1. Introduction
Chiari malformations (CM) are considered as a group of disorders characterized by a downward displacement of cerebellar tonsils or vermis into the cervical spinal canal. In 1891 Chiari reported the first detailed case of malformation. [
1] CMs can be distinguished into four type (1 to 4). [
2] Chiari malformation type 1 (CM-1) is characterized by the caudal displacement of one or both cerebellar tonsils through the foramen magnum, using the diagnostic cut off of 5 mm from McRae line. [
3] In Chiari malformation type 1.5 (CM-1.5), a more severe variant of CM-1, both cerebellar tonsils and brainstem are herniated below the foramen magnum. [
2]
The variety of symptoms of CM are due to the obstruction of cerebrospinal fluid (CSF) circulation for the anatomical abnormalities and to the compression of the neural structures or the presence of syringomyelia. The most common symptoms are headache, with a retro-ocular or generalized location affecting up to 80% of children [
4,
5], and cervical pain, present in 60-70% of children and typically induced by neck extension or Valsalva maneuver [
6]. Compression of cerebellum and brain stem may result in ataxia (20–40%), nystagmus (23–70%), dysfunction of lower cranial nerves (15-26%), sensory losses (30-92%) motor deficits and signs of upper motor neuron lesions. In 20-30% of patient affected by CM-1 progressive scoliosis is present. [
7]
The most common finding in CM-1 or CM-1.5 is syringomyelia, reported in 67-80% of patients. [
8,
9] The portion of the dilatated central canal is usual the cervical and/or the thoracic one and it represents an indication for surgery. Other anomalies such as hydrocephalus, present in 10 % of CM-1, or craniosynostosis or tethered spinal cord may be present. [
10] These conditions may be addressed firstly by surgical treatment because it may resolve CM-1 related symptoms avoiding the need of decompression.
Radiological evaluation, clinical history and precise neurological examination are essential to patient selection for surgical intervention. [
11] The recognized diagnostic cut-off of the caudal displacement of cerebellar tonsils through the foramen magnum is 5 mm from McRae line but up to one third of patient with radiological diagnosis of CM-1 may be asymptomatic. [
12] Therefore, more importance is given to the presence of symptoms and compression of neuronal structures at the level of foramen magnum. [
11] In asymptomatic patients’ surgical treatment is not indicated, and since the natural history of CM-1 is still not clear, the treatment is limited to radiological and clinical follow-up. The presence of syringomyelia has been proven to be a criterion for surgical decompression while there is not shared consensus on operating symptomatic individuals without syringomyelia. [
3]
Analyzing patients’ clinical features, surgical techniques, and outcomes, we report our experience and the role of in ultra-operative ultrasonography in planning the surgical decompression with or without dural opening and and duraplasty.
2. Diagnostic Work-Up, Surgical Procedure and pPostoperative Course
In all patients with evidence of CM-1 or MC-1.5 at MRI a full spine Magnetic Resonance Imaging (MRI) was routinely performed to detect syringomyelia or other associated anomalies. We did not routinely use neurophysiological studies in the process of diagnosis and surgical management.
All patients were positioned prone with bolsters under the shoulders and hips. Bony and ligamentous decompression with suboccipital craniectomy and C1 laminectomy alone or with the combination of dural delamination or with duraplasty with autologous graft were the possible surgical options. Tonsils’ coagulation was not standardly performed.
We tailored the procedure of choice on single patient. In the presence of spinal syringomyelia we always performed a dural and osteo-ligamentous decompression, while in the other situations we performed a first bony and ligamentous decompression and use the intra-operative ultrasonography to evaluate the CSF dynamic and the pulsation pattern of the cerebellar tonsils. If the flow of cerebrospinal fluid in the foramen magnum region was recovered after the bony decompression, the duraplasty would not be carried out. A sufficient decompression was testified by the presence of CSF layer behind the tonsils and an antero-posterior pattern of pulsation of tonsils, controversially the need of dural opening was demonstrated by the absence of CSF flux and a pattern of movement of tonsils like a “piston” with vertical pulsations.
