Submitted:
24 November 2023
Posted:
26 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
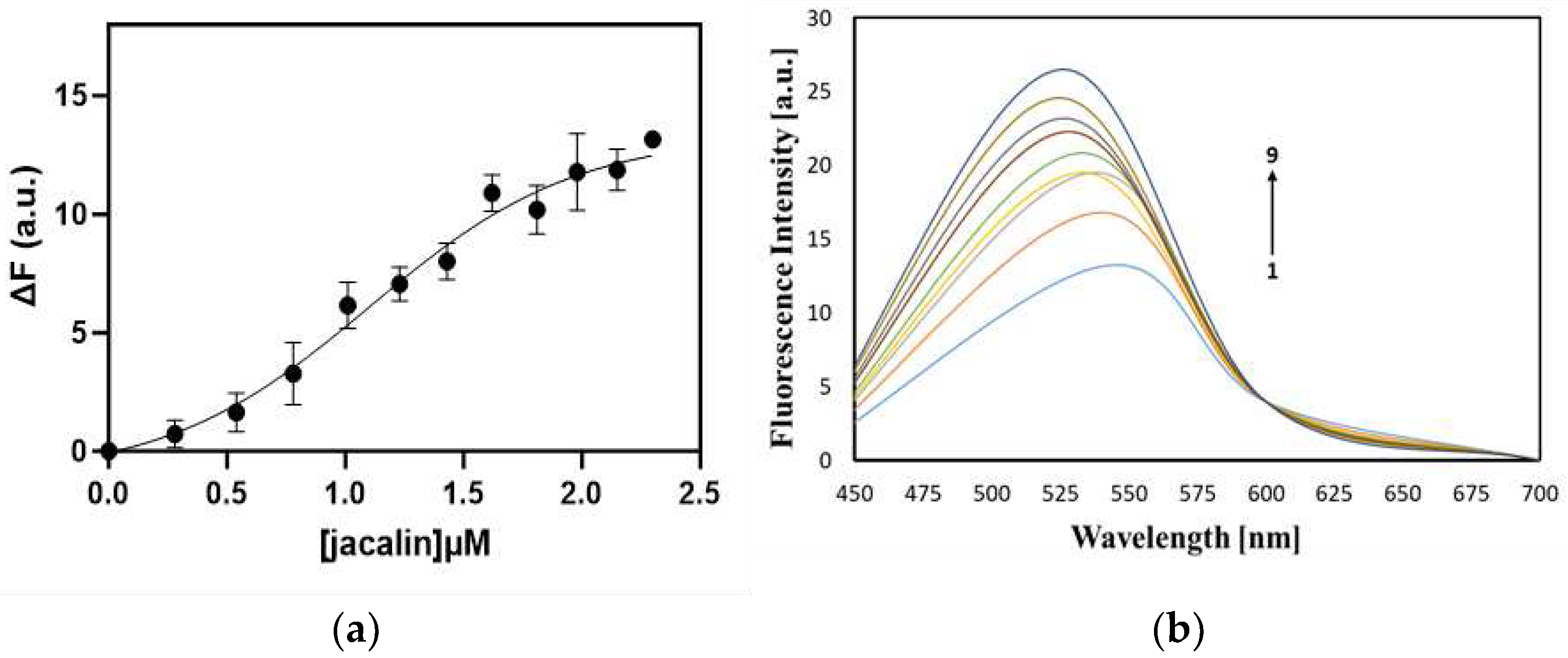
2.1. Interaction of jacalin with curcumin, registered by steady state fluorescence
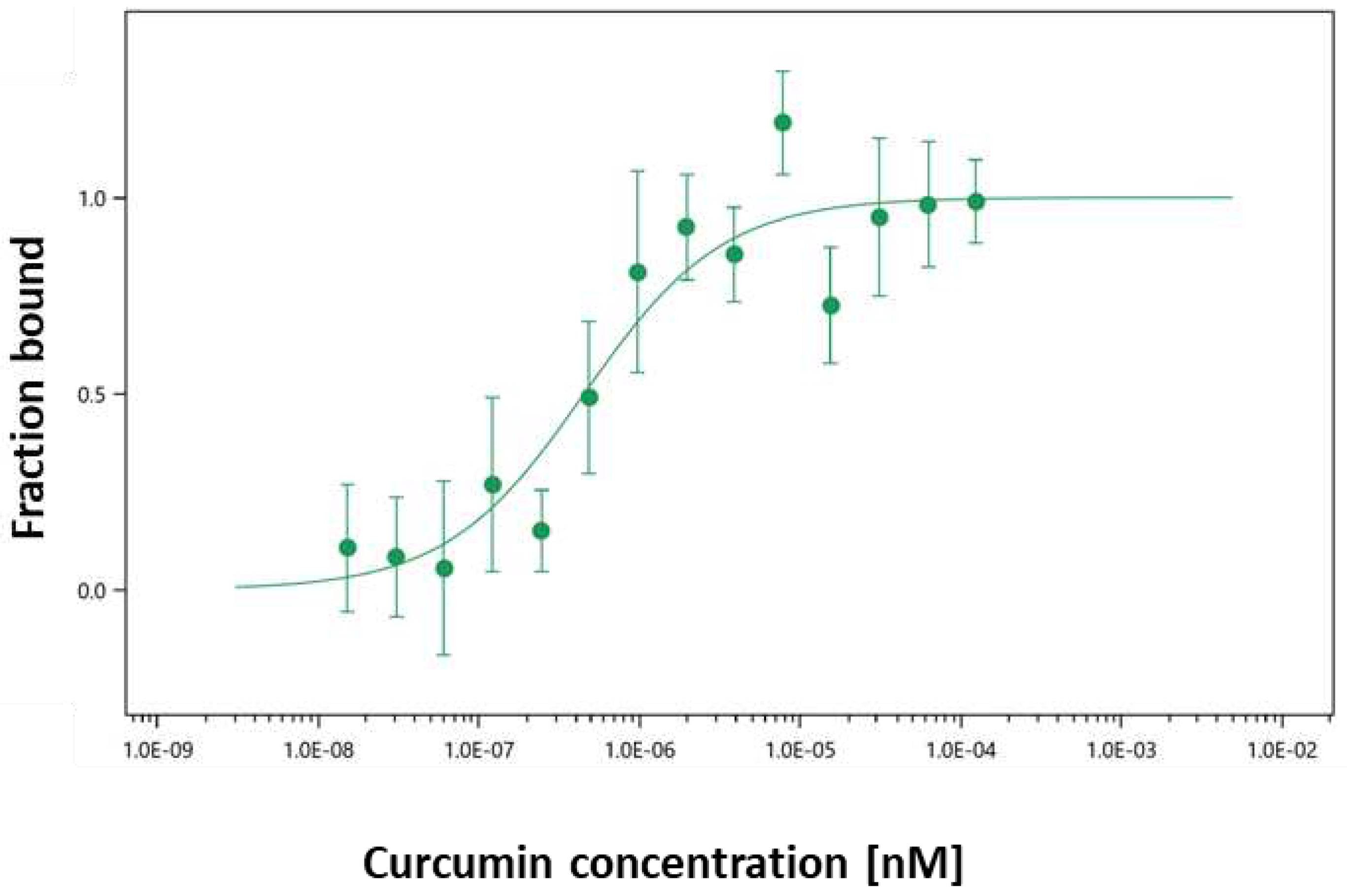
2.2. Interaction of curcumin with fluorescently labeled jacalin
2.3. Cell viability results
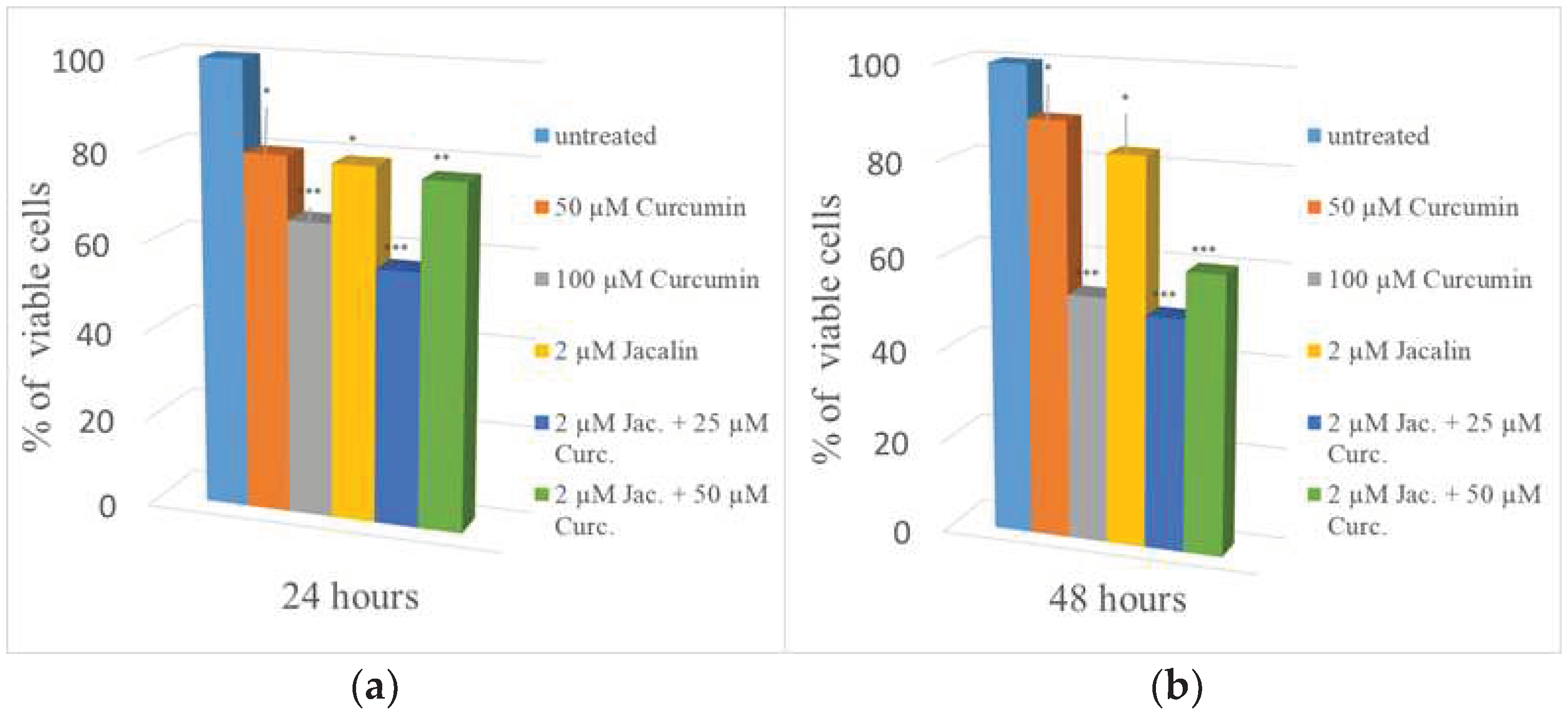
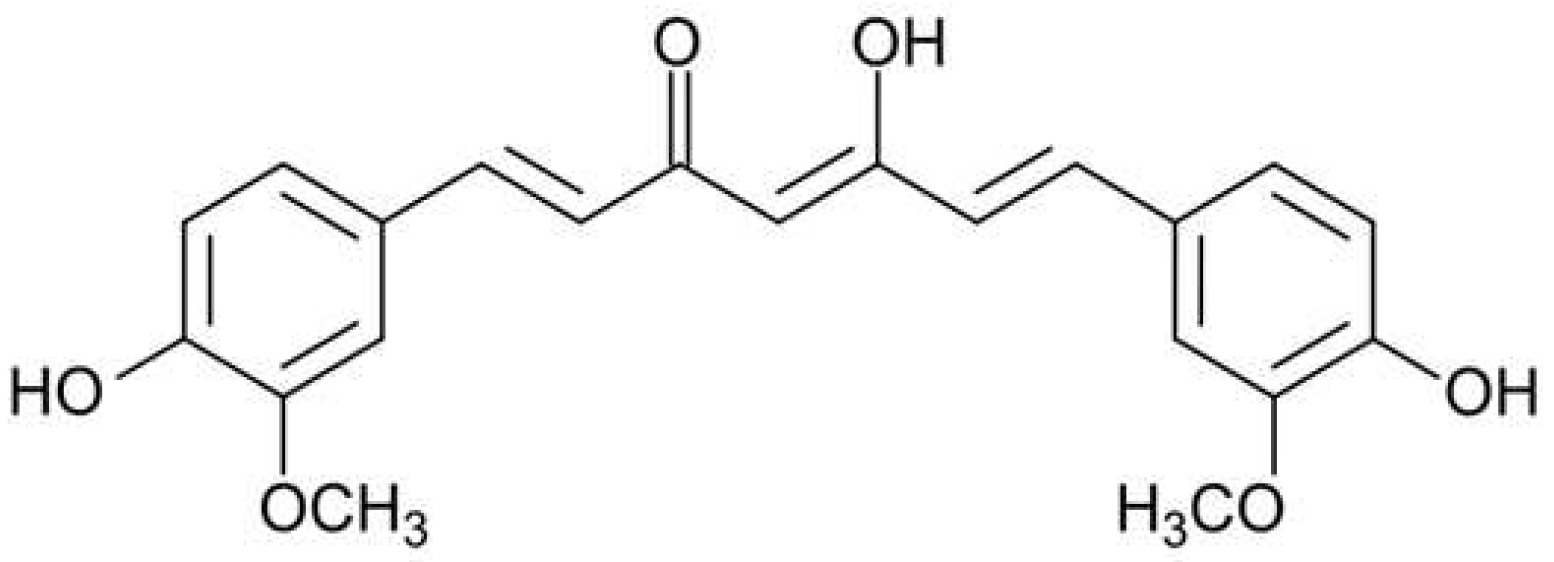
3. Discussion and conclusions
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Purification of Jacalin
4.4. Steady state fluorescence
4.5. Jacalin-Curcumin Interaction Studies Using Microscale Thermophoresis
4.6. Mitochondrial dehydrogenase assay (MTT assay)
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer. Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, A.A.; Ahmed, N.; Krishnasastry, M.V. Stress-Induced Phosphorylation of Caveolin-1 and P38, and down-Regulation of EGFr and ERK by the Dietary Lectin Jacalin in Two Human Carcinoma Cell Lines. Cell Stress Chaperones 2006, 11, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Chatterjee, B.P. Further Characterization and Immunochemical Studies on the Carbohydrate Specificity of Jackfruit (Artocarpus integrifolia) Lectin. J. Biol. Chem. 1989, 264, 9365–9372. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S. The Isolation and Characterisation of Jacalin [Artocarpus Heterophyllus (Jackfruit) Lectin] Based on Its Charge Properties. Int. J. Biochem. Cell Biol. 1995, 27, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.V.; Banarjee, P.; Patanjali, S.R.; Swamy, M.J.; Swarnalatha, G.V.; Surolia, A. Analysis of Saccharide Binding to Artocarpus Integrifolia Lectin Reveals Specific Recognition of T-Antigen (Beta-D-Gal(1----3)D-GalNAc). J. Biol. Chem. 1986, 261, 11726–11733. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-M.; Karsten, U.; Goletz, S.; Cheng, R.