1. Introduction
The oxidative decarboxylation of pyruvate to acetyl-CoA and CO
2 is a central biochemical reaction that links glycolysis to the tricarboxylic acid cycle. Under aerobic conditions, it is catalyzed by the pyruvate dehydrogenase complex [
1]. In most anaerobic and microaerophilic microorganisms, however, pyruvate is decarboxylated by pyruvate:ferredoxin oxidoreductase (PFOR; E.C. 1.2.7.1) [
2,
3]. PFOR is an oxygen-sensitive iron-sulfur protein of the 2-oxo acid ferredoxin oxidoreductase (OFOR) superfamily [
4]. The fermentative decarboxylation of pyruvate by PFOR generates electrons that are transferred to either ferredoxin or flavodoxin [
5,
6]. Due to the reversibility of this reaction, PFOR is also called pyruvate synthase. PFOR is of high interest to (at least) three different research areas: (i) to biochemistry as a means for oxidation of pyruvate under anaerobic conditions; (ii) to pharmacology because it can reduce and thereby activate nitro-based prodrugs such as metronidazole; (iii) to evolutionary biology as it is an ancient enzyme that stems from the times when life on earth was anaerobic.
PFOR orthologs are present in the archaea, many anaerobic bacteria, and some anaerobic eukaryotes [
7]. In amitochondriate protists, the enzyme has varying localizations [
2,
3]. Based on the localization of PFOR, amitochondriate protists can be classified into type 1 and type 2 [
8]. In type 1 protists such as
Giardia duodenalis,
Entamoeba histolytica and
Cryptosporidium parvum, PFOR is localized in the cytosol [
9]. The PFOR in type 2 protists is localized in the hydrogenosome, as observed in the trichomonads [
10,
11]. The hydrogenosome is a double-membrane-bounded organelle [
12,
13] that shares common ancestry with mitochondria [
14,
15,
16]. It is a powerhouse for the fermentative decarboxylation of pyruvate to hydrogen, carbon dioxide, and ATP in the absence of molecular oxygen [
17,
18,
19]. Interestingly, the free-living, mitochondriate algae
Chlamydomonas reinhardtii is able to switch from aerobic to anaerobic metabolism by upregulating the expression of PFOR, which localizes to and functions in the chloroplastic stroma [
20,
21]. However, the N-terminus of PFOR of the facultative anaerobic, photosynthetic protist
Euglena gracilis localizes in the mitochondrion [
22]. Due to the patchy occurrence and diverse localizations of PFOR in eukaryotes, bacterial origins have been proposed [
8,
22,
23]. One plausible mechanism to explain this phenomenon is by horizontal gene transfer (HGT).
The role of HGT in the acquisition of virulence and multiple drug resistance genes within prokaryotes is well established [
24,
25,
26]. However, its involvement in the transfer of genetic material from prokaryotes to eukaryotes or within eukaryotes remains controversial [
19,
20,
21,
22,
23,
24,
25,
26,
27]. HGT was proposed to be an essential evolutionary mechanism by which unicellular eukaryotes acquire functional genetic material from bacteria that reside in close proximity, such as in the host gastrointestinal or genito-urinary tract [
30,
31]. It was also proposed that some protozoan parasites residing in the same host environment with bacteria can receive foreign DNA fragments through phagocytic feeding of bacteria [
32]. The acquisition of exogenous genetic material may facilitate the adaptation, pathogenicity, and survival of protozoa in the face of strong selective pressures [
30,
33,
34,
35,
36,
37]. So protozoa may have acquired genes encoding bacterial redox enzymes such as PFOR via HGT, which facilitated their survival in deteriorating environments [
40]. A previous study that utilized phylogenetic methods of the whole genome sequences of
E. histolytica and
T. vaginalis inferred 68 and 153 cases of HGT, respectively. Most of the transferred genes were found to encode enzymes involved in prokaryote intermediary metabolism, and the study concluded that prokaryotes that reside in close proximity to
E. histolytica and
T. vaginalis were potential gene donors [
41].
The presence of PFOR sensitizes anaerobic microorganisms to nitro-based antimicrobials such as metronidazole or tinidazole. These nitro-heterocyclic and nitro-aromatic compounds are prodrugs that need to be activated by chemical reduction of the nitro group, which leads to the formation of potent nitro radicals (Fleck et al. 2014). In analogy to Paul Ehrlich's concept of the magic bullet, such drugs can be thought of as magic bombs that need to be triggered by electron transfer [
42].
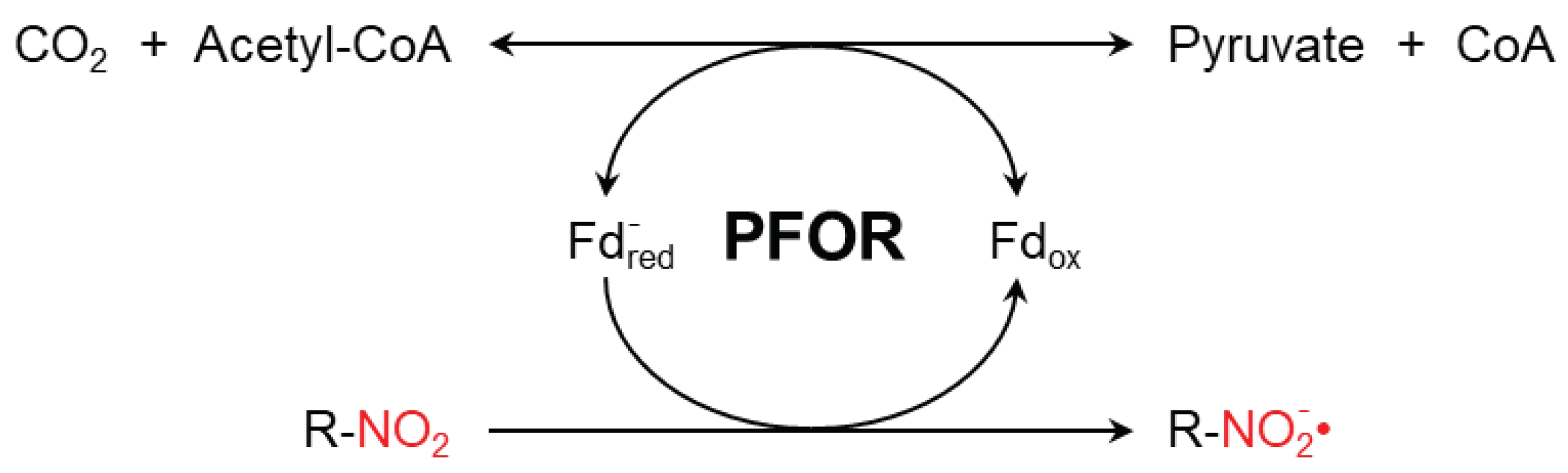
Figure 1.
PFOR-mediated fermentative decarboxylation of pyruvate (reversible) and reductive activation of nitro-based prodrugs (irreversible due to cascade of redox reaction converting the nitroso radical to hydroxylamine).
Figure 1.
PFOR-mediated fermentative decarboxylation of pyruvate (reversible) and reductive activation of nitro-based prodrugs (irreversible due to cascade of redox reaction converting the nitroso radical to hydroxylamine).
