1. Introduction
Polymeric scaffolds have been studied with increasing interest for tissue engineering applications, thanks to the availability of a wide range of biocompatible and biodegradable polymers and to the development of several convenient processes for manufacturing interconnected porous structures, e.g., by means of porogenic agents, electrospinning or 3D-printing [
1,
2]. These biomaterials act as three-dimensional scaffolds able to mimic the functionality of the native extracellular matrix (ECM) in the human body and to support cells and guide them towards a specific tissue construct [
1]. Aiming to mimic the physicochemical properties and biological activity of native extracellular matrix, both synthetic and natural-based materials have been employed. Given the complex functionalities of the ECM, the most promising results have been achieved with composite scaffolds combining multiple material classes. In fact, the mechanical and degradation behavior of synthetic polymers is typically easier to control and tailor according to the specific target tissue; on the other hand, bioactivity can be greatly improved by using natural polymers, particularly in the form of hydrogel macromolecular networks [
3,
4,
5].
Especially when dealing with mineralized tissues, it is important to provide adequate mechanical support until new tissue is formed [
6]. Matching the mechanical properties of native tissue is also believed to stimulate proper cell differentiation [
7,
8]. For these reasons, several scaffolds have been realized with stiff thermoplastics, above all biocompatible and biodegradable polyesters such as poly(lactic acid) (PLA) and poly(ε-caprolactone) (PCL) [
9]. These materials have been combined with bioceramics (
e.g., tricalcium phosphate, hydroxyapatite) and/or hydrogels (
e.g., gelatin, chitosan, alginate, hyaluronic acid) to enhance the scaffold bioactivity through multiple solutions, including surface-modified polymers [
10], embedded particles/nanoparticles [
11,
12], co-axial fibers [
13,
14], interpenetrating/semi-interpenetrating networks [
15] and other composite systems [
16,
17,
18]. Moreover, tissue regeneration can be promoted by adding cells and growth factors to the scaffolds [
19].
Mesenchymal stromal cells (MSCs) are the most frequently used stem cells in regenerative medicine approaches thanks to their immunomodulation, tissue regeneration, and protective functions [
20]. MSCs can be derived from different sources, such as bone marrow, adipose tissue, and oral tissues, and they can differentiate into various mesodermal cells, such as bone and cartilage lineages [
21]. It is noteworthy that the paracrine activity of MSCs is one of the most important features that contribute to tissue regeneration [
22]. To consider these cells in different clinical situations, MSCs must fulfill the definition criteria requested by the International Society for Cellular Therapy (ISCT) [
23].
In a previous work [
24], the authors developed a solvent-free manufacturing process for the realization of composite scaffolds for bone regeneration, grafting a stiff PLA-PCL porous core [
25] with a bioactive gelatin-chitosan hydrogel shell [
26], which was found to form a thin layer even into the innermost pores. While the PLA-based core allowed obtaining stiffness and strength comparable with those of mineralized tissues, the shell was grafted on its surface with the aim of promoting cell spreading, proliferation and osteogenic differentiation. Noteworthily, in vitro experiments with human bone marrow mesenchymal stromal cells (BM-hMSCs) proved that also the composite scaffolds could host viable cells and support cell proliferation and osteogenic differentiation.
Based on the recent rise of additive manufacturing (AM) in tissue engineering, the authors deemed it interesting to improve the possibility to tailor their core-shell scaffolds by producing the core through fused deposition modeling (FDM). Indeed, AM technologies allow accurate reproduction of anatomical shapes as well as easy customization of the scaffold internal structure [
27,
28,
29], but few geometrical configurations have been so far studied (0°/90° lay-up [
30,
31], simple cubic cells [
32,
33], and a few alternative designs [
34,
35]). In particular, the design optimization of core-shell scaffolds incorporating a hydrogel within a stiff polymer core would require deeper knowledge of their structure-property correlations [
36,
37,
38]. Therefore, to study the influence of the core structural arrangement on scaffold properties, the authors produced 3D-printed PLA cores with several lattice structures and integrated them with hydrogel [
39]. The influence of lattice geometrical parameters on the hydrogel content, stiffness and strength of the scaffolds was investigated, also considering anisotropic effects due to external shape, lattice strut arrangement and printing direction. The tunability of the scaffold properties was highlighted and the results appeared especially promising in view of bone tissue engineering.
In this work, composite scaffolds with a PLA core and a gelatin-chitosan hydrogel shell were prepared by FDM of PLA lattice structures and subsequent grafting of the bioactive hydrogel, presenting highly interconnected pores obtained by freeze-drying. Such hybrid structures were developed aiming at mimicking the hierarchical structure of bone tissue. Three different types of lattices, hosting different amounts of hydrogel, were investigated. Special attention was dedicated to the study of mechanical properties provided by the core and of the effect of hydrolytic degradation on the scaffold strength, stiffness, and hydrogel stability. Finally, the composite materials were seeded with hBM-MSCs and subjected to cell viability, proliferation, morphology and mineralization assessments. Thanks to the core-shell design, these scaffolds may ensure long-term temporary mechanical support, owing to their stiff core, as well as a favorable environment for cell growth and colonization throughout the hydrogel, which is destined to be faster replaced by new tissue during the regeneration process. Moreover, the overall performance and resorbability of the scaffolds can be easily modulated by properly tailoring their 3D-printed core structure and the associated core-shell proportions.
2. Materials and methods
2.1. Materials
Poly(L-lactic acid) (Raise3D PLA Premium filament, density 1.2 g/cm) was kindly supplied by Raise 3D Technologies, Inc. (Irvine, CA, United States).
Pharmaceutical grade type A gelatin (280 bloom, viscosity 4.30 mPs) was provided by Italgelatine (Cuneo, Italy). Poly(ethylene glycol) diglycidyl ether (PEGDGE) (molecular weight 526 Da) and chitosan (molecular weight 50,000÷190,000 Da, degree of deacetylation 75–85%) were supplied by Sigma-Aldrich Co (Milan, Italy). Ethylene diamine (EDA) and acetic acid were provided by Fluka (Milan, Italy).
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Sigma- Aldrich, Italy.