-C.; Cao, Y. Expression of CD176 (Thomsen-Friedenreich Antigen) on Lung, Breast and Liver Cancer-Initiating Cells. Int. J. Exp. Pathol. 2011, 92, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Targeting the Oncofetal Thomsen-Friedenreich Disaccharide Using Jacalin-PEG Phthalocyanine Gold Nanoparticles for Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2012, 51, 6158–6162. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer Targeting with Biomolecules: A Comparative Study of Photodynamic Therapy Efficacy Using Antibody or Lectin Conjugated Phthalocyanine-PEG Gold Nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Geraldino, T.H.; Modiano, P.; Veronez, L.C.; Flória-Santos, M.; Garcia, S.B.; Pereira-da-Silva, G. Jacalin Has Chemopreventive Effects on Colon Cancer Development. BioMed. Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Fukase, Y.; Katagiri, T.; Ishikawa, N.; Irie, S.; Sato, T.-A.; Ito, H.; Nakayama, H.; Miyagi, Y.; Tsuchiya, E.; et al. Targeted Serum Glycoproteomics for the Discovery of Lung Cancer-Associated Glycosylation Disorders Using Lectin-Coupled ProteinChip Arrays. Proteomics 2009, 9, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Ayaz Ahmed, K.B.; Mohammed, A.S.; Veerappan, A. Interaction of Sugar Stabilized Silver Nanoparticles with the T-Antigen Specific Lectin, Jacalin from Artocarpus Integrifolia. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 145, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Danella Polli, C.; Pereira Ruas, L.; Chain Veronez, L.; Herrero Geraldino, T.; Rossetto De Morais, F.; Roque-Barreira, M.C.; Pereira-da-Silva, G. Jacalin-Activated Macrophages Exhibit an Antitumor Phenotype. BioMed. Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shu, W.; Chen, W.; Wu, Q.; Liu, H.; Cui, G. Curcumin, Both Histone Deacetylase and P300/CBP-Specific Inhibitor, Represses the Activity of Nuclear Factor Kappa B and Notch 1 in Raji Cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From Kitchen to Clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23 (1A), 363–398. [Google Scholar]
