlot, P.; Patra, S.K.; Kanwar, N.; Kanwal, A. Mitochondrial Modulations, Autophagy Pathways Shifts in Viral Infections: Consequences of COVID-19. Int J Mol Sci 2021, 22(15). [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, P.; Ma, W.; Wang, X.; Wang, H.; Yu, Z.; Chang, H.; Wang, T.; Jin, S.; Wang, X.; Wang, W.; Zhao, Y.; Zhao, Y.; Xu, C.; Ma, X.; Gao, Y.; He, H. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell Mol Immunol 2022, 19(1), 67–78. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Das, N.; Mukherjee, P. Picking up a Fight: Fine Tuning Mitochondrial Innate Immune Defenses Against RNA Viruses. Front Microbiol 2020, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Gvozdjakova, A.; Kucharska, J.; Sumbalova, Z.; Rausova, Z.; Chladekova, A.; Komlosi, M.; Szamosova, M.; Mojto, V. The importance of coenzyme Q10 and its ratio to cholesterol in the progress of chronic kidney diseases linked to non-communicable diseases. Bratisl Lek Listy 2020, 121(10), 693–699. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.A.; Marino, G.; Spagnolo, B.; Bianchi, F.P.; Falappone, P.C.F.; Spagnolo, L.; Gatti, P. Coenzyme Q10 + alpha lipoic acid for chronic COVID syndrome. Clin Exp Med 2023, 23(3), 667–678. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.S.; Mogensen, T.H.; Agergaard, J.; Schiottz-Christensen, B.; Ostergaard, L.; Vibholm, L.K.; Leth, S. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur 2023, 24, 100539. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Diaz-Parrado, E.; Carrion, A.M.; Alfonsi, S.; Sanchez-Alcazar, J.A.; Bullon, P.; Battino, M.; de Miguel, M. Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxid Redox Signal 2013, 18(7), 800–7. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; Yokota, S.; Yamamoto, Y. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep 2013, 18(1), 12–9. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gomez, E.; de Miguel, M.; Culic, O.; Carrion, A.M.; Alvarez-Suarez, J.M.; Bullon, P.; Battino, M.; Fernandez-Rodriguez, A.; Sanchez-Alcazar, J.A. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid Redox Signal 2013, 19(12), 1356–61. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gomez, E.; Sanchez-Alcazar, J.A.; Cordero, M.D. Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: results of a small clinical trial. J Clin Psychopharmacol 2014, 34(2), 277–8. [Google Scholar] [CrossRef]
- Alcocer-Gomez, E.; Culic, O.; Navarro-Pando, J.M.; Sanchez-Alcazar, J.A.; Bullon, P. Effect of Coenzyme Q(10) on Psychopathological Symptoms in Fibromyalgia Patients. CNS Neurosci Ther 2017, 23(2), 188–189. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Cano-Garcia, F.J.; Alcocer-Gomez, E.; De Miguel, M.; Sanchez-Alcazar, J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: coenzyme Q10 effect on clinical improvement. PLoS One 2012, 7(4), e35677. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Rossi, A.; Consensi, A.; Giacomelli, C.; Bazzichi, L. Role for a water-soluble form of CoQ10 in female subjects affected by fibromyalgia. A preliminary study. Clin Exp Rheumatol 2017, 35 Suppl 105(3), 20–27. [Google Scholar] [PubMed]
- Sawaddiruk, P.; Apaijai, N.; Paiboonworachat, S.; Kaewchur, T.; Kasitanon, N.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, N.; Chattipakorn, S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic Res 2019, 53(8), 901–909. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.E. An open, pilot study to evaluate the potential benefits of coenzyme Q10 combined with Ginkgo biloba extract in fibromyalgia syndrome. J Int Med Res 2002, 30(2), 195–9. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, V.; Secchettin, E.; Castellani, C.; Martini, A.; Mazzocchi, E.; Picelli, A.; Polati, E.; Donadello, K.; Valenti, M.T.; Dalle Carbonare, L. Comparison between Acupuncture and Nutraceutical Treatment with Migratens((R)) in Patients with Fibromyalgia Syndrome: A Prospective Randomized Clinical Trial. Nutrients 2020, 12(3). [Google Scholar] [CrossRef]