2.2. Preparation of core-shell composite scaffolds
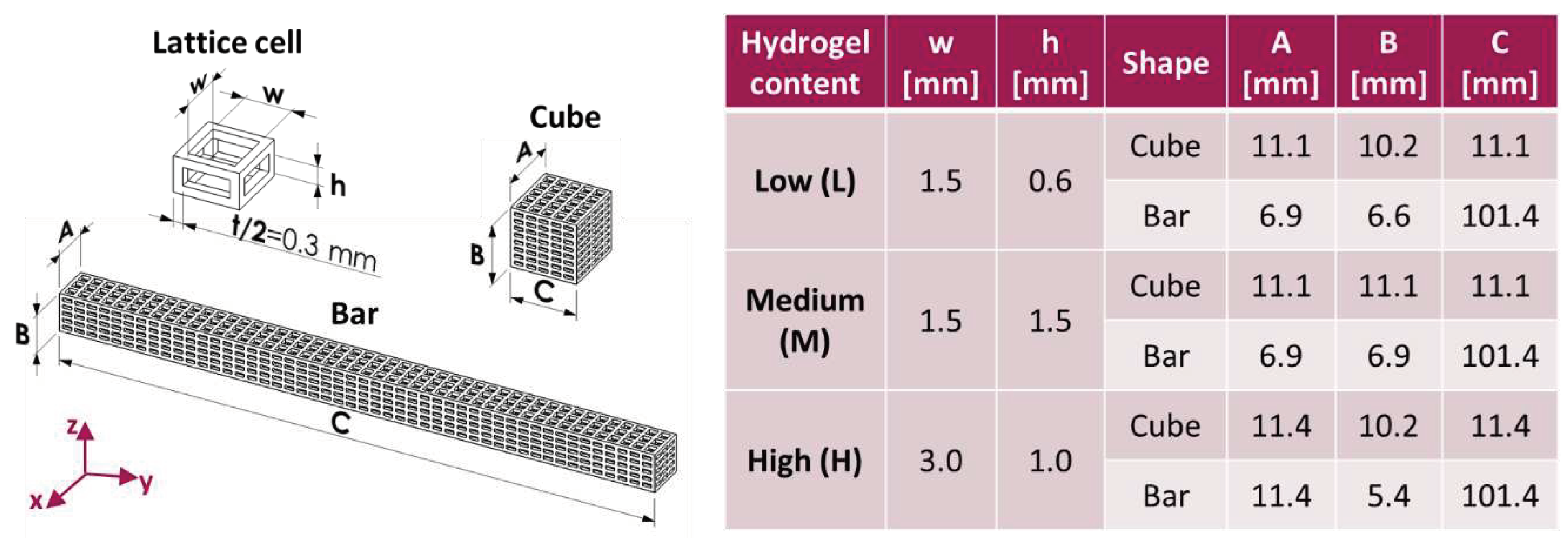
Three types of lattice core structures were designed by means of the software Solidworks (Dassault Systèmes, Vélizy-Villacoublay, France), adopting the cartesian coordinate system in
Figure 1. All structures had the same strut thickness (t = 0.6 mm) but different hole width (w) along x and y and different hole height (h) along z, properly chosen in order to control the amount of hydrogel that may be hosted by the lattice (L: low; M: medium; H: high). Cubic specimens were employed in physical-mechanical characterizations and degradation experiments, while bar specimens were produced for biological characterizations. The outer dimensions of the specimens (A, B, C), as well as the lattice parameters (w, h, t) are all summarized in
Figure 1 for the different types of structures realized.
The core specimens were realized by FDM, using the PLA filament, a slicing software (IdeaMaker) and a 3D-printer (Raise3D Pro2), all provided by Raise 3D Technologies. The slicing of the CAD models was performed adopting z as build direction, with a layer thickness of 0.1 mm and without adding any supports. A 0.2 mm nozzle was employed, and the nozzle temperature and the bed temperature were set to 205 °C and 60 °C, respectively.
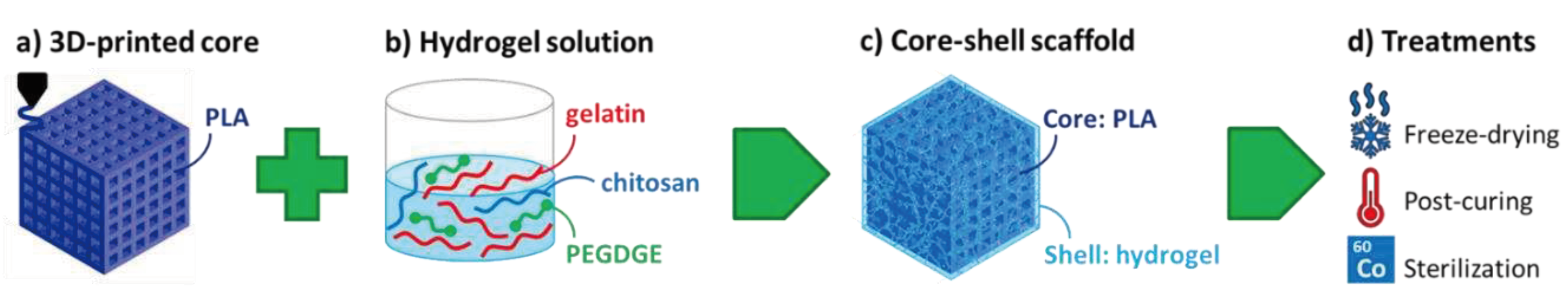
For the hydrogel shell preparation, 6 g of gelatin were dissolved in 65 mL of distilled water at 40 °C under mild magnetic stirring, followed by 1.4 g of PEDGE, 32.5 g of a chitosan solution (2 wt% in acetic acid 1%), and 70 mg of EDA. The reactants were left dissolving and reacting at 40 °C under stirring for about 10 minutes, during which grafting/crosslinking began through condensation reactions between the epoxy groups of PEGDGE and the amino groups of gelatin and chitosan. According to the amounts of ingredients employed, the nominal composition of the dry hydrogel was 74.3 wt% gelatin, 17.6 wt% PEGDGE and 8.1 wt% chitosan.
The lattice core structures were immersed in the hydrogel forming solution at 40 °C, and penetration of the hydrogel into their holes was ensured by applying three cycles of vacuum/air, which helped preventing entrapped air bubbles. After about 40 min at 40 °C followed by 20 min at room temperature, the scaffolds were subjected to freezing and freeze-drying in a lyophilizer (HyperCOOL HC3055, LabTech Srl, Italy). Dry core-shell specimens were freed from the excess hydrogel and crosslinking reactions were completed by post-curing in oven at 45 °C under vacuum for 2 h. Unreacted reagents were finally removed by washing with distilled water, a further freeze-drying treatment was carried out to prevent hydrolysis during storage and the final hydrogel content was evaluated by weight.
2.3. Physical and mechanical characterization
The scaffolds were observed under an optical microscope (Leica DMS 300, Leica Microsystems, Wetzlar, Germany), both in dry conditions and after immersion in distilled water for 24 h.
The theoretical void volume (V
v,th) of the core lattices was obtained from CAD models, while their experimental void volume (V
v,exp) was evaluated as follows:
where V
c is the core volume and V is the core total volume (
i.e., A x B x C, according to
Figure 1). V
c corresponds to m
c/ρ
c, where m
c is the core mass (measured by laboratory balance: Gibertini E42-B, Gibertini Elettronica, Novate Milanese, Italy) and ρ
c is the density of Raise3D PLA Premium.
Theoretical and experimental values of hydrogel content (H
th, i.e., the maximum amount that may be incorporated; H
exp, i.e., the amount actually incorporated) in the scaffolds were calculated by weight through the following equations:
where ρ
s is the density of the hydrogel and m
cs,dry is the mass of the core-shell specimens in dry conditions.
The water uptake (W) of the core-shell specimens was obtained as:
where m
cs,wet is the mass of the core-shell specimens after immersion in water for 24 h.
Compression tests were carried out at room temperature on cubic core and core-shell specimens by means of an electromechanical dynamometer (Instron 3366, Illinois Tool Works Inc., Norwood, MA, United States), equipped with a 10 kN load cell. The load was applied along the transverse direction of the 3D-printed lattices (
i.e., x- or y-direction in
Figure 1), setting the crosshead speed to 2 mm/min. From load (P) and displacement (u) data, apparent stress (σ
app) and apparent strain (ε
app) were calculated through the following equations:
where S is the total area of the specimen cross-section (
i.e., A x B, according to
Figure 1) and A is the cubic specimen height along the compression direction. The term apparent indicates that stress and strain are here evaluated as macromechanical parameters for a homogeneous equivalent material, while local stress and strain are not uniform due to the non-homogeneous structure of the specimens. Finally, compressive stiffness and strength were expressed in terms of apparent modulus (E
app, corresponding to the initial slope of σ
app vs. ε
app curves) and apparent stress at failure (σ
app,f, evaluated at the first peak or knee of the curves), respectively.
