1. Introduction
Legumes are commonly used in sustainable agroecosystems because of their ability to tolerate low N fertilizer input due to their capacity to use atmospheric N2 through biological nitrogen fixation (BNF). Advantage of using legumes in agroecosystems is not limited to protecting soils from pollution caused by chemical fertilizers [1] because once well-established legumes progressively fertilize the soil [2]. If legumes, such as Pea (Pisum sativum) are still mainly used as annual protein-rich crops, introduction of legumes in sustainable cropping systems is becoming an objective to reach for breeders and growers. As such, legumes would provide ecological services i.e., limiting usage of N fertilizer and decreasing herbicides input by competing with weeds for soil water, mineral nutrients and light thus limiting their development [3,4].
Competitive genotypes to fulfil this role should be selected on the basis of their ability to colonize efficiently the soil with a deep-foraging, fast-growing and highly branched root systems. These traits are known to be under the control of rhizosphere factors among which nitrate as a signal molecule, sensed by various nitrate transporters such as NPF (Nitrate Transporter1/Peptide transporter Family) and NRT2 (Nitrate Transporter 2), play a major role [5–8]. Paradoxically, if nitrate is necessary to ensure legumes seedling establishment before BNF starts, it is also a negative regulator of nodulation and BNF if it is provided at high concentrations [9]. For these reasons increasing our knowledge of molecular aspects pertaining to nitrate sensing via nitrate transporters and signaling in legumes is a corner stone for selecting genetically competitive genotypes suitable for ecological intercropping systems.
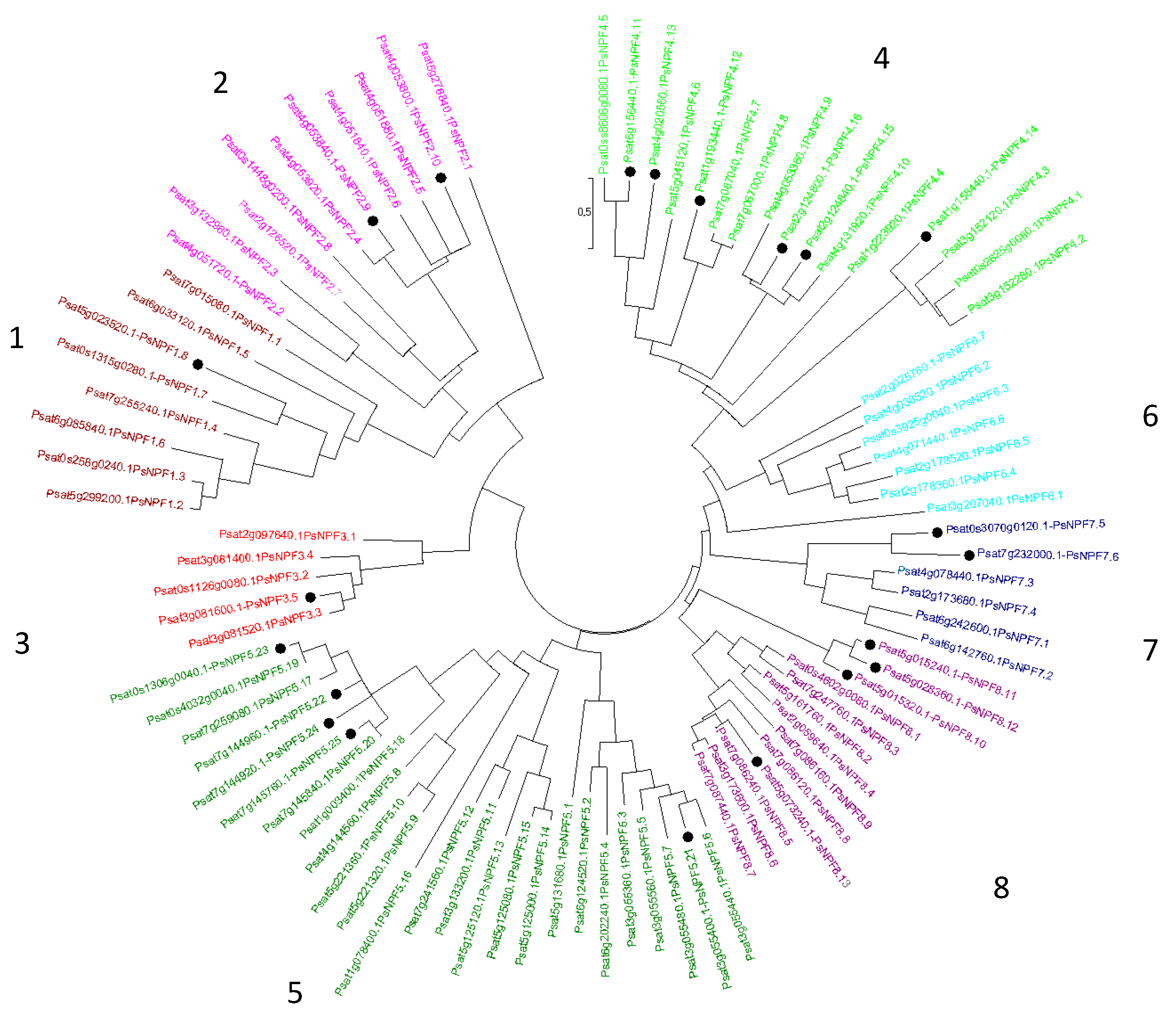
Recently, a particular interest has been given to the role of nitrate transporters in the functioning of nodules, some transporters having a positive and/or a negative role in nodule functioning, depending on nitrate concentration; this review updates the results obtained in the two model legumes, Lotus japonicus and Medicago truncatula. In addition, we have identified the complete PsNPF family in Pea by using P. sativum v1a genomic assembly [10]. Thus, we were able to find 90 putative PsNPF sequences among which, we not only found the 69 previously described in the literature [11] but identified 21 new sequences that we have annotated according to the two-number code [12]. Furthermore, we have also exploited available transcriptomic data in the literature generated in this species [13] to identify transporters, belonging to either NPF or NRT2 families, expressed in nodules that would be involved in positive or negative regulation in relation to nitrate concentration.
