Submitted:
22 November 2023
Posted:
26 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
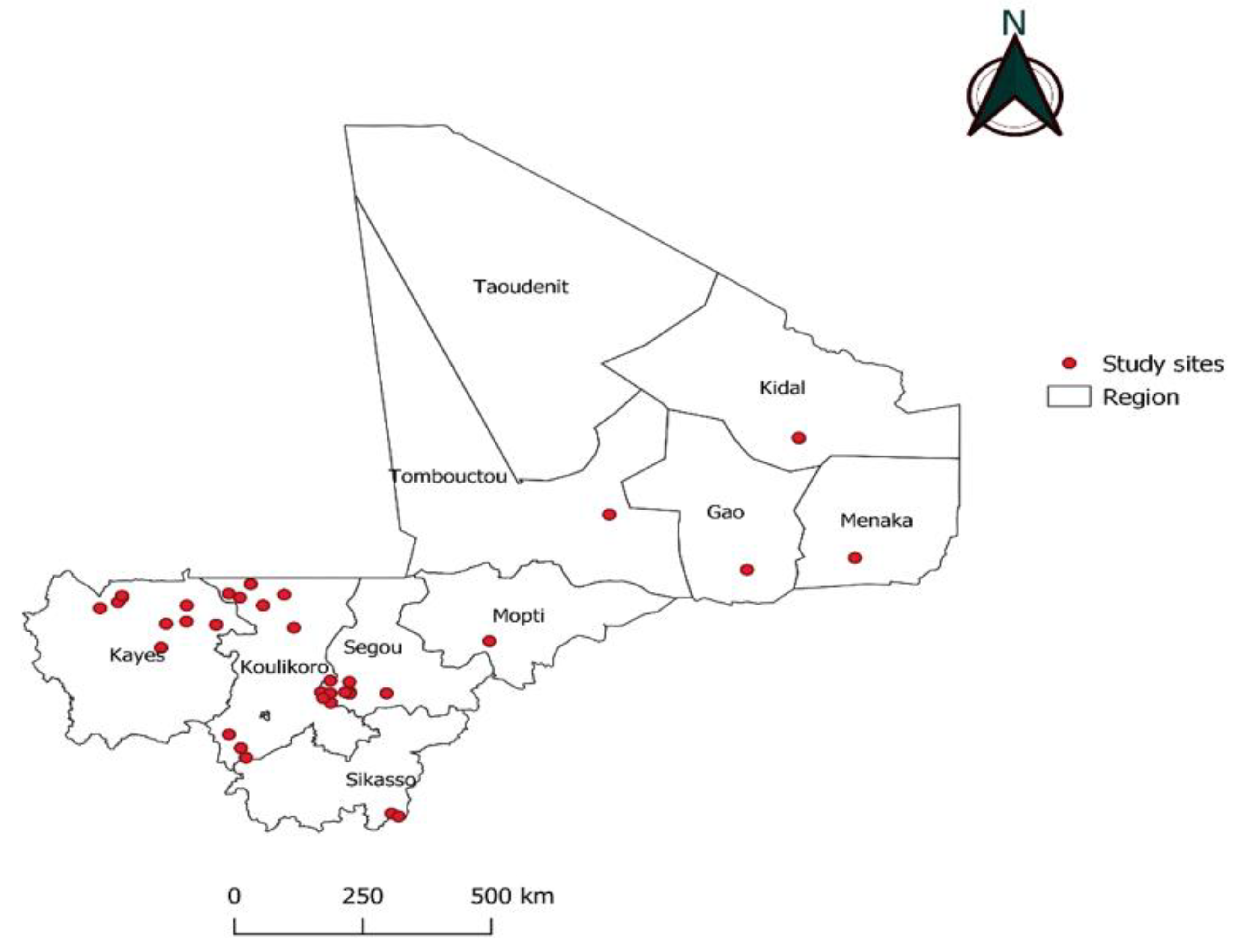
2.1. Study sites and period of sample collection
2.2. Rapid diagnostic test (RDT):
2.3. DNA extraction technique:
2.4. Plasmodium species detection by qPCR
2.5. Case definition
2.6. Data analyses
3. Results
4. Discussion:
5. Conclusions
Authors Contributions
Acknowledgments
Ethical considerations
Competing of Interest
References
- Global messaging: World malaria report 2022 https://www.who.int/publications/m/item/WHO-UCN-GMP-2022.07. Available online: https://www.who.int/publications/m/item/WHO-UCN-GMP-2022.07 (accessed on 5 March 2023).
