Submitted:
24 November 2023
Posted:
25 November 2023
You are already at the latest version
Abstract
Keywords:
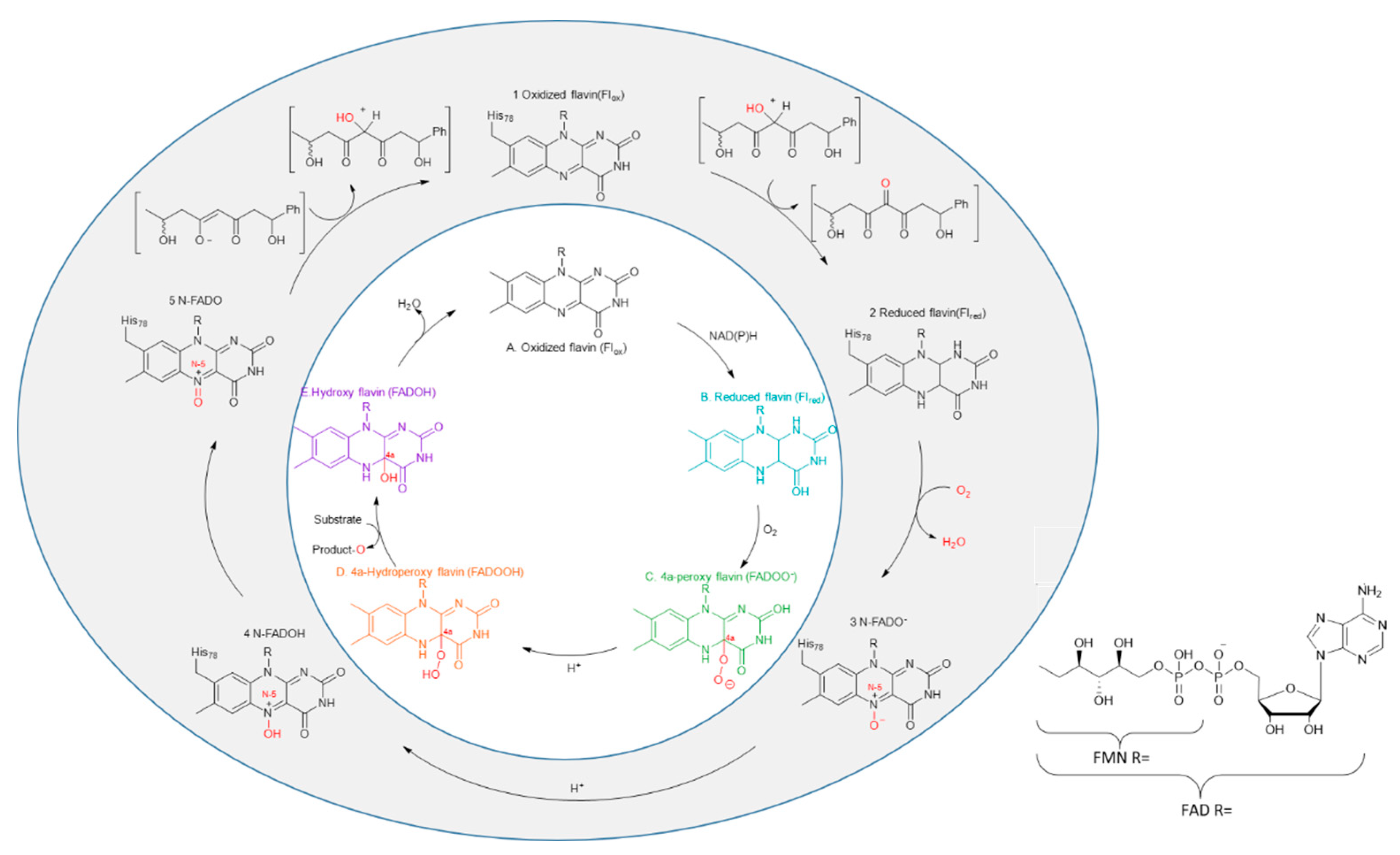
1. Introduction
2. Applications of FMOs in the Biomedical Field
2.1. Applications of FMOs in Antibiotic Research
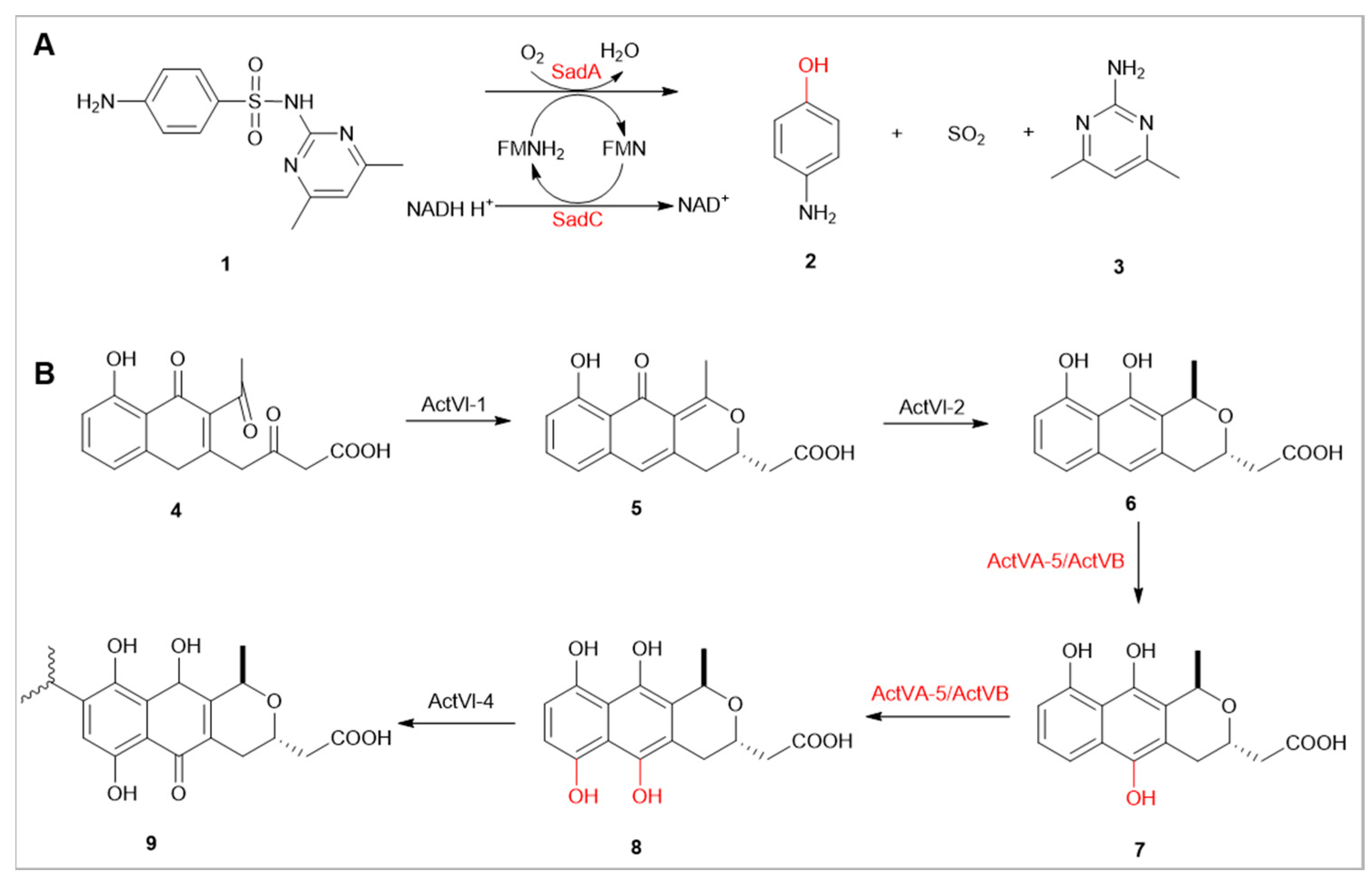
2.2. Applications of FMOs as Pharmaceutical Targets
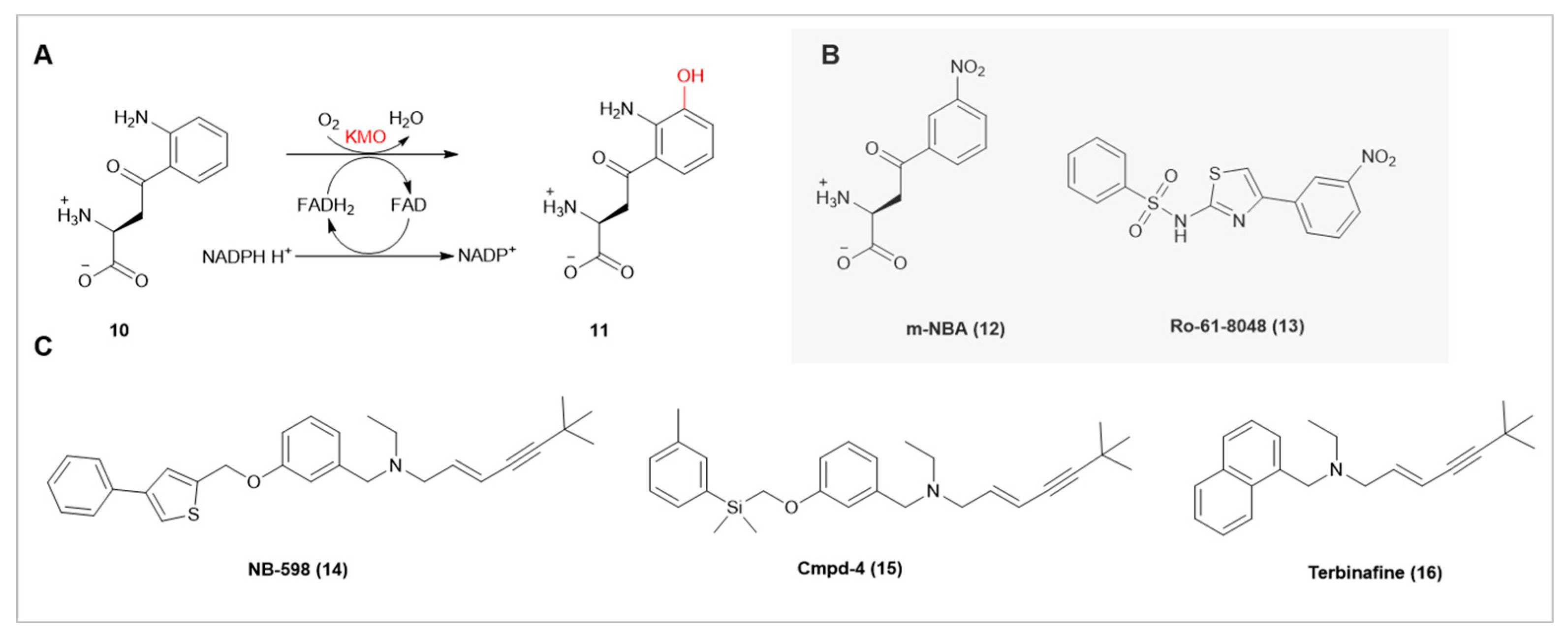
2.3. Applications of FMOs in Drug Synthesis
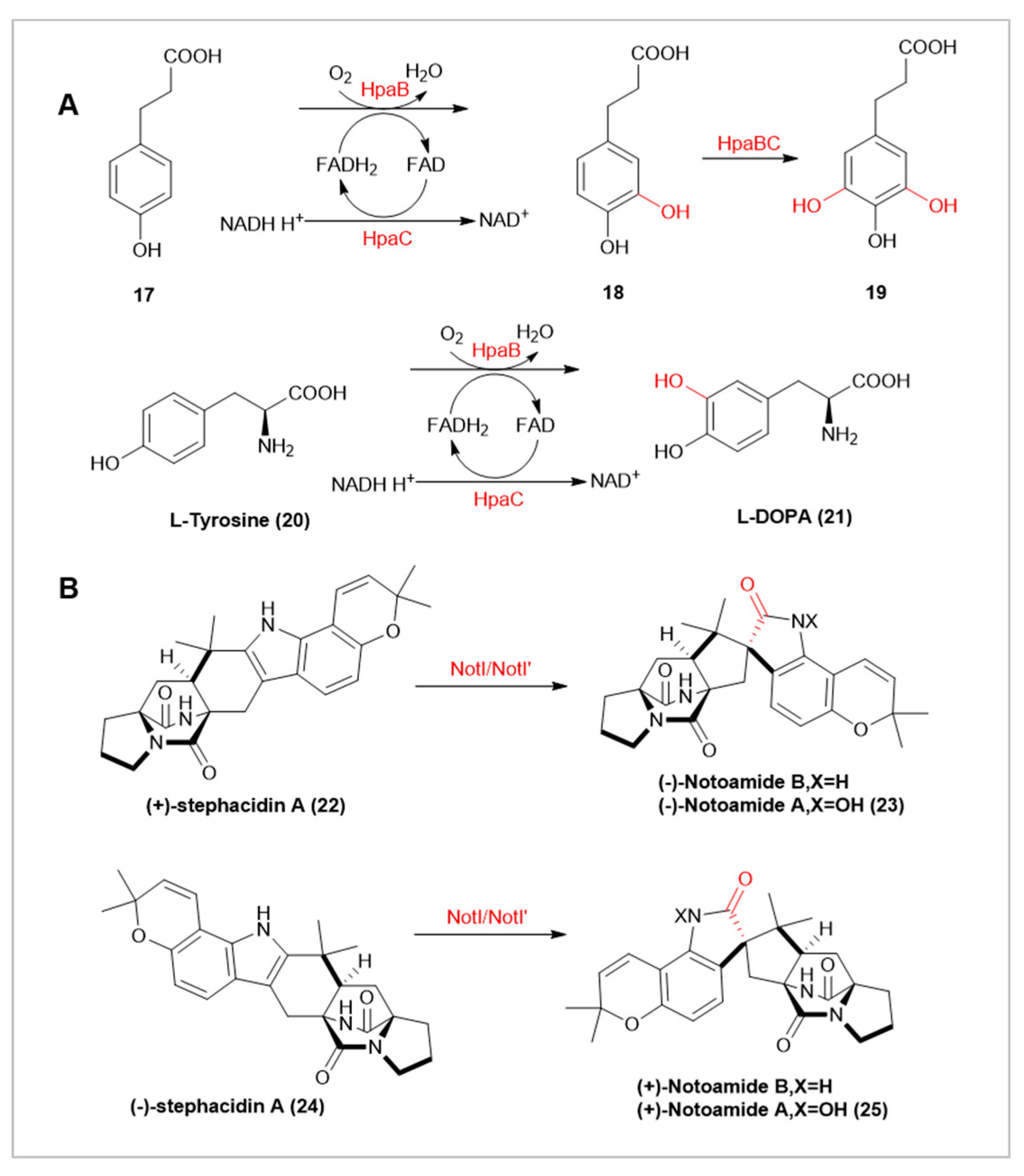
3. Applications of FMOs in the Biomedical Field
3.1. Application of FMOs in the Processing of Pesticides
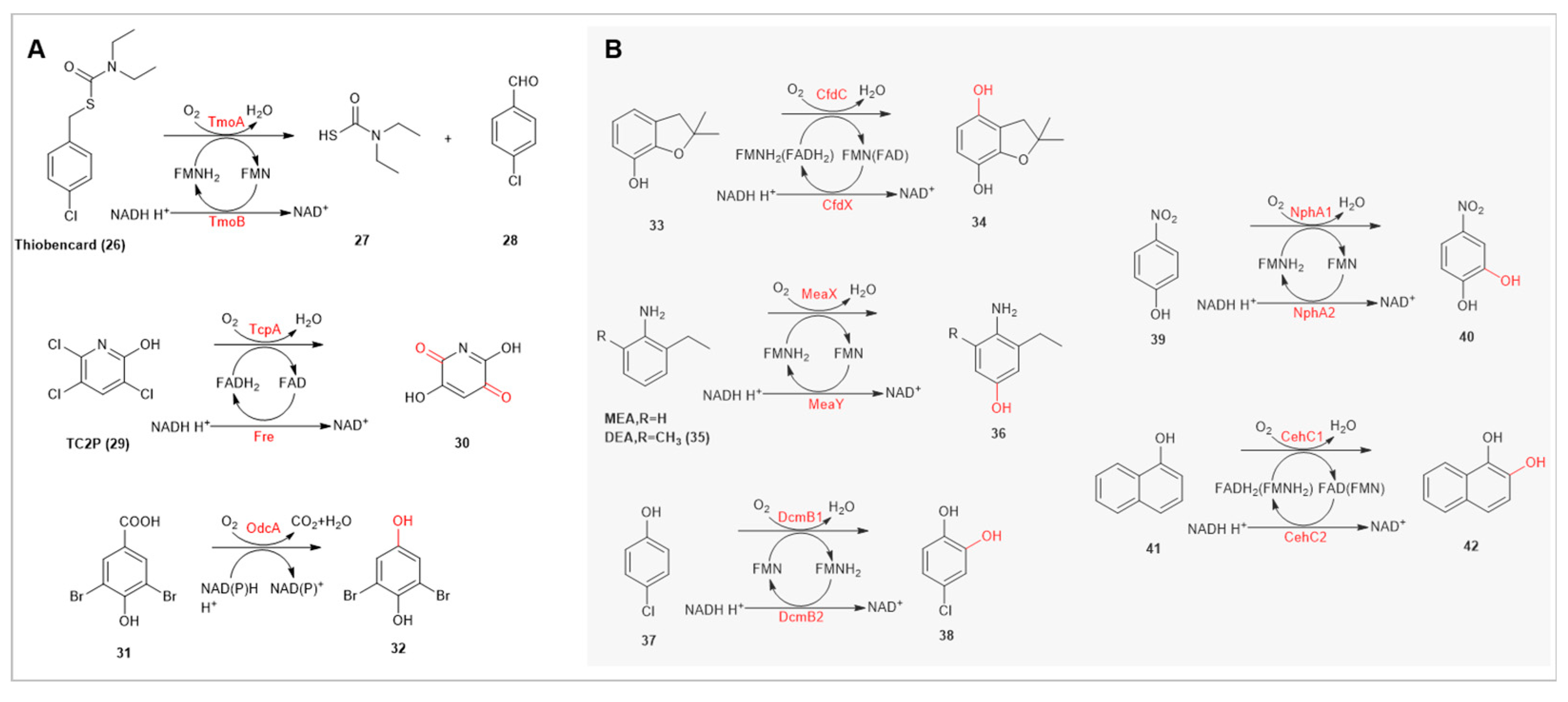
3.2. Application of FMOs in the Processing of Pesticides
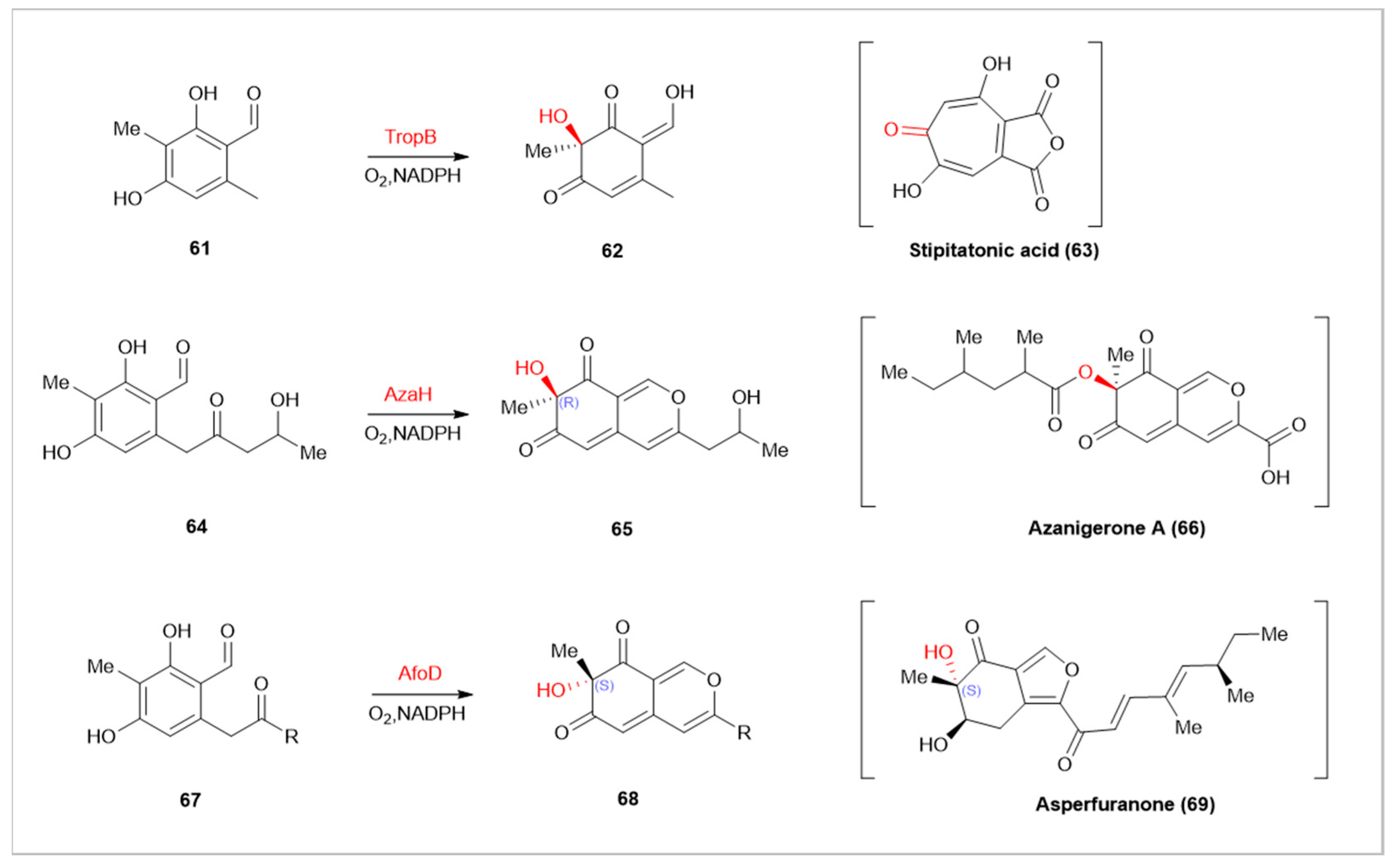
4. Applications of FMOs in the Natural Product Synthesis
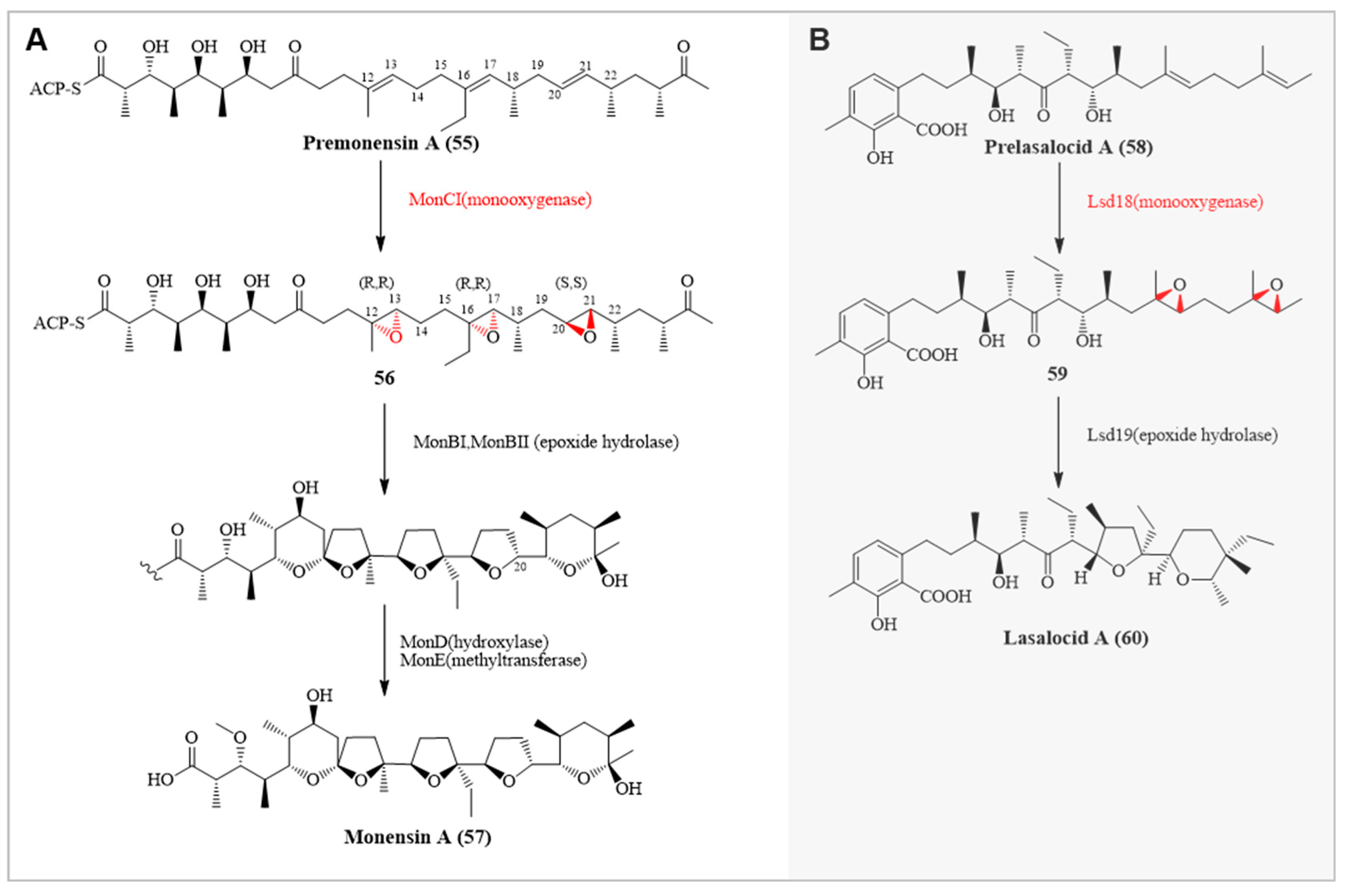
4.1. Application of FMOs in the Biosynthesis of Polyether through epoxidations
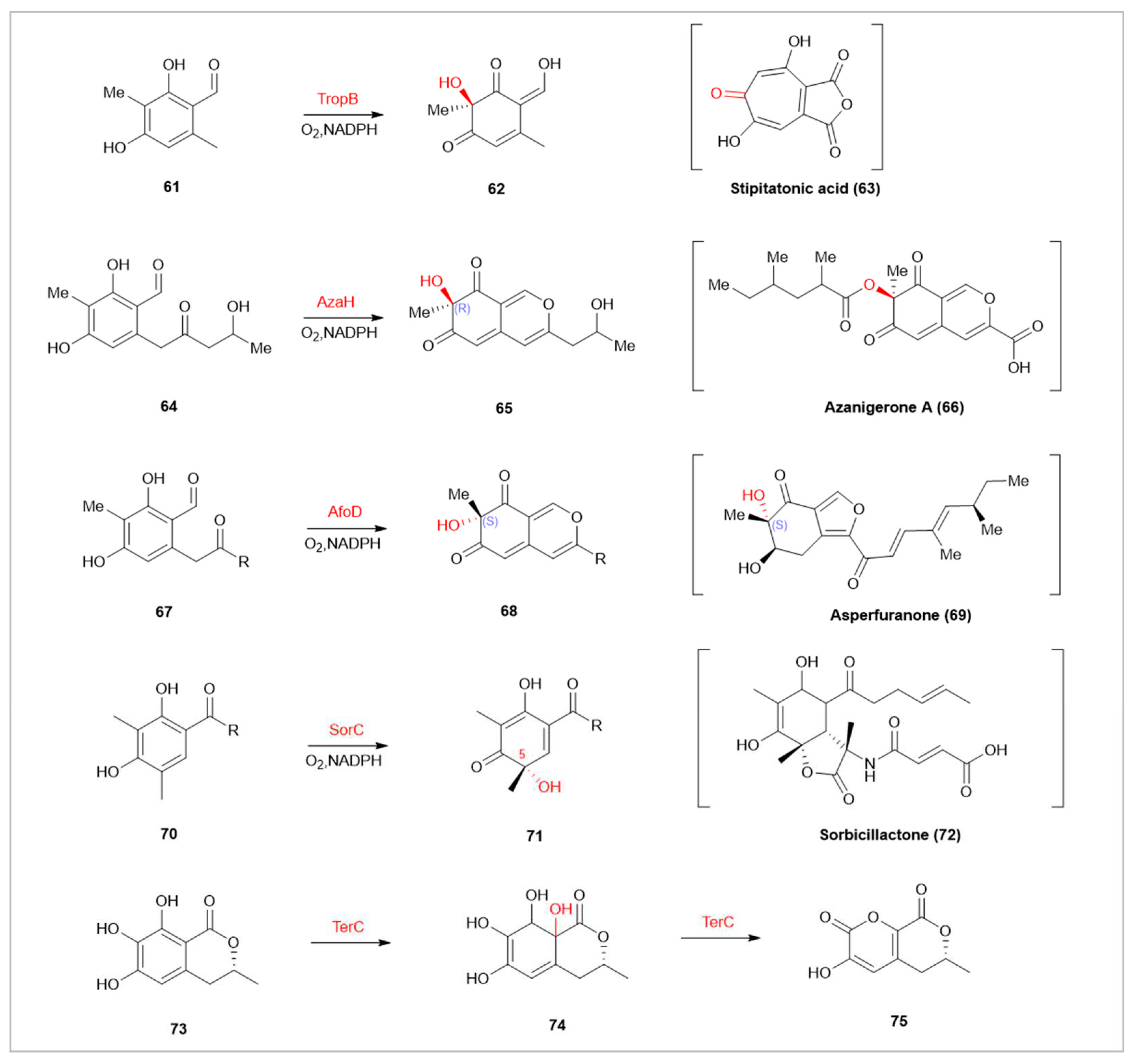
4.2. Application of FMOs in the Biosynthesis of Natural Products through Dearomatization
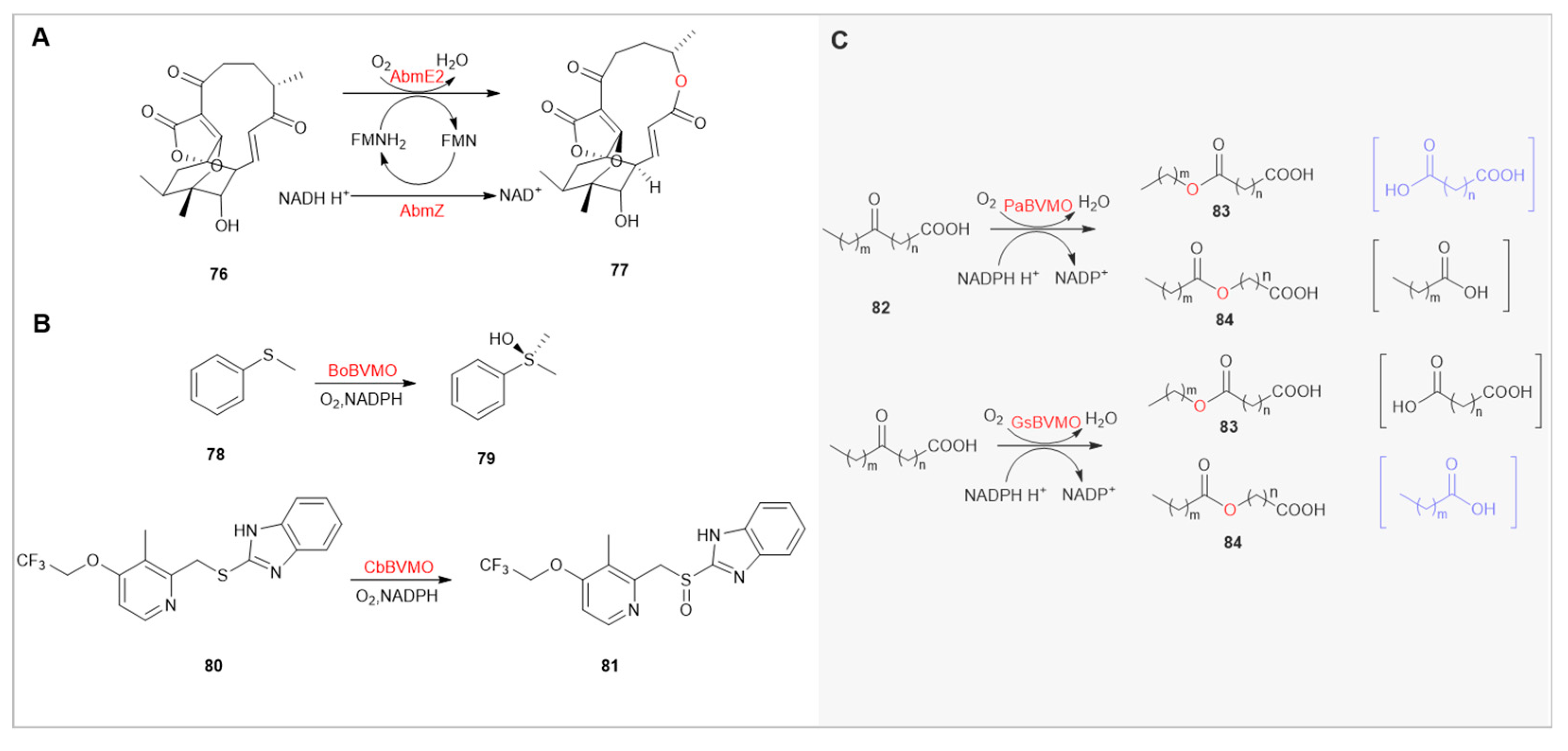
4.3. Application of Baeyer-Villiger Monooxygenases in Natural Product Synthesis
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Macheroux, P.; Kappes, B.; et al. Flavogenomics—A genomic and structural view of flavin-dependent proteins. Febs Journal 2011, 278, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chemical Reviews 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, N.; et al. Triepoxide formation by a flavin-dependent monooxygenase in monensin biosynthesis. Nature communications 2023, 14, 6273. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.M.E.; Montersino, S.; et al. Flavin dependent monooxygenases. Archives of Biochemistry and Biophysics 2014, 544, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angewandte Chemie-International Edition 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.M.; Zhou, Q.; et al. Properties and Mechanisms of Flavin-Dependent Monooxygenases and Their Applications in Natural Product Synthesis. International Journal of Molecular Sciences 2022, 23, 2622. [Google Scholar] [CrossRef] [PubMed]
- Teufel, R.; Miyanaga, A.; et al. Flavin-mediated dual oxidation controls an enzymatic Favorskii-type rearrangement. Nature 2013, 503, 552. [Google Scholar] [CrossRef] [PubMed]
- Eswaramoorthy, S.; Bonanno, J.B.; et al. Mechanism of action of a flavin-containing monooxygenase. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 9832–9837. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.R. Some distinctions between flavin-containing and cytochrome P450 monooxygenases. Biochemical and Biophysical Research Communications 2005, 338, 599–604. [Google Scholar] [CrossRef]
- Krueger, S.K.; Williams, D.E. Mammalian flavin-containing monooxygenases: Structure/function, genetic polymorphisms and role in drug metabolism. Pharmacology & Therapeutics 2005, 106, 357–387. [Google Scholar] [CrossRef]