Metronidazole is a synthetic nitro-imidazole derivative effective against most obligate anaerobic bacteria and protozoan parasites such as
G. duodenalis,
E. histolytica,
T. vaginalis [
43,
44]. It exerts its antimicrobial effect by interfering with nucleic acid synthesis resulting in DNA damage in the target pathogens [
45]. Electrons from the initial redox reaction catalyzed by PFOR are transferred to ferredoxin, which has a sufficiently low redox potential to reductively activate metronidazole to its toxic radical metabolites. Through a series of electron transfer reactions, different radical intermediates are formed that act primarily as alkylating agents that disrupt DNA synthesis and growth of invading pathogens [
46]. Strains of
G. duodenalis and
E. histolytica with decreased or absent expression of
pfor exhibited increased metronidazole resistance [
47]. However, some recent studies suggest the involvement of additional enzymes that also play central roles in the activation of, and resistance to, metronidazole [
48,
49,
50,
51]. For example,
T. vaginalis has been shown in some studies to display a significant resistance to metronidazole only after both, PFOR and NAD-dependent malic enzyme, are inactivated in the hydrogenosome [
52,
53].
Here we analyze the distribution of PFOR in selected protozoan parasites, re-investigating the question whether the genes have been acquired from prokaryotes by horizontal transfer, and if so, which other genes came along with PFOR.
2. Materials and Methods
2.1. Protein Sequences
The protein sequences were retrieved from the UniProt protein database (
www.uniprot.org). In addition to PFOR, three eukaryote 'housekeeping' proteins were included as controls: glyceraldehayde-3-phosphate dehydrogenase (GAPDH) (EC 1.2.1.12), tubulin alpha chain (TUBa), and DNA-directed RNA polymerase II subunit beta I (RPB1) (EC 2.7.7.6). See supplementary table 1 for accession numbers.
2.2. Sequence Alignment
Sequence similarity queries were performed using the proteins of interest against the NCBI non-redundant protein sequence database with the basic local alignment search tool program (BLASTP version 2.12.0+) from the National Center for Biotechnology Information (NCBI) website (
https://blast.ncbi.nlm.nih.gov/Blast) [
54]. Initially, the queries were performed using the control proteins of each parasite. The same procedure was performed for the corresponding redox enzymes using an exploratory BLAST "pilot approach" with the following algorithm parameters. Expected threshold: 1e
-15 , Word size: 6, Matrix: BLOSUM62, Gap Costs: Existence 11, Extension 1 and Compositional adjustments: Conditional compositional score matrix adjustments [
55]. Proteome-wide blastp searches were run locally on a Linux PC and the results, in tabular format, were parsed with self-made Perl scripts.
2.3. Phylogenetic Analyses
Multiple sequence alignments and phylogenetic trees were made with the Molecular Evolutionary Genetics Analysis (MEGA) version X software using the Muscle algorithm with default parameters [
56], followed by manual trimming of the loose, unaligned ends of the alignment. The evolutionary distances were computed using the Jones-Taylor-Thornton (JTT) substitution model. This phylogenetic trees were constructed using the neighbor-joining algorithm [
57]. Bootstrapping was performed with 1,000 rounds of replication [
58].
2.4. Screening Selected Proteomes Against HMM Profile Libraries
Multiple sequence alignments were converted to position-dependent scoring matrices using the command hmmbuild of the HMMer 3.0 package. The resulting profiles were concatenated and converted to HMM profile libraries using hmmpress. Complete proteomes of representative organisms from each eukaryotic supergroup and selected bacteria were downloaded from ensemblgenomes database (ftp://ftp.ensemblgenomes.org/pub/). The HMM profile libraries were used to screen the downloaded proteomes with hmmscan of the HMMer 3.0 package. All the steps were executed with self-made Perl scripts on a Linux computer.
2.5. Gene Enrichment Analysis
The gene IDs of the proteins that were exclusively present in the selected protozoan species and
D. vulgaris were transferred to the Gene Ontology (GO) website
(http://geneontology.org/) for gene enrichment analysis. The analyses were performed using the PANTHER Overrepresentation Test (released on 13-10-2022) and the GO database DOI: 10.5281/zenodo.6799722 (PANTHER version 17.0, released on 01-07-2022). The PANTHER Pathway for biological process was used for the annotation data set and Fisher's exact test with false discovery rate (FDR) correction for the statistical analysis. Only results with p-values < 0.05 were included.
3. Results
3.1. BLAST Pilot Experiment
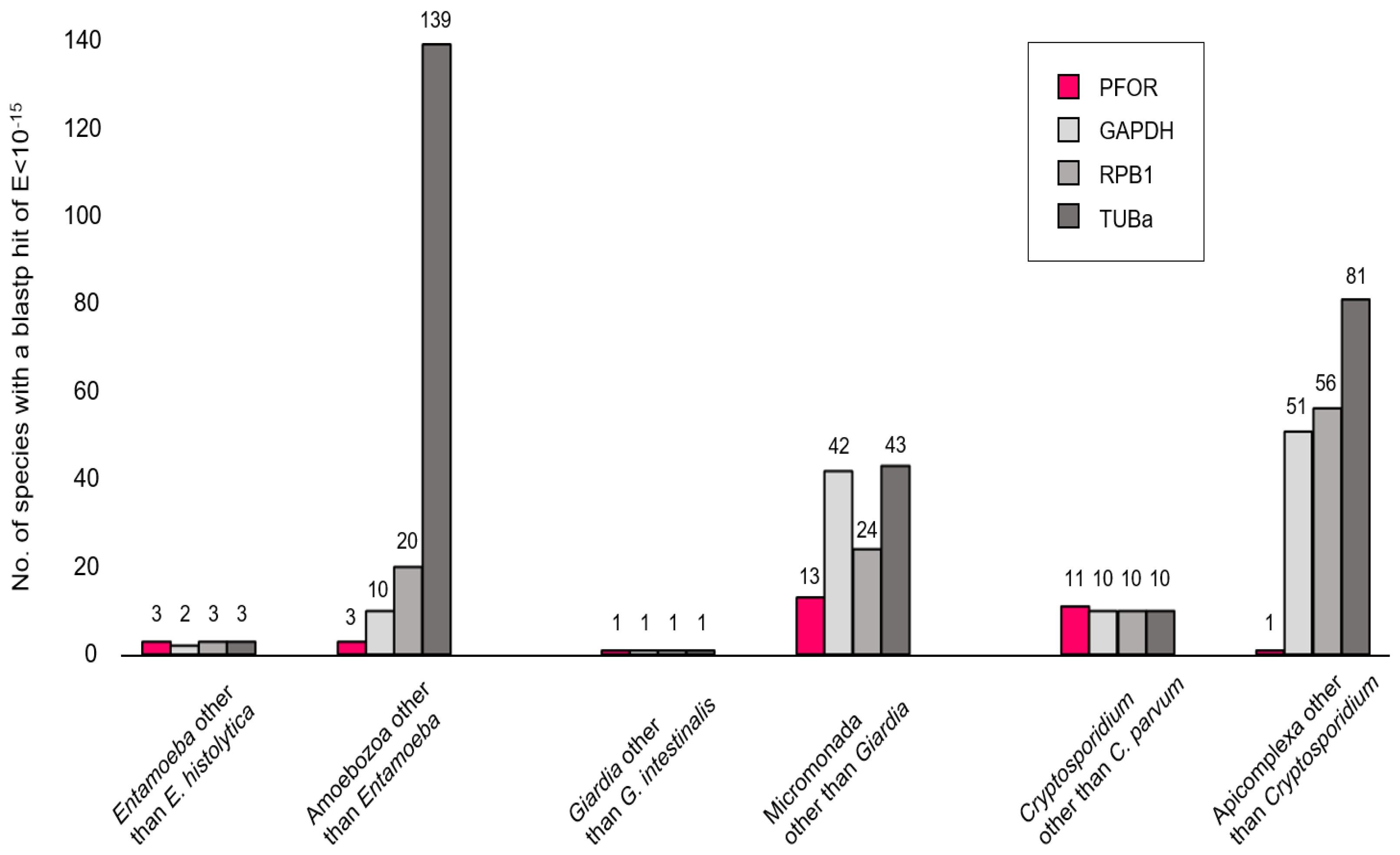
In a first exploratory attempt towards understanding the phylogeny of PFOR, we compared its performance to that of eukaryotic housekeeping proteins when used as query in blastp searches. The searches were performed online at NCBI, profiting from the 'organism' feature that allows to in- or exclude particular clades of the tree of life. We would expect the number of hits returned for a given query to rise with increasing search space, e.g. when moving up from the level of genus to a higher taxonomic order. This was the case for the control proteins glyceraldehayde-3-phosphate dehydrogenase (GAPDH), RNA polymerase II subunit B1 (RPB1), and α-tubulin (TUBa;