- OMS World Health Organization World Malaria Report 2020; World Health Organization: Geneva, 2021; ISBN 978-92-4-004049-6.; 2021; p. 322;
- CPS/SS-DS-PF-Annuaire SNIS 2021.
- Institut National de La Statistique (INSTAT) Enquête Demographique et de Santé:Http://Www.Sante.Gov.Ml/Docs/EDSM VI.Pdf.
- Enquête Sur Les Indicateurs Du Paludisme Au Mali En 2021.
- PNLP PSE-MALI 2018-2022 2018.
- Conférence ministérielle sur le paludisme (1992 ; Amsterdam).
- WHO_CDS_RBM_2000.25.Pdf.
- OMS Stratégie Technique Mondiale de lutte contre le Paludisme 2016–2030. 2016, 91, 9–10. 91.
- Ministère de La Santé et Du Développement Social, Programme National de Lutte Contre Le Paludisme. Directives Nationales Pour La Prise En Charge de Cas de Paludisme Au Mali. Bamako: Ministère de La Santé et Du Développement Social; 2020.
- Bell, D.; Wongsrichanalai, C.; Barnwell, J.W. Ensuring Quality and Access for Malaria Diagnosis: How Can It Be Achieved? Nat Rev Microbiol 2006, 4, S7–20. [Google Scholar] [CrossRef] [PubMed]
- Lohfeld, L.; Kangombe-Ngwenya, T.; Winters, A.M.; Chisha, Z.; Hamainza, B.; Kamuliwo, M.; Miller, J.M.; Burns, M.; Bridges, D.J. A Qualitative Review of Implementer Perceptions of the National Community-Level Malaria Surveillance System in Southern Province, Zambia. Malar J 2016, 15, 400. [Google Scholar] [CrossRef]
- Randriatsarafara, F.M.; Mandrosovololona, V.; Andrianirinarison, J.C.; Rakotondrandriana, A.N.; Randrianarivo-Solofoniaina, A.E.; Ratsimbasoa, A.; Rakotomanga, J.; de, D.M. [Adherence of private sector providers to uncomplicated malaria management policy in Madagascar]. Pan Afr Med J 2019, 32, 79. [Google Scholar] [CrossRef] [PubMed]
- Mouatcho, J.C.; Goldring, J.P.D. Malaria Rapid Diagnostic Tests: Challenges and Prospects. Journal of Medical Microbiology 2013, 62, 1491–1505. [Google Scholar] [CrossRef]
- Kozycki, C.T.; Umulisa, N.; Rulisa, S.; Mwikarago, E.I.; Musabyimana, J.P.; Habimana, J.P.; Karema, C.; Krogstad, D.J. False-Negative Malaria Rapid Diagnostic Tests in Rwanda: Impact of Plasmodium Falciparum Isolates Lacking Hrp2 and Declining Malaria Transmission. Malar J 2017, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, M.L.; Aidoo, M.; Kachur, S.P. Malaria Rapid Diagnostic Tests in Elimination Settings—Can They Find the Last Parasite? Clinical Microbiology and Infection 2011, 17, 1624–1631. [Google Scholar] [CrossRef]
- Motshoge, T.; Ababio, G.K.; Aleksenko, L.; Read, J.; Peloewetse, E.; Loeto, M.; Mosweunyane, T.; Moakofhi, K.; Ntebele, D.S.; Chihanga, S.; et al. Molecular Evidence of High Rates of Asymptomatic P. Vivax Infection and Very Low P. Falciparum Malaria in Botswana. BMC Infect Dis 2016, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Feleke, S.M.; Gidey, B.; Mohammed, H.; Nega, D.; Dillu, D.; Haile, M.; Solomon, H.; Parr, J.B.; Tollera, G.; Tasew, G.; et al. Field Performance of Plasmodium Falciparum Lactate Dehydrogenase Rapid Diagnostic Tests during a Large Histidine-Rich Protein 2 Deletion Survey in Ethiopia. Malar J 2022, 21, 236. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Choi, L.; Johnson, S.; Takwoingi, Y. Rapid Diagnostic Tests for Plasmodium Vivax Malaria in Endemic Countries. Cochrane Database of Systematic Reviews 2020, 2020. [Google Scholar] [CrossRef]
- Mens, P.F.; Schoone, G.J.; Kager, P.A.; Schallig, H.D. Detection and Identification of Human Plasmodium Species with Real-Time Quantitative Nucleic Acid Sequence-Based Amplification. Malar J 2006, 5, 80. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High Sensitivity of Detection of Human Malaria Parasites by the Use of Nested Polymerase Chain Reaction. Mol Biochem Parasitol 1993, 61, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Doumbo, O.; Koita, O.; Traore, S.F.; Sangare, O.; Coulibaly, A.; Robert, V.; Soula, G.; Quilici, M.; Toure, Y.T. Les Aspects Parasitologiques de l’épidémiologie Du Paludisme Dans Le Sahara Malien.