2.4. Hydrolytic degradation experiments
The degradation of core-shell scaffolds was studied in distilled water at 37 °C for a total of 7 weeks. The distilled water medium was changed every week.
The evolution of mechanical properties with degradation time was investigated by compression tests on specimens withdrawn at different time points (1 day, 21 days, 35 days, 49 days). Compressive stiffness and strength along degradation time were obtained as described in the previous paragraph.
The mass loss of the specimens was also recorded on scaffolds picked up from the bath at different time points (1 day, 7 days, 21 days, 35 days, 49 days) and dried under vacuum in oven at 40 °C. It was expressed both as percentage of the initial mass of the dry core-shell specimen and as percentage of the initial mass of the dry hydrogel shell:
where m
deg is the mass of the specimens after degradation and drying.
2.5. In vitro characterization, optical and electron microscopy
The bar-shaped core-shell specimens were cut along their length into about 20 slices each, obtaining scaffolds with a thickness of about 5 mm. The scaffolds were packed under vacuum into polypropylene bags and sterilized by gamma irradiation with a dose of 25 kGy of Cobalt 60 gamma rays, according to UNI EN ISO 11137 standard for the sterilization of health care products.
2.5.1. BM-hMSC culture, seeding and osteogenic differentiation
For the purpose of the study, commercial BM-hMSCs (PromoCell, Germany) were expanded, as previously described [
41]. All experiments were conducted with cells between passage 3 and 4.
Dry scaffolds were placed in 24-well non-adherent plate (Corning, Sigma-Aldrich, USA) for cell seeding. The cell concentration was adapted to have the required number of cells in a volume of 50μl of growth medium (GM), consisting of DMEM medium supplemented with FBS. A small drop (7x105/50μl viable BM-hMSCs) was slowly deposited on the top of each dry scaffold, waiting for 2 hours at 37°C for the complete absorption of the drop. After this time, 1 ml of GM was added to each well. The cell culture medium was changed three times per week. Each construct was analyzed on day 28 for cell viability and cell proliferation.
Additionally, we seeded BM-hMSCs in the PLA-CH(L), PLA-CH(M) and PLA-CH(H) to investigate the osteogenic differentiation capability of the scaffolds at day 28, following the protocol previously published [
41]. Particularly, BM-hMSCs were grown in osteogenic medium (OM) for 28 days.
2.5.2. BM-hMSC viability and proliferation assay
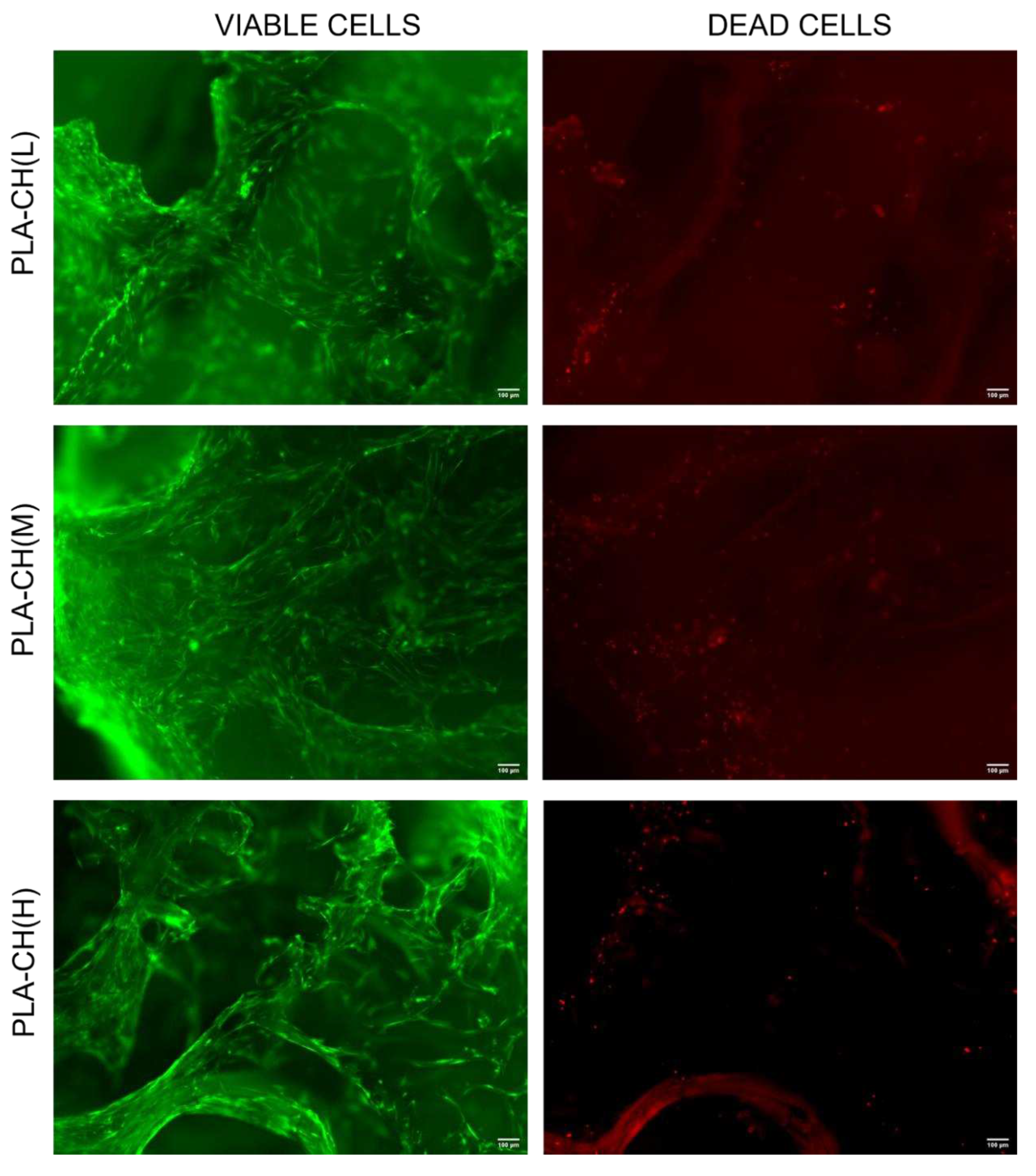
A Live/Dead kit for mammalian cells (ThermoFisher, USA) was used to evaluate cell viability of BM-hMSCs in the scaffolds. The samples were washed with DPBS and incubated for 30-45 min at room temperature in DPBS with 2 μM of calcein AM and 4 μM of ethidium homodimer-1 (EthD-1). NucBlue® Live reagent (2 drops/mL) for nuclei staining was added to the cultures. An analysis of live (stained in green with calcein AM) and dead (stained in red with EthD-1) cells was then performed using a Zeiss Observer Z1 fluorescence microscope.