Figure 2, grays); however, it was not evident for PFOR (
Figure 2, red). For instance,
E. histolytica PFOR returned hits from three
Entamoeba species other than
E. histolytica (i.e.
E. dispar,
E. invadens,
E.nutalli), same as for the control proteins. Increasing the search space to include all amoebozoa produced hits only from three additional species (
Acanthamoeba castellanii,
Mastigamoeba balamuthi,
Pelomyxa schiedti) with PFOR, but clearly more with the control proteins. Similarly, only thirteen micromonads other than
Giardia spp. possessed a PFOR ortholog, and only one apicomplexan (
Porospora gigantea, a gregarine intestinal parasite of lobsters) other than
Cryptosporidium spp. (
Figure 2). While this simple approach may be biased due to differences in coverage and number of sequences submitted to GenBank, it nevertheless showed that PFOR in the amoebozoa, micromonada, and apicomplexa does not occur ubiquitously but punctually, only in particular genera.
3.2. Distribution of PFOR and Control Proteins along the Tree of Life
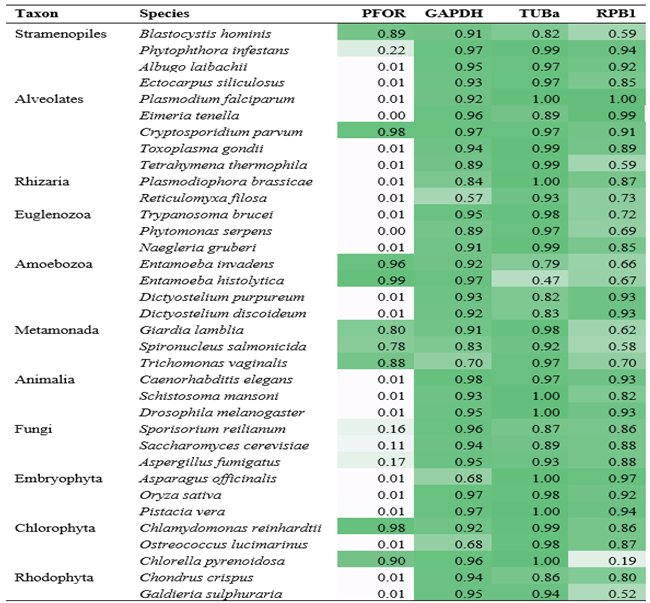
To explore the distribution of PFOR orthologs in an unbiased way, we had to concentrate on species for which whole genome sequence data were available. We constructed hidden Markov model-based profiles for the different query proteins, i.e. PFOR, GAPDH, RPB1 and TUBa. These profiles were then implemented for genome-wide surveys, searching the predicted proteomes of different eukaryotes. The score of the best hit was noted for each species, and the scores for each profile were normalized to a maximum value of 1 for the best overall hit (to account for the fact that longer profiles return a higher maximal score than shorter ones). This survey confirmed an overall scarcity of PFOR orthologues in eukaryotes, with an apparent absence from animals, land plants, euglenozoa and fungi, a very punctual distribution in the alveolates, stramenopiles, amoebozoa and chlorophytes, and a wider occurrence in all the metamonades for which predicted proteomes were available (
Table 1). There were only two rhizarian species with available proteome sequences in the ENSEMBL genomes database, neither of which had a hit for PFOR (
Table 1). All the species in the genus
Cryptosporidium returned hits for PFOR. However, the closely related parasites in the genera
Eimeria and
Cyclospora did not. The presence of a strong PFOR hit in
Blastocystis hominis was suprising and, to the best of our knowledge, has not been reported before. A further surprise was the presence of PFOR hits in the nematodes
Necator americanus and
