Submitted:
24 November 2023
Posted:
27 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Trail Design
2.3. Test Supplement and Dosage
2.4. Dry Eye Syndrome (DES)
2.4.1. Tear Break-Up Time (TBUT)
2.4.2. Strip Meniscometry Test
2.5. Visual Function
2.7. Questionnaire of Subjective Symptoms associated with Ocular Comfort
2.8. Memory Function
2.9. Statisical Analysis
3. Results
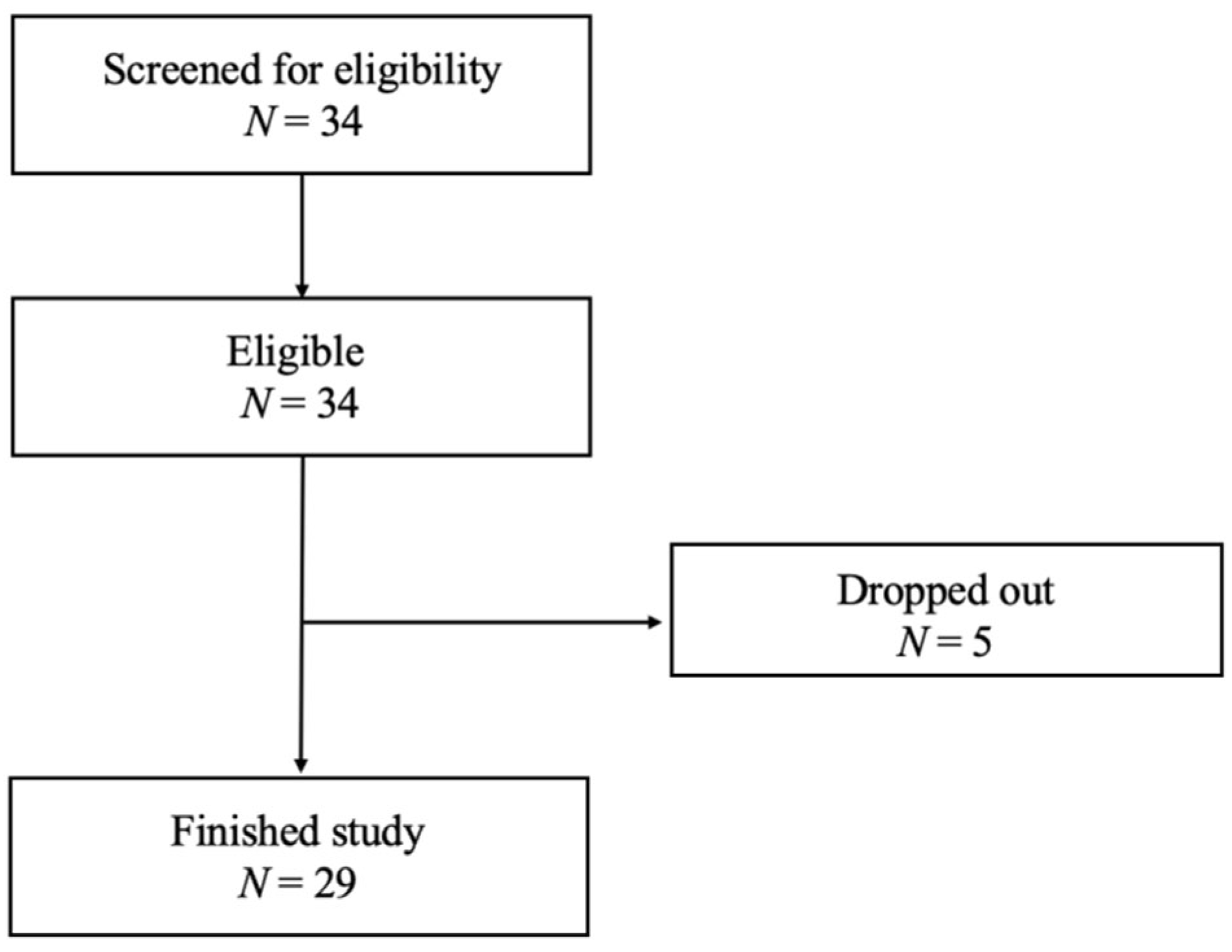
3.1. Subject Characteristics and Participation
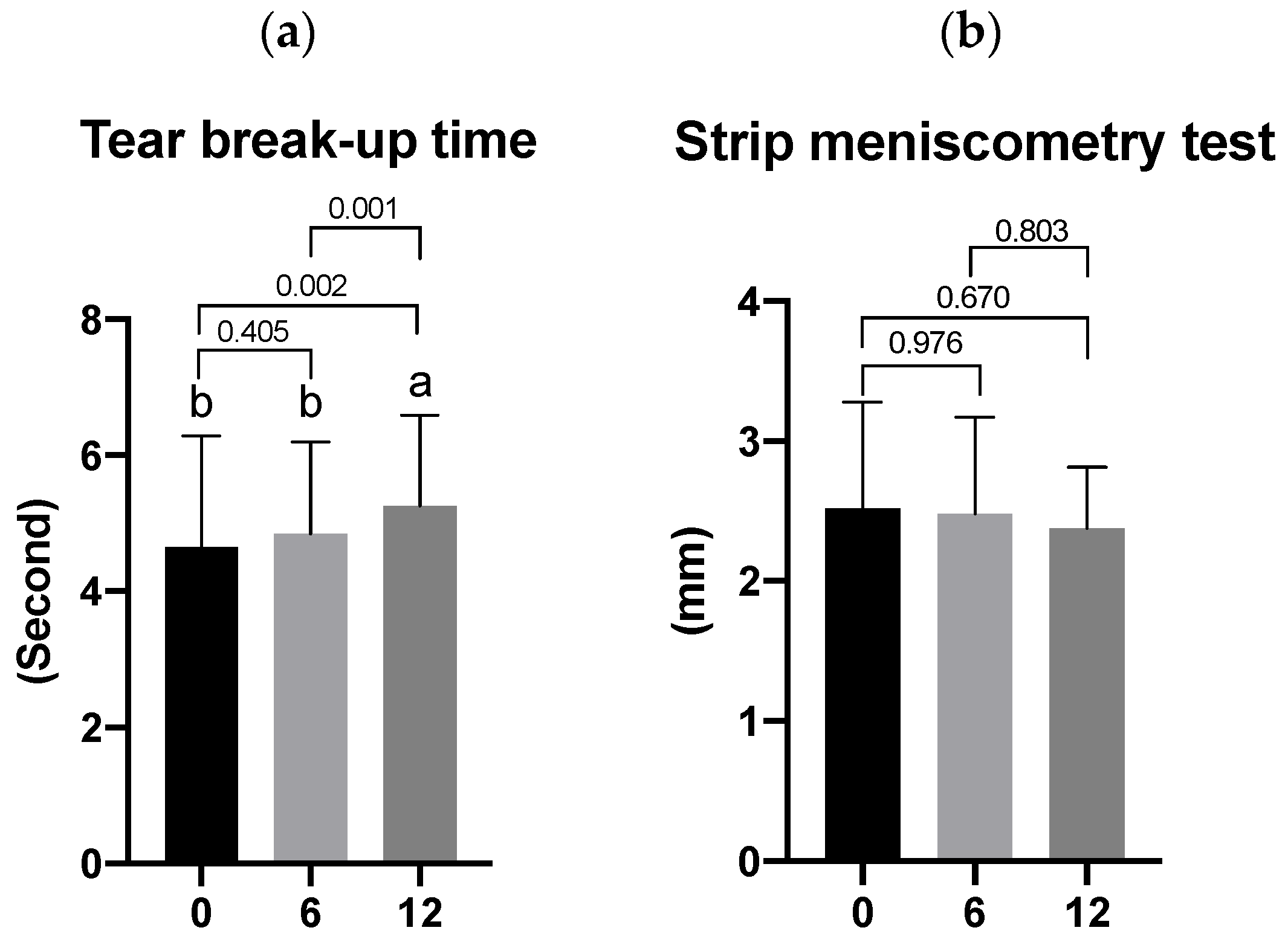
3.2. Effects of Lutein/DHA Complex Supplementation on DES in Healthy Subjects
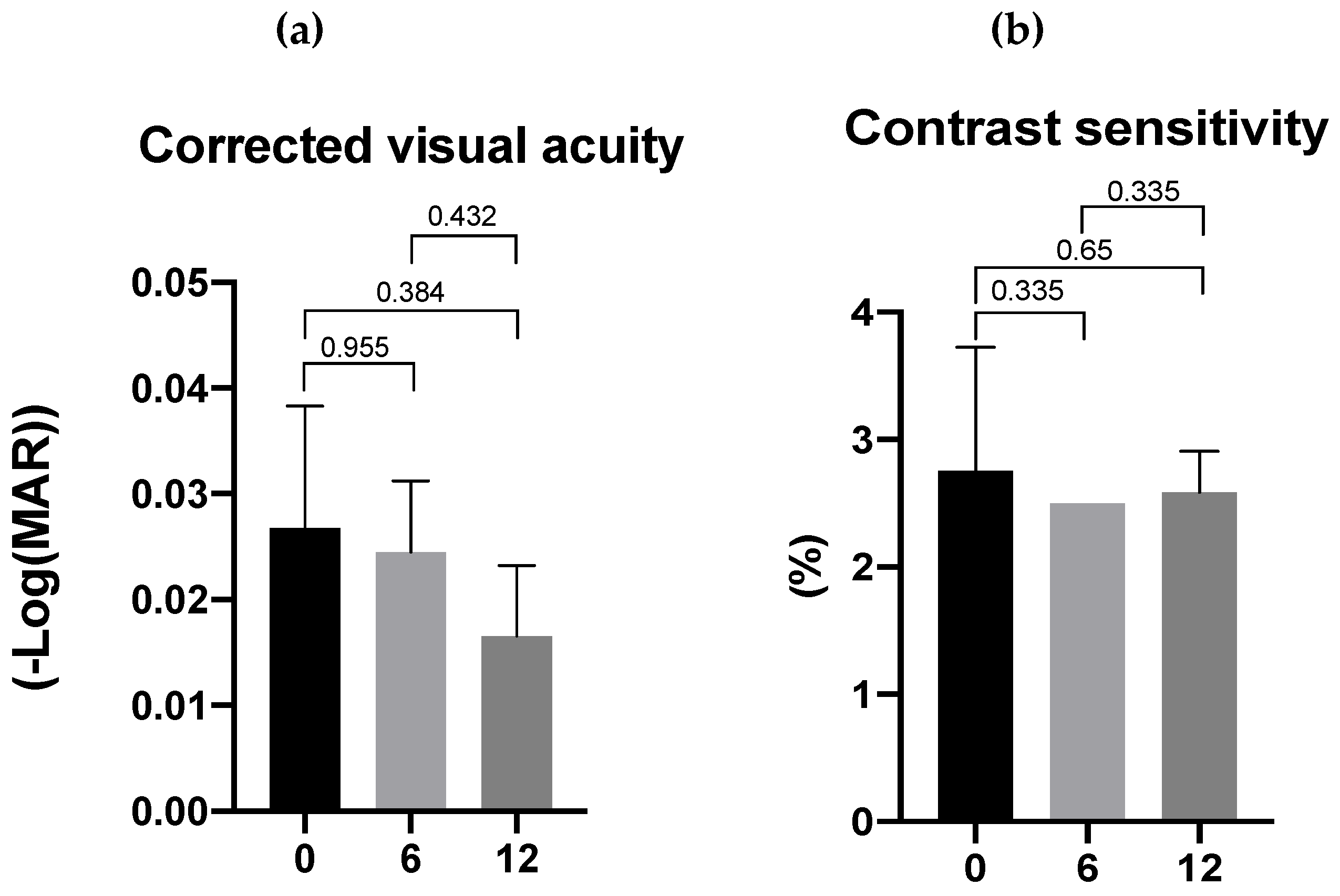
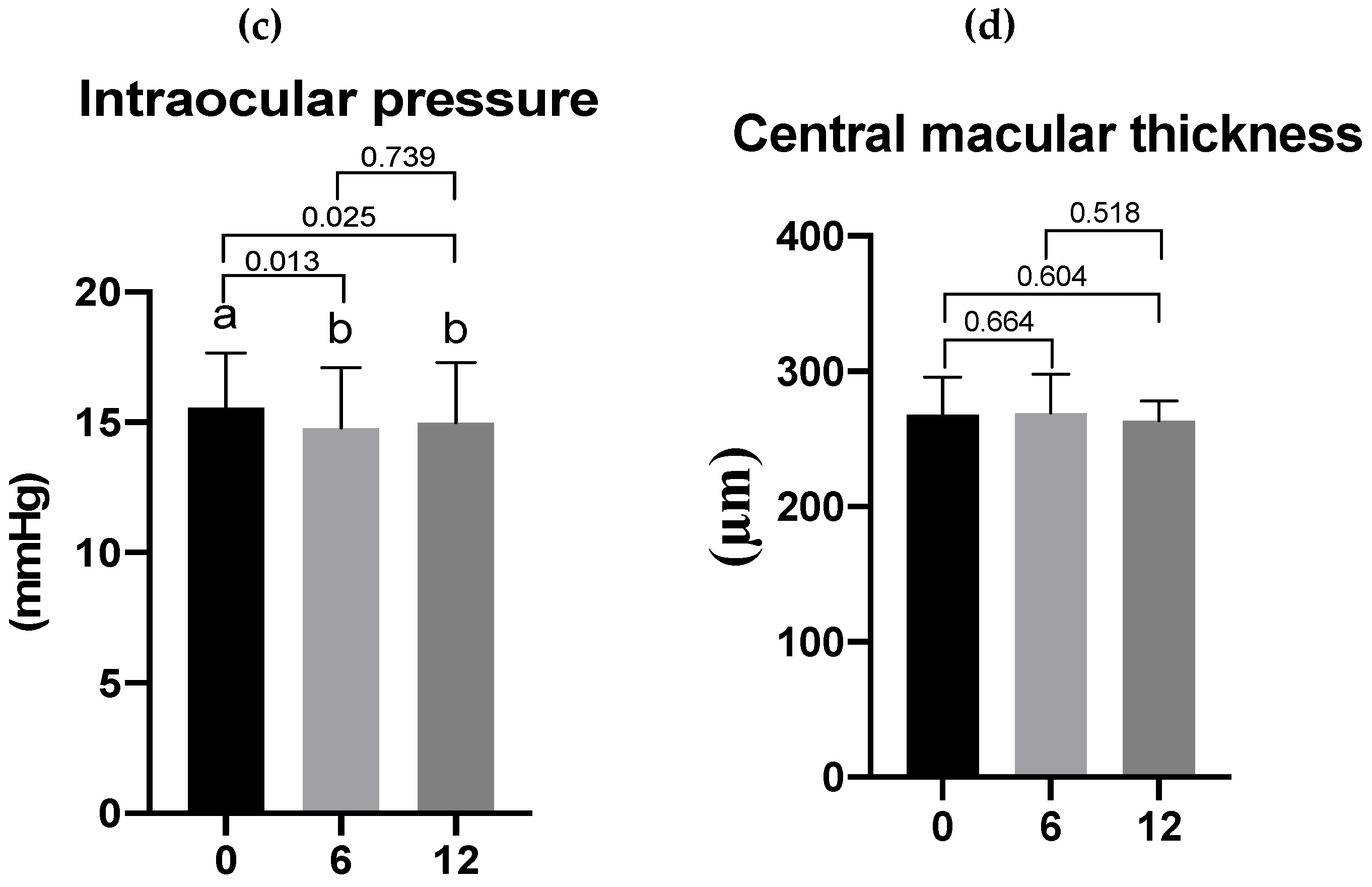
3.3. Effects of Lutein/DHA Complex Supplementation on Visual Function in Healthy Subjects
3.4. Effects of Lutein/DHA Complex Supplementation on Subjective Symptoms Associated with Dry Eyes and Eye Fatigue in Healthy Subjects as Assessed by a Questionnaire
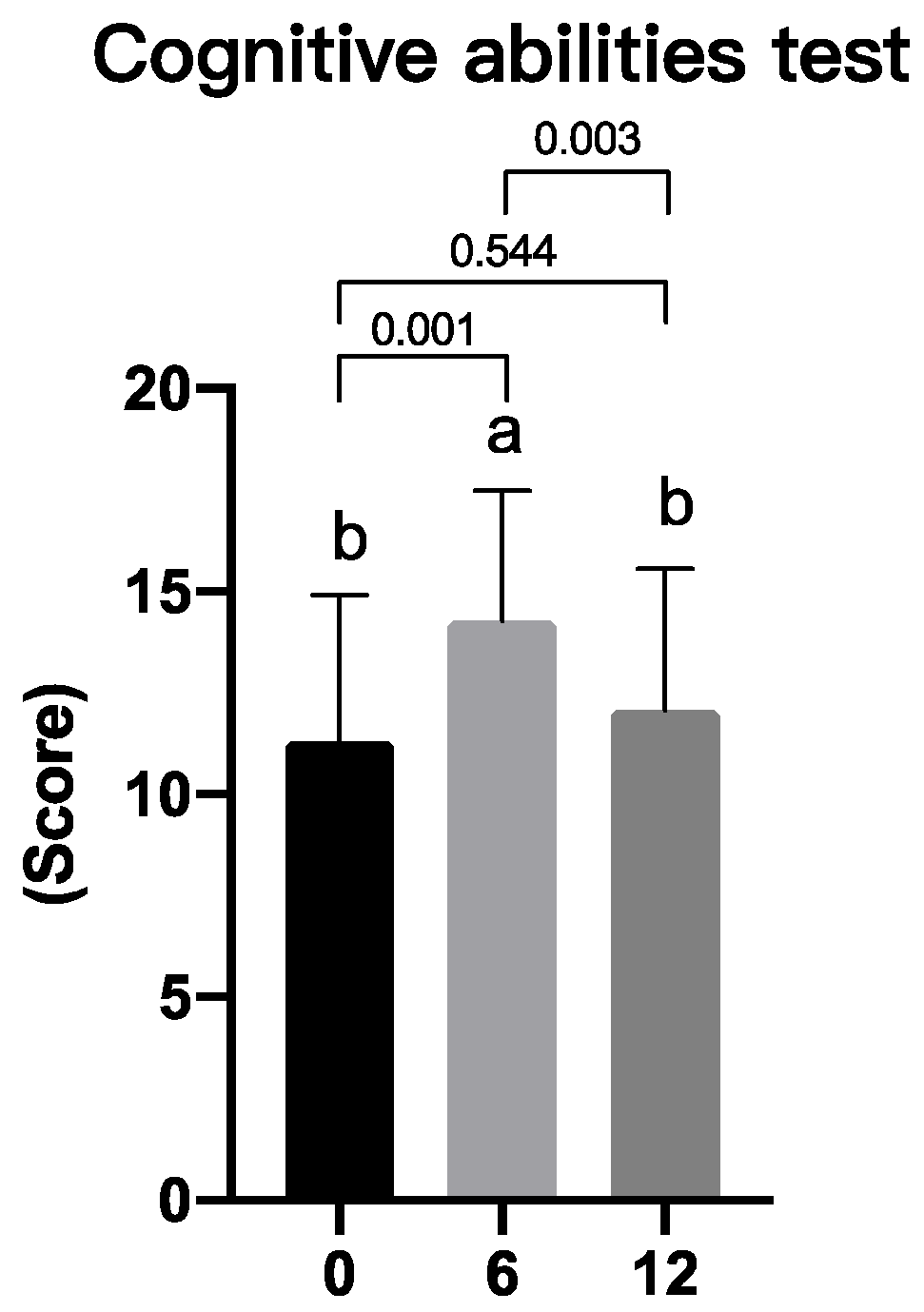