- Israel, A.; Schaffer, A.A.; Cicurel, A.; Feldhamer, I.; Tal, A.; Cheng, K.; Sinha, S.; Schiff, E.; Lavie, G.; Ruppin, E. Identification of drugs associated with reduced severity of COVID-19: A case-control study in a large population. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Moreno Fernandez-Ayala, D.J.; Navas, P.; Lopez-Lluch, G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp Gerontol 2020, 142, 111147. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M.; Pedersen, J.Z.; Incerpi, S. Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone. J Infect Public Health 2020, 13(12), 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022, 28(3), 583–590. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; Saad, J.M.; Han, Y.; Alahdab, F.; Malahfji, M.; Nabi, F.; Mahmarian, J.J.; Cooke, J.P.; Zoghbi, W.A.; Al-Mallah, M.H. Coronary Microvascular Health in Patients With Prior COVID-19 Infection. JACC Cardiovasc Imaging 2022, 15(12), 2153–2155. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.J.; Sykes, R.; McIntosh, A.; Kamdar, A.; Bagot, C.; Bayes, H.K.; Blyth, K.G.; Briscoe, M.; Bulluck, H.; Carrick, D.; Church, C.; Corcoran, D.; Findlay, I.; Gibson, V.B.; Gillespie, L.; Grieve, D.; Hall Barrientos, P.; Ho, A.; Lang, N.N.; Lennie, V.; Lowe, D.J.; Macfarlane, P.W.; Mark, P.B.; Mayne, K.J.; McConnachie, A.; McGeoch, R.; McGinley, C.; McKee, C.; Nordin, S.; Payne, A.; Rankin, A.J.; Robertson, K.E.; Roditi, G.; Ryan, N.; Sattar, N.; Allwood-Spiers, S.; Stobo, D.; Touyz, R.M.; Veldtman, G.; Watkins, S.; Weeden, S.; Weir, R.A.; Welsh, P.; Wereski, R.; Consortium, C.-.; Mangion, K.; Berry, C. A multisystem, cardio-renal investigation of post-COVID-19 illness. Nat Med 2022, 28 (6), 1303-1313. [CrossRef]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and Immune Function: An Overview. Antioxidants (Basel) 2021, 10(5). [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.R.; Mantle, D. COVID-19, Coenzyme Q10 and Selenium. Adv Exp Med Biol 2021, 1327, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Bjornstedt, M.; Rosen, A.; Dahlstrom, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol 2013, 167(5), 1860–6. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Investigators, Q.S.S. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail 2014, 2(6), 641–9. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; Nielsen, H.; Grabowski, P.; Sekulic, S.; Sepulveda, N.; Estevez-Lopez, F.; Zalewski, P.; Pheby, D.F.H.; Castro-Marrero, J.; Sakkas, G.K.; Capelli, E.; Brundsdlund, I.; Cullinan, J.; Krumina, A.; Bergquist, J.; Murovska, M.; Vermuelen, R.C.W.; Lacerda, E.M. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina (Kaunas) 2021, 57(5). [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nojima, J.; Kajimoto, O.; Yamaguti, K.; Nakatomi, Y.; Kuratsune, H.; Watanabe, Y. Ubiquinol-10 supplementation improves autonomic nervous function and cognitive function in chronic fatigue syndrome. Biofactors 2016, 42(4), 431–40. [Google Scholar] [CrossRef] [PubMed]
- Todic, M. Dossier for marketing authorization in the European Union. Bosn J Basic Med Sci 2003, 3(1), 56–60. [Google Scholar] [CrossRef] [PubMed]
- Sardella, M.; Belcher, G.; Lungu, C.; Ignoni, T.; Camisa, M.; Stenver, D.I.; Porcelli, P.; D'Antuono, M.; Castiglione, N.G.; Adams, A.; Furlan, G.; Grisoni, I.; Hall, S.; Boga, L.; Mancini, V.; Ciuca, M.; Chonzi, D.; Edwards, B.; Mangoni, A.A.; Tuccori, M.; Prokofyeva, E.; De Gregorio, F.; Bertazzoli Grabinski Broglio, M.; van Leeuwen, B.; Kruger, P.; Rausch, C.; Le Louet, H. Monitoring the manufacturing and quality of medicines: a fundamental task of pharmacovigilance. Ther Adv Drug Saf 2021, 12, 20420986211038436. [Google Scholar] [CrossRef] [PubMed]
- Kressmann, S.; Muller, W.E.; Blume, H.H. Pharmaceutical quality of different Ginkgo biloba brands. J Pharm Pharmacol 2002, 54(5), 661–9. [Google Scholar] [CrossRef] [PubMed]
- Czigle, S.; Toth, J.; Jedlinszki, N.; Haznagy-Radnai, E.; Csupor, D.; Tekelova, D. Ginkgo biloba Food Supplements on the European Market - Adulteration Patterns Revealed by Quality Control of Selected Samples. Planta Med 2018, 84(6-07), 475–482. [Google Scholar] [CrossRef]