2. Nitrogen acquisition by legumes
Most of the nitrogen taken-up by higher plants is in inorganic form with nitrate as the major source. In their natural habitat plants are exposed to frequent changes in mineral nutrients availability. In particular, to respond to variation of nitrate availability in the soil, plants absorption mechanism of nitrate have evolved into two transport systems, the low-affinity transport system (LATS) and the high-affinity transport system (HATS) [14]. LATS proteins are mainly represented by NPF and HATS proteins are mainly represented by NRT2 [7]. NPF members belong to a large family of 92 MtNPF in M. truncatula, 86 LjNPF in L. japonicus [15–17]. A study using genomic data of 31 plant species, including M. truncatula, showed that NPFs belong to eight subfamilies; this distribution was confirmed for the NPFs of L. japonicus [12,17]. By using heterologous expression system, often Xenopus oocytes, some NPFs have been shown to be nitrate transporters but others are likely to transport substrates like peptides, amino acids, glucosinolates, IAA or ABA for example, some NPFs were shown to be able to transport two different substrates [12]. NRT2s belong to a smaller family than NPF, in M. truncatula it includes three members [18]: MtNRT2.1 (Medtr4g057890), MtNRT2.2 (Medtr4g057865) and MtNRT2.3 (Medtr8g069775). In L. japonicus this family consists of four members [19,20]: LjNRT2.1 (Lj3g3v3069030), LjNRT2.2 (Lj3g3v3069050), LjNRT2.3 (Lj4g3v1085060) and LjNRT2.4 (Lj1g3v3646440). However, LjNRT2.2 was shown to be not functional in some L. japonicus ecotypes; a stop codon interrupts the reading phase and results in a truncated protein [21]. Thus, it is reasonable to consider that NRT2 family of L. japonicus consists of three functional genes. All NRT2-type transporters transport only nitrate, the transport of this substrate requires in most cases the interaction of NRT2 with another protein, NAR2. Two NAR2 genes were identified in M. truncatula while only a single NAR2 gene was identified in L. japonicus [8,22].
In addition to absorbing nitrate from the soil, legumes can form symbioses with bacteria, called rhizobia. The formation of root nodules allows legumes to perform atmospheric nitrogen (N2) fixation. In root nodule cells rhizobacteria are enclosed in symbiosomes, which are structures surrounded by a peribacteroidal membrane (PBM) of plant origin. Bacteria differentiated into bacteroids acquire the ability to fix atmospheric N2 through nitrogenase enzymatic activity. Nitrogen fixation is a process requiring carbon energy supplied by the plant in the form of photosynthesis products, and oxygen for respiration to generate ATP and reducing equivalents for the reduction of N2 to NH3. Paradoxically, if mitochondria require normal level of O2 (normoxic condition) for respiration, nitrogenase is inactivated by oxygen. This potential problem is solved thanks to the presence of leghemoglobin (Lb). This oxygen-carrying protein plays an important role; due to its high-affinity for oxygen it efficiently delivers oxygen to mitochondria of the bacteroids while by buffering free oxygen it decreases its level in the vicinity of nitrogenase [23]. Furthermore, Lbs protect nitrogenase as a scavenger of nitric oxide (NO), which is an inhibitor of its activity [24]. Proteins of the plant play a role in the infection and organogenesis among which the NODULE INCEPTION (NIN), one of the most important nodulation proteins. NINs are transcription factors that positively regulate rhizobial infection, nodule organogenesis, N fixation [25,26]. NIN also control nodule number by inducing expression of CLAVATA3/ENDOSPERM SURROUNDING REGION (CLE) peptides involved in communication between root and shoot [27].
Symbiotic nitrogen fixation and nodule formation are energetically costly for the plant. Therefore, legumes have developed mechanisms regulating nodulation in response to the amount of nitrate in the soil [9]; in the presence of high nitrate concentrations, nodulation is inhibited. The responsiveness to high nitrate concentrations (5-10 mM) of nodule functioning has been associated with a decrease in functional leghemoglobin and nitrogenase activity [28]. In M. truncatula and L. japonicus, it has also been shown that NIN-LIKE PROTEIN (NLP) transcription factors play a central role in inhibiting nodulation under high nitrate [29–32]. On the contrary, low nitrate concentrations stimulate nodulation and nitrogen fixation. C-TERMINALLY ENCODED PEPTIDEs (CEPs) are signaling molecules that enhance nodulation [33,34]. In M. truncatula, MtCEP1 is induced under low nitrogen and expresses during nodule formation [33]. MtCEP1 has been shown to interact with its putative CRA2 (COMPACT ROOT ARCHITECTURE 2) receptor to mediate nodulation [34]. Those examples of mechanisms regulating nodulation in response to the amount of nitrate in the soil allow the legumes to switch from soil nitrogen acquisition to symbiotic nitrogen fixation.
While the inhibitory effect of nitrate at high concentration has often been studied on the formation, development and functioning of nodules, few studies have been dedicated to the positive effect of nitrate at low concentration. Omics studies have shown that the expression of many NPF genes is upregulated in mature nodules [35–37]. Some nitrate transporters of L. japonicus or M. truncatula have been shown to play a role in the functioning of nodules; some transporters have a positive and/or a negative role in nodule functioning, depending on nitrate concentration.
3. NPFs playing a role in nodule functioning
In L. japonicus, an in-silico analysis showed that the expression of eight LjNPF genes is upregulated in mature N2-fixing nodules [36]. Two of these eight NPFs, LjNPF8.6 and LjNPF3.1, were studied in depth [38,39]. LjNPF8.6, whose expression is strongly induced in nodules compared to roots, is the first NPF for which a specific and positive role on nodule functioning has been shown [38]. LjNPF8.6 was found to be located in the central infection zone where N fixation takes place [35]. In addition, after inoculation of Ljnpf8.6 mutants by M. loti, an increase in nodular superoxide content in the nodules accompanied by a reduction in N-fixation activity was observed with an accumulation of anthocyanin in stems and roots [38]. Anthocyanin accumulation in stems has been reported as a phenotype associated with nitrogen starvation condition associated with impaired nodule function or lack of nodulation [39] and references therein). These observations suggest that LjNPF8.6 would play a role in the control of nodule functioning rather than in development. Furthermore, this transporter was shown to have a nitrate transport activity, it is thus tempting to suggest that LjNPF8.6 plays a role in the control of nodule functioning through the modulation of nitrate flux trough the peribacteroidal membrane [38]. Another interesting transporter in L. japonicus is LjNPF3.1 [36] which promoter was shown to be active in the cortical cells of inoculated hairy roots and at the base of the nodules [39]. Actually, its expression was more than 10-fold higher in nodules than in roots while it is also expressed in leaves and mature flowers. In addition, inoculated Ljnpf3.1 mutants showed increased nodule biomass and anthocyanin accumulation in the stems, phenotypes that can be explained by a slight but significant decrease in the measured nitrogenase activity. Thus, LjNPF3.1 plays a positive role in efficient nodule functioning, possibly by transporting nitrate from the roots or from outside to the nodules [39]. However, the role of LjNPF3.1 would be limited to conditions of low external nitrate concentration that are not inhibitory for BNF.