3.5. Effects of Lutein/DHA Complex Supplementation on Memory Function in Healthy Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y., et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46 Suppl 1, S2-s13. [CrossRef]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int 2015, 112, 71-81; quiz 82. [CrossRef]
- Papas, E.B. The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol Opt 2021, 41, 1254-1266. [CrossRef]
- Lin, P.Y.; Tsai, S.Y.; Cheng, C.Y.; Liu, J.H.; Chou, P.; Hsu, W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 2003, 110, 1096-1101. [CrossRef]
- Kuo, Y.K.; Lin, I.C.; Chien, L.N.; Lin, T.Y.; How, Y.T.; Chen, K.H.; Dusting, G.J.; Tseng, C.L. Dry Eye Disease: A Review of Epidemiology in Taiwan, and its Clinical Treatment and Merits. J Clin Med 2019, 8. [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu Rev Nutr 2016, 36, 571-602. [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14. [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9. [CrossRef]
- Neelam, K.; Goenadi, C.J.; Lun, K.; Yip, C.C.; Au Eong, K.G. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol 2017, 101, 551-558. [CrossRef]
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: from bench to bedside. Crit Rev Biotechnol 2016, 36, 829-839. [CrossRef]
- Al-Ahmary, K.M. The carotenoids of some food stuffs in Saudi Arabia. Int J Food Sci Nutr 2010, 61, 823-828. [CrossRef]
- Nagao, A. Absorption and metabolism of dietary carotenoids. Biofactors 2011, 37, 83-87. [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci Nutr 2021, 9, 5229-5243. [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 2012, 3, 1-7. [CrossRef]
- Carver, J.D.; Benford, V.J.; Han, B.; Cantor, A.B. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull 2001, 56, 79-85. [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S., et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [CrossRef]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum Psychopharmacol 2014, 29, 133-144. [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565-573. [CrossRef]