- Ziegler, D.M. An overview of the mechanism, substrate specificities, and structure of FMOs. Drug Metabolism Reviews 2002, 34, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Finefield, J.M.; Frisvad, J.C.; et al. Fungal Origins of the Bicyclo 2.2.2 diazaoctane Ring System of Prenylated Indole Alkaloids. Journal of Natural Products 2012, 75, 812–833. [Google Scholar] [CrossRef]
- Sehlmeyer, S.; Wang, L.Z.; et al. Flavin-Dependent Monooxygenases as a Detoxification Mechanism in Insects: New Insights from the Arctiids (Lepidoptera). PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Yurimoto, H.; et al. Novel acetone metabolism in a propane-utilizing bacterium, Gordonia sp strain TY-5. Journal of Bacteriology 2007, 189, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Volkers, G.; Palm, G.J.; et al. Structural basis for a new tetracycline resistance mechanism relying on the TetX monooxygenase. Febs Letters 2011, 585, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Rudra, P.; Hurst-Hess, K.; et al. High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase. Antimicrobial Agents and Chemotherapy 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, P. Resistance to trimethoprim-sulfamethoxazole. Clinical Infectious Diseases 2001, 32, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.R.; Moore, I.F.; et al. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. Journal of Biological Chemistry 2004, 279, 52346–52352. [Google Scholar] [CrossRef]
- Hashimoto, M.; Taguchi, T.; et al. Unveiling Two Consecutive Hydroxylations: Mechanisms of Aromatic Hydroxylations Catalyzed by Flavin-Dependent Monooxygenases for the Biosynthesis of Actinorhodin and Related Antibiotics. Chembiochem 2020, 21, 623–627. [Google Scholar] [CrossRef]
- Mole, D.J.; Webster, S.P.; et al. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nature Medicine 2016, 22, 202–209. [Google Scholar] [CrossRef]
- Chiang, Y.L.; Lei, H.L.; et al. KMO as a novel diagnostic and prognostic biomarker in canine mammary gland tumors. Cancer Research 2012, 72. [Google Scholar] [CrossRef]
- Huang, T.T.; Tseng, L.M.; et al. Kynurenine 3-monooxygenase upregulates pluripotent genes through β-catenin and promotes triple-negative breast cancer progression. Ebiomedicine 2020, 54. [Google Scholar] [CrossRef]
- Jacobs, K.R.; Guillemin, G.J.; et al. Development of a Rapid Fluorescence-Based High-Throughput Screening Assay to Identify Novel Kynurenine 3-Monooxygenase Inhibitor Scaffolds. Slas Discovery 2018, 23, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.S.; Iradukunda, E.C.; et al. Modulation of Enzyme Activity in the Kynurenine Pathway by Kynurenine Monooxygenase Inhibition. Frontiers in Molecular Biosciences 2019, 6. [Google Scholar] [CrossRef]
- Padyana, A.K.; Gross, S.; et al. Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nature Communications 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Chua, N.K.; et al. The shape of human squalene epoxidase expands the arsenal against cancer. Nature Communications 2019, 10. [Google Scholar] [CrossRef]
- Santos, A.G.; da Rocha, G.O.; et al. Occurrence of the potent mutagens 2-nitrobenzanthrone and 3-nitrobenzanthrone in fine airborne particles. Scientific Reports 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Kino, K. Catalytic activity of the two-component flavin-dependent monooxygenase from Pseudomonas aeruginosa toward cinnamic acid derivatives. Applied Microbiology and Biotechnology 2014, 98, 1145–1154. [Google Scholar] [CrossRef]