After dural closure, if necessary, multiple Valsalva maneuvers were performed to check the continence of dura mater.
Traditional postoperative pain control regimens relied on continuous opioid analgesia and Ketorolac, usually in patient or nurse-controlled analgesia for 3-4 days, switching then to Paracetamol on demand.
A Computed Tomography (TC) scan was performed in all the patients the day after the surgery, to analyze the surgical results and eventually detect possible complications. Three months after surgery all patients underwent a cerebral and spinal MRI. The follow up consisted in an MRI each 12 months for the first few years and was repeated earlier than expected in case of onset of new symptoms.
3. Materials and Methods
We retrospectively examined all clinical charts and selected patients affected by CM-1 and CM-1.5 who were managed between 2006-2020 (age 0-18 years old) at Giannina Gaslini Hospital (Genoa, Italy). Exclusion criteria were the association with achondroplasia and with spina bifida, in case of Chiari 2 (CM-2).
The descriptive analysis was performed retrospectively, considering demographic details, radiological findings, surgical treatment details, and outcomes.
4. Results
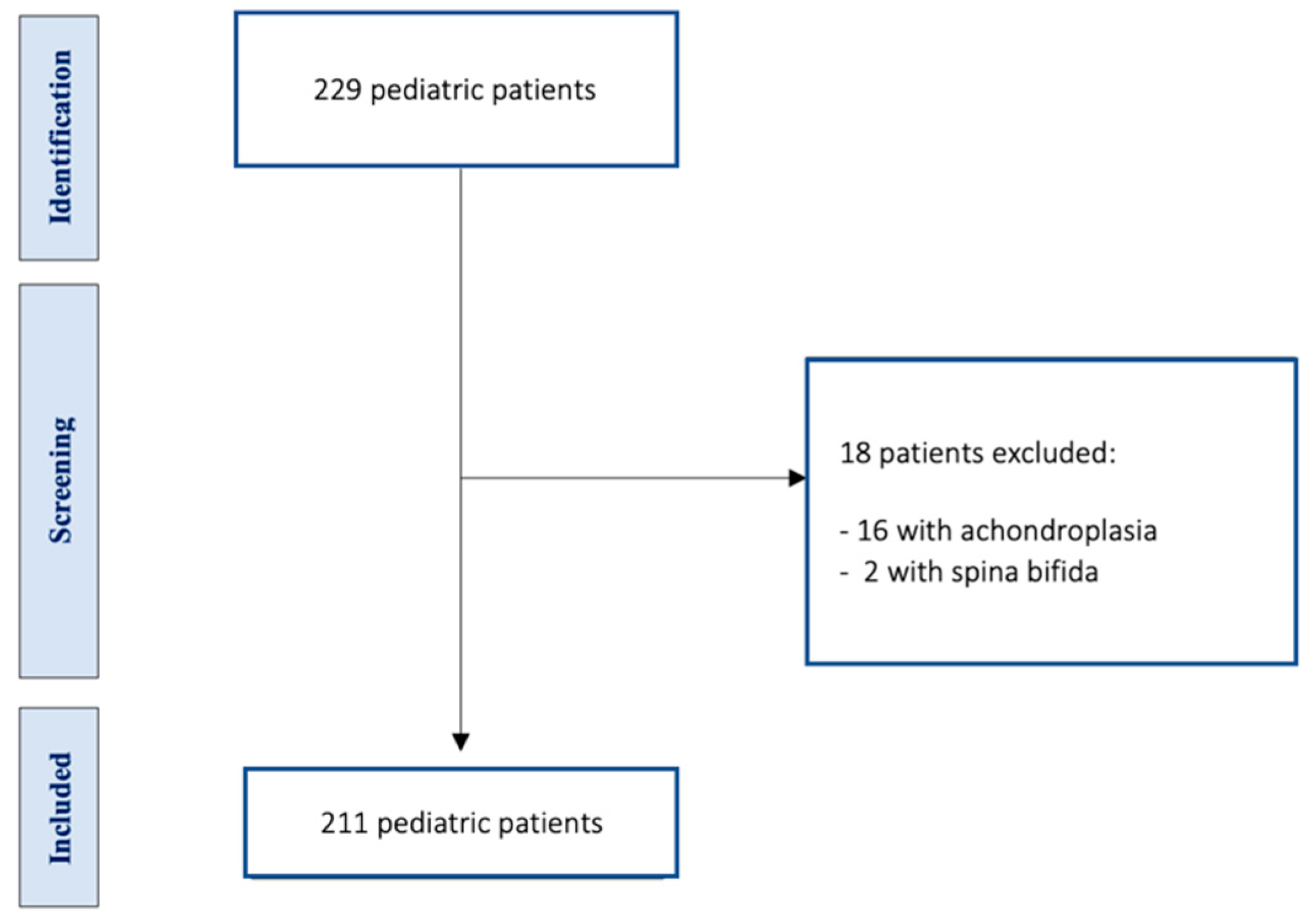
We identified 229 pediatric patients with caudal displacement of cerebellar tonsils through the foramen magnum (more than 5mm from McRae line), who were managed between 2006 and 2017 at the neurosurgery department of our pediatric center. Among them 18 individuals were excluded, 16 had achondroplasia in association with CM and 2 a diagnosis of CM-2 (with the presence of spina bifida), with a total of 211 patients for evaluation (see
Figure 1).
The group included 122 men and 89 women, aged between 2 months and 18 years (mean age: 8.1 years).
Children affected by CM-1 accounted for 83.9% and by CM-1.5 16.1%.
Syringomyelia was present in 28.4% of cases, while hydrocephalus in 8% (see
Table 1). In 6 of 17 patients with diagnosis of hydrocephalus, first treatment of this pathology was sufficient to relieve the symptoms associated to CM-1 or CM1.5.