- Chi, S.C.; Tuan, H.I.; Kang, Y.N. Effects of Polyunsaturated Fatty Acids on Nonspecific Typical Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11. [CrossRef]
- Jackson, D.C.; Zeng, W.; Wong, C.Y.; Mifsud, E.J.; Williamson, N.A.; Ang, C.S.; Vingrys, A.J.; Downie, L.E. Tear Interferon-Gamma as a Biomarker for Evaporative Dry Eye Disease. Invest Ophthalmol Vis Sci 2016, 57, 4824-4830. [CrossRef]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P.A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009, 28, 1023-1027. [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002, 56, 365-379. [CrossRef]
- Li, N.; He, J.; Schwartz, C.E.; Gjorstrup, P.; Bazan, H.E. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther 2010, 26, 431-439. [CrossRef]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev 2019, 12, Cd011016. [CrossRef]
- Tian, L.; Wen, Y.; Li, S.; Zhang, P.; Wang, Y.; Wang, J.; Cao, K.; Du, L.; Wang, N.; Jie, Y. Benefits and Safety of Astaxanthin in the Treatment of Mild-To-Moderate Dry Eye Disease. Front Nutr 2021, 8, 796951. [CrossRef]
- Nolan, J.M.; Loughman, J.; Akkali, M.C.; Stack, J.; Scanlon, G.; Davison, P.; Beatty, S. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision Res 2011, 51, 459-469. [CrossRef]
- Zanón-Moreno, V.; Domingo Pedrol, J.C.; Sanz-González, S.M.; Raga-Cervera, J.; Salazar-Corral, J.; Pinazo-Durán, M.D. Feasibility Study of a Docosahexaenoic Acid-Optimized Nutraceutical Formulation on the Macular Levels of Lutein in a Healthy Mediterranean Population. Ophthalmic Res 2021, 64, 1068-1076. [CrossRef]
- Liu, R.; Wang, T.; Zhang, B.; Qin, L.; Wu, C.; Li, Q.; Ma, L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci 2014, 56, 252-258. [CrossRef]
- Dietze, J.; Blair, K.; Havens, S.J. Glaucoma. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
- Machiele, R.; Motlagh, M.; Patel, B.C. Intraocular Pressure. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