- Nakagawa, A.; Nakamura, S.; et al. Selection of the optimal tyrosine hydroxylation enzyme for (S)-reticuline production in Escherichia coli. Applied Microbiology and Biotechnology 2021, 105, 5433–5447. [Google Scholar] [CrossRef]
- Deng, Y.F.; Faivre, B. Structural and Functional Characterization of 4-Hydroxyphenylacetate 3-Hydroxylase from Escherichia coli. Chembiochem 2020, 21, 163–170. [Google Scholar] [CrossRef]
- Herrmann, S.; Dippe, M.; et al. Engineered Bacterial Flavin-Dependent Monooxygenases for the Regiospecific Hydroxylation of Polycyclic Phenols. Chembiochem 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Fraley, A.E.; Tran, H.T.; et al. Flavin-Dependent Monooxygenases NotI and NotI′ Mediate Spiro-Oxindole Formation in Biosynthesis of the Notoamides. Chembiochem 2020, 21, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.G.; Chen, D.; et al. Oxygenases as Powerful Weapons in the Microbial Degradation of Pesticides. Annual Review of Microbiology 2022, 76, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.W.; Liu, B.; et al. A Novel Aerobic Degradation Pathway for Thiobencarb Is Initiated by the TmoAB Two-Component Flavin Mononucleotide-Dependent Monooxygenase System in Acidovorax sp Strain T1. Applied and Environmental Microbiology 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.J.; Zhou, X.Y. Experiment and Simulation on Adsorption of 3,5,6-Trichloro-2-Pyridinol in Typical Farmland of Purple Soil, Southwestern China. Soil & Sediment Contamination 2017, 26, 345–363. [Google Scholar] [CrossRef]
- Fang, L.C.; Shi, T.Z.; et al. Kinetics and Catabolic Pathways of the Insecticide Chlorpyrifos, Annotation of the Degradation Genes, and Characterization of Enzymes TcpA and Fre in Cupriavidus nantongensis X1T. Journal of Agricultural and Food Chemistry 2019, 67, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mu, Y.; et al. Comparative Transcriptome Analysis Reveals the Mechanism Underlying 3,5-Dibromo-4-Hydroxybenzoate Catabolism via a New Oxidative Decarboxylation Pathway. Applied and Environmental Microbiology 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jin, W.; et al. Hydrolase CehA and Monooxygenase CfdC Are Responsible for Carbofuran Degradation in Sphingomonas sp Strain CDS-1. Applied and Environmental Microbiology 2018, 84. [Google Scholar] [CrossRef]
- Cheng, M.G.; Meng, Q.; et al. The Two-Component Monooxygenase MeaXY Initiates the Downstream Pathway of Chloroacetanilide Herbicide Catabolism in Sphingomonads. Applied and Environmental Microbiology 2017, 83. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; et al. Two dcm Gene Clusters Essential for the Degradation of Diclofop-methyl in a Microbial Consortium of Rhodococcus sp JT-3 and Brevundimonas sp JT-9. Journal of Agricultural and Food Chemistry 2018, 66, 12217–12226. [Google Scholar] [CrossRef]