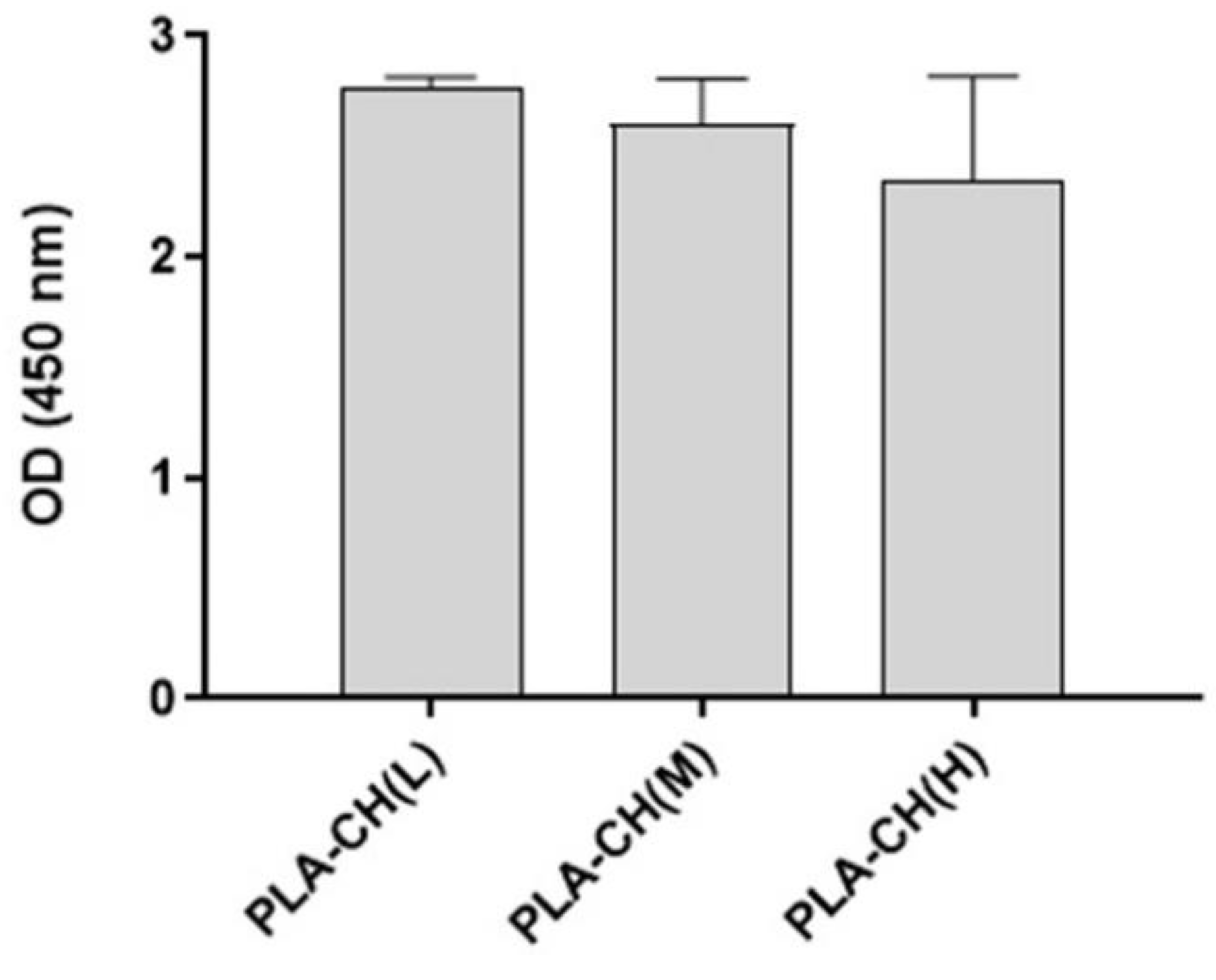
Cell proliferation was determined using the Cell Counting Kit-8 (CCK-8, Sigma-Aldrich, USA) on day 28 of cell culture, following manufacturer instructions. Briefly, the cell-cultured samples (three replicates) were moved to a new cell culture plate and incubated with a fresh culture medium containing the CCK-8 reagent (ratio 1:10) at 37 °C for 2 h 30 min. Then, the absorbance of 100 μL of supernatant transferred to a new cell culture plate was measured at 450 nm with an Infinite 200 PRO plate reader (Tecan, Switzerland). Absorbance at 450 nm is proportional to the number of viable cells in each sample.
2.5.3. Histomorphological analysis at optical microscope
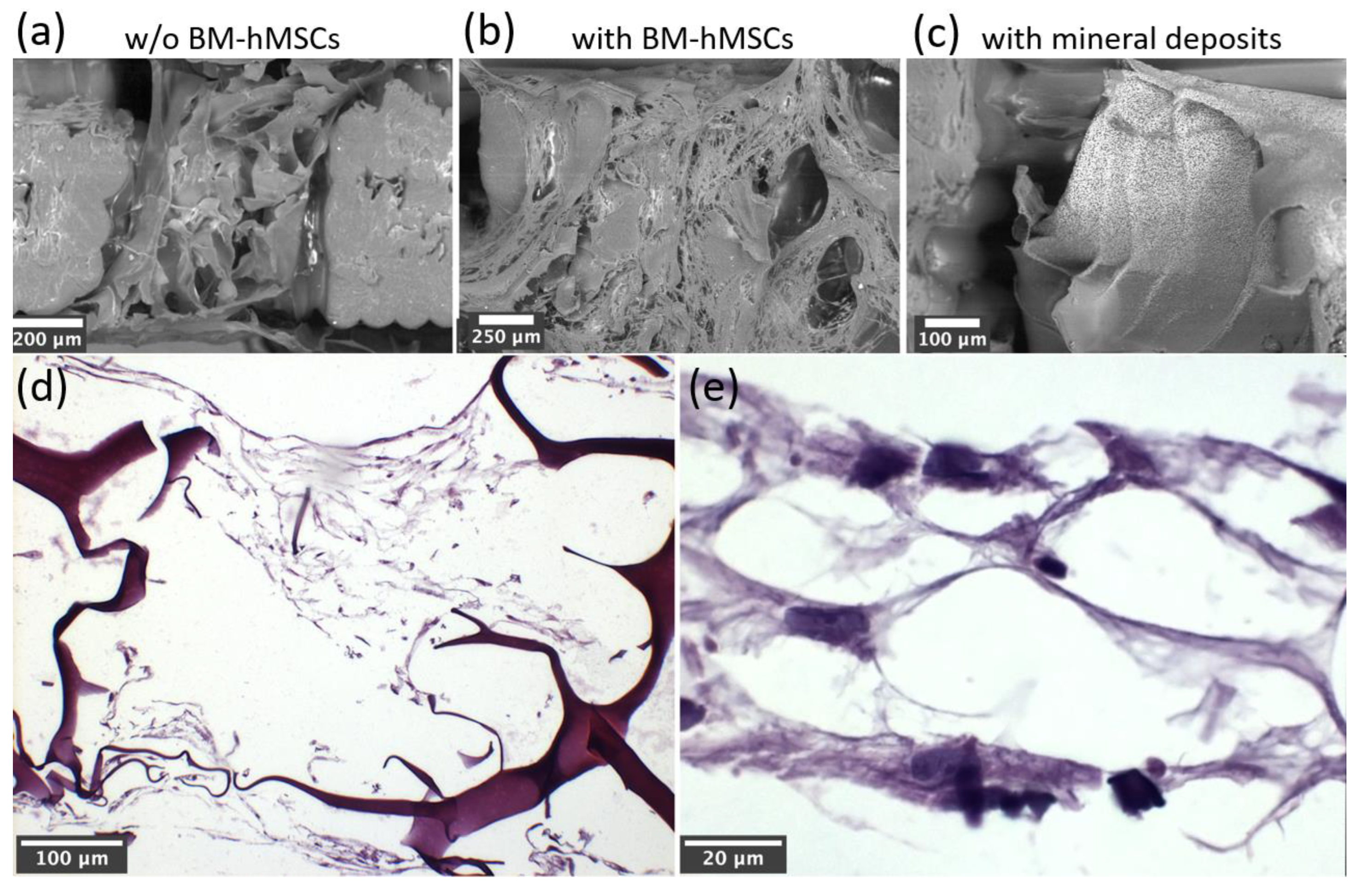
Scaffolds were embedded in paraffin using an automatic processor Donatello series 2 (Diapath S.p.A., Bergamo, Italy). Serial paraffin sections (5 µm thick) of each sample were cut with a semiautomatic microtome Galileo semi-series 2 (Diapath S.p.A., Bergamo, Italy). Alternate sections were deparaffinized, and rehydrated, according to standard procedures and stained with hematoxylin–eosin stain (automatic stainer Giotto; Diapath S.p.A., Bergamo, Italy) for general morphology. The micrographs were recorded using a camera equipped with an image analysis system (Image-Pro Premier 9.1; 2018, Immagini e Computer, Milan, Italy).