- Doumbo, O.; Koita, O.; Traore, S.F.; Sangare, O.; Coulibaly, A.; Robert, V.; Soula, G.; Quilici, M.; Toure, Y.T. LES ASPECTS PARASITOLOGIQUES DE L’EPIDEMIOLOGIE DU PALUDISME. Médecine d’Afrique Noire, 1991; 5. [Google Scholar]
- Koita, O.A.; Sangaré, L.; Sango, H.A.; Dao, S.; Keita, N.; Maiga, M.; Mounkoro, M.; Fané, Z.; Maiga, A.S.; Traoré, K.; et al. Effect of Seasonality and Ecological Factors on the Prevalence of the Four Malaria Parasite Species in Northern Mali. Journal of Tropical Medicine 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Konaté, A.M. Dynamique de l’infection palustre dans une étude de cohorte d’enfant à Bandiagara. Mali. Thesis, 2020. [Google Scholar]
- DNA Extraction: Preparing Rapid Diagnostic Tests. Available online: https://www.wwarn.org/tools-resources/procedures/dna-extraction-preparing-rapid-diagnostic-tests (accessed on 20 March 2022).
- Sazed, S.A.; Kibria, M.G.; Alam, M.S. An Optimized Real-Time QPCR Method for the Effective Detection of Human Malaria Infections. Diagnostics 2021, 11, 736. [Google Scholar] [CrossRef]
- JavaStat -- 2-Way Contingency Table Analysis. Available online: https://statpages.info/ctab2x2.html (accessed on 8 February 2023).
- Un Coefficient d’accord Pour Les Échelles Nominales - Jacob Cohen, 1960. Available online: https://journals.sagepub.com/doi/abs/10.1177/001316446002000104?journalCode=epma (accessed on 19 February 2023).
- Coulibaly, D.; Rebaudet, S.; Travassos, M.; Tolo, Y.; Laurens, M.; Kone, A.K.; Traore, K.; Guindo, A.; Diarra, I.; Niangaly, A.; et al. Spatio-Temporal Analysis of Malaria within a Transmission Season in Bandiagara, Mali. Malar J 2013, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, M.; Gomez-Perez, G.P.; Sissoko, S.; Niambélé, M.B.; Haibala, A.A.; Sanz, A.; Théra, M.A.; Fernandez-Becerra, C.; Traoré, K.; Alonso, P.L.; et al. Plasmodium Vivax Malaria in Mali: A Study from Three Different Regions. Malar J 2012, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Njie, F.; Cairns, M.; Bojang, K.; Coulibaly, S.O.; Kayentao, K.; Abubakar, I.; Akor, F.; Mohammed, K.; Bationo, R.; et al. Non-Falciparum Malaria Infections in Pregnant Women in West Africa. Malar J 2016, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Dao, F.; Dembele, L.; Diarra, B.; Sogore, F.; Marin-Menendez, A.; Goita, S.; Haidara, A.S.; Barre, Y.N.; Sangare, C.P.O.; Kone, A.; et al. The Prevalence of Human Plasmodium Species during Peak Transmission Seasons from 2016 to 2021 in the Rural Commune of Ntjiba, Mali. Trop Med Infect Dis 2023, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Eiam-Ong, S. Malarial Nephropathy. Semin Nephrol 2003, 23, 21–33. [Google Scholar] [CrossRef]