- Yang, M.; Wang, Y. Recent Advances and the Mechanism of Astaxanthin in Ophthalmological Diseases. J Ophthalmol 2022, 2022, 8071406. [CrossRef]
- Cort, A.; Ozturk, N.; Akpinar, D.; Unal, M.; Yucel, G.; Ciftcioglu, A.; Yargicoglu, P.; Aslan, M. Suppressive effect of astaxanthin on retinal injury induced by elevated intraocular pressure. Regul Toxicol Pharmacol 2010, 58, 121-130. [CrossRef]
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J Ophthalmol 2018, 2018, 8259371. [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8. [CrossRef]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr 2013, 97, 1134-1143. [CrossRef]

| Ingredient | Amount per serving | Daily intake (2 servings) |
|---|---|---|
| Free-form lutein | 5 mg | 10 mg |
| Docosahexaenoic acid (DHA) | 100 mg | 200 mg |
| Red algae extract (astaxanthin) | 15 mg | 30 mg |
| Phosphatidylserine (PS) | 25 mg | 50 mg |
| Vitamin A | 70 μg RE | 140 μg RE |
| All subjects (N=29) | |
|---|---|
| Male | 14 |
| Female | 15 |
| Age (years) | |
| 40~50 | 15 |
| 51~60 | 14 |
| Average age of subjects (years) | 50.03 ± 4.77 |