Not considering anatomical anomalies of the posterior cranial fossa (such as platybasia) or of supratentorial structures, 35 patients presented other associated neuroaxial alteration: 8 non-syndromic craniostenosis, 4 of which were treated surgically by skull remodeling, 11 craniovertebral junction malformations, 6 scoliosis and 10 spinal dysraphism with tethered cord and in only 2 of them surgical detethering was sufficient to relieve symptoms.
According to CSF dynamic and pulsation of cerebellar tonsils at the intra-operative ultrasonography, bony and ligamentous decompression alone was sufficient in 119 patients (59.8%), subsequent simple opening of dura mater was performed in 6 patients (3%) and associated duraplasty with autologous graft in 74 patients (27.1%), in 3 of them the cerebellar tonsils were coagulated.
One of the patients, who underwent the osteo-dural decompression with plasty, had already been operated in another center and our surgery consisted in an expansion of the previous bony decompression with dural opening. Unfortunately, the day after the surgery he presented a CSF leakage through the wound necessitating an external lumbar drain for few days.
Seven patients experience post-operative complications. In 3 patients a surgery was performed in emergency: an evacuation of compressive subdural hematoma, a positioning of EVD (external ventricular drain) for acute hydrocephalus and a removal of a broken subfascial drainage. Of the remaining 4 patients, 2 had a CSF fistula (in one patient an external lumbar CSF drain was sufficient to solve the situation, while in the other one two surgeries to repair the leakage were mandatory), and 2 postoperative pseudomeningoceles, that disappeared at follow-up.
As expected, in dural and osteo-ligamentous decompression with or without autologous duraplasty post-operative complications were significantly higher than in bony-ligamentous decompression (p<0.05, see
Table 2).
The median follow-up period was 4.2 years (range 2 month–10 years), and 4 patients were lost at follow-up.