- Takeo, M.; Murakami, M.; et al. Mechanism of 4-Nitrophenol Oxidation in Rhodococcus sp Strain PN1: Characterization of the Two-Component 4-Nitrophenol Hydroxylase and Regulation of Its Expression. Journal of Bacteriology 2008, 190, 7367–7374. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Ke, Z.J.; et al. Hydrolase CehA and a Novel Two-Component 1-Naphthol Hydroxylase CehC1C2 are Responsible for the Two Initial Steps of Carbaryl Degradation in Rhizobium sp. X9. Journal of Agricultural and Food Chemistry 2020, 68, 14739–14747. [Google Scholar] [CrossRef]
- Riebel, A.; de Gonzalo, G.; et al. Expanding the biocatalytic toolbox of flavoprotein monooxygenases from Rhodococcus jostii RHA1. Journal of Molecular Catalysis B-Enzymatic 2013, 88, 20–25. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; et al. Baeyer-Villiger monooxygenases in the biosynthesis of microbial secondary metabolites. Sheng wu gong cheng xue bao = Chinese journal of biotechnology 2019, 35, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Castellanos, J.R.G.; et al. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chemical Reviews 2018, 118, 1742–1769. [Google Scholar] [CrossRef]
- Schlaich, N.L. Flavin-containing monooxygenases in plants: Looking beyond detox. Trends in Plant Science 2007, 12, 412–418. [Google Scholar] [CrossRef]
- Thodberg, S.; Neilson, E.J.H. The “Green” FMOs: Diversity, Functionality and Application of Plant Flavoproteins. Catalysts 2020, 10, 329. [Google Scholar] [CrossRef]
- Fraaije, M.W.; Kamerbeek, N.M. Identification of a Baeyer-Villiger monooxygenase sequence motif. Febs Letters 2002, 518, 43–47. [Google Scholar] [CrossRef]
- Malito, E.; Alfieri, A.; et al. Crystal structure of a Baeyer-Villiger monooxygenase. Proceedings of the National Academy of Sciences of the United States of America 2004, 101, 13157–13162. [Google Scholar] [CrossRef] [PubMed]
- Beneventi, E.; Niero, M.; et al. Discovery of Baeyer-Villiger monooxygenases from photosynthetic eukaryotes. Journal of Molecular Catalysis B-Enzymatic 2013, 98, 145–154. [Google Scholar] [CrossRef]
- Mascotti, M.L.; Ayub, M.J.; et al. Chopping and Changing: The Evolution of the Flavin-dependent Monooxygenases. Journal of Molecular Biology 2016, 428, 3131–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.D.; Christensen, S.K.; et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jung, J.H.; et al. Activation of a flavin monooxygenase gene YUCCA7 enhances drought resistance in Arabidopsis. Planta 2012, 235, 923–938. [Google Scholar] [CrossRef]
- Won, C.; Shen, X.L.; et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 18518–18523. [Google Scholar] [CrossRef] [PubMed]
- Mashiguchi, K.; Tanaka, K.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 2011, 108, 18512–18517. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.L.; et al. The Roles of Auxin Biosynthesis YUCCA Gene Family in Plants. International Journal of Molecular Sciences 2019, 20, 6343. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.R.; Chen, J.B. Genome-Wide Identification, Expression Analysis, and Potential Roles under Abiotic Stress of the YUCCA Gene Family in Mungbean (Vigna radiata L.). International Journal of Molecular Sciences 2023, 24, 1603. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.H.; Mashiguchi, K.; et al. The Biochemical Mechanism of Auxin Biosynthesis by an Arabidopsis YUCCA Flavin-containing Monooxygenase. Journal of Biological Chemistry 2013, 288, 1448–1457. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kim, W.Y.; et al. A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nature Communications 2015, 6. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kim, M.R.; et al. The Thiol Reductase Activity of YUCCA6 Mediates Delayed Leaf Senescence by Regulating Genes Involved in Auxin Redistribution. Frontiers in Plant Science 2016, 7. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Saito, K. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. Journal of Experimental Botany 2019, 70, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Onuma, M.; et al. Identification of a flavin-containing S-oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant Journal 2015, 83, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mishina, T.E.; Zeier, J. The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiology 2006, 141, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Zeier, T.; et al. Flavin Monooxygenase-Generated N-Hydroxypipecolic Acid Is a Critical Element of Plant Systemic Immunity. Cell 2018, 173, 456. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.R.; Bertin, M.J.; et al. Retrospective analysis of natural products provides insights for future discovery trends. Proceedings of the National Academy of Sciences of the United States of America 2017, 114, 5601–5606. [Google Scholar] [CrossRef]
- Tintore, M.; Vidal-Jordana, A.; et al. Treatment of multiple sclerosis—Success from bench to bedside. Nature Reviews Neurology 2019, 15, 53–58. [Google Scholar] [CrossRef]
- Waltenberger, B.; Mocan, A.; et al. Natural Products to Counteract the Epidemic of Cardiovascular and Metabolic Disorders. Molecules 2016, 21, 807. [Google Scholar] [CrossRef]
- Hu, Z.F.; Gu, A.D.; et al. Construction and optimization of microbial cell factories for sustainable production of bioactive dammarenediol-II glucosides. Green Chemistry 2019, 21, 3286–3299. [Google Scholar] [CrossRef]
- Li, B.J.; Wang, H.; et al. Improving 10-deacetylbaccatin III-10-β-O-acetyltransferase catalytic fitness for Taxol production. Nature Communications 2017, 8, 15544. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Vogt, M.; et al. Construction of a Corynebacterium glutamicum platform strain for the production of stilbenes and (2S)-flavanones. Metabolic Engineering 2016, 38, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Araújo, R.G.; et al. Production of curcuminoids from tyrosine by a metabolically engineered Escherichia coli using caffeic acid as an intermediate. Biotechnology Journal 2015, 10, 599–U315. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Liu, H.; et al. Engineering the Biosynthesis of Caffeic Acid in Saccharomyces cerevisiae with Heterologous Enzyme Combinations. Engineering 2019, 5, 287–295. [Google Scholar] [CrossRef]