2.5.3. Scanning Electron Microscopy observation
Scanning Electron Microscopy (SEM) was used to evaluate both the adhesion and the osteogenic differentiation of the cells. For SEM observation, samples have been progressively dehydrated through immersion in alcohol solutions, with no additional preparation and without deposition of a conductive metal coating. The SEM (EVO LS-10 manufactured by ZEISS) was operated in environmental mode, i.e., at 0.1-0.2 Torr pressure at the specimen. The electron beam was accelerated at 15 keV, and the imaging detector was sensitive to electrons backscattered by the specimen.
2.6. Statistical analysis
Statistical analysis for cell proliferation assay data was performed using two-way ANOVA with Tukey’s multiple comparisons test. Numerical results are presented as mean ± standard deviation (SD). Graphical results were produced using GraphPad Prism (version 7). Three replicates of each sample were used. Statistical significance was accepted at the probability level p < 0.05.
4. Conclusions
In this paper, bioresorbable scaffolds with innovative core-shell architecture were realized by grafting a bioactive hydrogel shell onto a stiff 3D-printed PLA core. The two components were selected in order to provide the scaffolds with complementary functions to better match the multiple requirements for bone tissue engineering applications.
The PLA core was designed with lattice structures having different void volume fractions to modulate its stiffness and strength, aiming at providing tailored mechanical support for specific target tissues. These properties resemble those of bone tissue and remain stable in the long term, as demonstrated by hydrolytic degradation experiments.
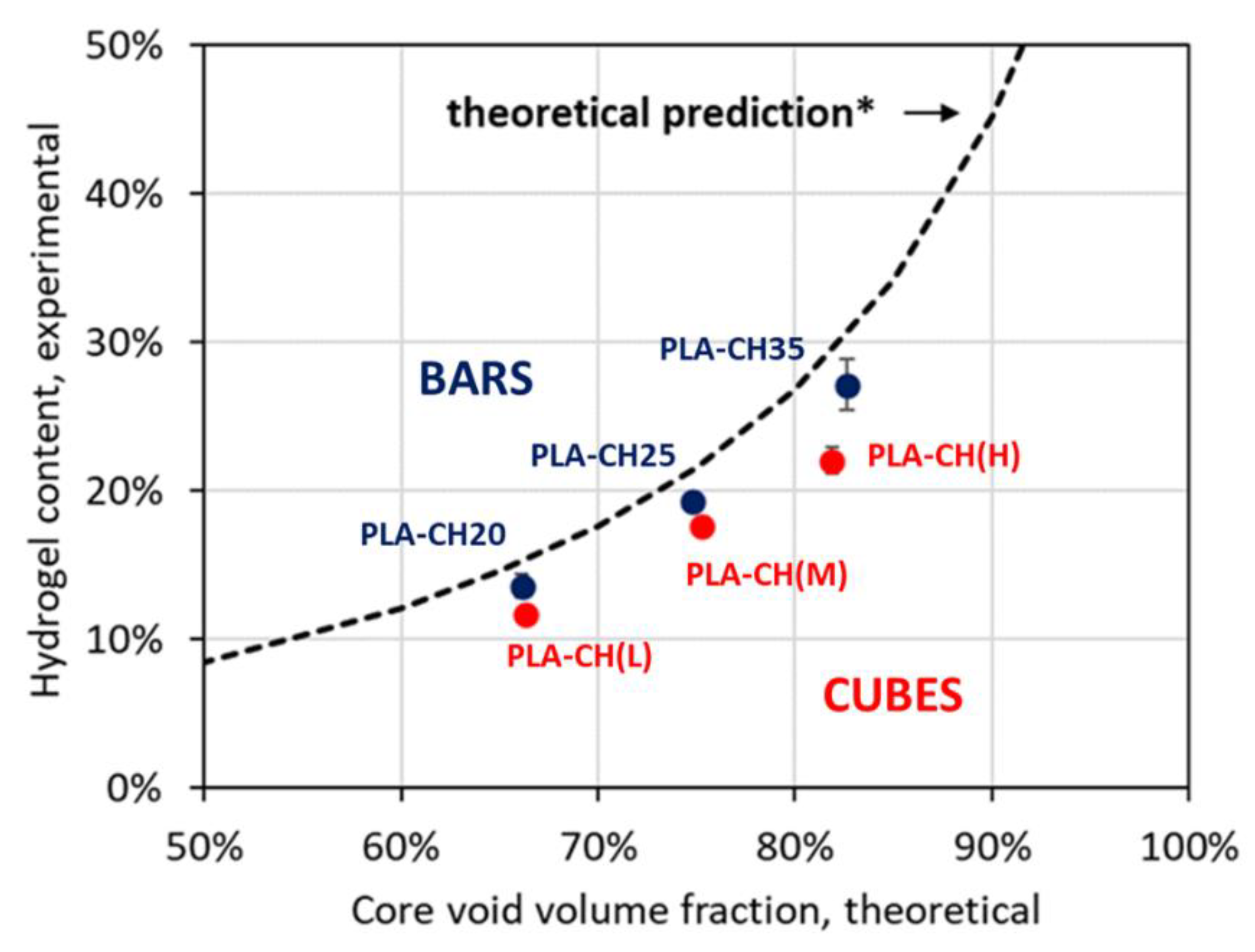
The hydrogel shell filled the entire void volume of the core lattices, reaching experimental contents in good agreement with the theoretical predictions. The hydrogel interconnected porous structure is preserved in the composite scaffolds and represents the preferred microenvironment for cell colonization and mineralization, as verified during in vitro experiments. Moreover, the hydrogel undergoes gradual degradation, leaving space to new bone tissue formation.