- Hendrickse, R.G.; Adeniyi, A.; Edington, G.M.; Glasgow, E.F.; White, R.H.; Houba, V. Quartan Malarial Nephrotic Syndrome. Collaborative Clinicopathological Study in Nigerian Children. Lancet 1972, 1, 1143–1149. [Google Scholar] [CrossRef]
- Langford, S.; Douglas, N.M.; Lampah, D.A.; Simpson, J.A.; Kenangalem, E.; Sugiarto, P.; Anstey, N.M.; Poespoprodjo, J.R.; Price, R.N. Plasmodium malariae Infection Associated with a High Burden of Anemia: A Hospital-Based Surveillance Study. PLOS Neglected Tropical Diseases 2015, 9, e0004195. [Google Scholar] [CrossRef]
- Dao, F.; Djonor, S.K.; Ayin, C.T.-M.; Adu, G.A.; Sarfo, B.; Nortey, P.; Akuffo, K.O.; Danso-Appiah, A. Burden of Malaria in Children under Five and Caregivers’ Health-Seeking Behaviour for Malaria-Related Symptoms in Artisanal Mining Communities in Ghana. Parasit Vectors 2021, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Cullen, K.A.; Kachur, S.P.; Arguin, P.M.; Baird, J.K. Severe Morbidity and Mortality Risk From Malaria in the United States, 1985–2011. Open Forum Infect Dis 2014, 1, ofu034. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.E.; Jeffery, G.M. Plasmodium Malariae: Parasite and Disease. Clin Microbiol Rev 2007, 20, 579–592. [Google Scholar] [CrossRef]
- Milner, D.A. Malaria Pathogenesis. Cold Spring Harb Perspect Med 2018, 8, a025569. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, G.G.; Böhme, U.; Sanders, M.; Reid, A.J.; Cotton, J.A.; Maiga-Ascofare, O.; Djimdé, A.A.; Apinjoh, T.O.; Amenga-Etego, L.; Manske, M.; et al. Plasmodium Malariae and P. Ovale Genomes Provide Insights into Malaria Parasite Evolution. Nature 2017, 542, 101–104. [Google Scholar] [CrossRef]
- Tebben, K.; Yirampo, S.; Coulibaly, D.; Koné, A.K.; Laurens, M.B.; Stucke, E.M.; Dembélé, A.; Tolo, Y.; Traoré, K.; Niangaly, A.; et al. Malian Children Infected with Plasmodium Ovale and Plasmodium Falciparum Display Very Similar Gene Expression Profiles. PLoS Negl Trop Dis 2023, 17, e0010802. [Google Scholar] [CrossRef]