- Campbell, A.; Bauchart, P.; et al. Engineering of a Nepetalactol-Producing Platform Strain of Saccharomyces cerevisiae for the Production of Plant Seco-Iridoids. Acs Synthetic Biology 2016, 5, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Galanie, S.; Thodey, K.; et al. SYNTHETIC BIOLOGY Complete biosynthesis of opioids in yeast. Science 2015, 349, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.A.G.; Li, H.; et al. New frontiers in flavin-dependent monooxygenases. Archives of Biochemistry and Biophysics 2021, 699. [Google Scholar] [CrossRef]
- Cane, D.E. Unified Stereochemical Model of Polyether Antibiotic Structure and Biogenesis. J. Am. Chem. SOC. 1983, 105, 3594–3600. [Google Scholar] [CrossRef]
- Leadlay, P.F.; Staunton, J.; et al. Engineering of complex polyketide biosynthesis -: Insights from sequencing of the monensin biosynthetic gene cluster. Journal of Industrial Microbiology & Biotechnology 2001, 27, 360–367. [Google Scholar] [CrossRef]
- Goodrich, R.D.; Garrett, J.E.; et al. Influence of monensin on the performance of cattle. Journal of animal science 1984, 58, 1484–1498. [Google Scholar] [CrossRef]
- Bhatt, A.; Stark, C.B.W.; et al. Accumulation of an E, E, E-triene by the monensin-producing polyketide synthase when oxidative cyclization is blocked. Angewandte Chemie-International Edition 2005, 44, 7075–7078. [Google Scholar] [CrossRef]
- Suzuki, G.; Minami, A.; et al. Analysis of Enantiofacial Selective Epoxidation Catalyzed by Flavin-containing Monooxygenase Lsd18 Involved in Ionophore Polyether Lasalocid Biosynthesis. Chemistry Letters 2014, 43, 1779–1781. [Google Scholar] [CrossRef]
- Pinkerton, D.M.; Banwell, M.G.; et al. Chemoenzymatic Access to Versatile Epoxyquinol Synthons. Organic Letters 2009, 11, 4290–4293. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Romero, E.O.; et al. Chemoenzymatic Total Synthesis of Natural Products. Accounts of Chemical Research 2021, 54, 1374–1384. [Google Scholar] [CrossRef]
- Davison, J.; al Fahad, A.; et al. Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 7642–7647. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Takada, S.; et al. In vitro and in vivo antimalarial activity of puberulic acid and its new analogs, viticolins A-C, produced by Penicillium sp FKI-4410. Journal of Antibiotics 2011, 64, 183–188. [Google Scholar] [CrossRef]
- Zabala, A.O.; Xu, W.; et al. Characterization of a Silent Azaphilone Gene Cluster from Aspergillus niger ATCC 1015 Reveals a Hydroxylation-Mediated Pyran-Ring Formation. Chemistry & Biology 2012, 19, 1049–1059. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Szewczyk, E.; et al. A Gene Cluster Containing Two Fungal Polyketide Synthases Encodes the Biosynthetic Pathway for a Polyketide, Asperfuranone, in Aspergillus nidulans. Journal of the American Chemical Society 2009, 131, 2965–2970. [Google Scholar] [CrossRef]
- al Fahad, A.; Abood, A.; et al. Oxidative dearomatisation: The key step of sorbicillinoid biosynthesis. Chemical Science 2014, 5, 523–527. [Google Scholar] [CrossRef]