Overall, the core-shell scaffolds proved to be biocompatible and promising for bone tissue regeneration and personalized medicine. In fact, their versatile multi-material design and the use of additive manufacturing allow fine tuning of their physical-mechanical and biological properties. In particular, it is fundamental to reach good balance in mechanical support and regenerative potential by properly designing the core-shell proportions and architecture.
Figure 1.
Hole dimensions and global dimensions for lattice-structured cubes and bars capable of hosting low, medium or high hydrogel content values.
Figure 1.
Hole dimensions and global dimensions for lattice-structured cubes and bars capable of hosting low, medium or high hydrogel content values.
Scheme 1.
Fabrication process of composite core-shell scaffolds.
Scheme 1.
Fabrication process of composite core-shell scaffolds.
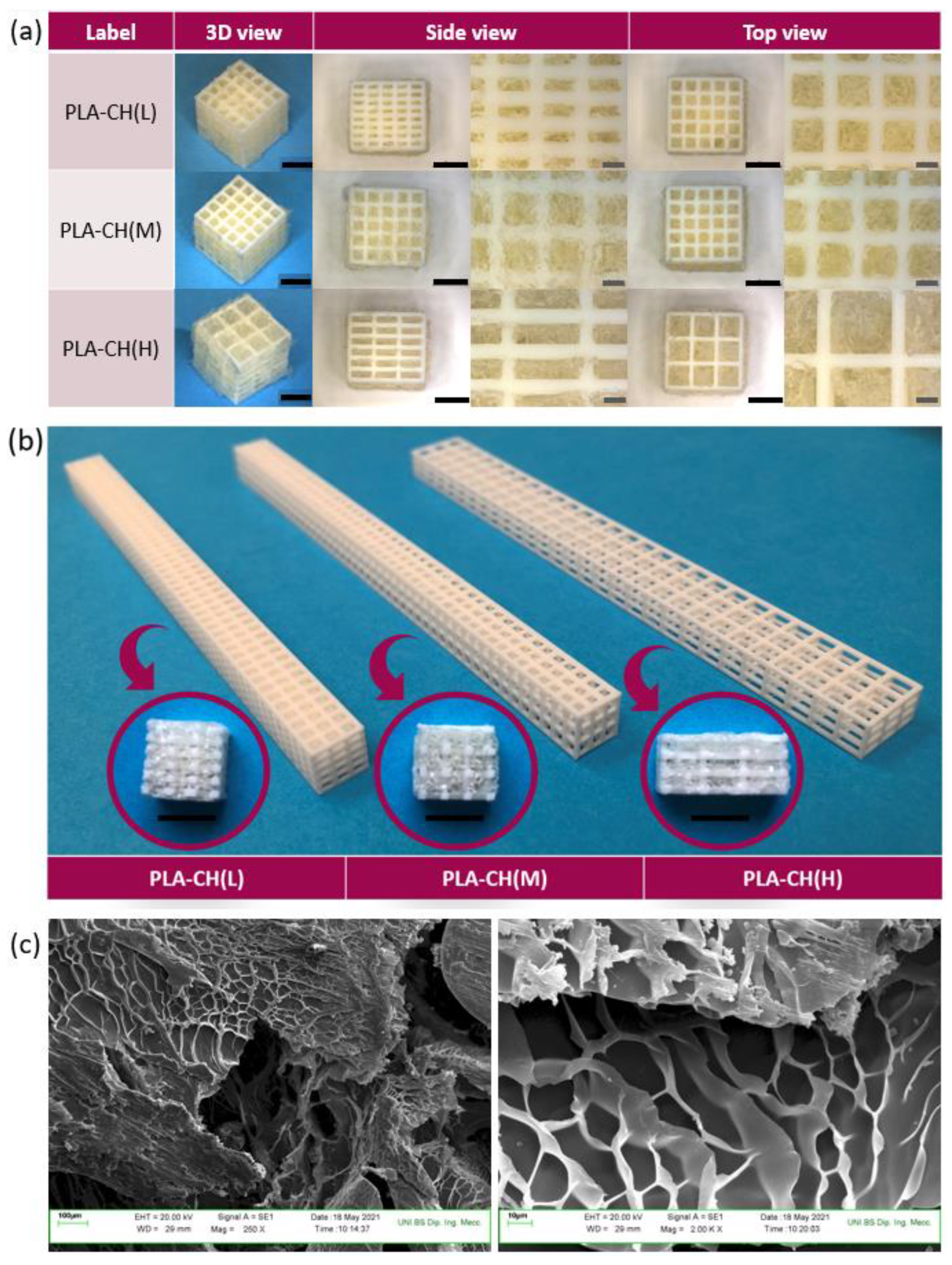
Figure 2.
Core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content: (a) wet cubic specimens (different viewpoints and magnifications); (b) dry slice specimens for in vitro studies, along with the bar-shaped core specimens from which they were obtained. Markers: 5 mm (black) and 1 mm (grey). (c) Porous structure of the hydrogel observed by SEM.
Figure 2.
Core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content: (a) wet cubic specimens (different viewpoints and magnifications); (b) dry slice specimens for in vitro studies, along with the bar-shaped core specimens from which they were obtained. Markers: 5 mm (black) and 1 mm (grey). (c) Porous structure of the hydrogel observed by SEM.
Figure 3.
Hydrogel content (weight fraction) versus core void volume fraction for composite scaffolds with either cubic or bar-shaped core; the dashed line represents the theoretical hydrogel content prediction.
Figure 3.
Hydrogel content (weight fraction) versus core void volume fraction for composite scaffolds with either cubic or bar-shaped core; the dashed line represents the theoretical hydrogel content prediction.