Trichuris trichiura, gastrointestinal human parasites. However, upon reciprocal blastp searches the two hits (GenBank XP_013306304 and CDW60499) turned out to be identical to PFOR sequences from bacteria (PVY31832 and WP_000628243, respectively). Hence, these sequences could also result from a contamination of nematode DNA with bacterial DNA and were therefore not included for further analysis.
3.3. Phylogenetic Tree of PFOR
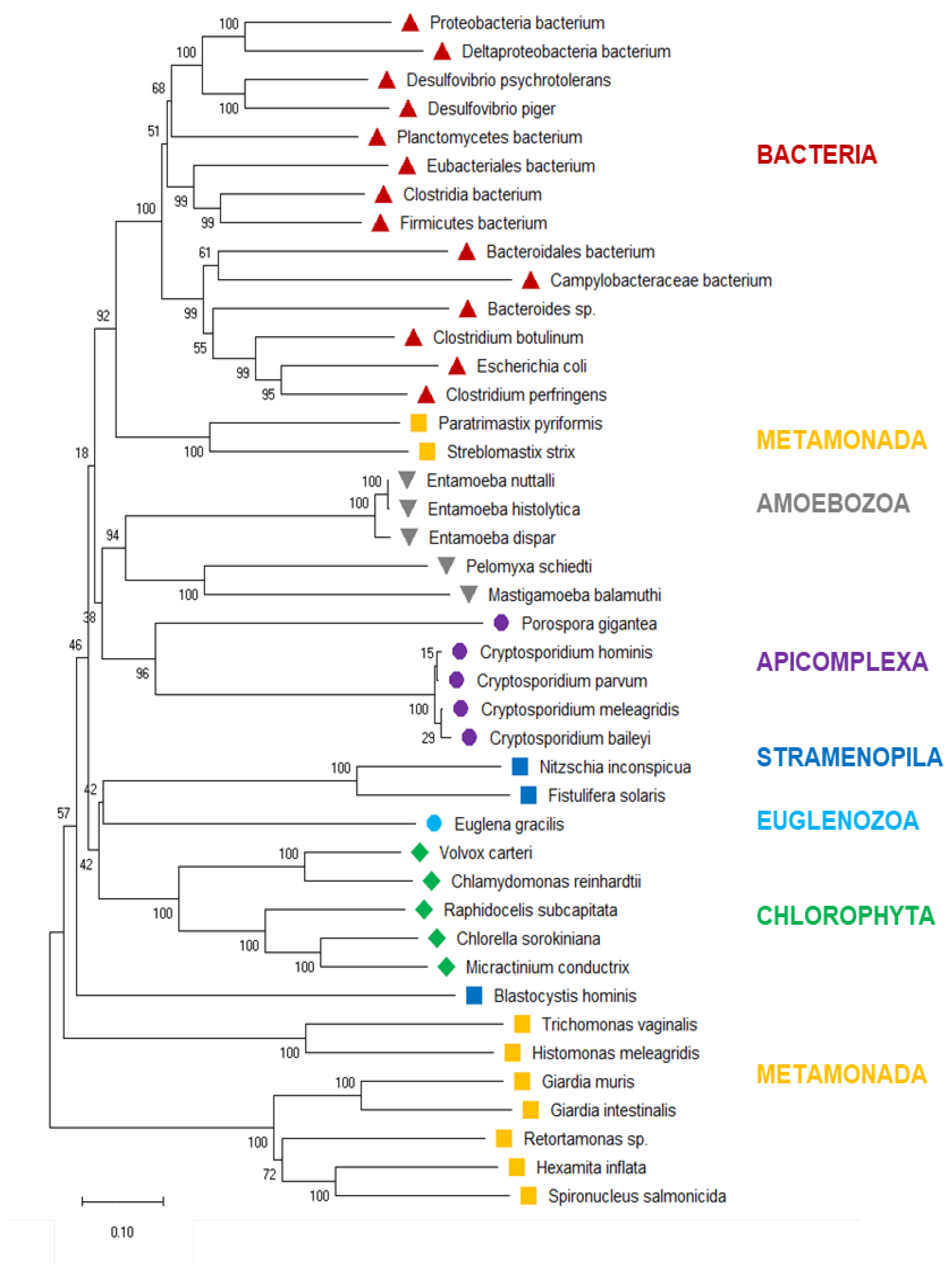
To investigate the evolutionary historyof eukaryotic PFOR further, we drew a phylogenetic tree of the PFOR sequences from eukaryotes identified with the profile searches and the blastp approach, supplemented with the most closely related sequences from prokaryotes as identified by blastp searches using the eukaryotic PFOR orthologs as queries. The hierarchy of the major branches of the PFOR tree did not unequivocally resolve, as indicated by the bootstrap numbers below 60% (
Figure 3). Nevertheless, it was evident that the PFOR sequences clustered according to their phylogeny: the sequences from the amoebozoa, apicomplexa, and green algae built their own clades, each with a high bootstrap support (
Figure 3). Among the sequences from metamonads, however, those from intestinal parasites of vertebrates formed their own clade, separate from that of the free-living
Paratrimastix pyriformis and the termite endosymbiont
Streblomastix strix. Eukaryote and prokaryote sequences did not intermix: all the bacterial sequences ended up in the same branch, with a bootstrap support of 100% (
Figure 3).
3.5. Proteome-Wide BLAST Surveys
The patchy distribution of PFOR orthologues in eukaryotes (
Table 1,
Figure 2) is in agreement with the hypothesis that PFOR was obtained by eukaryotes via horizontal gene transfer from prokaryotes. To further explore this hypothesis, we asked the question whether – if PFOR was indeed acquired horizontally – any other bacterial genes came along with it. We first addressed this question using PFOR from
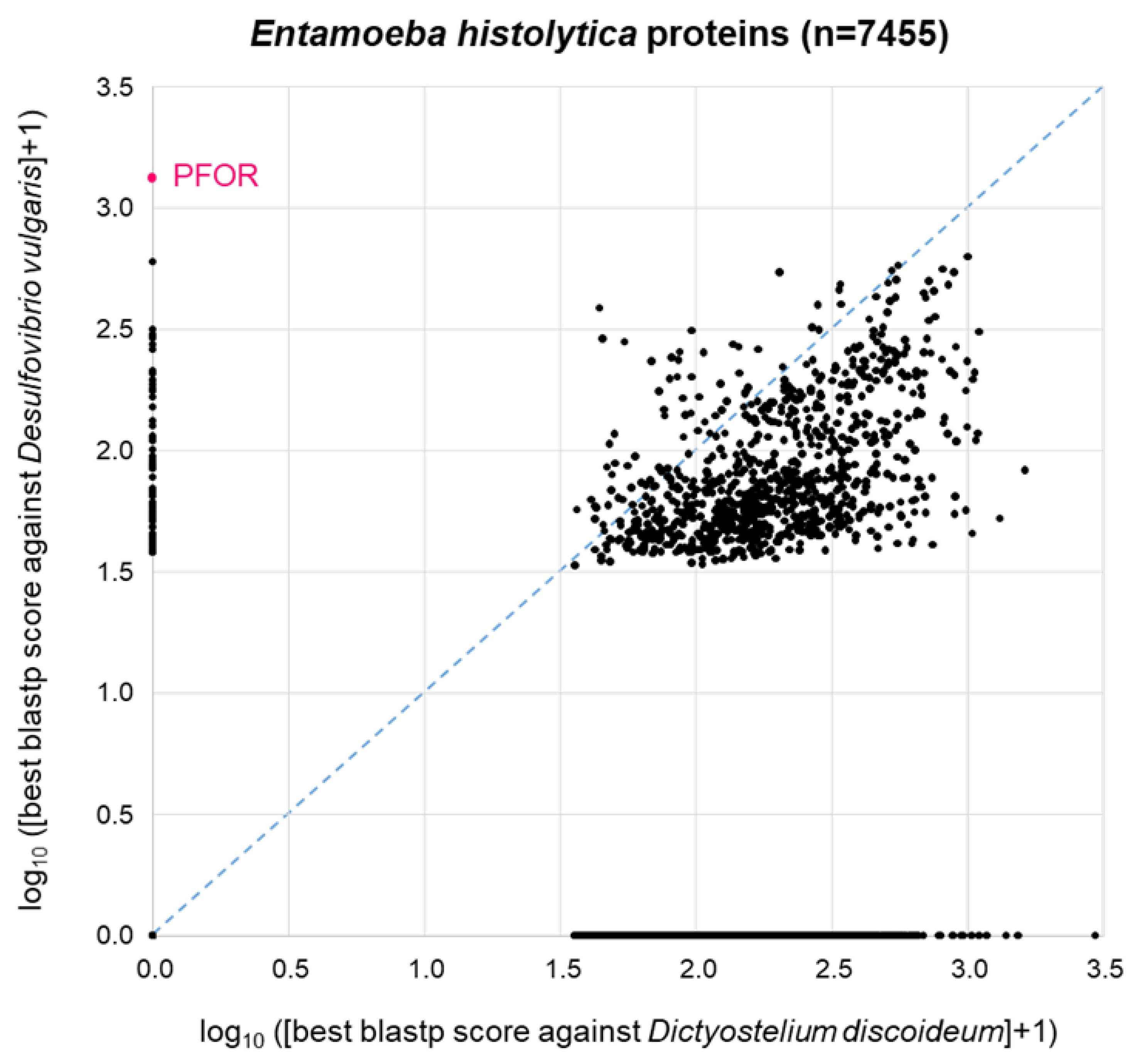
E. histolytica. Its most similar ortholog from prokaryotes as determined by blastp is the one from
Desulfovibrio vulgaris, a gram-negative anaerobic bacterium that occurs in the environment and in the mammalian gut. Hence we conducted a proteome-wide BLAST survey, searching every protein of
E. histolytica (n=7,455) against the proteome of
D. vulgaris (n=3880). In parallel, we performed the same search of all
E. histolytica proteins against the proteome of
Dictyostelium discoideum (n=13,233), a free-living amoeba that lacks PFOR (
Table 1).
