Submitted:
24 November 2023
Posted:
26 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
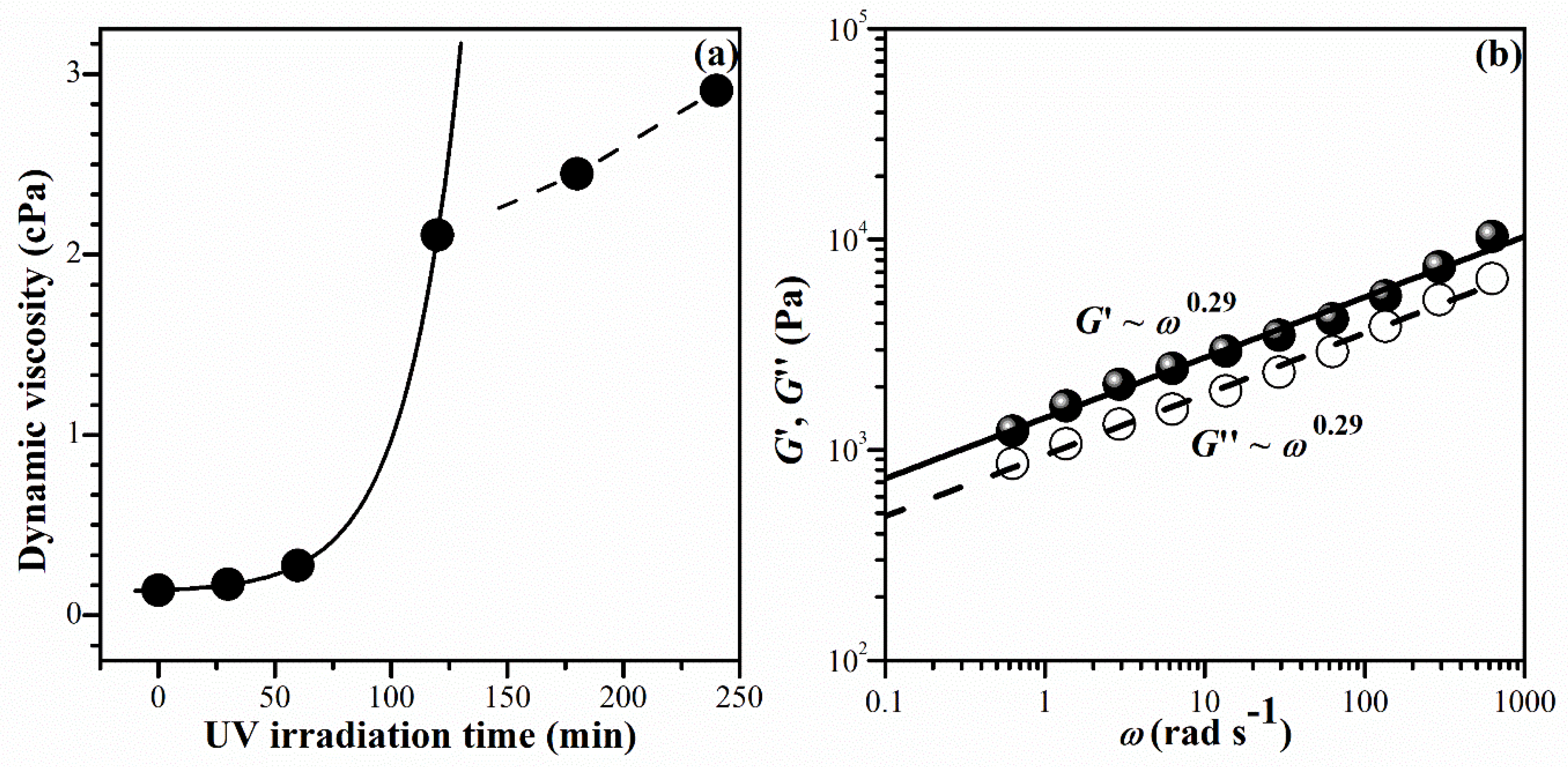
2.1. UV-induced sol to gel transition of PNiPAM solution
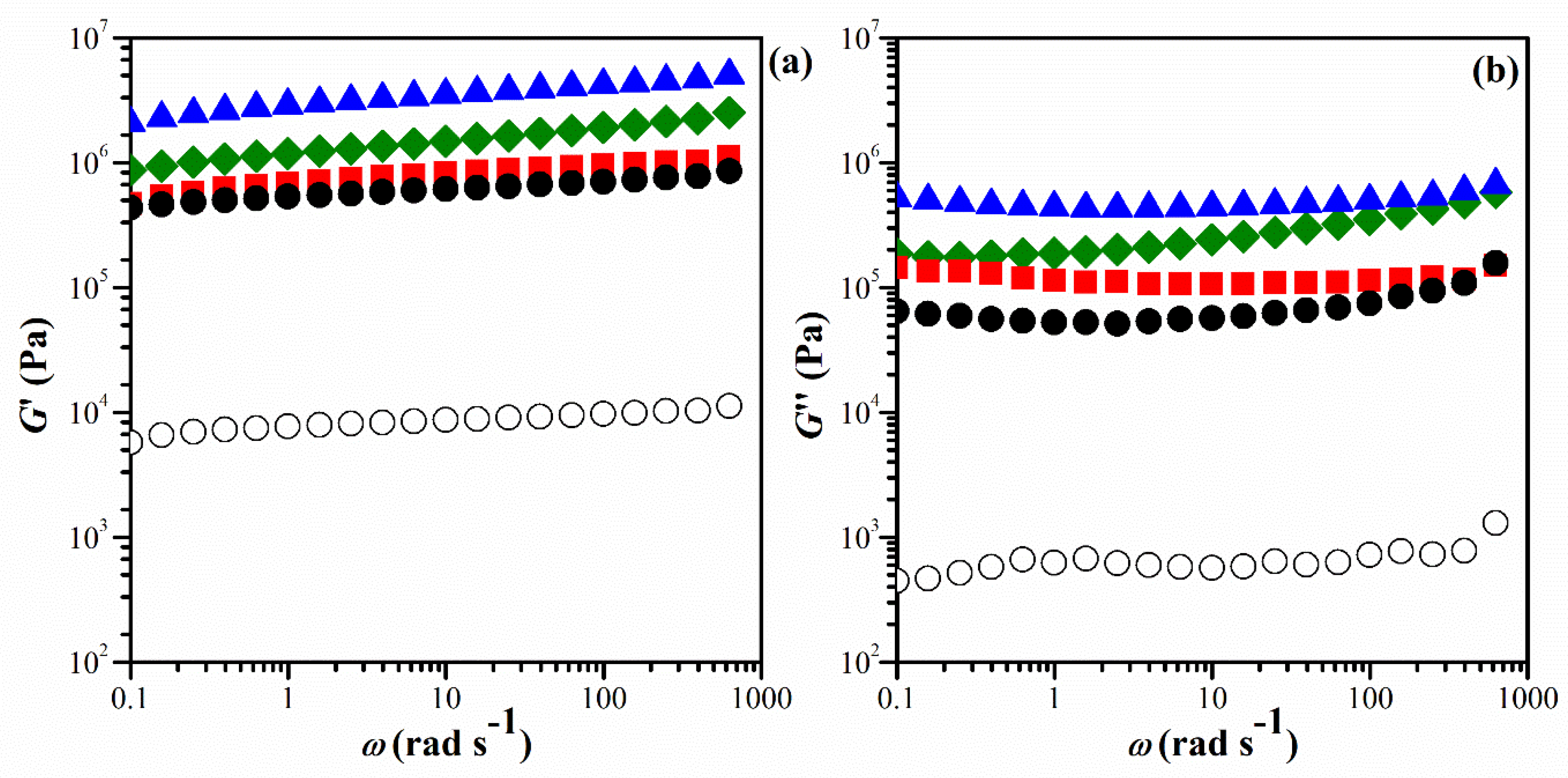
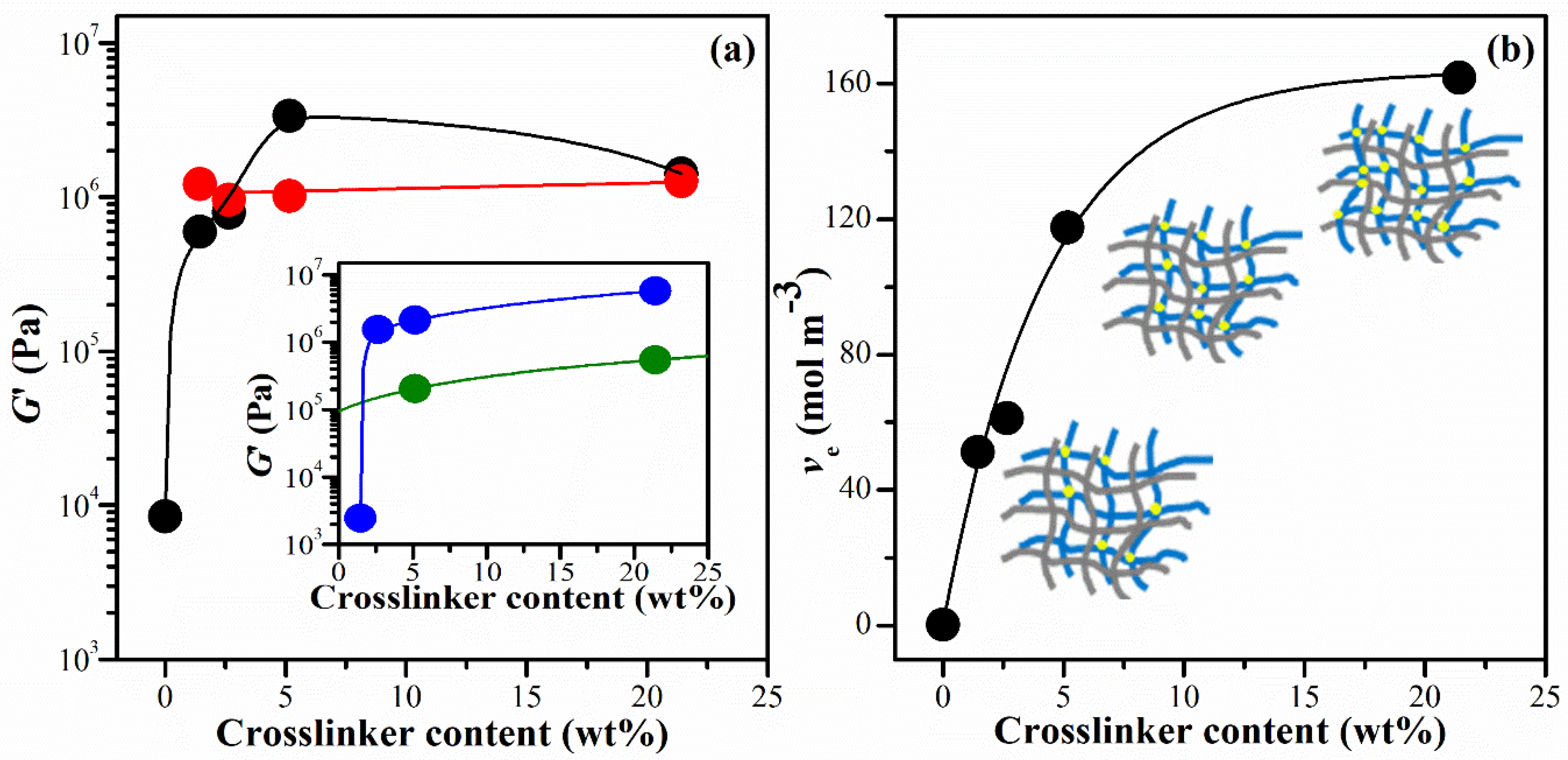
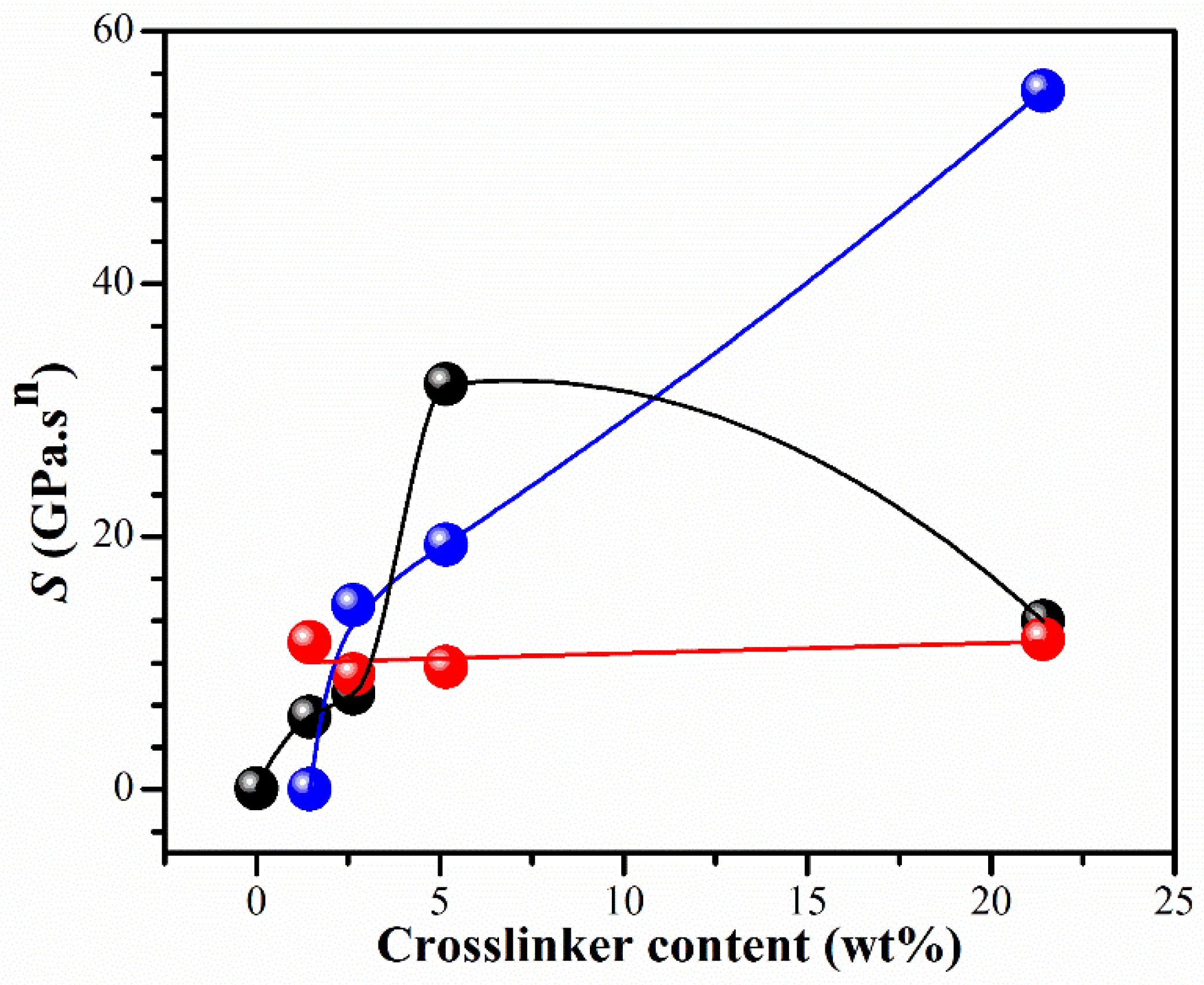
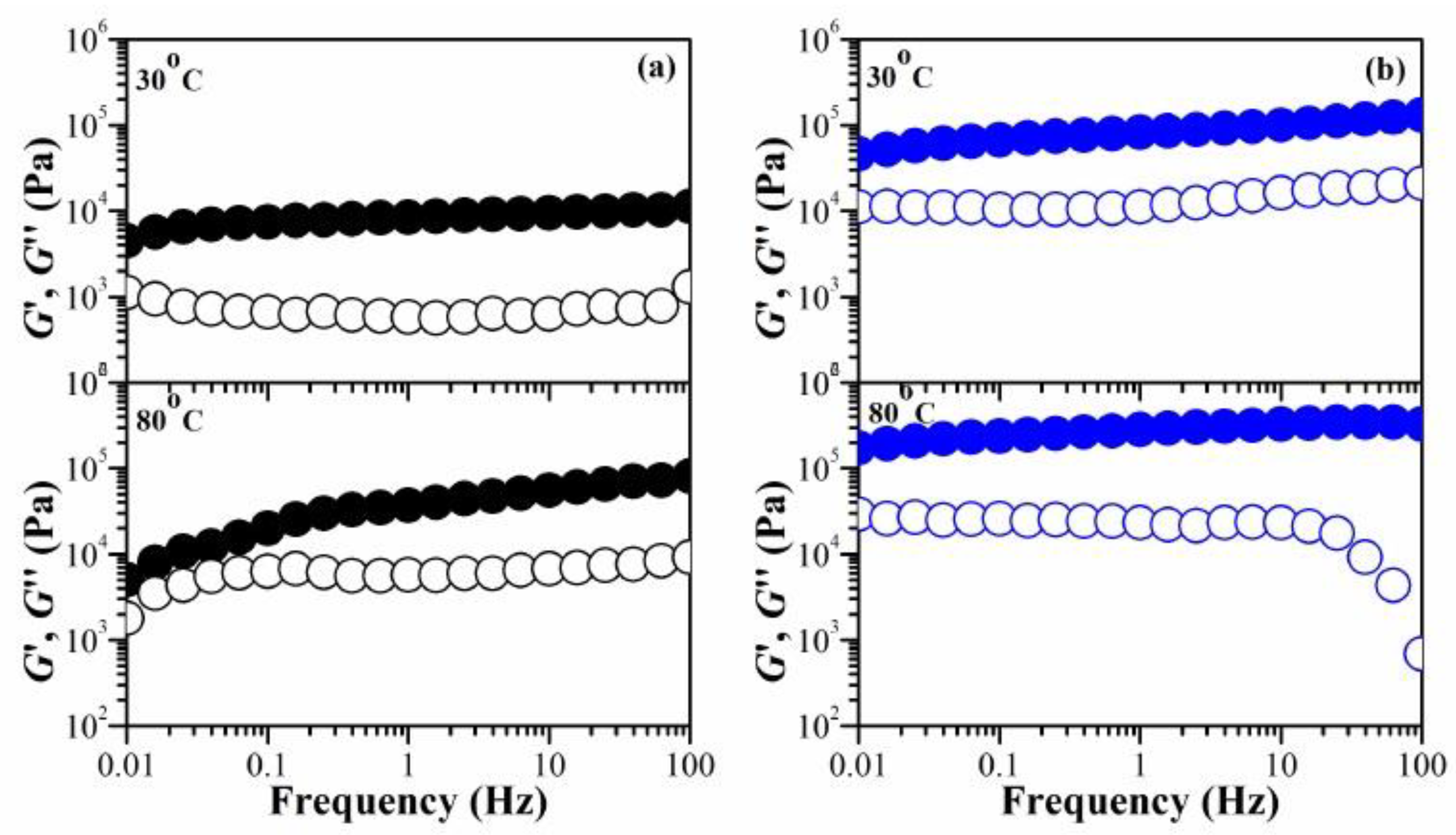
2.2. Effect of crosslinker concentration on viscoelastic and mechanical behaviors of single and interpenetrating polymer networks
2.3. Temperature dependence on mechanical stability of swollen hydrogel networks
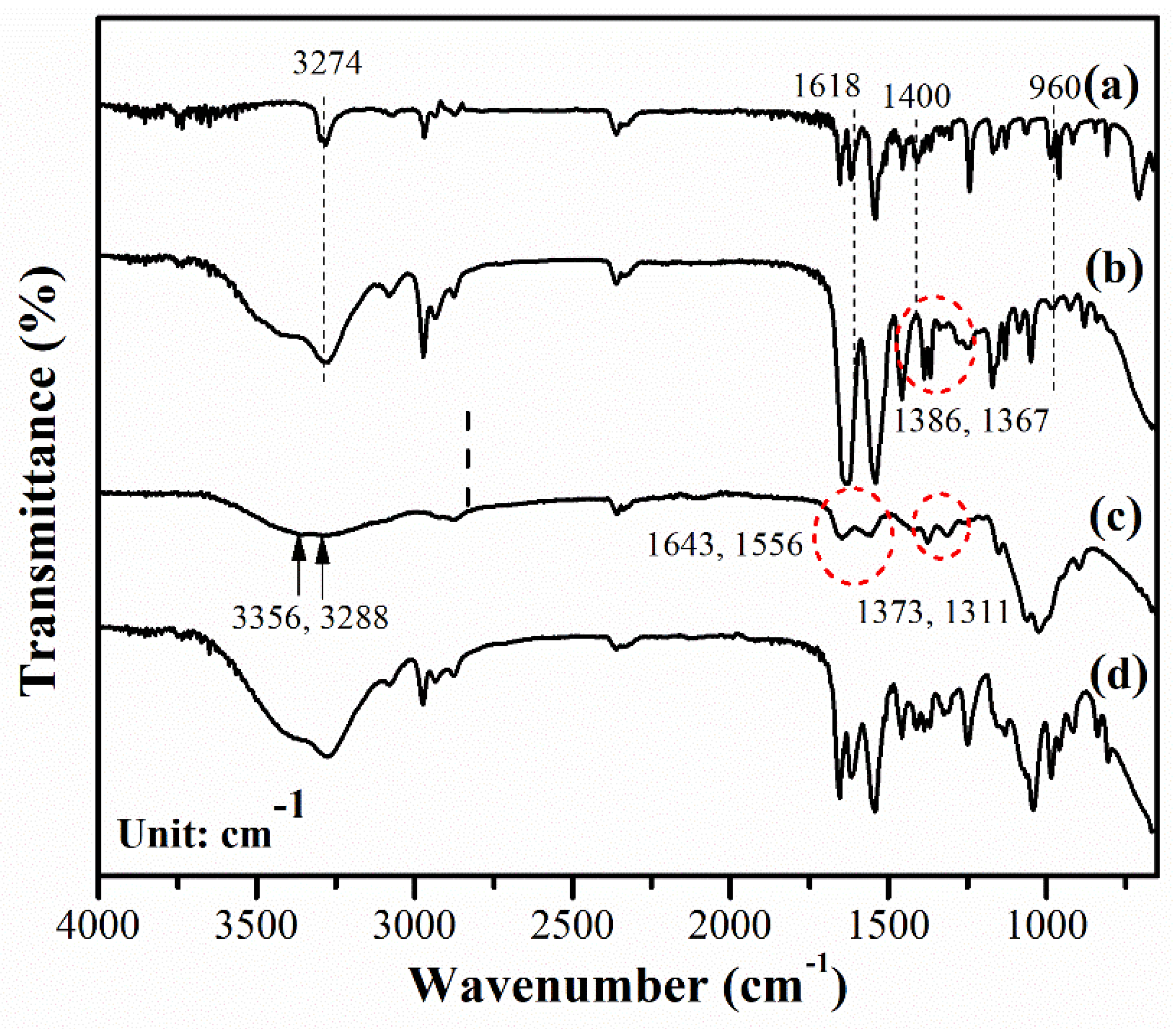
2.4. Chemical structure of single and IPN hydrogels
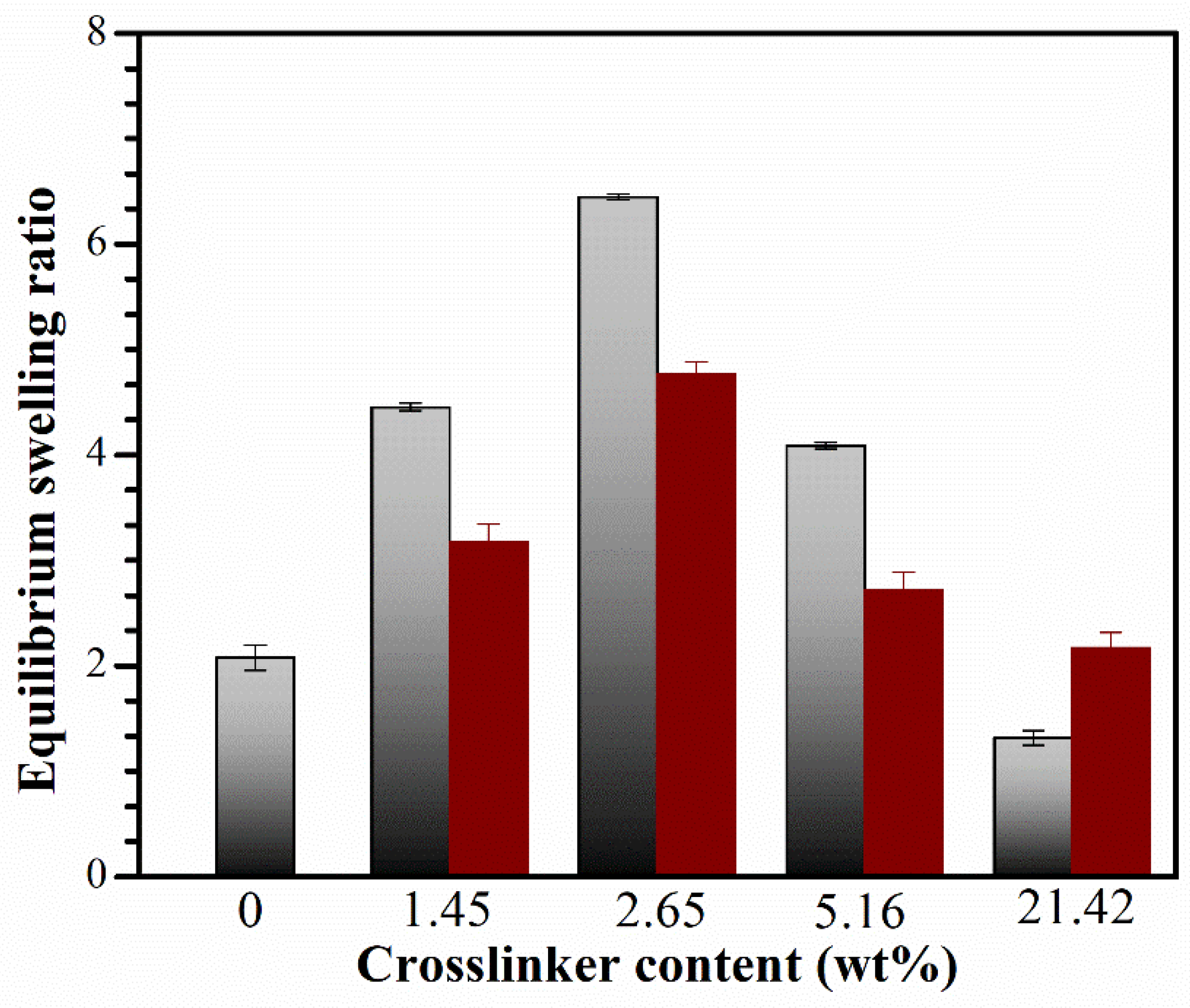
2.5. Equilibrium swelling ratio (SRe) of hydrogel networks
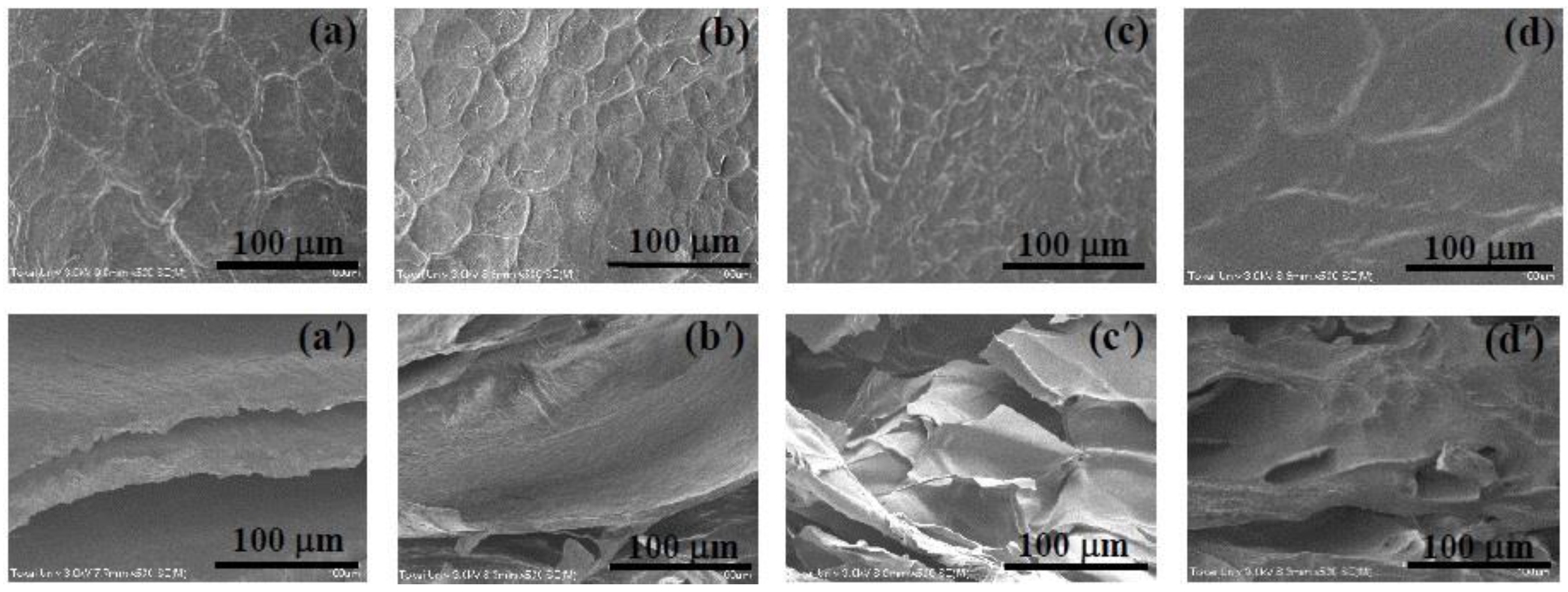
2.6. Microstructure of the CS and IPN hydrogels
2.7. Wettability of hydrogel surface
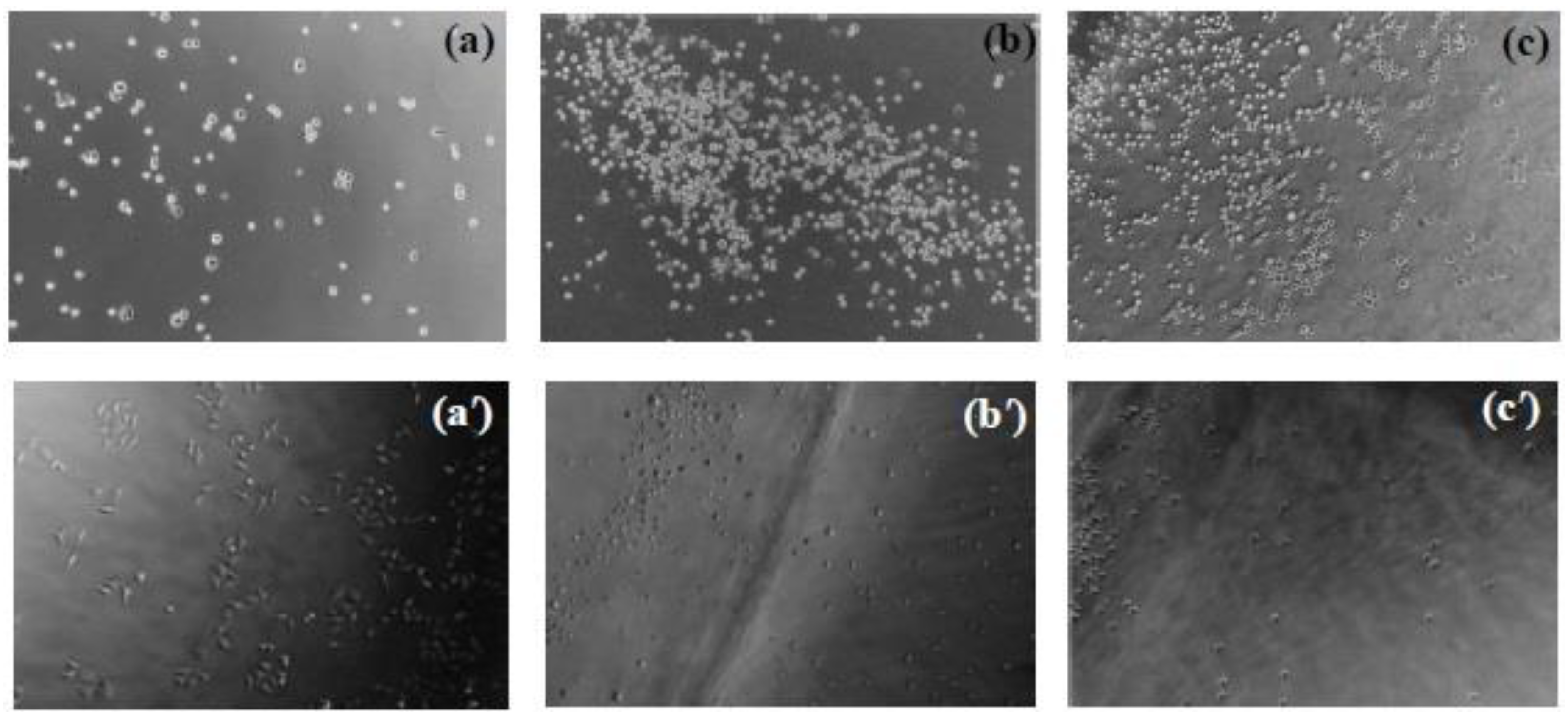
2.8. HeLa cell adhesion and proliferation on the hydrogels’ surface
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterization of the samples
4.2.1. Fourier-transform infrared spectroscopy (FT-IR)
4.2.2. Rheological test