- Shu, X.; Wei, G.Z.; et al. TerC Is a Multifunctional and Promiscuous Flavoprotein Monooxygenase That Catalyzes Bimodal Oxidative Transformations. Organic Letters 2021, 23, 8947–8951. [Google Scholar] [CrossRef]
- Leisch, H.; Morley, K.; et al. Baeyer-Villiger Monooxygenases: More Than Just Green Chemistry. Chemical Reviews 2011, 111, 4165–4222. [Google Scholar] [CrossRef]
- Tolmie, C.; Smit, M.S.; et al. Native roles of Baeyer-Villiger monooxygenases in the microbial metabolism of natural compounds. Natural Product Reports 2019, 36, 326–353. [Google Scholar] [CrossRef]
- van Berkel, W.J.H.; Kamerbeek, N.M.; et al. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. Journal of Biotechnology 2006, 124, 670–689. [Google Scholar] [CrossRef]
- Yachnin, B.J.; Sprules, T.; et al. The Substrate-Bound Crystal Structure of a Baeyer-Villiger Monooxygenase Exhibits a Criegee-like Conformation. Journal of the American Chemical Society 2012, 134, 7788–7795. [Google Scholar] [CrossRef]
- Isupov, M.N.; Schröder, E.; et al. The oxygenating constituent of 3,6-diketocamphane monooxygenase from the CAM plasmid of Pseudomonas putida: The first crystal structure of a type II Baeyer-Villiger monooxygenase. Acta Crystallographica Section D-Biological Crystallography 2015, 71, 2344–2353. [Google Scholar] [CrossRef]
- Song, Y.X.; Li, Q.L.; et al. Neoabyssomicins A-C, polycyclic macrolactones from the deep-sea derived Streptomyces koyangensis SCSIO 5802. Tetrahedron 2017, 73, 5366–5372. [Google Scholar] [CrossRef]
- Wang, Q.; Song, F.H.; et al. Abyssomicins from the South China Sea Deep-Sea Sediment Verrucosispora sp.: Natural Thioether Michael Addition Adducts as Antitubercular Prodrugs. Angewandte Chemie-International Edition 2013, 52, 1231–1234. [Google Scholar] [CrossRef]
- Tu, J.J.; Li, S.T.; et al. Characterization and heterologous expression of the neoabyssomicin/abyssomicin biosynthetic gene cluster from Streptomyces koyangensis SCSIO 5802. Microbial Cell Factories 2018, 17. [Google Scholar] [CrossRef]
- Ji, X.Q.; Tu, J.J.; et al. A Luciferase-Like Monooxygenase and Flavin Reductase Pair AbmE2/AbmZ Catalyzes Baeyer-Villiger Oxidation in Neoabyssomicin Biosynthesis. Acs Catalysis 2020, 10, 2591–2595. [Google Scholar] [CrossRef]
- Fass, R.; Frazier, R. The role of dexlansoprazole modified-release in the management of gastroesophageal reflux disease. Therapeutic Advances in Gastroenterology 2017, 10, 243–251. [Google Scholar] [CrossRef]
- Mejia, A.; Kraft, W.K. Acid peptic diseases: Pharmacological approach to treatment. Expert review of clinical pharmacology 2009, 2, 295–314. [Google Scholar] [CrossRef]
- Robinson, M. Proton pump inhibitors: Update on their role in acid-related gastrointestinal diseases. International Journal of Clinical Practice 2005, 59, 709–715. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; et al. Discovery of Two Native Baeyer-Villiger Monooxygenases for Asymmetric Synthesis of Bulky Chiral Sulfoxides. Applied and Environmental Microbiology 2018, 84. [Google Scholar] [CrossRef]
- Liu, F.; Shou, C.; et al. A Baeyer-Villiger monooxygenase from Cupriavidus basilensis catalyzes asymmetric synthesis of (R)-lansoprazole and other pharmaco-sulfoxides. Applied Microbiology and Biotechnology 2021, 105, 3169–3180. [Google Scholar] [CrossRef]