Entamoeba is much more closely related to
Dictyostelium than to
Desulfovibrio; furthermore,
Dicytostelium has a larger proteome than
Desulfovibrio. So clearly, we would expect
Entamoeba proteins, in general, to return a higher-scoring best hit from
Dictyostelium than from
Desulfovibrio. This was indeed the case: the majority of points deviated from the diagonal towards the lower right corner of the 2D plot (
Figure 4). Of the 7455
E. histolytica proteins, 3310 (44%) did not return a hit from either
D. discoideum or
D. vulgaris (zero point of the 2D plot). PFOR did not have a hit in
D. discoideum, but it was the highest scoring of all
E. histolytica proteins in
D. vulgaris (
Figure 4). Interestingly, several other E. histolytica proteins also had a good-scoring blastp hit in
Desulfovibrio but not in
Dictyostelium (
Figure 4).
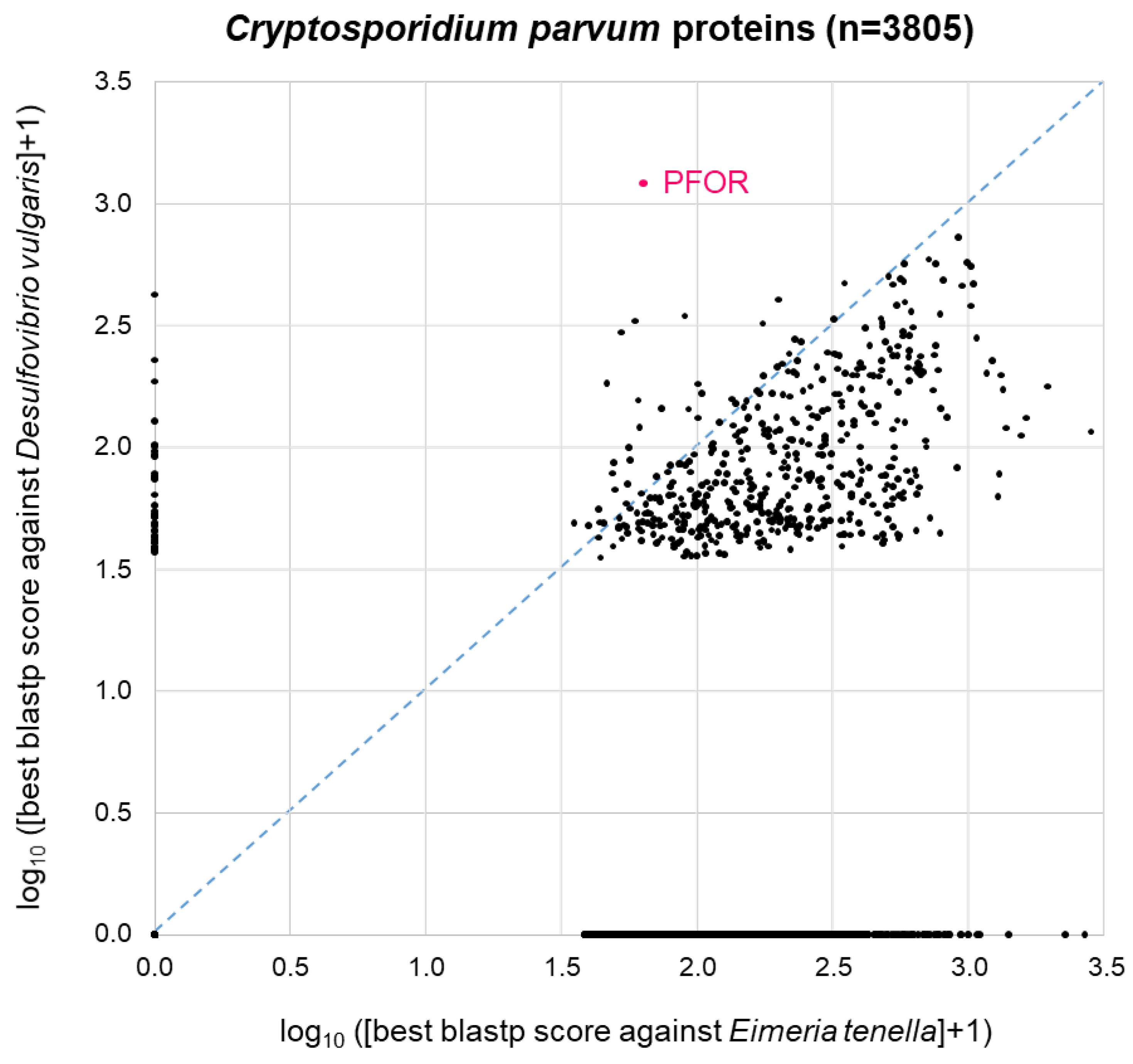
A similar picture was obtained when the same approach was carried out with
C. parvum (
Figure 5). Here, we used
Eimeria tenella (n=8,599 proteins) as a related apicoplastid parasite that does not possess PFOR (
Table 1). As with
E. histolytica, 44% of the
C. parvum proteins (1689 of n=3805 total proteins) did not return a blastp hit from either proteome. The remainder of the
C. parvum proteins showed a distribution that was, as expected, clearly skewed towards the lower right corner, i.e. towards higher similarity to
Eimeria than to
Desulfovibrio (
Figure 5). Again,
C. parvum PFOR stood out as the protein with a highest scoring hit in
Desulfovibrio, followed by a couple of other
C. parvum proteins at the ordinate of the 2D diagram (
Figure 5).
3.6. GO Term Enrichment Analysis
Concentrating on the subset of proteins from anaerobic protozoa that, like PFOR, had a higher similarity to bacterial than to eukaryotic proteins, we investigated what – if anything – this subset of sequences had in common. For this purpose, we performed a gene ontology (GO) term enrichment analysis, profiting from the good level of annotation of the
E. histolytica proteome [
59,
60]. Biological Process was used as annotation data category to analyze the gene list corresponding to the 40
E. histolytica proteins of highest similarity to
D. vulgaris but not to
D. discoideum. Enrichment of GO terms was calculated with reference to the complete set of 7,959
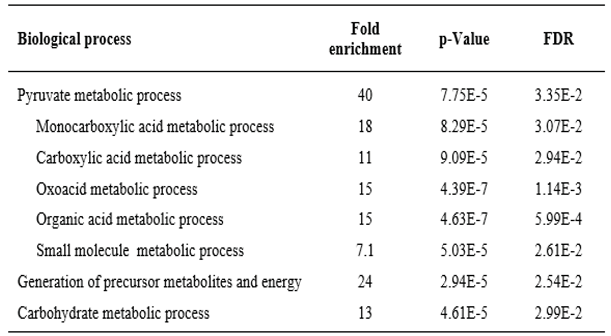
E. histolytica genes. Nine of the 40 query genes were unmapped, hence the remaining 31 genes were used for the enrichment analysis. The significantly enriched biological processes are summarized in
Table 2. Pyruvate metabolism was overrepresented by a factor of 40, followed by related but less specific categories. The general GO categories overrepresented in the set of 31
E. histolytica genes also dealt with energy metabolism, i.e. the carbohydrate metabolic process and pathways involved in the generation of precursor metabolites and energy (
Table 2). Interestingly, the list of genes with mapped GO terms that were enriched included pyruvate phosphate dikinase (XP_657332), the enzyme that converts pyruvate to phosphoenolpyruvate in a reaction typical for C4 plants. The surprising presence of pyruvate phosphate dikinase in
E. histolytica has been noted before [
61]. The enrichment of GO terms in the subset of
E. histolytica genes whose products have a higher resemblance to bacterial than to eukaryotic proteins suggests that these genes do not comprise a random selection. Rather, they share a common purpose related to the metabolism of pyruvate.