4.2.3. Field emission-SEM (FE-SEM)
4.2.4. Measurement of equilibrium swelling ratio (SRe)
4.2.5. Wettability test; water contact angle (WCA)
4.2.6. Cell adhesion test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.Y.; Cao, Z.Q. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent developments in antimicrobial and antifouling coatings to reduce or prevent contamination and cross-contamination of food contact surfaces by bacteria. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3093–3134. [Google Scholar] [CrossRef]
- Dueramae, I.; Nishida, M.; Nakaji-Hirabayashi, T.; Matsumura, K.; Kitano, H. Biodegradable shape memory polymers functionalized with anti-biofouling interpenetrating polymer networks. J. Mater. Chem. B 2016, 4, 5394–5404. [Google Scholar] [CrossRef]
- Bauman, L.; Zhao, B. Multi-thermo responsive double network composite hydrogel for 3D printing medical hydrogel mask. J. Colloid Interface Sci. 2023, 638, 882–892. [Google Scholar] [CrossRef]
- Du, J.; Ma, Q.; Wang, B.; Sun, L.; Liu, L. Hydrogel fibers for wearable sensors and soft actuators. iScience 2023, 26, 106796. [Google Scholar] [CrossRef]
- Jian, Y.; Wu, B.; Yang, X.; Peng, Y.; Zhang, D.; Yang, Y.; Qiu, H.; Lu, H.; Zhang, J.; Chen, T. Stimuli-responsive hydrogel sponge for ultrafast responsive actuator. Supramol. Mater. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H. High electrochemical and mechanical performance of zinc conducting-based gel polymer electrolytes. Sci. Rep. 2021, 11, 13268. [Google Scholar] [CrossRef]
- Aruchamy, K.; Ramasundaram, S.; Divya, S.; Chandran, M.; Yun, K.; Oh, T.W. Gel polymer electrolytes: Advancing solid-state batteries for high-performance applications. Gel 2023, 9, 585. [Google Scholar] [CrossRef]
- Creton, C. 50th Anniversary perspective: Networks and gels: Soft but dynamic and tough. Macromolecules 2017, 50, 8297–8316. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh, K.P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Dueramae, I.; Yoneyama, N.; Shinyashiki, S.; Yagihara, S.; Kita, R. Self-assembly of acetylated dextran with various acetylation degrees in aqueous solutions: Studied by light scattering. Carbohydr. Polym. 2017, 159, 171–177. [Google Scholar] [CrossRef]