In M. truncatula, expression of several MtNPFs is up-regulated in nodules [15]; however only two NPFs playing a role in nodule functioning, MtNPF1.7 and MtNPF7.6, have been deeply studied in M. truncatula. MtNPF1.7 (also known as LATD/NIP) was functionally characterized as a high-affinity nitrate transporter [40], involved in root development [41,42] with also an essential role in the formation and maintenance of nodule meristems and in rhizobial invasion [42]. Studies of different mutants, affected in MtNPF1.7 have shown that MtNPF1.7 is not necessary for the initial stages of rhizobial invasion into host roots but is required for rhizobial infection during nodulation [43–45]. Since MtNPF1.7 is expressed and required in both lateral root and nodule meristems, the corresponding protein could play a key role in the balance between development of lateral roots and nodules [42].
MtNPF7.6 is a NPF of M. truncatula studied in detail, specifically expressed in nodule vasculature, localized in the plasma membrane of nodule transfer cells (NTCs) [46]. By using knockout mtnpf7.6 mutants, it has been shown that MtNPF7.6 modulates Lb expression, endogenous NO homeostasis and nitrogenase activity. MtNPF7.6 has been shown to play a role in nitrate-mediated regulation during root nodule symbiosis under both low and high nitrate conditions [46]. Under low-nitrate (0.2 mM), MtNPF7.6, demonstrated as being a high-affinity nitrate transporter, functions in nitrate uptake from the environment and from the host root and in nitrate transport to NTCs promoting nodule growth. Under high-nitrate condition (20 mM), MtNPF7.6 expression was induced and an over-accumulation of nitrate due to MtNPF7.6-nitrate-transport inhibits nodule functioning. Interestingly, comparing the transcriptome of wild-type and mtnpf7.6 nodules, it has been shown that the expression patterns of four genes, encoding MtNRT2.1, MtNRT2.2, MtNRT2.3 and MtNPF6.5, were altered in the mutants, suggesting that MtNPF6.5 and MtNRT2s may be involved in the nutrient or signal exchange in nodule [46].
Concerning
P. sativum, , 69 PsNPFs were identified [11]. In addition, a full-length Unigene set of expressed sequences has been developed in
P. sativum by sequencing 20 cDNA libraries produced from various plant organs harvested at various developmental stages from plants grown under different conditions [13],
https://urgi.versailles.inra.fr/download/pea/Pea_PSCAM_transcriptome) study in which, some NPFs mentioned were not identified previously [11]. Thus, to identify the complete PsNPF family in
P. sativum, we performed a blastp search using PsNPF6.7 (Psat2g025760) as query against
P. sativum v1a genomic assembly [10]. We were able to find 90 putative PsNPF sequences (
Supplementary Table 1) among which we found the 69 previously identified [11] and 21 new ones distributed in the 8 clades (
Figure 1) previously described [11].
The new sequences are distributed as follows: one sequence belongs to the clade 1, two to the clade 2, one to the clade 3, six to the clade 4, five to the clade 5, two to the clade 7 and four to the clade 8. New PsNPF were annotated according to the two-number code previously established [12]. Then we have exported the expression data of the 90
PsNPF genes from the full-length Unigene set of
P. sativum [13]
Supplementary Table 2). It should be noted that the length of PsNPF proteins are ranging from 93 to 637 amino acids (
Supplementary Table 1), some protein sequences being much shorter than those of NPFs already described in the literature: they have been retained in this study because the corresponding genes are expressed (except
PsNPF5.23), and sometimes, very significantly as seen for
PsNPF4.16 (233 amino acids) which is very strongly expressed in the peduncles of the C stage [13] (
Supplementary Table 2).
In [13], 842 genes whose expression was preferentially or specifically up-regulated in nodules were identified. Among them, 66 contigs encodes transporters of various families of which 6 belongs to the NPF family (
Table 1A). In the same study, other PsNPFs have significant expression in nodules but are also expressed in other organs; we have grouped them in
Table 1B, bringing to nine the number of
PsNPFs specifically or strongly expressed in nodules in
P. sativum. One of them, PsNPF7.1, is the ortholog of MtNPF7.6 [46] (
Supplementary Table 3). PsNPF7.1 is specifically and very strongly expressed in nodules (
Table 1, Alves-Carvalho et al., 2015). In a recent study, we investigated whether
Rhizobium-derived signals interfere with nitrate signaling in
P. sativum [47]. It appeared that
PsNPF7.1 expression was induced in 12-day-old seedlings only in the presence of Rhizobium. In addition,
PsNPF7.1 expression was up-regulated by 1 mM nitrate and down-regulated by 10 mM. A possible role of PsNPF7.1 in nodule functioning dependent on environmental nitrate concentration would be interesting to study further. The orthologous genes of
MtNPF1.7,
LjNPF8.6 and
LjNPF3.1 in
P. sativum,
PsNPF1.5,
PsNPF8.4 and
PsNPF3.1 respectively, would also be interesting to study (
Supplementary Table 3). It should be noted that some
NPF genes produce different transcripts (
Supplementary Table 1) as seen for
AtNPF5.5 [48]. Among the interesting genes mentioned above,
PsNPF1.5 produces two different transcripts, that it would be interesting to investigate whether the corresponding proteins are functional and have same or different roles.
4. NRT2s playing a role in nodule functioning
LjNRT2.4 was the first NRT2 to be thoroughly studied in L. japonicus. In contrast to the other LjNRT2 genes, a strong induction of LjNRT2.4 expression was observed in nodules compared to roots [19,20]. A positive role of LjNRT2.4 was reported in a nitrate-mediated nodule functioning pathway [20]. In fact, two Ljnrt2.4 mutants were impaired in nitrate content and nitrogenase activity in nodules. LjNRT2.4, whose tissue localization was shown to be the nodule vascular bundles and subcellular localization the plasma membrane, would transport nitrate into the N2-fixing cells of the nodule. Nitrate can be reduced to nitrite by nitrate reductase in the cytoplasm of the cell, nitrite which, transported to the mitochondria, functions as an electron acceptor in the respiratory chain, thus contributing to ATP synthesis [9,49,50]. Nitrate can also be reduced to nitrite by nitrate reductase in the bacteroid. LjNPF8.6, localized in the peribacteroidal membrane, would play a role in the regulation of nitrate flux between the plant cell and the bacteroid [38]. Thus, the model proposed in nodule functioning involves LjNRT2.4 and LjNPF8.6 in a complementary manner [20].