- Sinha, D.; Biswas, J.; Sung, B.; B. Aggarwal, B.; Bishayee, A. Chemopreventive and Chemotherapeutic Potential of Curcumin in Breast Cancer. Curr. Drug Targets 2012, 13, 1799–1819. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.-D.; Liu, X.-P.; Zhao, W.-J.; Dong, Q.; Li, F.-N.; Wang, H.-B.; Kong, B. Curcumin Induces Apoptosis in Breast Cancer Cells and Inhibits Tumor Growth in Vitro and in Vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818–2824. [Google Scholar] [PubMed]
- Zargan, S.; Salehi Barough, M.; Zargan, J.; Shayesteh, M.; Haji Noor Mohammadi, A.; Mousavi, M.; Keshavarz Alikhani, H. Evaluation of the Anti-Cancer Effect of Curcumin on MCF-7 Cells in 3D Culture Conditions to Increase the Efficacy of Breast Cancer Treatment. J. Appl. Biotechnol. Rep. 2022, 9. [Google Scholar] [CrossRef]
- D’Auria, S.; Petrova, L.; John, C.; Russev, G.; Varriale, A.; Bogoeva, V. Tumor-Specific Protein Human Galectin-1 Interacts with Anticancer Agents. Mol. Biosyst. 2009, 5, 1331. [Google Scholar] [CrossRef] [PubMed]
- Bogoeva, V.P.; Petrova, L.P.; Ivanov, I.B.; Kulina, H.N.; Buchvarov, I. Ch. Characterization of Metalloanticancer Capacity of an Agglutinin from Wheat. Mol. Biosyst. 2012, 8, 2633. [Google Scholar] [CrossRef]
- Bogoeva, V.; Petrova, L.; Kubát, P. Binding of Palladium (II) 5, 10, 15, 20-Tetrakis (4-Sulfonatophenyl) Porphyrin to a Lectin for Photosensitizer Targeted Delivery. J. Photochem. Photobiol. B 2015, 153, 276–280. [Google Scholar] [CrossRef]