- Dueramae, I.; Yoneyama, N.; Shinyashiki, S.; Yagihara, S.; Kita, R. Thermal diffusion of aqueous solution of acetylated dextran: The effect of hydrophobicity using optical beam deflection technique. Int. J. Heat Mass Transf. 2019, 132, 997–1003. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiassaon, B. Smart polymers: physical forms and bioengineering applications. Prog. Poly. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuo, R.; Cui, J.; Zhang, J. A novel thermo-responsive drug delivery System with positive controlled release. Int. J. Pharm. 2002, 235, 43–50. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery. Sci. Rep. 2020, 10, 12587. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, J.; Song, X.; Wen, Y.; Li, J. Smart hydrogel formed by alginate-g-poly(N-isopropylacrylamide) and chitosan through polyelectrolyte complexation and its controlled release properties. Gels 2022, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Afloarea, O.-T.; Cheaburu Yilmaz, C.N.; Verestiuc, L.; Bibire, N. Development of vaginal carriers based on chitosan-grafted-PNIPAAm for progesterone administration. Gels 2022, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Takatsuka, M.; Kita, R.; Shinyashiki, N.; Yagihara, S.; Rathinasabapathy, S. Dynamics of the poly(N-Isopropylacrylamide) microgel aqueous suspension investigated by dielectric relaxation spectroscopy. Macromolecules 2022, 55, 1218–1229. [Google Scholar] [CrossRef]
- Fukai, T.; Shinyashiki, N.; Yagihara, S.; Kita, R.; Tanaka, F. Phase behavior of co-nonsolvent systems: Poly(N-isopropylacrylamide) in mixed solvents of water and methanol. Langmuir 2018, 34, 3003–3009. [Google Scholar] [CrossRef]
- Xu, L.; Liang, X.; You, L.; Yang, Y.; Fen, G.; Gao, Y.; Cui, X. Temperature-sensitive poly(N-isopropylacrylamide)-chitosan hydrogel for fluorescence sensors in living cells and its antibacterial application. Int. J. Biol. Macromol. 2021, 189, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Jo, S.-H.; Phan, Q.-T.; Park, H.; Park, S.-H.; Oh, C.-W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using "click" chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef]
- Romero, M.R.; Coser, N.A.; P´erez, M.A.; Gomez, C.G. Poly(N-isopropylacrylamide)-interpenetrated chitosan coils working as nanoreactors for controlled silver nanoparticle growth. Carbohydr. Polym. 