LjNRT2.1 has also been studied in depth. By using Ljnrt2.1 mutants, it has been shown how LjNRT2.1 control root nodule symbiosis in a nitrate-rich environment in L. japonicus [21]. The authors proposed a model in which LjNRT2.1 acts in the same signaling pathway as LjNLP1 and LjNLP4 for the nitrate-induced control of nodulation. In the presence of nitrate, LjNLP1 transcription factor induced LjNRT2.1 expression. LjNRT2.1 transports nitrate from the soil to the root. The increase of nitrate in the root triggers the nuclear localization of LjNLP4 which inhibits nodulation through the regulation of gene expression. As LjNLP1 is activated by nitrate, it has been suggested that another nitrate transporter than LjNRT2.1 should be involved in the model to allow the first step which is nitrate transport and LjNLP1 activation [21]. In addition, LjNIN, a positive regulator of nodulation, whose expression is induced by rhizobial infection [51,52], would negatively regulate the expression of LjNRT2.1 resulting in a reduction of nitrate uptake. Thus LjNRT2.1 would be at the center of a strategy used by the plant regarding nitrate acquisition, switching from dependence on soil nitrate to symbiotic fixation [21].
Among the three MtNRT2 of M. truncatula, only the role of MtNRT2.1 in nodulation has been addressed [53]; it shows some similarities with LjNRT2.1. In fact, MtNRT2.1 expression like that of LjNRT2.1 was activated by MtNLP1. By using Mtnrt2.1 mutants, it has been shown that MtNRT2.1 encodes a high-affinity nitrate transporter responsible for the majority of nitrate taken up by the plant in the 0.5-5 mM nitrate concentration range [53]. MtNRT2.1 was also shown to play a dual role in nitrate regulation of nodulation in M. truncatula as it is required for nodule establishment under low-nitrate conditions and necessary for repression of nodulation under high-nitrate conditions [53]. Accordingly, a model has been proposed in which low nitrate induces MtCEP1expression, which systemically induces MtNRT2.1 expression through MtCRA2 resulting in an enhancement in nodulation and nitrate uptake. MtNLP1, whose localization in the nucleus was limited under low nitrate, is increased by high nitrate in the nucleus leading to the activation of the expression of CLE5, which negatively regulates nodulation [53].
In the pea genome, only one full-length
PsNRT2, named
PsNRT2.3 (Ps4g113000), was identified [11]. Two more
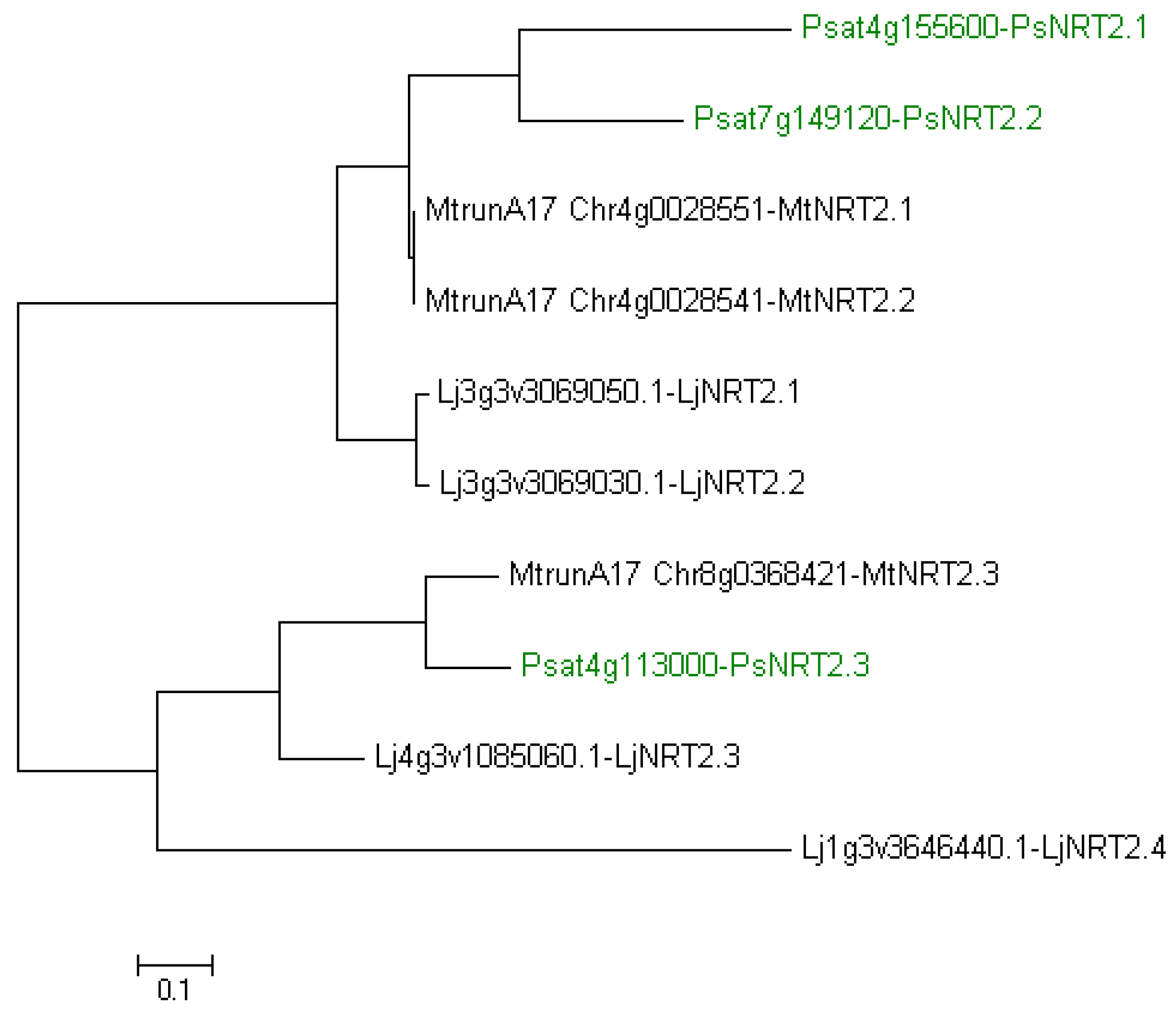
PsNRT2 genes exist, PsNRT2.1 (Psat4g155600.1) and PsNRT2.2 (Psat7g149120.1), but both corresponding proteins are short with only three transmembrane domains against eight in NRT2 in general. A possible loss of nitrate transport function has been suggested for these two proteins [11]. We have made a phylogenetic tree to establish PsNRT2 relationship with NRT2 of
M. truncatula and
L. japonicus (
Figure 2). It shows a clustering of PsNRT2.1/2.2 with MtNRT2.1/2.2 and LjNRT2.1/2.2 on the one hand and a clustering of PsNRT2.3 with MtNRT2.3 and LjNRT2.3 on the other hand. We confirm that LjNRT2.4 appears isolated in the phylogenetic tree, having no ortholog in
M. truncatula [20] and having no ortholog in
P. sativum either (
Figure 2).