- Bogoeva, V.; Petrova, L.; Bouckaert, J.; Yordanova, A.; Ivanov, I.; Vanderesse, R.; Frochot, C. Dual Function of Lectins — New Perspectives in Targeted Photodynamic Therapy. J. Porphyr. Phthalocyanines 2019, 23 (11n12), 1241–1250. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Pandey, R.; Priyadarsini, K.I. Transport of Liposomal and Albumin Loaded Curcumin to Living Cells: An Absorption and Fluorescence Spectroscopic Study. Biochim. Biophys. Acta BBA - Gen. Subj. 2006, 1760, 1513–1520. [Google Scholar] [CrossRef]
- Ayaz Ahmed, K.B.; Raja, M.R. C.; Mahapatra, S.K.; Anbazhagan, V. Interaction of Cadmium Sulfide Quantum Dots with Jacalin for Specific Recognition of Cancer Cells. J. Lumin. 2017, 182, 283–288. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence Study of the Curcumin−Casein Micelle Complexation and Its Application as a Drug Nanocarrier to Cancer Cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef]
- Komath, S.S.; Kavitha, M.; Swamy, M.J. Beyond Carbohydrate Binding: New Directions in Plant Lectin Research. Org. Biomol. Chem. 2006, 4, 973. [Google Scholar] [CrossRef]
- Komath, S.S.; Bhanu, K.; Maiya, B.G.; Swamy, M.J. Binding of Porphyrins by the Tumor-Specific Lectin, Jacalin [Jack Fruit (Artocarpus Integrifolia) Agglutinin]. Biosci. Rep. 2000, 20, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.M.; Esko, J.D. The Sweet and Sour of Cancer: Glycans as Novel Therapeutic Targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Glavey, S.V.; Huynh, D.; Reagan, M.R.; Manier, S.; Moschetta, M.; Kawano, Y.; Roccaro, A.M.; Ghobrial, I.M.; Joshi, L.; O’Dwyer, M.E. The Cancer Glycome: Carbohydrates as Mediators of Metastasis. Blood Rev. 2015, 29, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Poiroux, G.; Barre, A.; Van Damme, E.; Benoist, H.; Rougé, P. Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2017, 18, 1232. [Google Scholar] [CrossRef]