4. Discussion
The distribution of PFOR compared to that of GAPDH along the taxonomic tree of the selected protozoan species confirmed the relatively limited occurrence of PFOR in eukaryotes. The BLAST results further confirmed that the distribution of PFOR is highly restricted to few organisms in the eukaryotic domain, which is potentially interesting and worth looking at in closer detail. Due to the absence of deviation observed in the total number of hits for PFOR compared to GAPDH in the genera Giardia, Entamoeba and Cryptosporidium, it can be inferred that all species in these genera possess PFOR. The expanded cladograms of Entamoeba and Cryptosporidium derived from the whole proteome analysis, confirmed the findings from the BLAST results, since all the selected species in these genera with available whole proteome sequences from the ensemblgenomes database had positive hits for PFOR. Taking a broader view of all the selected protozoan species in the alveolate clade, only C. parvum showed a positive hit for PFOR. This is an interesting observation in support of a possible horizontal acquisition of pfor because the expanded cladogram of the genus Cryptosporidium showed that the closely related species in the genera Eimeria and Cyclospora had no hits for PFOR. A similar pattern observed in the expanded cladogram of all the available species of the genus Entamoeba and those of the related genera Dictyostelium, Cavenderia, Tiegemostilium and Planoprotostelium, further substantiated the hypothesis that pfor was most likely horizontally acquired. However, due to the limited availability of additional species with whole proteome sequences in the metamonad clade, it was not possible to generate an expanded cladogram for the genus Giardia and its closely related genera to support the horizontal transfer hypothesis of pfor in G. duodenalis. Consequently, all further analyses to support the hypothesis were limited to C. parvum and E. histolytica.
The phylogenic tree generated from the protein sequences of PFOR using the neighbor joining method showed some degree of incongruence with the expected phylogeny of eukaryotes. Yet the statistical support for the most inner nodes was generally weak indicating strong divergence of PFOR sequences between distantly related groups. This phylogenetic incongruence could indicate multiple sources of the acquisition of pfor in protozoa; however, the bacterial and protozoan species were clearly separated in distinct clades. This pattern is in agreement with a single horizontal transfer event from bacteria to eukaryotes.
It is striking that PFOR in eukaryotes occurs in gut endosymbionts or endoparasites that live, inside their host, in close contact with bacteria. This suggests two alternative hypotheses: (1) the proximity to bacteria facilitated the acquisition of PFOR by horizontal gene transfer; or (2) the anaerobic environment enforced maintenance of an ancestral PFOR gene, whereas free-living protists have lost the gene. The two scenarios are not necessarily mutually exclusive, and both are in agreement with the observed phylogenetic tree. However, the fact that there are groups of anaerobic eukaryotes that do not possess PFOR, such as trypanosomatid parasites of insects, may speak against hypothesis 2.
In view of the proximity hypothesis that supports the horizontal transfer of genes from bacteria, the bacterium with the highest normalized similarity score of 1.0 for PFOR, D. vulgaris, qualifies as the most suitable model of the putative gene donor or representative of an extant bacterial lineage of gene donors. D. vulgaris is a gram-negative, anaerobic, non-spore forming, curved rod-shaped, sulfate-reducing bacterium capable of producing hydrogen sulfide in the host gastrointestinal tract and as such dwells in close proximity with some invading protozoan parasites. The comparative whole proteome BLAST analysis of E. histolytica and C. parvum against their corresponding related control species, D. discoideum and E. tenella, and the bacterium D. vulgaris demonstrated significantly high hits for some proteins that are exclusively present in D. vulgaris but absent in the more closely related protozoan control species.
Out of the forty selected genes in E. histolytica and D. vulgaris, eleven genes predicted to be involved in biological processes related to small molecule metabolism, generation of precursor metabolites, and carbohydrate metabolism were significantly over-represented. The pyruvate metabolic process is a biological process related to the small molecule metabolic pathway, under which three out of the forty exclusively expressed proteins are functionally categorized. The findings suggest that the eleven genes that were significantly enriched did not occur by chance compared to the reference genome of E. histolytica. Furthermore, the small p-values indicate that the outcome of the gene enrichment analysis was non-random and worth looking at in further detail.
The significantly enriched genes can be analyzed further using syntenic approaches to give substantial information on how they are structurally related according to their relative position at the chromosomal level. If it can be established that these genes are located close to each other at the chromosomal level, this would provide a stronger evidence in support of the hypothesis that they were most likely horizontally transferred together with pfor as a genetic fragment from bacteria. Moreover, if these findings can be reproduced in the other PFOR-possessing protozoan species, this will provide more evidence in support of the horizontal gene transfer hypothesis.
5. Conclusions
The redox enzyme PFOR plays significant roles in the energy metabolism and growth of pathogens that dwell predominantly in oxygen-deprived niches. From the BLAST pilot analysis as well as the whole proteome analysis, it can be inferred that the presence of PFOR is limited to few protozoan species in the eukaryotic domain whereas a relatively large proportion of prokaryotes possess PFOR. Protozoa belonging to the genera
Cryptosporidium,
Giardia,
Entamoeba and
Blastocystis are anaerobic parasites that reside predominantly in close proximity with bacterial species in the mammalian host's gastrointestinal tract [
62]. Whereas, those belonging to the genera
Trichomonas and
Tritrichomonas are sexually transmitted extracellular anaerobic parasites that also dwell in close proximity with bacteria in the host genito-urinary tract [
63,
64].
A plausible explanation for the restricted occurrence of PFOR in the above-mentioned protozoa is based on the hypothesis that bacterial species serve as potential sources for acquisition of genes that enhance the optimal adaptation of these protozoa in hostile host environments. The expanded cladograms of Entamoeba and Cryptosporidium with their closely related genera substantiated this hypothesis. Although the phylogeny based on the protein sequences of PFOR demonstrated some degree of phylogenetic incongruence, the PFOR of none of the protozoa was monophyletic with that of bacteria. The observed monophyletic relationship of the PFOR of E. invadens with Giardia species, and that of E. gracilis with Cryptosporidium species, is suggestive that these two distinct groups of protozoa may have acquired pfor from common ancestral lineage. The exclusively expressed proteins obtained from E. histolytica and the putative bacterial gene donor, D. vulgaris, showed an over-representation of eleven genes involved in small molecule metabolism, generation of precursor metabolites, and carbohydrate metabolism. If these results obtained from E. histolytica and D. vulgaris can be reproduced in other PFOR-possessing protozoan species, it would provide more evidence to support the horizontal transfer of pfor from bacteria. Subsequent syntenic analyses of the significantly enriched genes would be required to provide further information regarding the positional relatedness of these genes at the chromosomal level.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org Table S1: Gene and accession numbers of the redox enzymes and control proteins from the UniProt™ Protein Database; Table S2: List of protozoa showing how their lifecycle supports the horizontal acquisition of PFOR from bacteria based on the proximity hypothesis.
Author Contributions
Conceptualization, Pascal Mäser and Daniela Brites; Methodology, Seth Duwor, Pascal Mäser and Daniela Brites; Software, Pascal Mäser and Daniela Brites; Validation, Pascal Mäser and Daniela Brites; Formal analysis, Seth Duwor; Investigation, Seth Duwor; Resources, Seth Duwor, Pascal Mäser and Daniela Brites; Data curation, Seth Duwor, Pascal Mäser and Daniela Brites; Writing – original draft, Seth Duwor; Writing – review & editing, Seth Duwor, Pascal Mäser and Daniela Brites; Visualization, Pascal Mäser. Seth Duwor and Daniela Brites; Supervision, Pascal Mäser and Daniela Brites; Project administration, Seth Duwor, Pascal Mäser and Daniela Brites.