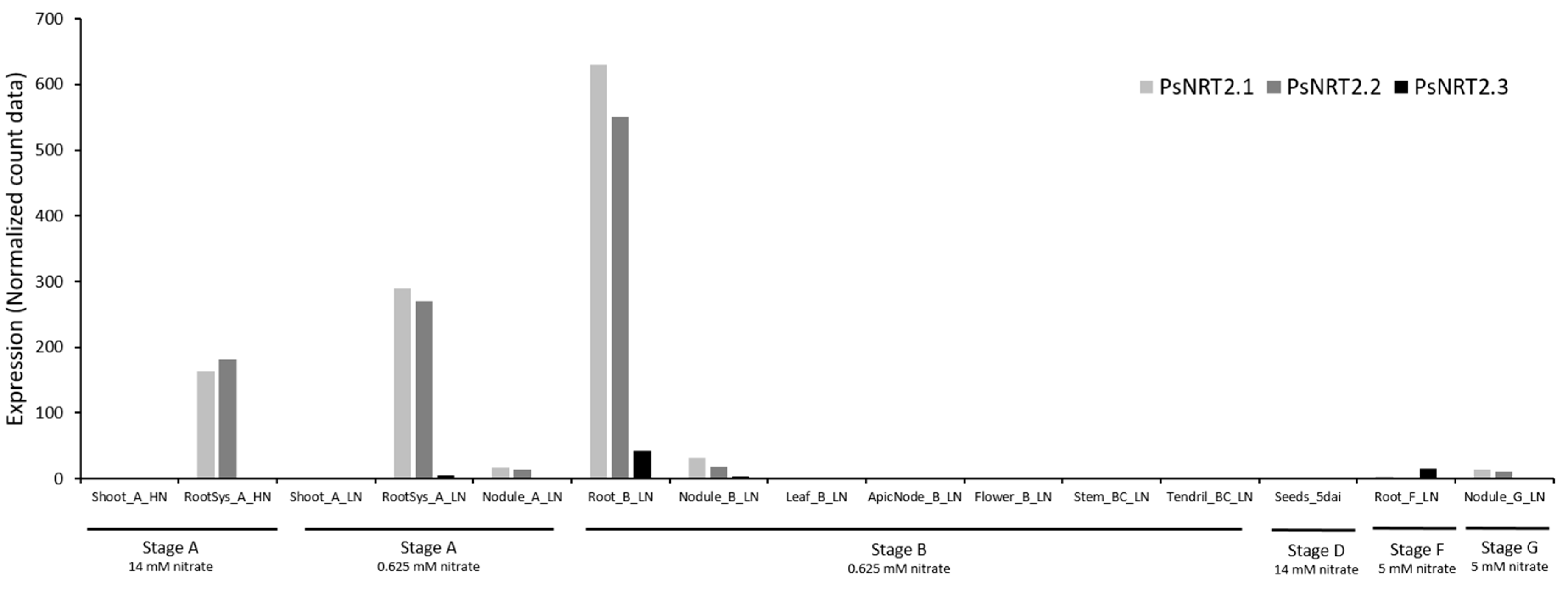
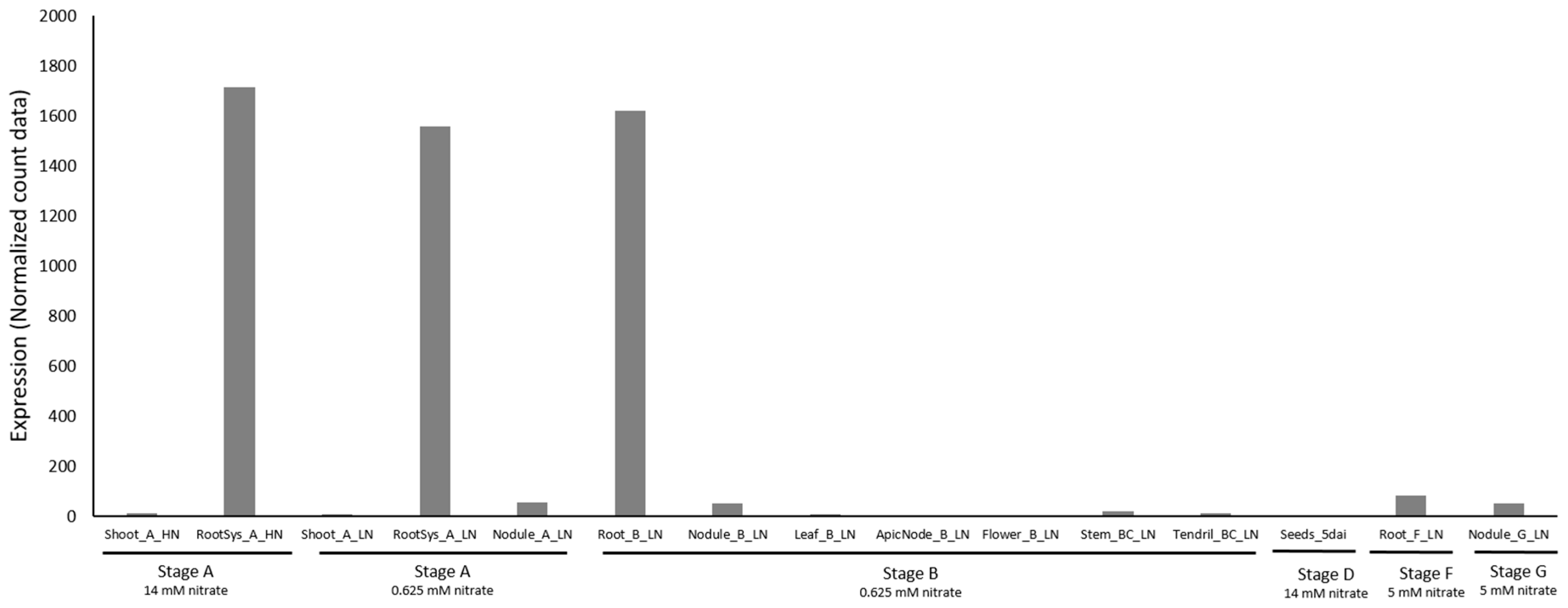
The omics data [13] allow visualization of the expression of the three
PsNRT2 genes and of the
PsNAR2 gene under different conditions in different tissues (
Figure 3 and
Figure 4).