| Subjective symptoms associated with ocular comfort (score) | Week 0 | Week 6 | Week 12 |
|---|---|---|---|
| Eye strain | 4.59 ± 1.84 | 4.93 ± 1.65 | 5.14 ± 1.83 |
| Heavy eyelids | 6.17 ± 1.67 | 5.90 ± 1.47 | 6.07 ± 1.60 |
| Eye dryness | 5.28 ± 1.83 | 5.24 ± 2.05 | 5.34 ± 1.93 |
| Itching eyes | 5.59 ± 1.64 | 5.76 ± 1.38 | 5.97 ± 1.57 |
| Thick eye discharges | 5.97 ± 1.57 | 5.72 ± 1.69 | 5.86 ± 1.71 |
| Foreign-matter sensation in the eyes | 5.79 ± 1.72 | 5.76 ± 1.55 | 5.83± 2.00 |
| Photophobia | 6.17 ± 1.49 | 5.93 ± 1.58 | 5.86 ± 1.75 |
| Blurred vision | 4.45 ± 1.80 | 4.76 ± 1.77 | 4.86 ± 1.79 |
| Swollen eyes | 6.34 ± 1.56 | 6.21 ± 1.50 | 6.38 ± 1.50 |
| Sore eyes | 5.76 ± 1.48 | 6.00 ± 1.65 | 5.66 ± 1.91 |
| Eyes tearing involuntarily | 6.03 ± 1.43 | 6.17 ± 1.39 | 5.86 ± 1.57 |
| Blurred vision at night | 4.83 ± 2.00 | 5.17 ± 2.22 | 5.14 ± 1.81 |
| Summary score | 66.97± 14.27 | 67.55± 14.92 | 67.97± 16.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