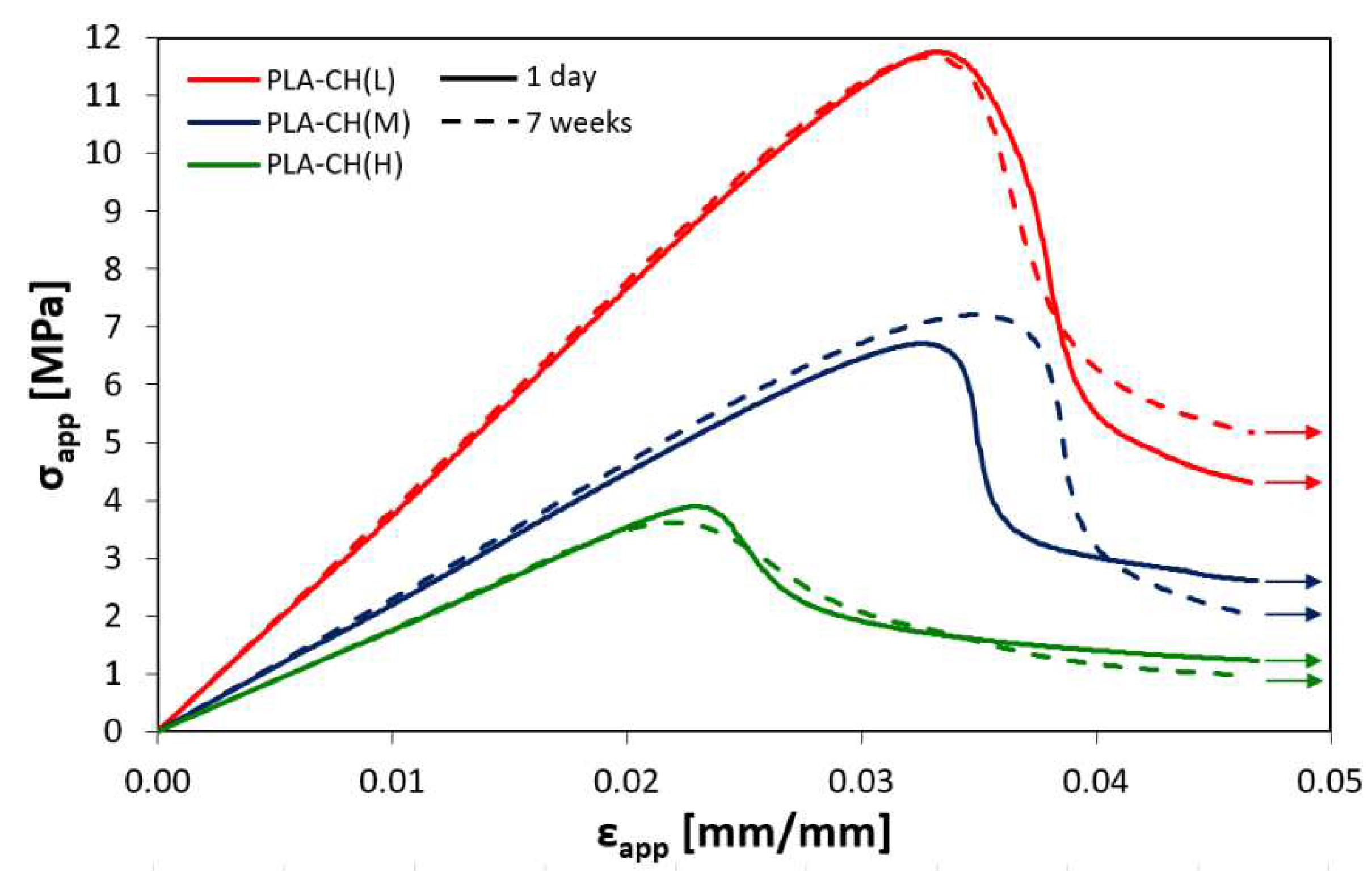
Figure 4.
Apparent stress versus apparent strain curves of core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content tested under compression after 1 day or 7 weeks in water at 37°C.
Figure 4.
Apparent stress versus apparent strain curves of core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content tested under compression after 1 day or 7 weeks in water at 37°C.
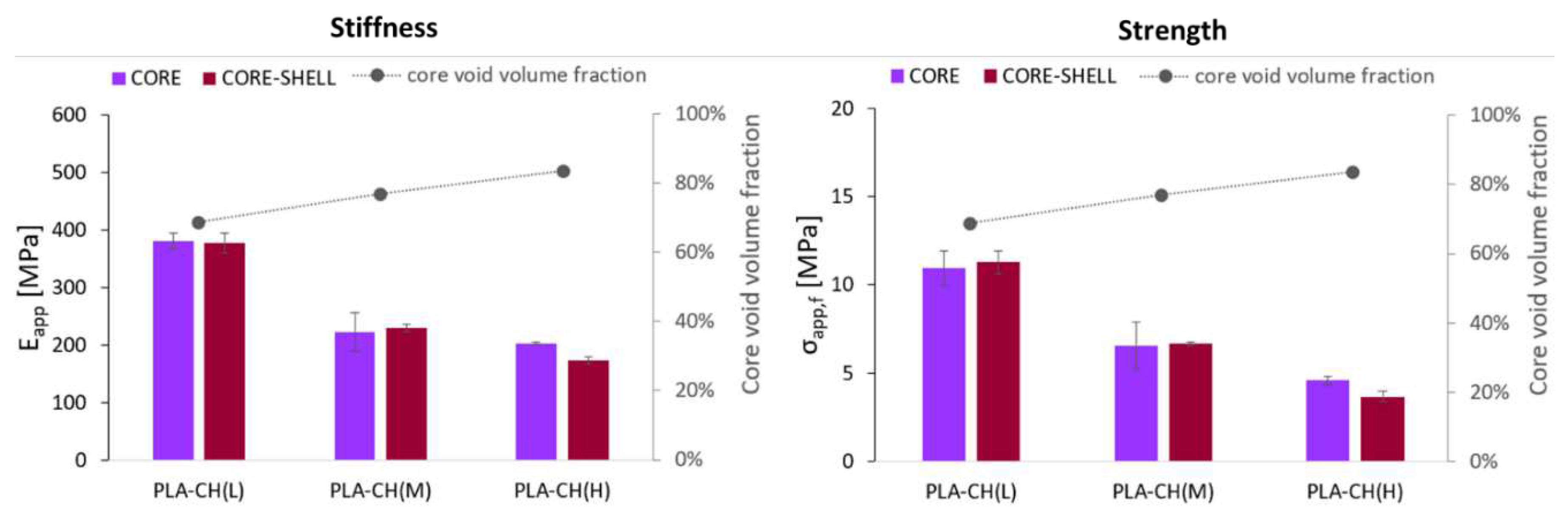
Figure 5.
Apparent modulus (Eapp) and apparent failure stress (σapp,f) for core and core-shell -specimens with low (L), medium (M) or high (H) hydrogel content. The core void volume fraction of the specimens is also reported in both graphs (in grey).
Figure 5.
Apparent modulus (Eapp) and apparent failure stress (σapp,f) for core and core-shell -specimens with low (L), medium (M) or high (H) hydrogel content. The core void volume fraction of the specimens is also reported in both graphs (in grey).
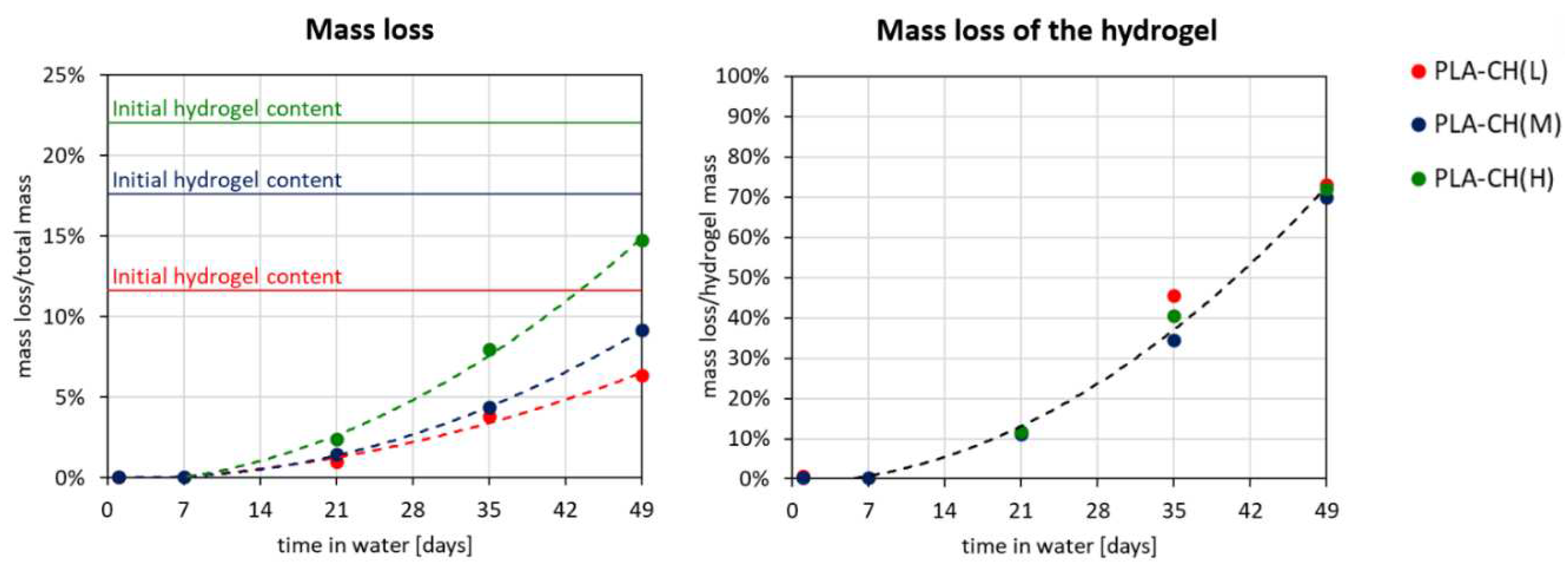
Figure 6.