It can be noted that despite the smaller size of PsNRT2.1 and PsNRT2.2 proteins, corresponding genes are expressed.
PsNRT2.1,
PsNRT2.2 and
PsNAR2 are very strongly expressed in the roots of the A and B stages (5-6 opened leaves and at the beginning of flowering, respectively) while
PsNRT2.3 is much less expressed at those stages. Nevertheless,
PsNRT2.3 is expressed around 30-fold more in roots than in other tissues at B stage. It can also be noted that
LjNRT2.3 is the
NRT2 gene most expressed in roots at the F stage (8 days after sowing) (
Figure 3). The results indicate that
PsNRT2.1 and
PsNRT2.2 are also expressed in nodules but much less than in roots (at least 18 times less) and
PsNRT2.3 is almost not expressed in nodules. Therefore, in
P. sativum, no
NRT2 gene is so strongly expressed in nodules as
LjNRT2.4 in
L. japonicus [19]. Anyway, further study would be necessary to see if either or both proteins, PsNRT2.1 and PsNRT2.2, have a role in the regulation of nodulation.
5. Conclusions
In order to shed light on the roles of nitrate transporters and their potential complementary roles in nodules functioning, it is necessary that a substantial number if not all transporters expressed in nodules be functionally and physiologically characterized. Up to now, however, in comparison to the large number of putative transporters identified by various genomic and transcriptomic approaches to be expressed in nodules [15,36,54] only few NPFs have been thoroughly studied
i.e. two in
M. truncatula, MtNPF1.7 [42], MtNPF7.6 [46] and two in
L. japonicus, LjNPF8.6 [38], LjNPF3.1 [39]. In
P. sativum,
PsNPFs identified in this study as specifically expressed in nodules (
Table 1A) or expressed in nodules and other organs (
Table 1B) are interesting candidates waiting for functional characterization and investigation of their roles in nodules. As to NRT2s, three have been shown to play a role in nodule functioning: LjNRT2.4 [20], LjNRT2.1 [21] and MtNRT2.1 [53]. It should be noted that the expression of
MtNRT2.3 was higher in nodules than that in roots [18] but until now no studies have been performed on the role of the encoded protein (MtNRT2.3) in nodules.
Identification of the roles of the myriad of nitrate transporters would open new avenues for better characterizing the involvement of nitrate and other substrates such as phytohormones transported by members of NPF and NRT2 families in nodules functioning. In fact, besides the well-illustrated role of nitrate as negative regulator of nodulation through local and systemic signaling pathways [9] nitrate plays an important role as a source of nitric oxide (NO). Interestingly both the plant and the symbiont were shown to use nitrate as a substrate for NO synthesis in functional nodules [49]. NO has been shown to be produced from early phases of plant-symbiont interaction to nodule senescence [55]. At early phases, NO contributes to the repression of plant defense reactions which favors the microbe penetration in plant tissue while in mature nodules NO participates to the modulation of nitrogen acquisition by inhibiting N2 fixation. Nitrate as a provider of NO contributes also to the energy status (ATP synthesis) in both nodules and bacteroids through the mitochondrial NO3−-NO respiration in invaded cells and the denitrification pathway in bacteroids [49]. It is thus of importance that the putative transporters of nitrate expressed in nodules be functionally characterized because their contribution seem essential to ensure nitrate trafficking between the root system and nodules and between invaded cells and bacteroids enclosed in symbiosomes [9,49,55]. In this context an integrative model could be drawn in L. japonicus, where complementary roles were proposed for two nitrate transporters. A high affinity transporter LjNRT2.4 to ensure nitrate allocation to the N2-fixing cells [20] and a low affinity transporter LjNPF8.6 that regulates nitrate flux between plant cell cytosol and bacteroid compartments [38]. Furthermore NPFs transport other substrates than nitrate such as phytohormones [56,57] that might be involved in nodule functioning. Auxin and ABA were found to play major roles in nodule formation [42] and GA was reported as a positive regulator of nodule functioning [58]. Thus, NPF transporters could couple nitrate and hormone signaling during root symbiosis.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
conceptualization, M-CM-LP, TC, AML; transcriptomic data analysis, M-CM-LP; phylogenetic tree design, TC; manuscript writing, M-CM-LP, AML; manuscript revision, M-CM-LP, TC, AML. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors are grateful to the University of Angers for its support to research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galloway, J.N.; Cowling, E.B. Reflections on 200 Years of Nitrogen, 20 Years Later: This Article Belongs to Ambio’s 50th Anniversary Collection. Theme: Eutrophication. Ambio 2021, 50, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M.; Lum, M.R.; Downie, J.A. What Makes the Rhizobia-Legume Symbiosis so Special? Plant Physiology 2001, 127, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving Intercropping: A Synthesis of Research in Agronomy, Plant Physiology and Ecology. New Phytologist 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Nazzicari, N.; Notario, T.; Monterrubio Martin, C.; Romani, M.; Ferrari, B.; Pecetti, L. Pea Breeding for Intercropping With Cereals: Variation for Competitive Ability and Associated Traits, and Assessment of Phenotypic and Genomic Selection Strategies. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Forde, B.G. Regulation of Arabidopsis Root Development by Nitrate Availability. Journal of Experimental Botany 2000, 51, 51–59. [Google Scholar] [CrossRef]
- Zhang, H.; Rong, H.; Pilbeam, D. Signalling Mechanisms Underlying the Morphological Responses of the Root System to Nitrogen in Arabidopsis Thaliana. Journal of Experimental Botany 2007, 58, 2329–2338. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate Transport, Signaling, and Use Efficiency. Annual Review of Plant Biology 2018, 69, 85–122. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Clochard, T.; Cukier, C.; Bourdin, C.; Juchaux, M.; Montrichard, F.; Thany, S.; Raymond, V.; Planchet, E.; Limami, A.M.; et al. The Nitrate Transporter MtNPF6.8 (MtNRT1.3) Transports Abscisic Acid and Mediates Nitrate Regulation of Primary Root Growth in Medicago Truncatula. Plant Physiology 2014, 166, 2152–2165. [Google Scholar] [CrossRef]