Funding
This research received no external funding
Institutional Review Board Statement
Ethical approval was not required for this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hucho F. The pyruvate dehydrogenase multienzyme complex. Angew Chem Int Ed Engl. 1975 Sep;14(9):591-601. PMID: 811134. [CrossRef]
- Chabrière, E., M. H. Charon, A. Volbeda, L. Pieulle, E. C. Hatchikian and J. C. Fontecilla-Camps (1999). "Crystal structures of the key anaerobic enzyme pyruvate:ferredoxin oxidoreductase, free and in complex with pyruvate." Nat Struct Biol 6(2): 182-190.
- Charon, M. H., A. Volbeda, E. Chabriere, L. Pieulle and J. C. Fontecilla-Camps (1999). "Structure and electron transfer mechanism of pyruvate:ferredoxin oxidoreductase." Curr Opin Struct Biol 9(6): 663-669. [CrossRef]
- Gibson MI, Chen PY, Drennan CL. A structural phylogeny for understanding 2-oxoacid oxidoreductase function. Curr Opin Struct Biol. 2016 Dec;41:54-61. Epub 2016 Jun 14. PMID: 27315560; PMCID: PMC5381805. [CrossRef]
- Marczak, R., T. E. Gorrell and M. Müller (1983). "Hydrogenosomal ferredoxin of the anaerobic protozoon, Tritrichomonas foetus." J Biol Chem 258(20): 12427-12433.
- Moreno, S. N. and R. Docampo (1985). "Mechanism of toxicity of nitro compounds used in the chemotherapy of trichomoniasis." Environ Health Perspect 64: 199-208.
- Blamey, J. M. and M. W. Adams (1993). "Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus." Biochim Biophys Acta 1161(1): 19-27. [CrossRef]
- Hrdý, I. and M. Müller (1995). "Primary structure and eubacterial relationships of the pyruvate:ferredoxin oxidoreductase of the amitochondriate eukaryote Trichomonas vaginalis." J Mol Evol 41(3): 388-396.
- Embley, T. M., M. van der Giezen, D. S. Horner, P. L. Dyal and P. Foster (2003). "Mitochondria and hydrogenosomes are two forms of the same fundamental organelle." Philos Trans R Soc Lond B Biol Sci 358(1429): 191-201; discussion 201-192. [CrossRef]
- Müller, M. (1993). "The hydrogenosome." J Gen Microbiol 139(12): 2879-2889.
- Kulda, J. (1999). "Trichomonads, hydrogenosomes and drug resistance." Int J Parasitol 29(2): 199-212. [CrossRef]
- Benchimol, M. (2009). "Hydrogenosomes under microscopy." Tissue Cell 41(3): 151-168. [CrossRef]
- Benchimol, M., J. C. Almeida and W. de Souza (1996). "Further studies on the organization of the hydrogenosome in Tritrichomonas foetus." Tissue Cell 28(3): 287-299. [CrossRef]
- Roger, A. J., C. G. Clark and W. F. Doolittle (1996). "A possible mitochondrial gene in the early-branching amitochondriate protist Trichomonas vaginalis." Proc Natl Acad Sci U S A 93(25): 14618-14622. [CrossRef]
- Hrdy, I., R. P. Hirt, P. Dolezal, L. Bardonová, P. G. Foster, J. Tachezy and T. M. Embley (2004). "Trichomonas hydrogenosomes contain the NADH dehydrogenase module of mitochondrial complex I." Nature 432(7017): 618-622. [CrossRef]
- Makiuchi, T. and T. Nozaki (2014). "Highly divergent mitochondrion-related organelles in anaerobic parasitic protozoa." Biochimie 100: 3-17. [CrossRef]
- Lindmark, D. G. and M. Müller (1973). "Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism." J Biol Chem 248(22): 7724-7728. [CrossRef]
- Hackstein, J. H., A. Akhmanova, B. Boxma, H. R. Harhangi and F. G. Voncken (1999). "Hydrogenosomes: eukaryotic adaptations to anaerobic environments." Trends Microbiol 7(11): 441-447. [CrossRef]
- Boxma, B., R. M. de Graaf, G. W. van der Staay, T. A. van Alen, G. Ricard, T. Gabaldón, A. H. van Hoek, S. Y. Moon-van der Staay, W. J. Koopman, J. J. van Hellemond, A. G. Tielens, T. Friedrich, M. Veenhuis, M. A. Huynen and J. H. Hackstein (2005). "An anaerobic mitochondrion that produces hydrogen." Nature 434(7029): 74-79. [CrossRef]
- Noth, J., D. Krawietz, A. Hemschemeier and T. Happe (2013). "Pyruvate:ferredoxin oxidoreductase is coupled to light-independent hydrogen production in Chlamydomonas reinhardtii." J Biol Chem 288(6): 4368-4377. [CrossRef]
- van Lis, R., C. Baffert, Y. Couté, W. Nitschke and A. Atteia (2013). "Chlamydomonas reinhardtii chloroplasts contain a homodimeric pyruvate:ferredoxin oxidoreductase that functions with FDX1." Plant Physiol 161(1): 57-71. [CrossRef]
- Rotte, C., F. Stejskal, G. Zhu, J. S. Keithly and W. Martin (2001). "Pyruvate : NADP+ oxidoreductase from the mitochondrion of Euglena gracilis and from the apicomplexan Cryptosporidium parvum: a biochemical relic linking pyruvate metabolism in mitochondriate and amitochondriate protists." Mol Biol Evol 18(5): 710-720. [CrossRef]
- Horner, D. S., R. P. Hirt and T. M. Embley (1999). "A single eubacterial origin of eukaryotic pyruvate: ferredoxin oxidoreductase genes: implications for the evolution of anaerobic eukaryotes." Mol Biol Evol 16(9): 1280-1291. [CrossRef]
- Juhas, M. (2015). "Horizontal gene transfer in human pathogens." Crit Rev Microbiol 41(1): 101-108. [CrossRef]
- Deng, Y., H. Xu, Y. Su, S. Liu, L. Xu, Z. Guo, J. Wu, C. Cheng and J. Feng (2019). "Horizontal gene transfer contributes to virulence and antibiotic resistance of Vibrio harveyi 345 based on complete genome sequence analysis." BMC Genomics 20(1): 761. [CrossRef]
- Wyres, K. L., R. R. Wick, L. M. Judd, R. Froumine, A. Tokolyi, C. L. Gorrie, M. M. C. Lam, S. Duchêne, A. Jenney and K. E. Holt (2019). "Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae." PLoS Genet 15(4): e1008114. [CrossRef]
- Keeling, P. J. and J. D. Palmer (2008). "Horizontal gene transfer in eukaryotic evolution." Nat Rev Genet 9(8): 605-618. [CrossRef]
- Lacroix, B. and V. Citovsky (2016). "Transfer of DNA from Bacteria to Eukaryotes." mBio 7(4).
- Lacroix, B. and V. Citovsky (2018). "Beyond Agrobacterium-Mediated Transformation: Horizontal Gene Transfer from Bacteria to Eukaryotes." Curr Top Microbiol Immunol 418: 443-462.
- Ricard, G., N. R. McEwan, B. E. Dutilh, J. P. Jouany, D. Macheboeuf, M. Mitsumori, F. M. McIntosh, T. Michalowski, T. Nagamine, N. Nelson, C. J. Newbold, E. Nsabimana, A. Takenaka, N. A. Thomas, K. Ushida, J. H. Hackstein and M. A. Huynen (2006). "Horizontal gene transfer from Bacteria to rumen Ciliates indicates adaptation to their anaerobic, carbohydrates-rich environment." BMC Genomics 7: 22.