- Walkowiak, A.; Wnuk, K.; Cyrankiewicz, M.; Kupcewicz, B. Discrimination of Adulterated Ginkgo Biloba Products Based on 2T2D Correlation Spectroscopy in UV-Vis Range. Molecules 2022, 27(2). [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Millichap, L.; Castro-Marrero, J.; Hargreaves, I.P. Primary Coenzyme Q10 Deficiency: An Update. Antioxidants (Basel) 2023, 12(8). [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants (Basel) 2020, 9(5). [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lluch, G.; Del Pozo-Cruz, J.; Sanchez-Cuesta, A.; Cortes-Rodriguez, A.B.; Navas, P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 2019, 57, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Lopez-Lluch, G.; Hargreaves, I.P. Coenzyme Q10 Metabolism: A Review of Unresolved Issues. Int J Mol Sci 2023, 24(3). [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Hargreaves, I.P. Mitochondrial Dysfunction and Neurodegenerative Disorders: Role of Nutritional Supplementation. Int J Mol Sci 2022, 23(20). [Google Scholar] [CrossRef] [PubMed]
| Population | Case criteria |
CoQ10 daily dose and duration | Sample size |
Study design |
Outcomes | Refs. |
|---|---|---|---|---|---|---|
| FM | 1990 ACR | 300 mg for 40 days |
20 | RCT | Reduced chronic pain and fatigue, improved mitochondrial bioenergetic function, reduced oxidative stress and inflammation | Cordero et al. 2013 [77] |
| FM | 2010 ACR | 300 mg for 40 days |
20 | RCT | Regulated serotonin levels in platelets and improved depression symptoms |
Alcocer-Gómez et al. 2014 & 2017 [78,79] |
| FM | 1990 ACR | 300 mg for 40 days |
20 cases 15 controls |
Case-control study | Significant negative correlations between CoQ10 or catalase levels in PBMCs and headache parameters, restored biochemical parameters and improved clinical symptoms | Cordero et al. 2012 [80] |
| FM | 1990 ACR | 2 x 200 mg for 6 months |
22 | Open label crossover study | Significantly improved most pain-related outcomes by 24-37%, including fatigue (by ~22%) and sleep disturbance (by ~33%) |
Di Pierro et al. 2017 [81] |
| FM | 2010 ACR | Pregabalin with CoQ10 or pregabalin with placebo for 40 days | 11 | RCT crossover study | Reduced greater pain, anxiety, inflammation, and mitochondrial oxidative stress and also increased GSH levels and superoxide dismutase levels | Sawaddiruk et al. 2019 [82] |
| FM | Not given | 200 mg CoQ10 and 200 mg Ginkgo biloba extract for 84 days | 25 | Open label pilot study | Improved quality-of-life and improved self-rating with 64% claiming to be better and only 9% claiming to feel worse | Lister et al. 2002 [83] |
| FM | 2016 ACR | 150 mg CoQ10 combined with vitamin D, alpha-lipoic acid, magnesium, and tryptophan or acupuncture treatment, both for 3 months | 55 | RCT | Reduced pain at one month after the start of therapy, strengthened after 3 months with the maintenance of treatment | Schweiger et al. 2020 [84] |
| Long COVID | eHRs from Israel Clalit Health Services provider | Not given |
6953 cases 6530 controls |
Retrospective case-control study | Case studies showing ubiquinone associated with significantly reduced odds for COVID-19 hospitalization (OR = 0.185, 95% CI (0.058 to 0.458), p < 0.001) | Israel et al 2021 [85] |
| Long COVID | 2015 IOM/NIH criteria for ME/CFS | 100 mg CoQ10 and 100 mg alpha-lipoic acid (n= 116) or placebo (n= 58) for 60 days | 174 |
Prospective observational study | Complete Fatigue Severity Scale response was reached more frequently in treatment group (53.5%) than in placebo (3.5%) | Barletta et al. 2023 [73] |
| Long COVID | 2021 WHO clinical case definition | 500 mg or placebo for 6 weeks, with cross-over treatment after a 4-week washout period | 119 | RCT crossover trial | No significant benefit on chronic COVID-19 symptoms | Hansen et al. 2023 [74] |
| ME/CFS | 1994 CDC/Fukuda definition | 200 mg CoQ10 and 20 mg of NADH (n = 104) or placebo (n = 103) for 12 weeks |
207 | RCT | Reduced cognitive fatigue perception and overall FIS-40 score and improved quality of life; improved sleep duration at 4 weeks and improved habitual sleep efficiency at 8 weeks | Castro-Marrero et al. 2021 [63] |
| ME/CFS | 1994 CDC/Fukuda definition | 400 mg CoQ10 and 200 mcg selenium for 8 weeks |
27 | Open label exploratory study | Improved overall fatigue severity and global quality of life but no significant effect on the sleep disturbances; increased total antioxidant capacity, reduced lipoperoxides levels, and decreased circulating cytokine levels | Castro-Marrero et al. 2022 [64] |
| ME/CFS | 1994 CDC/Fukuda | 150 mg ubiquinol or placebo for 3 months | 20 | RCT | Improved autonomic function and cognitive function | Fukuda et al. 2016 [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