In all patients with syringomyelia, the spinal cavity was reduced in dimensions at the last spinal MRI of follow-up, except for 4 patients in which it remained stable and in 1 one where it augmented without the need of surgical intervention.
For insufficient decompression at follow-up, in 3 patients a further operation with dural decompression and plasty was performed after the first bony and ligamentous opening and in 3 other ones an expansion of the previous osteo-dural decompression with coagulation of tonsils was carried out.
5. Discussion
Since symptoms and signs of CM are related to CSF obstruction and compression of cerebellum, brainstem and spinal nerves, the standard surgical treatment consists in decompression of posterior cranial fossa, releasing the cranial-cervical interface. [
13] It is possible to conduct an osteo-dural decompression, with opening of the dura mater and performing the duraplasty at the main time in order to reduce surgical complications. [
14]
The degree of decompression in pediatric population has always been a matter of controversy. Some neurosurgeons suggest that bony decompression alone is sufficient to restore the CSF flow [
15], while others strongly believe in the superiority of osteo-dural decompression and duraplasty. [
16] Regardless of the type of surgery used, the purpose is to restore the CSF dynamics and circulation through the foramen magnum.
Although a conspicuous number of theories have emerged to explain syringomyelia in Chiari, a CSF ineffective circulation seem to be at the basis of cervical syringomyelia. [
17] And this is the reason why in the presence of syringomyelia an osteodural decompression with graft is always preferred. In our series, indeed, in the majority of patients with syringomyelia who underwent surgery, the spinal cavity decreased in dimensions or completely disappeared at the MRI of follow-up. This concept is underlined also by few publications where the authors showed how placing a stent from the fourth ventricle to cervical subarachnoid space could promote circulation of CSF and improve syringomyelia. [
18,
19,
20]
If we consider this endpoint, the restoration of CSF flux, it would be rational to propose to all patients, affected by CM-1 or CM-1.5, an osteo-dural decompression as primary treatment. However, as also demonstrated by our case series, the dural opening is usually associated to a higher number of post-operative complications compared to only-bone-decompression. Therefore, there is the need for a dynamic tool to measure CSF circulation to tailor decompression on single case.
Although MRI can depict CSF dynamics, as showed by Liu B et al with electrocardiography-gated phase-contrast MRI [
21], applying this technology in the operating room to choose the proper surgical strategy brings considerable difficulties. Intra-operative ultrasound is an efficient and inexpensive alternative to MRI and its intraoperative use is much more feasible.
In our experience, the intraoperative decision not to perform opening of the dura mater and duraplasty was based on each surgeon’s subjective interpretation of the ultrasonography findings. In addition to the fact that this decision was based on non-quantitative and therefore non-systematic variables, the analysis of the presence of CSF layer behind the tonsils and their pulsation’ pattern represented a huge simplification of the pathophysiology of CM.
As previous studies on animals, conducted with invasive methods, have shown, indeed, CSF flow is bi-directional: directly related to cardiac circle. In the systolic phase, since the cerebral flow increases with consequent cerebral expansion, CSF flows from brain to vertebral canal, while, when the hearth muscle relaxes itself, the venous return is augmented, and the cerebral blood reduced causing the CSF flowing toward the cranial compartment. Breathing, also, may slightly influence CSF flow: during inhalation the thoracic pressure decreases causing the blood to flow out and the CSF to flow in the brain, while on exhalation the CSF in the cerebral ventricle flow in the caudal direction through the aqueduct.