2022, 288, 119374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Chen, W.; Liao, L.; Xie, P.; Zhang, T.C.; Wu, X.; Feng, X. Comparative adsorption of emerging contaminants in water by functional designed magnetic poly(N-isopropylacrylamide)/chitosan hydrogels. Sci. Total Environ. 2019, 671, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-T.; Zeng, K.; Fuhrmann, B.; Woelk, C.; Zhang, K.; Groth, T. Engineering of stable cross-linked multilayers based on thermo-responsive PNIPAM-grafted chitosan/heparin to tailor their physiochemical properties and biocompatibility. ACS Appl. Mater. Interfaces 2022, 14, 29550–29562. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Luo, L.-J. Chitosan-g-poly(N-isopropylacrylamide) copolymers as delivery carriers for intracameral pilocarpine administration. Eur J Pharm Biopharm 2017, 113, 140–148. [Google Scholar] [CrossRef]
- Kopač, T.; Ručigaj, A.; Krajnc, M. The mutual effect of the crosslinker and biopolymer concentration on the desired hydrogel properties. Int. J. Biol. Macromol. 2020, 159, 557–569. [Google Scholar] [CrossRef]
- Dueramae, I.; Ishiyama, T.; Torigaki, A.; Nakano, S.; Sasaki, K.; Kita, R.; Shinyashiki, N.; Yagihara, S.; Katsumoto, Y.; Yoneyama, M. Separation of micro-Brownian motion and side-group rotational motion for poly(N-isopropylacrylamide) in 1,4-dioxane studied by dielectric relaxation spectroscopy. Macromolecules 2023, 56, 4041–4048. [Google Scholar] [CrossRef]
- Dueramae, I.; Fukuzawa, S.; Shinyashiki, N.; Yagihara, S.; Kita, R. Dynamics of amyloid -like aggregation and gel formation of hen egg-white lysozyme in highly concentrated ethanol solution. J. Biorheology 2017, 31, 21–28. [Google Scholar] [CrossRef]
- Rimdusit, S.; Punson, K.; Dueramae, I.; Somwangthanaroj, A.; Tiptipakorn, S. Rheological and thermomechanical characterizations of fumed silica-filled polybenzoxazine nanocomposites. Eng. J. 2011, 15, 27–38. [Google Scholar] [CrossRef]
- Carmona, P.; Tasici, A.M.; Sande, S.A.; Knudsen, K.D.; Nyström, B. Glyceraldehyde as an efficient chemical crosslinker agent for the formation of chitosan hydrogels. Gels 2021, 7, 186. [Google Scholar] [CrossRef]
- Joly, J.-P.; Aricov, Ludmila.; Balan, G.-A.; Popescu, E.I.; Mocanu, S.; Leonties, A.R.; Matei, I.; Marque, S.R.A.; Ionita, G. Formation of alginate/chitosan interpenetrated networks revealed by EPR spectroscopy. Gels 2023, 9, 231.