- Oguike, M.C.; Betson, M.; Burke, M.; Nolder, D.; Stothard, J.R.; Kleinschmidt, I.; Proietti, C.; Bousema, T.; Ndounga, M.; Tanabe, K.; et al. Plasmodium Ovale Curtisi and Plasmodium Ovale Wallikeri Circulate Simultaneously in African Communities. Int J Parasitol 2011, 41, 677–683. [Google Scholar] [CrossRef]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat Rev Dis Primers 2017, 3, 17050. [Google Scholar] [CrossRef]
- Martiáñez-Vendrell, X.; Skjefte, M.; Sikka, R.; Gupta, H. Factors Affecting the Performance of HRP2-Based Malaria Rapid Diagnostic Tests. Tropical Medicine and Infectious Disease 2022, 7. [Google Scholar] [CrossRef]
- Harris, I.; Sharrock, W.W.; Bain, L.M.; Gray, K.-A.; Bobogare, A.; Boaz, L.; Lilley, K.; Krause, D.; Vallely, A.; Johnson, M.-L.; et al. A Large Proportion of Asymptomatic Plasmodium Infections with Low and Sub-Microscopic Parasite Densities in the Low Transmission Setting of Temotu Province, Solomon Islands: Challenges for Malaria Diagnostics in an Elimination Setting. Malar J 2010, 9, 254. [Google Scholar] [CrossRef]
- Maltha, J.; Gillet, P.; Cnops, L.; van den Ende, J.; van Esbroeck, M.; Jacobs, J. Malaria Rapid Diagnostic Tests: Plasmodium Falciparum Infections with High Parasite Densities May Generate False Positive Plasmodium Vivax PLDH Lines. Malar J 2010, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Dembele, P.; Cissoko, M.; Kone, A.; Diarra, A.; Doumbia, L.; Magassa, M.; Mehadji, M.; Thera, M.; Ranque, S. Performance Des Tests de Diagnostic Rapide Pour Le Diagnostic Du Paludisme Au Mali. Revue d’Épidémiologie et de Santé Publique 2023, 71, 101795. [Google Scholar] [CrossRef]
- Agarwal, R.; Choi, L.; Johnson, S.; Takwoingi, Y. Rapid Diagnostic Tests for Plasmodium Vivax Malaria in Endemic Countries. Cochrane Database Syst Rev 2020, 11, CD013218. [Google Scholar] [CrossRef] [PubMed]
- Briolant, S.; Pradines, B.; Basco, L.K. Place de la primaquine dans la lutte contre le paludisme en Afrique francophone. Bull. Soc. Pathol. Exot. 2017, 110, 198–206. [Google Scholar] [CrossRef] [PubMed]
| Target Species | Primers and Probes | Sequences |
|---|---|---|
| Plasmodium spp | VAR ATS-F | CCCATACACAACCAAYTGGA |
| VAR ATS-R | TTCGCACATATCTCTATGTCTATCT | |
| Var ATS-Probe | FAM-TRTTCCATAAATGGT | |
| Plasmodium falciparum | Pf-F | TAGCATATATTAAAATTGTTGCAG |
| Pf-R | GTTATTCCATGCTGTAGTATTCA | |
| Pf-probe | 6FAM-CGGGTAGTCATGATTGAGTTCATTC | |
| Plasmodium malariae | Pm-F | TAGCATATATTAAAATTGTTGCAG |