Cellular particles and proteins, that contained inside CSF, can determine Doppler signals at the ultrasonography. Cui LG et al showed how Doppler ultrasonography may be used as a precise and helping tool in detecting CSF flow velocity and its variation during posterior fossa decompression. [
22]
Children with CM-1 or CM-1.5 may present a heterogeneous spectrum of symptoms and signs. In our pediatric center, we offered the surgical treatment to all symptomatic patients. The mean age of diagnosis is 8 years old, as in our patients’ series, although the range is wide and cover all the pediatric age. [
23] The prevalence of syringomyelia reported by us is inferior to the one of literature: 28.4% versus 57%. [
24] Hydrocephalus, also, was less reported, 8% compared to the 10% of the series published by Koueik et al. [
25] These two pathologies can often coexist without clear understanding of the cause-and-effect relationship. In the absence of other associated etiologies, neurosurgeons tend to treat the hydrocephalus first: in our series 6 patients that were treated firstly for hydrocephalus did not need the following decompressive surgery.
Looking at the outcome, for us the success of the surgical treatment resided in symptom relief or disappearance independently from radiological improvement, since there are no well-defined criteria relating to it.
6. Conclusions
Our experience shows how real-time ultrasonography can provide intraoperative guidance regarding mode selection for posterior cranial fossa decompression and duraplasty, and assist in the establishment of the CSF circulation path. It can be used as a fundamental tool in limiting the need for re-operation, avoiding insufficient decompression of posterior cranial fossa. Nevertheless, there is a need to decline this method through quantitative variables, eventually with the use of Doppler, to make it applicable in a systematic way and to correlate it to post-operative radiological findings.
References
- Koehler, P.J. Chiari's description of cerebellar ectopy (1891). With a summary of Cleland's and Arnold's contributions and some early observations on neural-tube defects. J Neurosurg. 1991, 75(5), 823–6. [Google Scholar] [CrossRef]
- Giallongo, A.; Pavone, P.; Tomarchio, S.P.; Filosco, F.; Falsaperla, R.; Testa, G.; Pavone, V. Clinicoradiographic data and management of children with Chiari malformation type 1 and 1.5: an Italian case series. Acta Neurol Belg. 2021, 121(6), 1547–1554, Epub 2020 Jun 10. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, A.; Consales, A.; Pavanello, M.; Rossi, A.; Lanteri, P.; Cama, A.; Piatelli, G. Management: opinions from different centers-the Istituto Giannina Gaslini experience. Childs Nerv Syst. 2019, 35(10), 1905–1909, Epub 2019 May 9. [Google Scholar] [CrossRef] [PubMed]
- Nohria, V.; Oakes, W.J. Chiari I malformation: a review of 43 patients. Pediatr Neurosurg. 1990-1991, 16(4-5), 222–7. [Google Scholar] [CrossRef] [PubMed]
- Steinbok, P. Clinical features of Chiari I malformations. Childs Nerv Syst. 2004, 20(5), 329–31, Epub 2004 Feb 14. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Beckman, J.; Naftel, R.P.; Chern, J.J.; Wellons JC 3rd Rozzelle, C.J.; Blount, J.P.; Oakes, W.J. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. J Neurosurg Pediatr. 2011, 7(3), 248–56. [Google Scholar] [CrossRef] [PubMed]
- Poretti, A.; Ashmawy, R.; Garzon-Muvdi, T.; Jallo, G.I.; Huisman, T.A.; Raybaud, C. Chiari Type 1 Deformity in Children: Pathogenetic, Clinical, Neuroimaging, and Management Aspects. Neuropediatrics. 2016, 47(5), 293–307, Epub 2016 Jun 23. [Google Scholar] [CrossRef]
- Milhorat, T.H.; Chou, M.W.; Trinidad, E.M.; Kula, R.W.; Mandell, M.; Wolpert, C.; et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999, 44, 1005–17. [Google Scholar] [CrossRef]