- Malca, S.H.; Scheps, D.; et al. Bacterial CYP153A monooxygenases for the synthesis of omega-hydroxylated fatty acids. Chemical Communications 2012, 48, 5115–5117. [Google Scholar] [CrossRef]
- Scheps, D.; Malca, S.H.; et al. Synthesis of ω-hydroxy dodecanoic acid based on an engineered CYP153A fusion construct. Microbial Biotechnology 2013, 6, 694–707. [Google Scholar] [CrossRef]
- Lu, W.H.; Ness, J.E.; et al. Biosynthesis of Monomers for Plastics from Renewable Oils. Journal of the American Chemical Society 2010, 132, 15451–15455. [Google Scholar] [CrossRef]
- Rehdorf, J.; Kirschner, A.; et al. Cloning, expression and characterization of a Baeyer-Villiger monooxygenase from Pseudomonas putida KT2440. Biotechnology Letters 2007, 29, 1393–1398. [Google Scholar] [CrossRef]
- Kirschner, A.; Altenbuchner, J.; et al. Cloning, expression, and characterization of a Baeyer-Villiger monooxygenase from Pseudomonas fluorescens DSM 50106 in E-coli. Applied Microbiology and Biotechnology 2007, 73, 1065–1072. [Google Scholar] [CrossRef]
- Yu, J.M.; Liu, Y.Y.; et al. Direct Access to Medium-Chain α,ω-Dicarboxylic Acids by Using a Baeyer-Villiger Monooxygenase of Abnormal Regioselectivity. Chembiochem 2018, 19, 2049–2054. [Google Scholar] [CrossRef]
- Zhang, G.X.; You, Z.N.; et al. Discovery and Engineering of a Novel Baeyer-Villiger Monooxygenase with High Normal Regioselectivity. Chembiochem 2021, 22, 1190–1195. [Google Scholar]
- Hansen, C.C.; Sorensen, M.; et al. Reconfigured Cyanogenic Glucoside Biosynthesis in Eucalyptus cladocalyx Involves a Cytochrome P450 CYP706C55. Plant Physiology 2018, 178, 1081–1095. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamamoto, K.; et al. Identification and characterization of CYP79D16 and CYP71AN24 catalyzing the first and second steps in L-phenylalanine-derived cyanogenic glycoside biosynthesis in the Japanese apricot, Prunus mume Sieb. et Zucc. Plant Molecular Biology 2014, 86, 215–223. [Google Scholar] [CrossRef]
- Toplak, M.; Matthews, A.; et al. The devil is in the details: The chemical basis and mechanistic versatility of flavoprotein monooxygenases. Archives of Biochemistry and Biophysics 2021, 698. [Google Scholar] [CrossRef]
- Toplak, M.; Saleem-Batcha, R.; et al. Catalytic Control of Spiroketal Formation in Rubromycin Polyketide Biosynthesis. Angewandte Chemie-International Edition 2021, 60, 26960–26970. [Google Scholar] [CrossRef]
- Hollmann, F.; Taglieber, A.; et al. A light-driven stereoselective biocatalytic oxidation. Angewandte Chemie-International Edition 2007, 46, 2903–2906. [Google Scholar] [CrossRef]
- Hollmann, F.; Hofstetter, K.; et al. Non-enzymatic regeneration of nicotinamide and flavin cofactors for monooxygenase catalysis. Trends in Biotechnology 2006, 24, 163–171. [Google Scholar] [CrossRef]
- Bunzel, H.A.; Anderson, J.L.R.; et al. Designing better enzymes: Insights from directed evolution. Current Opinion in Structural Biology 2021, 67, 212–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