Mass loss versus degradation time for core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content, expressed both as percentage of the initial mass of the dry core-shell specimen and as percentage of the initial mass of the dry hydrogel shell. The trends are fitted with second-order polynomial curves (dashed lines).
Figure 6.
Mass loss versus degradation time for core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content, expressed both as percentage of the initial mass of the dry core-shell specimen and as percentage of the initial mass of the dry hydrogel shell. The trends are fitted with second-order polynomial curves (dashed lines).
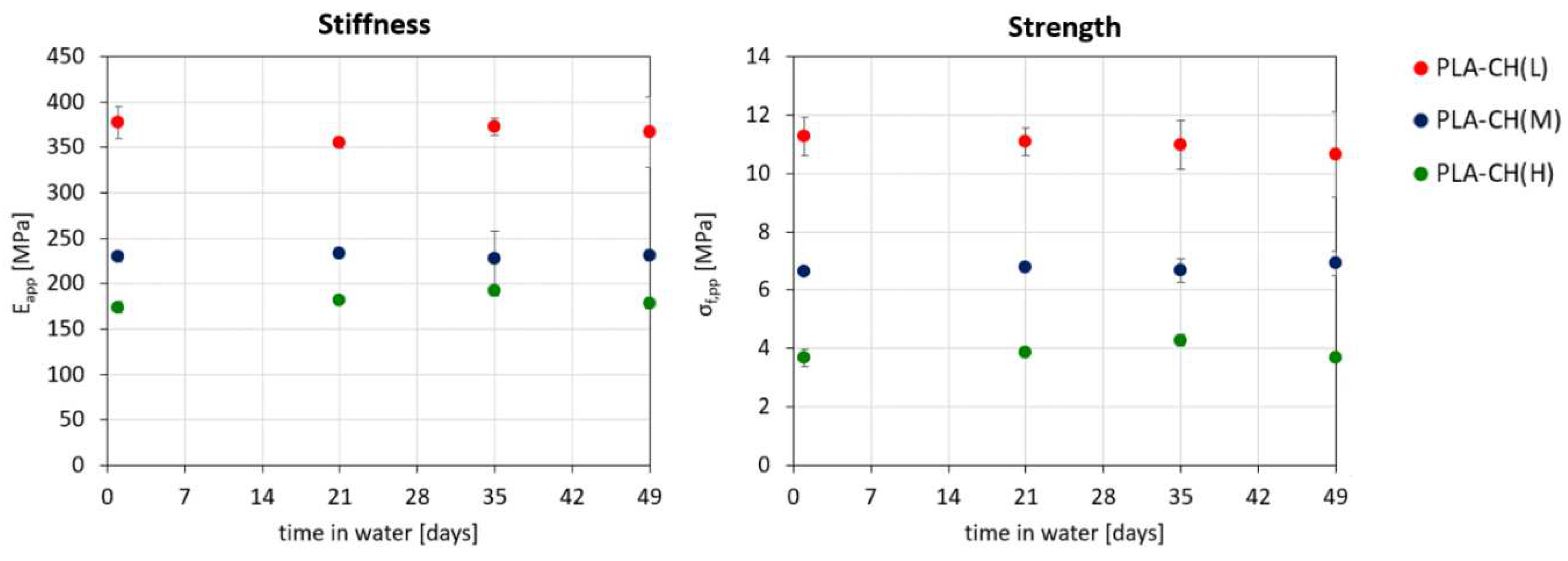
Figure 7.
Stiffness (Eapp) and strength (σapp,f) as a function of degradation time for core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content.
Figure 7.
Stiffness (Eapp) and strength (σapp,f) as a function of degradation time for core-shell scaffolds with low (L), medium (M) or high (H) hydrogel content.
Figure 8.
Live/dead staining of BM-hMSCs cultivated in PLA-CH(L), PLA-CH(M), PLA-CH(H) for 28 days in the GM. Scale bar: 100 μm.
Figure 8.
Live/dead staining of BM-hMSCs cultivated in PLA-CH(L), PLA-CH(M), PLA-CH(H) for 28 days in the GM. Scale bar: 100 μm.
Figure 9.
3D culture proliferation of BM-hMSCs cultivated in PLA-CH(L), PLA-CH(M), PLA-CH(H) in the GM at 28 days measured by the CCK8 assay.
Figure 9.
3D culture proliferation of BM-hMSCs cultivated in PLA-CH(L), PLA-CH(M), PLA-CH(H) in the GM at 28 days measured by the CCK8 assay.
Figure 10.
SEM images of PLA-CH(M) without cells (a), with BM-hMSCs in the GM (b), with BM-hMSCs in the OM and characterized by the production of mineral deposits (c) at 28 days and histomorphological analysis at the optical microscope of PLA-CH(M) with BM-hMSCs in the GM at different magnifications (d,e).
Figure 10.
SEM images of PLA-CH(M) without cells (a), with BM-hMSCs in the GM (b), with BM-hMSCs in the OM and characterized by the production of mineral deposits (c) at 28 days and histomorphological analysis at the optical microscope of PLA-CH(M) with BM-hMSCs in the GM at different magnifications (d,e).
Table 1.
Dimensions of core holes (w x h), core void volume fraction (theoretical and experimental), hydrogel content (weight fraction) and water uptake of cubic composite scaffolds with low (L), medium (M) or high (H) hydrogel content.
Table 1.
Dimensions of core holes (w x h), core void volume fraction (theoretical and experimental), hydrogel content (weight fraction) and water uptake of cubic composite scaffolds with low (L), medium (M) or high (H) hydrogel content.
| Label |
w x h
[mm] |
Core void volume fraction, th [%] |
Core void volume fraction, exp [%] |
Hydrogel content, exp [%] |
Water uptake (24 h) [%] |
| PLA-CH(L) |
1.5 x 0.6 |
66.3 |
68.8 ± 0.3 |
11.6 ± 0.4 |
102.5 ± 7.4 |
| PLA-CH(M) |
1.5 x 1.5 |
75.3 |
77.0 ± 0.3 |
17.6 ± 0.4 |
137.2 ± 4.3 |
| PLA-CH(H) |
3.0 x 1.0 |
81.9 |
83.4± 0.2 |
22.0 ± 0.9 |
201.4 ± 8.9 |