- Pandey, G.; Fatma, T.; Cowsik, S.M.; Komath, S.S. Specific Interaction of Jacalin with Phycocyanin, a Fluorescent Phycobiliprotein. J. Photochem. Photobiol. B 2009, 97, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.D.; Goldstein, I.J. Binding of Hydrophobic Ligands to Plant Lectins: Titration with Arylaminonaphthalenesulfonates. Arch. Biochem. Biophys. 1983, 224, 479–484. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Astoul, C.H.; Zamboni, V.; Peumans, W.J.; Menu-Bouaouiche, L.; Van Damme, E.J.M.; Barre, A.; Rougé, P. Structural Basis for the Unusual Carbohydrate-Binding Specificity of Jacalin towards Galactose and Mannose. Biochem. J. 2002, 364 (Pt 1) Pt 1, 173–180. [Google Scholar] [CrossRef]
- Arockia Jeyaprakash, A.; Jayashree, G.; Mahanta, S.K.; Swaminathan, C.P.; Sekar, K.; Surolia, A.; Vijayan, M. Structural Basis for the Energetics of Jacalin–Sugar Interactions: Promiscuity Versus Specificity. J. Mol. Biol. 2005, 347, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Enhanced in vitro Anti-Cancer Activity of Curcumin Encapsulated in Hydrophobically Modified Starch. Food Chem. 2010, 119, 669–674. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; Zhang, T.; Yang, D.; Wang, C.; Chen, Q.; Tang, L.; Fan, L.; Liu, C.; Shen, J.; et al. Development of Targeted Therapy Therapeutics to Sensitize Triple-Negative Breast Cancer Chemosensitivity Utilizing Bacteriophage Phi29 Derived Packaging RNA. J. Nanobiotechnology 2021, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Bint Abdul Jabbar, H.; Malik, H.; Sadaf, H.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Atezolizumab and Pembrolizumab in Triple-Negative Breast Cancer: A Meta-Analysis. Expert Rev. Anticancer Ther. 2022, 22, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Shao, S.; Zhang, Y.; Luo, A.; Zhao, L.; Zhang, L.; Gu, S.; Zhao, X. BRD4 Inhibition Suppresses PD-L1 Expression in Triple-Negative Breast Cancer. Exp. Cell Res. 2020, 392, 112034. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, D.; Kreft, M.E.; Romih, R. Selective Binding of Lectins to Normal and Neoplastic Urothelium in Rat and Mouse Bladder Carcinogenesis Models. Protoplasma 2014, 251, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zuraidah, M.A.; John, B.A.; Kamaruzzaman, Y. Cytotoxicity on mcf7 cell lines exposed to an extract of the jacalin from jackfruit seed. Sci. Herit. J. 2017, 1, 16–18. [Google Scholar] [CrossRef]
- Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.-T.; Shin, H.; Ramesh, M. Plant Derived Bioactive Compounds, Their Anti-Cancer Effects and In Silico Approaches as an Alternative Target Treatment Strategy for Breast Cancer: An Updated Overview. Cancers 2021, 13, 6222. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP–Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.L.A.; Polinati, R.M.; Ferreira, C.; Dos Santos, N.A.N.; Lima, D.G.V.; Da Silva, J.L.; Fialho, E. Curcumin and Melphalan Cotreatment Induces Cell Cycle Arrest and Apoptosis in MDA-MB-231 Breast Cancer Cells. Sci. Rep. 2023, 13, 13446. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Smith, B.D.; Mohney, B.G. Ocular Side Effects Following Intravitreal Injection Therapy for Retinoblastoma: A Systematic Review. Br. J. Ophthalmol. 2014, 98, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.A.; Waseem, M.; Jamal, S.; Ahmed, N. Effects of Jacalin- a Galactose Binding Lectin on MDA-MB-468, a Triple-Negative Breast Cancer Cell Line, and Its Combinatorial Effect with Taxol; preprint, in Review. 2022. [Google Scholar] [CrossRef]
- Liu, J.-L.; Pan, Y.-Y.; Chen, O.; Luan, Y.; Xue, X.; Zhao, J.-J.; Liu, L.; Jia, H.-Y. Curcumin Inhibits MCF-7 Cells by Modulating the NF-κB Signaling Pathway. Oncol. Lett. 2017, 14, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