- Lepetit, M.; Brouquisse, R. Control of the Rhizobium–Legume Symbiosis by the Plant Nitrogen Demand Is Tightly Integrated at the Whole Plant Level and Requires Inter-Organ Systemic Signaling. Frontiers in Plant Science 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Kreplak, J.; Madoui, M.A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A Reference Genome for Pea Provides Insight into Legume Genome Evolution. Nature Genetics 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Gu, B.; Chen, Y.; Xie, F.; Murray, J.D.; Miller, A.J. Inorganic Nitrogen Transport and Assimilation in Pea (Pisum Sativum). Genes 2022, 13. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A Unified Nomenclature of Nitrate Transporter 1/Peptide Transporter Family Members in Plants. Trends in Plant Science 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Alves-Carvalho, S.; Aubert, G.; Carrère, S.; Cruaud, C.; Brochot, A.L.; Jacquin, F.; Klein, A.; Martin, C.; Boucherot, K.; Kreplak, J.; et al. Full-Length de Novo Assembly of RNA-Seq Data in Pea (Pisum Sativum L.) Provides a Gene Expression Atlas and Gives Insights into Root Nodulation in This Species. Plant Journal 2015, 84, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, Allocation and Signaling of Nitrate. Trends in Plant Science 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Pellizzaro, A.; Alibert, B.; Planchet, E.; Limami, A.M.; Morère-Le Paven, M.C. Nitrate Transporters: An Overview in Legumes. Planta 2017, 246, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.; Miles, N.W.; Dickstein, R.; Harris, J.M. Genome Mining of Plant NPFs Reveals Varying Conservation of Signature Motifs Associated With the Mechanism of Transport. Frontiers in Plant Science 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Sol, S.; Valkov, V.T.; Rogato, A.; Noguero, M.; Gargiulo, L.; Mele, G.; Lacombe, B.; Chiurazzi, M. Disruption of the Lotus Japonicus Transporter LjNPF2.9 Increases Shoot Biomass and Nitrate Content without Affecting Symbiotic Performances. BMC Plant Biology 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Clochard, T.; Planchet, E.; Limami, A.M.; Morère-Le Paven, M.C. Identification and Molecular Characterization of Medicago Truncatula NRT2 and NAR2 Families. Physiologia Plantarum 2015, 154, 256–269. [Google Scholar] [CrossRef]
- Criscuolo, G.; Valkov, V.T.; Parlati, A.; Alves, L.M.; Chiurazzi, M. Molecular Characterization of the Lotus Japonicus NRT1(PTR) and NRT2 Families. Plant, Cell and Environment 2012, 35, 1567–1581. [Google Scholar] [CrossRef]
- Valkov, V.T.; Sol, S.; Rogato, A.; Chiurazzi, M. The Functional Characterization of LjNRT2.4 Indicates a Novel, Positive Role of Nitrate for an Efficient Nodule N2-Fixation Activity. New Phytologist 2020, 228, 682–696. [Google Scholar] [CrossRef]
- Misawa, F.; Ito, M.; Nosaki, S.; Nishida, H.; Watanabe, M.; Suzuki, T.; Miura, K.; Kawaguchi, M.; Suzaki, T. Nitrate Transport via NRT2.1 Mediates NIN-LIKE PROTEIN-Dependent Suppression of Root Nodulation in Lotus Japonicus. Plant Cell 2022, 34, 1844–1862. [Google Scholar] [CrossRef] [PubMed]
- Rogato, A.; Valkov, V.T.; Chiurazzi, M. LjNRT2.3 Plays a Hierarchical Role in the Control of High Affinity Transport System for Root Nitrate Acquisition in Lotus Japonicus. Frontiers in Plant Science 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Appleby, C.A. Leghemoglobin and Rhizobium Respiration. Annual Review of Plant Physiology 1984, 35, 443–478. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Frendo, P.; Brouquisse, R. Nitric Oxide Signaling, Metabolism and Toxicity in Nitrogen-Fixing Symbiosis. Journal of Experimental Botany 2019, 70, 4505–4520. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. The Plant cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Feng, J.; Lee, T.; Schiessl, K.; Oldroyd, G.E.D. Processing of NODULE INCEPTION Controls the Transition to Nitrogen Fixation in Root Nodules. Science 2021, 374, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Soyano, T.; Hirakawa, H.; Sato, S.; Hayashi, M.; Kawaguchi, M. NODULE INCEPTION Creates a Long-Distance Negative Feedback Loop Involved in Homeostatic Regulation of Nodule Organ Production. Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 14607–14612. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Koester, B.; Liese, R.; Lingner, A.; Baumgarten, V.; Dirks, J.; Salinas-Riester, G.; Pommerenke, C.; Dittert, K.; Schulze, J. An RNA Sequencing Transcriptome Analysis Reveals Novel Insights into Molecular Aspects of the Nitrate Impact on the Nodule Activity of Medicago Truncatula. Plant Physiology 2014, 164, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Lin, Js.; Li, X.; Luo, Z.; Mysore, K.S.; Wen, J.; Xie, F. NIN Interacts with NLPs to Mediate Nitrate Inhibition of Nodulation in Medicago Truncatula. Nature Plants 2018, 4, 942–952. [Google Scholar] [CrossRef]
- Luo, Z.; Lin, J. shun; Zhu, Y.; Fu, M.; Li, X.; Xie, F. NLP1 Reciprocally Regulates Nitrate Inhibition of Nodulation through SUNN-CRA2 Signaling in Medicago Truncatula. Plant Communications 2021, 2, 100183. [Google Scholar] [CrossRef]
- Nishida, H.; Tanaka, S.; Handa, Y.; Ito, M.; Sakamoto, Y.; Matsunaga, S.; Betsuyaku, S.; Miura, K.; Soyano, T.; Kawaguchi, M.; et al. A NIN-LIKE PROTEIN Mediates Nitrate-Induced Control of Root Nodule Symbiosis in Lotus Japonicus. Nature Communications 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Nosaki, S.; Suzuki, T.; Ito, M.; Miyakawa, T.; Nomoto, M.; Tada, Y.; Miura, K.; Tanokura, M.; Kawaguchi, M.; et al. Different DNA-Binding Specificities of NLP and NIN Transcription Factors Underlie Nitrate-Induced Control of Root Nodulation. Plant Cell 2021, 33, 2340–2359. [Google Scholar] [CrossRef] [PubMed]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The Peptide-Encoding CEP1 Gene Modulates Lateral Root and Nodule Numbers in Medicago Truncatula. Journal of Experimental Botany 2013, 64, 5395–5409. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Radzman, N.A.; Binos, S.; Truong, T.T.; Imin, N.; Mariani, M.; Djordjevic, M.A. Novel MtCEP1 Peptides Produced in Vivo Differentially Regulate Root Development in Medicago Truncatula. Journal of Experimental Botany 2015, 66, 5289–5300. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, K.; Takahashi, H.; Sakurai, N.; Sugiyama, A.; Suzuki, H.; Shibata, D.; Nakazono, M.; Yazaki, K. Tissue-Specific Transcriptome Analysis in Nodules of Lotus Japonicus. Molecular Plant-Microbe Interactions 2012, 25, 869–876. [Google Scholar] [CrossRef]
- Valkov, V.T.; Chiurazzi, M. Nitrate Transport and Signaling. In S Tabata, J Stougaard, eds, The Lotus japonicus Genome: Compendium of Plant Genomes. Springer-Verlag, Berlin; 2014; pp. 125–136.