| Pm-R | GTTATTCCATGCTGTAGTATTCA | |
| Pm-probe | 6FAM- TGCATGGGAATTTTGTTACTTTGAGT | |
| Plasmodium ovale | Po-F | TAGCATATATTAAAATTGTTGCAG |
| Po-F R | GTTATTCCATGCTGTAGTATTCA | |
| Po-probe | 6VIC- TGCATTCCTTATGCAAAATGTGTTC | |
| Plasmodium vivax | Pv-F | AGCATATATTAAAATTGTTGCAG |
| Pv-R | GTTATTCCATGCTGTAGTATTCA | |
| Pv-probe | 6VIC- CGACTTTGTGCGCATTTTGC |
| Area | Health district | RDT collection site | Result | Total | ||
|---|---|---|---|---|---|---|
| Positive N (%) | Negative N (%) | |||||
| Kayes | Diéma | Torodo | 6 (2.2) | 272(97.8) | 278 | |
| Lakamané | 0 (0.0) | 89 (100) | 89 | |||
| Lattakaf | 0 (0.0) | 47 (100) | 47 | |||
| Débomassassi | 0 (0.0) | 48 (100) | 48 | |||
| Koungo | 3 (75.0) | 1 (25.0) | 4 | |||
| Lambidou | 0 (0.0) | 13 (100) | 13 | |||
| Yélimané | kodié | 12 (19.0) | 51(81.0) | 63 | ||
| Csréf | 6 (4,1) | 139 (95.9) | 145 | |||
| Dogofry | 0 (0.0) | 23 (100.0) | 23 | |||
| Bandiougoula | 0 (0.0) | 11 (100.0) | 11 | |||
| sub total | 27 (3.7) | 694 (96.3) | 721 | |||
| Koulikoro | Nara | Bagoini | 0 (0.0) | 50 (100.0) | 50 | |
| Mourdiah | 0 (0.0) | 68 (100.0) | 68 | |||
| Kassakaré | 4 (12.1) | 29 (87.9) | 33 | |||
| Alasso | 1 (3.4) | 28 (96.6) | 29 | |||
| Tiapato | 3 (14.3) | 18 (85.7) | 21 | |||
| Waourou | 0 (0.0) | 13 (100.0) | 13 | |||
| Kangaba | Naréna | 2 (1.6) | 126 (98.4) | 128 | ||
| Cscom Central | 14 (28.0) | 36 (72.0) | 50 | |||
| Séléfougou | 0 (0.0) | 30 (100.0) | 30 | |||
| sub total | 24 (5.7) | 398 (94.3) | 422 | |||
| Sikasso | Kadiolo | cscom central | 110 (59.5) | 75 (40.5) | 185 | |
| Zégoua | 28 (38.89) | 44 (61.1) | 72 | |||
| sub total | 138 (53.7) | 119 (46.3) | 257 | |||
| Segou | Barouéli | Cscom Central | 5 (41.7) | 7 (58.3) | 12 | |
| Dioforogo | 13 (65.0) | 7 (35.0) | 20 | |||
| Tamani | 7 (35.0) | 13 (65.0) | 20 | |||
| NGara | 16 (80.0) | 4 (20.0) | 20 | |||
| Tigui | 20 (100.0) | 0 (0.0) | 20 | |||
| bananido | 7 (50.0) | 7 (50.0) | 14 | |||
| N’Gossola | 5 (31.3) | 11(68.75) | 16 | |||
| Nianzana | 20 (66.7) | 10 (33.3) | 30 | |||
| yerebougou | 16 (80.0) | 4 (20.0) | 20 | |||
| Csréf | 8 (40.0) | 12 (60.0) | 20 | |||
| Ndjila | 10 (50.0) | 10 (50.0) | 20 | |||
| sub total | 127 (59.9) | 85 (40.1) | 212 | |||
| Mopti | Mopti | Soufouroulaye | 79 (34.3) | 151 (65.7) | 230 | |
| Fatoma | 0 (0.0) | 35 (100.0) | 35 | |||
| Sévaré II | 136 (91.9) | 12 (8.1) | 148 | |||