- Ellenbogen, R.G.; Armonda, R.A.; Shaw, D.W.; Winn, H.R. Toward a rational treatment of Chiari I malformation and syringomye- lia. Neurosurg Focus 2000, 8, E6. [Google Scholar] [CrossRef]
- George, T.M.; Higginbotham, N.H. Defining the signs and symptoms of Chiari malformation type I with and without syringomyelia. Neurol Res. 2011, 33(3), 240–6. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, C.; Jallo, G.I. Chiari 1 deformity in children: etiopathogenesis and radiologic diagnosis. Handb Clin Neurol. 2018, 155, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Aitken, L.A.; Lindan, C.E.; Sidney, S.; Gupta, N.; Barkovich, A.J.; Sorel, M.; Wu, Y.W. Chiari type I malformation in a pediatric popula- tion. Pediatr Neurol 2009, 40(6), 449–454. [Google Scholar] [CrossRef]
- Cui, L.G.; Jiang, L.; Zhang, H.B.; Liu, B.; Wang, J.R.; Jia, J.W.; Chen, W. Monitoring of cerebrospinal fluid flow by intraoperative ultrasound in patients with Chiari I malformation. Clin Neurol Neurosurg. 2011, 113(3), 173–6, Epub 2010 Nov 13. [Google Scholar] [CrossRef]
- Shou, J.X.; Ma, L.; Song, L.J. Surgical treatment of Chiari I malformation with syringomyelia. Chin J Clin Neurol 2007, 5, 99–101. [Google Scholar]
- Li, S.F.; Zhou, M.D.; Jia, D.Z. Pathogenic mechanism and surgical treatment of Chiari I malformation with syringomyelia. Chin J Nerv Ment Dis 2003, 29, 68–9. [Google Scholar]
- Tubbs, R.S.; Webb, D.B.; Oakes, W.J. Persistent syringomyelia following pedi- atric Chiari I decompression: radiological and surgical findings. J Neurosurg 2004, 100, 460–4. [Google Scholar]
- Sekula, R.F., Jr.; Arnone, G.D.; Crocker, C.; Aziz, K.M.; Alperin, N. The pathogenesis of Chiari I malformation and syringomyelia. Neurol Res. 2011, 33(3), 232–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhou, M.; Liu, Y.; Du, J.; Zeng, G. Fourth ventricle stent placement for treatment of type I Chiari malformation in children. Childs Nerv Syst. 2023, 39(3), 671–676, Epub 2022 Dec 26. [Google Scholar] [CrossRef]
- Han, R.K.; Medina, M.P.; Giantini-Larsen, A.M.; Chae, J.K.; Cruz, A.; Garton, A.L.A.; Greenfield, J.P. Fourth ventricular subarachnoid stent for Chiari malformation type I-associated persistent syringomyelia. Neurosurg Focus. 2023, 54(3), E10. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhou, M.; Liu, Y.; Du, J.; Zeng, G. Fourth ventricle stent placement for treatment of type I Chiari malformation in children. Childs Nerv Syst. 2023, 39(3), 671–676, Epub 2022 Dec 26. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Z.Y.; Xie, J.C.; Han, H.B.; Pei, X.L. Cerebrospinal fluid dynamics in Chiari malformation associated with syringomyelia. Chin Med J (Engl). 2007, 120(3), 219–23. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.G.; Jiang, L.; Zhang, H.B.; Liu, B.; Wang, J.R.; Jia, J.W.; Chen, W. Monitoring of cerebrospinal fluid flow by intraoperative ultrasound in patients with Chiari I malformation. Clin Neurol Neurosurg. 2011, 113(3), 173–6, Epub 2010 Nov 13. [Google Scholar] [CrossRef] [PubMed]
- Albert, G.W. Chiari Malformation in Children. Pediatr Clin North Am. 2021, 68(4), 783–792. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Griessenauer, C.J.; Oakes, W.J. Chiari malformations. In Principles and practice of pediatric neurosurgery, 3rd ed.; Albright, A.L., Pollack, I.F., Adelson, P.D., Eds.; Thieme Publishers: New York, 2015; pp. 192–204. [Google Scholar]
- Koueik, J.; DeSanti, R.L.; Iskandar, B.J. Posterior fossa decompression for children with Chiari I malformation and hydrocephalus. Childs Nerv Syst. 2022, 38(1), 153–161, Epub 2021 Oct 20. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).