- Winter, H.H. Can the gel point of a cross-linking polymer be detected by the G′ – G″ crossover? Polym. Eng. Sci. 1987, 27, 698–1702. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Chen, L.A.; McDowell, C.; Khan, S.A.; Hwang, R.; White, S. Rheological study of crosslinking and gelation in chlorobutyl elastomer systems. Polymer 1996, 37, 5869–5875. [Google Scholar] [CrossRef]
- Hassan, A.; Niazi, M.B.K.; Hussain, A.; Earrukh, S.; Ahmad, T. Development of anti-bacterial PVA/starch based hydrogel emmbrane for wound dressing. J. Polym. Environ. 2018, 26, 235–243. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Dong, W.; Zhang, J. Fabrication of Salecan/poly(AMPS-co-HMAA) semi-IPN hydrogels for cell adhesion. Carbohydr. Polym. 2017, 174, 171–181. [Google Scholar] [CrossRef]
- Nie, W.; Yuan, X.; Zhao, J.; Zhou, Y.; Bao, H. Rapidly in situ forming chitosan/ɛ-polylysine hydrogels for adhesive sealants and hemostatic material. Carbohydr. Polym. 2013, 96, 342–348. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Linear viscoelasticity at the gel point of a crosslinking PDMS with imbalanced stoichiometry. J. Rheol. 1987, 31, 683–697. [Google Scholar] [CrossRef]
- Muthukumar, M. Screening effect on viscoelasticity near the gel point. Macromolecules 1989, 22, 4656–4658. [Google Scholar] [CrossRef]
- Scanlan, J.C.; Winter, H.H. Composition dependence of the viscoelasticity of end-linked poly(dimethylsiloxane) at the gel point. Macromolecules 1991, 24, 47–54. [Google Scholar] [CrossRef]
- Kurecic, M.; Sfiligoj-Smole, M.; Stana-Kleinschek, K. UV polymerization of poly(N-isopropylacrylamide) hydrogel. Mater. Technol. 2012, 46, 87–91. [Google Scholar]
- Maity, J.; Ray, S.K. Enhanced adsorption of methyl violet and congo red by using semi and full IPN of polymethacrylic acid and chitosan. Carbohydr. Polym. 2014, 104, 8–16. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly(acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohyd. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H. Controlled release of diclofenac sodium from pH-responsive carrageenan-g-poly(acrylic acid) superabsorbent hydrogel. J. Chem. Sci. 2010, 122, 651–659. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T. Description of a new surface morphology for chitin extracted from wings of cockroach (Periplaneta americana). Int. J. Biol. Macromol. 2015, 75, 7–12. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers. 2016, 8, 167. [Google Scholar] [CrossRef]
- Natarajan, N.; Shashirekha, V.; Noorjahan, S.E.; Rameshkumar, M.; Rose, C.; Sastry, T.P.J. Fibrin–chitosan–gelatin composite film: preparation and characterization. J. Macromol. Sci. A 2005, 42, 945–953. [Google Scholar] [CrossRef]
- Fuciños, C.; Fuciños, P.; Pastrana, L.M.; Rúa, M.L. Functional characterization of poly(N-isopropylacrylamide) nanohydrogels for the controlled release of food preservatives. Food Bioprocess Technol. 2014, 7, 3429–3441. [Google Scholar] [CrossRef]
- Pasmore, M.; Todd, P.; Smith, S.; Baker, D.; Silverstein, J.; Coons, D.; Bowman, C.N. Effects of ultrafiltration membrane surface properties on Pseudomonas aeruginosa biofilm initiation for the purpose of reducing biofouling. J. Membr. Sci. 2001, 194, 15–32. [Google Scholar] [CrossRef]
- Kolodin, A.N.; Bulavchenko, A.I. Contact angle and free surface energy of CdS films on polystyrene substrate. Appl. Surf. Sci. 2019, 463, 820–828. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Xiong, S.; Shi, Y.S.; Zhu, J.; Hu, Q.L.; Liu, J.; Wang, Y. Antifouling enhancement of polyimide membrane by grafting DEDA-PS zwitterions. Chemosphere 2018, 198, 30–39. [Google Scholar] [CrossRef]
- Santos, P.A.; Rocha, C.S.; Baptista, M.S. Adhesion and proliferation of HeLa and fibroblast cells onchemically-modified gold surfaces. Colloids. Surf. B. 2014, 123, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.P.; Fang, N. Adhesion dynamics, morphology, and organization of 3T3 fibroblast on chitosan and its derivative: the effect of O-carboxymethylation. Biomacromolecules 2005, 6, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, J. Hydrogel brushes grafted from stainless steel via surface-initiated atom transfer radical polymerization for marine antifouling. Appl. Surf. Sci. 2016, 382, 202–216. [Google Scholar] [CrossRef]
- Pelham, R.J.; Wang, Y.L. Cell locomotion and focal adhesions are regulated bysubstrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 2009, 3, 277–284. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Bae, T.H.; Kim, I.C.; Tak, T.M. Preparation and characterization of fouling-resistant TiO2 self-assembled nanocomposite membranes. J. Membr. Sci. 2006, 275, 1–5. [Google Scholar] [CrossRef]
| Name | NiPAM (M) |
BIS (M, wt%) |
photo-initiator /I2959 (M) |
Prepared solvent | Cell content (103 cell/cm2)a | Contact angle (degree) |
|---|---|---|---|---|---|---|
| Single polymer network | ||||||
|
CS (Chitosan) PNiPAME PNiPAMW |
0 1 1 |
0 0.04(5.16) 0.04(5.16) |
0 0.02 0.02 |
Ethanol Water |
4.50 ± 0.54 N/A N/A |
62.7 ± 5.9 |
| Interpenetrated polymer network of CS/PNiPAM prepared in ethanol | ||||||
| IPNE1 | 1 | 0.01 (1.45) | 0.02 | Ethanol | 3.70 ± 0.29 | 58 ± 11 |
| IPNE2 | 1 | 0.02 (2.65) | 0.02 | 3.30 ± 0.50 | 56 ± 7.3 | |
| IPNE3 | 1 | 0.04 (5.16) | 0.02 | 2.40 ± 0.52 | 61 ± 5.3 | |
| IPNE4 | 1 | 0.20 (21.4) | 0.02 | 2.40 ± 0.43 | 61 ± 8.2 | |
| Interpenetrated polymer network of CS/PNiPAM prepared in water | ||||||
| IPNw1 | 1 | 0.01 (1.45) | 0.02 | Water (DI) |
N/A | |
| IPNw2 | 1 | 0.02 (2.65) | 0.02 | |||
| IPNw3 | 1 | 0.04 (5.16) | 0.02 | |||
| IPNw4 | 1 | 0.20 (21.4) | 0.02 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