| sub total | 215 (52.1) | 198 (47.9) | 413 | |||
| Tombouctou | Gourma Rharous | 400 (66.9) | 198 (33.1) | 598 | ||
| Gao | Gao | Csref d’Ansongo | 30 (13.4) | 194 (86.6) | 224 | |
| Menaka | Ménaka | Menaka | 21 (36.8) | 36 (63.2) | 57 | |
| Kidal | Kidal | Cscom d’Aliou | 0 (0.0) | 54 (100.0) | 54 | |
| CSRéf de Kidal | 20 (10.3) | 120 (85.7) | 140 | |||
| sub total | 20 (10.3) | 174 (89.7) | 194 | |||
| Total | 1002 (32.3) | 2096 (67.7) | 3098 | |||
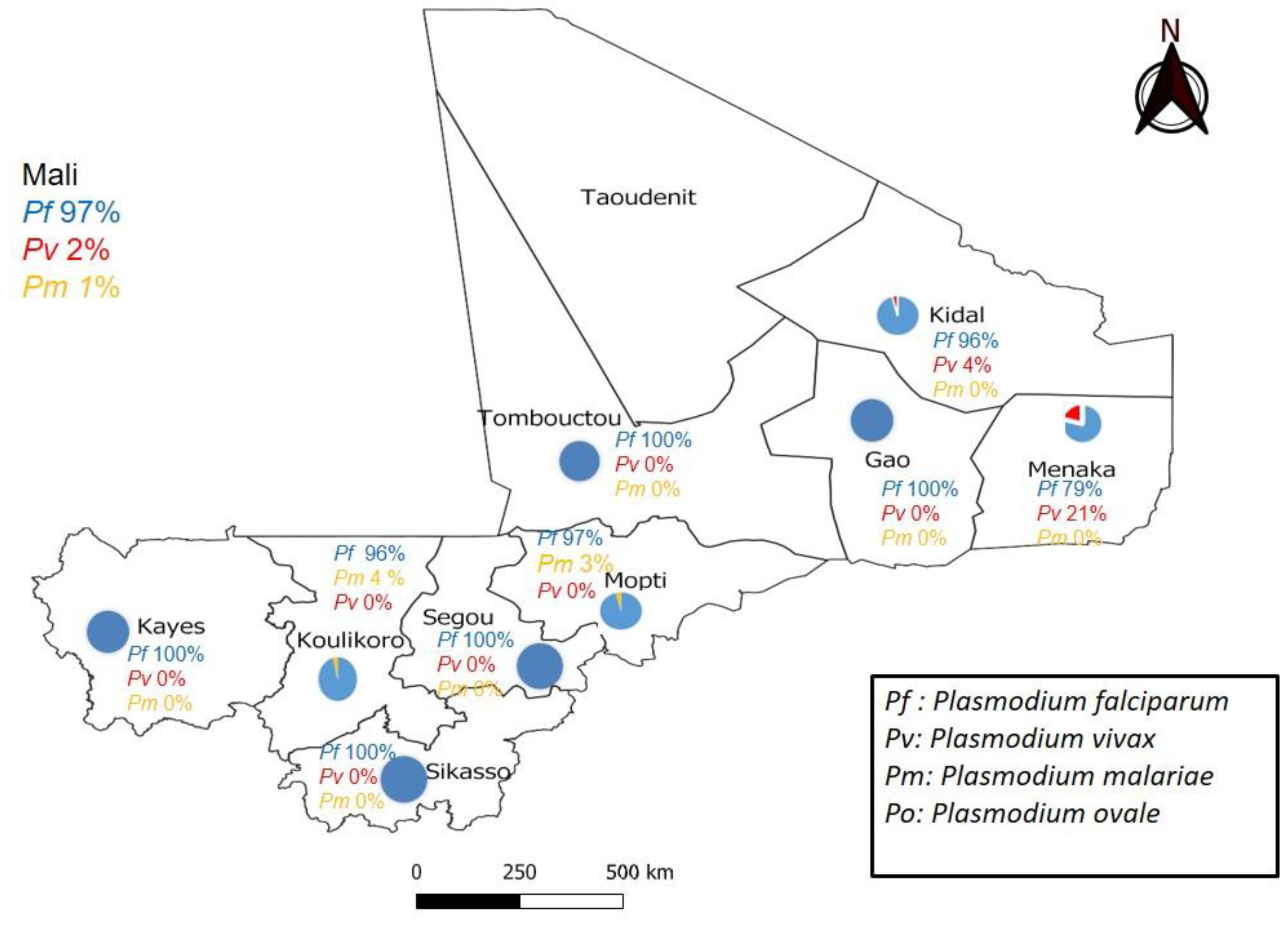
| Regions | P.f | P.v | P.m | P.o | Mixed Pf+P.m | Mixed Pf+P.v | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Kayes | 33 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Koulikoro | 28 | 100 | 0 | 0 | 1 | 3.57 | 0 | 0 | 1 | 3.57 | 0 | 0 | 28 |
| Sikasso | 30 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 |
| Ségou | 42 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 |
| Mopti | 33 | 97.05 | 0 | 0 | 1 | 2.90 | 0 | 0 | 0 | 0 | 0 | 0 | 34 |
| Tombouctou | 30 | 96.77 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 |
| Gao | 18 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
| Kidal | 22 | 95.65 | 1 | 4.34 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4.34 | 23 |
| Ménaka | 15 | 79 | 4 | 21.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 |
| Total | 251 | 97.29 | 5 | 1.94 | 2 | 0.78 | 0 | 0 | 1 | 0.39 | 1 | 0.39 | 258 |
| Regions | Sensitivity (Se) | Specificity (Sp) | Youden Index | Kappa |
|---|---|---|---|---|
| Kayes | 87.9 % [76.9 - 90.7] | 99.3 % [96.9 - 100] | 0.87 [0.74 - 0.91] | 0.90 [0.77 - 0.94] |
| Koulikoro | 96.4 % [83.9- 99.8] | 98 % [95.7 - 98.6] | 0.95 [0.80 - 0.99] | 0.92 [0.77 - 0.96] |
| Sikasso | 83.3 % [69.2 - 92] | 95.1 % [90.9 -97.6] | 0.78 [0.60 - 0.90] | 0.78 [0.60 - 0.90] |
| Segou | 71.4 % [61.9- 73.7] | 98.9 % [94.4 - 99.9] | 0.70 [0.56 - 0.74] | 0.76 [0.60 - 0.79] |
| Mopti | 88.2 % [79 - 88] | 100 % [0.98 - 100] | 0.88 [0.76 - 0.88] | 0.92 [0.80 - 0.92] |
| Tombouctou | 85.3 % [74.4 - 88.1] | 99.3 % [96.8 - 100] | 0.85 [0.71 - 0.88] | 0.89 [0.75 - 0.92] |
| Gao | 94.7 % [74.6 - 99.7 | 92.5 % [90.2 - 93.1] | 0.87 [0.65 - 0.93] | 0.70 [0.52 - 0.74] |
| Kidal | 65.2 % [45.8 - 808] | 91.8 % [88.7 - 94] | 0.57 [0.35 - 0.75] | 0.53 [0.32 - 0.70] |
| Ménaka | 89.5 % [70.9 - 98] | 89.5 % [80.2 - 93.7] | 0.79 [0.51 - 0.92] | 0.77 [0.50 - 89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