- Wang, C.; Yu, H.; Luo, L.; Duan, L.; Cai, L.; He, X.; Wen, J.; Mysore, K.S.; Li, G.; Xiao, A.; et al. NODULES WITH ACTIVATED DEFENSE 1 Is Required for Maintenance of Rhizobial Endosymbiosis in Medicago Truncatula. The New phytologist 2016, 212, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Valkov, V.T.; Rogato, A.; Alves, L.M.; Sol, S.; Noguero, M.; Léran, S.; Lacombe, B.; Chiurazzi, M. The Nitrate Transporter Family Protein LjNPF8.6 Controls the N-Fixing Nodule Activity. Plant Physiology 2017, 175, 1269–1282. [Google Scholar] [CrossRef]
- Vittozzi, Y.; Nadzieja, M.; Rogato, A.; Radutoiu, S.; Valkov, V.T.; Chiurazzi, M. The Lotus Japonicus NPF3.1 Is a Nodule-Induced Gene That Plays a Positive Role in Nodule Functioning. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, R.; Salehin, M.; Adeyemo, O.S.; Salazar, C.; Shulaev, V.; Sherrier, D.J.; Dickstein, R. Functional Assessment of the Medicago Truncatula NIP/LATD Protein Demonstrates That It Is a High-Affinity Nitrate Transporter. Plant Physiology 2012, 160, 906–916. [Google Scholar] [CrossRef]
- Yendrek, C.R.; Lee, Y.C.; Morris, V.; Liang, Y.; Pislariu, C.I.; Burkart, G.; Meckfessel, M.H.; Salehin, M.; Kessler, H.; Wessler, H.; et al. A Putative Transporter Is Essential for Integrating Nutrient and Hormone Signaling with Lateral Root Growth and Nodule Development in Medicago Truncatula. Plant Journal 2010, 62, 100–112. [Google Scholar] [CrossRef]
- Harris, J.M.; Dickstein, R. Control of Root Architecture and Nodulation by the LATD/NIP Transporter. Plant Signaling and Behavior 2010, 5, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Veereshlingam, H.; Haynes, J.G.; Penmetsa, R.V.; Cook, D.R.; Sherrier, D.J.; Dickstein, R. Nip, a Symbiotic Medicago Truncatula Mutant That Forms Root Nodules with Aberrant Infection Threads and Plant Defense-like Response. Plant physiology 2004, 136, 3692–3702. [Google Scholar] [CrossRef] [PubMed]
- Bright, L.J.; Liang, Y.; Mitchell, D.M.; Harris, J.M. The LATD Gene of Medicago Truncatula Is Required for Both Nodule and Root Development. Molecular Plant-Microbe Interactions 2005, 18, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Teillet, A.; Garcia, J.; De Billy, F.; Gherardi, M.; Huguet, T.; Barker, D.G.; De Carvalho-Niebel, F.; Journet, E.P. Api, a Novel Medicago Truncatula Symbiotic Mutant Impaired in Nodule Primordium Invasion. Molecular Plant-Microbe Interactions 2008, 21, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.F.; et al. Transfer Cells Mediate Nitrate Uptake to Control Root Nodule Symbiosis. Nature Plants 2020, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Boeglin, L.; Morère Le-Paven, M.C.; Clochard, T.; Fustec, J.; Limami, A.M. Pisum Sativum Response to Nitrate as Affected by Rhizobium Leguminosarum-Derived Signals. Plants 2022, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leran, S.; Garg, B.; Boursiac, Y.; Corratge-Faillie, C.; Brachet, C.; Tillard, P.; Gojon, A.; Lacombe, B. AtNPF5.5, a Nitrate Transporter Affecting Nitrogen Accumulation in Arabidopsis Embryo. Scientific Reports 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both Plant and Bacterial Nitrate Reductases Contribute to Nitric Oxide Production in Medicago Truncatula Nitrogen-Fixing Nodules. Plant Physiology 2011, 155, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Limami, A.M.; Diab, H.; Lothier, J. Nitrogen Metabolism in Plants under Low Oxygen Stress. Planta 2014, 239, 531–541. [Google Scholar] [CrossRef]
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A Plant Regulator Controlling Development of Symbiotic Root Nodules. Nature 1999, 402, 191–195. [Google Scholar] [CrossRef]
- Suzaki, T.; Kim, C.S.; Takeda, N.; Szczyglowski, K.; Kawaguchi, M. TRICOT Encodes an AMP1-Related Carboxypeptidase That Regulates Root Nodule Development and Shoot Apical Meristem Maintenance in Lotus Japonicus. Development (Cambridge) 2013, 140, 353–361. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Li, F.; Lu, Y.; Fang, Z.; Fu, M.; Mysore, K.S.; Wen, J.; Gong, J.; Murray, J.D.; et al. The Small Peptide CEP1 and the NIN-like Protein NLP1 Regulate NRT2.1 to Mediate Root Nodule Formation across Nitrate Concentrations. The Plant cell 2023, 35, 776–794. [Google Scholar] [CrossRef]
- Clarke, V.C.; Loughlin, P.C.; Gavrin, A.; Chen, C.; Brear, E.M.; Day, D.A.; Smith, P.M.C. Proteomic Analysis of the Soybean Symbiosome Identifies New Symbiotic Proteins. Molecular and Cellular Proteomics 2015, 14, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Boscari, A.; Castella, C.; Rovere, M.; Puppo, A.; Brouquisse, R. Nitric Oxide: A Multifaceted Regulator of the Nitrogen-Fixing Symbiosis. Journal of Experimental Botany 2015, 66, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-Regulated Auxin Transport by NRT1.1 Defines a Mechanism for Nutrient Sensing in Plants. Developmental Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Hanada, A.; Chiba, Y.; Ichikawa, T.; Nakazawa, M.; Matsui, M.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of an Abscisic Acid Transporter by Functional Screening Using the Receptor Complex as a Sensor. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 9653–9658. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A. V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins Inhibit Nodule Senescence and Stimulate Nodule Meristem Bifurcation in Pea (Pisum Sativum L.). Frontiers in Plant Science 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).