- Eme, L., E. Gentekaki, B. Curtis, J. M. Archibald and A. J. Roger (2017). "Lateral Gene Transfer in the Adaptation of the Anaerobic Parasite Blastocystis to the Gut." Curr Biol 27(6): 807-820.
- Doolittle, W. F. (1998). "You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes." Trends Genet 14(8): 307-311. [CrossRef]
- Field, J., B. Rosenthal and J. Samuelson (2000). "Early lateral transfer of genes encoding malic enzyme, acetyl-CoA synthetase and alcohol dehydrogenases from anaerobic prokaryotes to Entamoeba histolytica." Mol Microbiol 38(3): 446-455.
- Hedges, S. B., H. Chen, S. Kumar, D. Y. Wang, A. S. Thompson and H. Watanabe (2001). "A genomic timescale for the origin of eukaryotes." BMC Evol Biol 1: 4. [CrossRef]
- Keeling, P. J. (2009). "Functional and ecological impacts of horizontal gene transfer in eukaryotes." Curr Opin Genet Dev 19(6): 613-619. [CrossRef]
- Schönknecht, G., W. H. Chen, C. M. Ternes, G. G. Barbier, R. P. Shrestha, M. Stanke, A. Bräutigam, B. J. Baker, J. F. Banfield, R. M. Garavito, K. Carr, C. Wilkerson, S. A. Rensing, D. Gagneul, N. E. Dickenson, C. Oesterhelt, M. J. Lercher and A. P. Weber (2013). "Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote." Science 339(6124): 1207-1210. [CrossRef]
- Husnik, F. and J. P. McCutcheon (2018). "Functional horizontal gene transfer from bacteria to eukaryotes." Nat Rev Microbiol 16(2): 67-79. [CrossRef]
- Sibbald, S. J., L. Eme, J. M. Archibald and A. J. Roger (2020). "Lateral Gene Transfer Mechanisms and Pan-genomes in Eukaryotes." Trends Parasitol 36(11): 927-941. [CrossRef]
- Van Etten, J. and D. Bhattacharya (2020). "Horizontal Gene Transfer in Eukaryotes: Not if, but How Much?" Trends Genet 36(12): 915-925.
- Nixon, J. E., A. Wang, J. Field, H. G. Morrison, A. G. McArthur, M. L. Sogin, B. J. Loftus and J. Samuelson (2002). "Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica." Eukaryot Cell 1(2): 181-190.
- Alsmark, U. C., T. Sicheritz-Ponten, P. G. Foster, R. P. Hirt and T. M. Embley (2009). "Horizontal gene transfer in eukaryotic parasites: a case study of Entamoeba histolytica and Trichomonas vaginalis." Methods Mol Biol 532: 489-500.
- Wittlin S, Mäser P. From Magic Bullet to Magic Bomb: Reductive Bioactivation of Antiparasitic Agents. ACS Infect Dis. 2021 Oct 8;7(10):2777-2786. Epub 2021 Sep 2. PMID: 3447283. [CrossRef]
- Upcroft, P. and J. A. Upcroft (2001). "Drug targets and mechanisms of resistance in the anaerobic protozoa." Clin Microbiol Rev 14(1): 150-164. [CrossRef]
- Pal, D., S. Banerjee, J. Cui, A. Schwartz, S. K. Ghosh and J. Samuelson (2009). "Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases)." Antimicrob Agents Chemother 53(2): 458-464.
- Lamp, K. C., C. D. Freeman, N. E. Klutman and M. K. Lacy (1999). "Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials." Clin Pharmacokinet 36(5): 353-373. [CrossRef]
- 46. Dingsdag SA, Hunter N. Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J Antimicrob Chemother. 2018 Feb 1;73(2):265-279. PMID: 29077920. [CrossRef]
- Dan, M., A. L. Wang and C. C. Wang (2000). "Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme." Mol Microbiol 36(2): 447-456. [CrossRef]
- Leitsch, D., A. G. Burgess, L. A. Dunn, K. G. Krauer, K. Tan, M. Duchêne, P. Upcroft, L. Eckmann and J. A. Upcroft (2011). "Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia." J Antimicrob Chemother 66(8): 1756-1765. [CrossRef]
- Ansell, B. R., L. Baker, S. J. Emery, M. J. McConville, S. G. Svärd, R. B. Gasser and A. R. Jex (2017). "Transcriptomics Indicates Active and Passive Metronidazole Resistance Mechanisms in Three Seminal Giardia Lines." Front Microbiol 8: 398. [CrossRef]
- Müller, J., S. Braga, M. Heller and N. Müller (2019). "Resistance formation to nitro drugs in Giardia lamblia: No common markers identified by comparative proteomics." Int J Parasitol Drugs Drug Resist 9: 112-119. [CrossRef]
- Lopes-Oliveira, L. A. P., M. Fantinatti and A. M. Da-Cruz (2020). "In vitro-induction of metronidazole-resistant Giardia duodenalis is not associated with nucleotide alterations in the genes involved in pro-drug activation." Mem Inst Oswaldo Cruz 115: e200303. [CrossRef]
- Rasoloson, D., S. Vanacova, E. Tomkova, J. Razga, I. Hrdy, J. Tachezy and J. Kulda (2002). "Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis." Microbiology (Reading) 148(Pt 8): 2467-2477. [CrossRef]
- Hrdý, I., R. Cammack, P. Stopka, J. Kulda and J. Tachezy (2005). "Alternative pathway of metronidazole activation in Trichomonas vaginalis hydrogenosomes." Antimicrob Agents Chemother 49(12): 5033-5036. [CrossRef]
- Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller and D. J. Lipman (1997). "Gapped BLAST and PSI-BLAST: a new generation of protein database search programs." Nucleic Acids Res 25(17): 3389-3402. [CrossRef]
- Altschul, S. F., J. C. Wootton, E. M. Gertz, R. Agarwala, A. Morgulis, A. A. Schäffer and Y. K. Yu (2005). "Protein database searches using compositionally adjusted substitution matrices." Febs j 272(20): 5101-5109. [CrossRef]
- Kumar, S., G. Stecher, M. Li, C. Knyaz and K. Tamura (2018). "MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms." Mol Biol Evol 35(6): 1547-1549. [CrossRef]
- Saitou, N. and M. Nei (1987). "The neighbor-joining method: a new method for reconstructing phylogenetic trees." Mol Biol Evol 4(4): 406-425. [CrossRef]
- Felsenstein, J. (1985). "Confidence limits on phylogenies: An approach using the bootstrap." Evolution 39(4): 783-791.
- 59. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000 May;25(1):25-9. PMID: 10802651; PMCID: PMC3037419. [CrossRef]
- 60. Aleksander SA, Balhoff J, Carbon S, Cherry JM, Drabkin HJ, Ebert D, Feuermann M, Gaudet P, Harris NL, Hill DP, Lee R, Mi H, Moxon S, Mungall CJ, Muruganugan A, Mushayahama T, Sternberg PW, Thomas PD, Van Auken K, Ramsey J, Siegele DA, Chisholm RL, Fey P, Aspromonte MC, Nugnes MV, Quaglia F, Tosatto S, Giglio M, Nadendla S, Antonazzo G, Attrill H, Dos Santos G, Marygold S, Strelets V, Tabone CJ, Thurmond J, Zhou P, Ahmed SH, Asanitthong P, Luna Buitrago D, Erdol MN, Gage MC, Ali Kadhum M, Li KYC, Long M, Michalak A, Pesala A, Pritazahra A, Saverimuttu SCC, Su R, Thurlow KE, Lovering RC, Logie C, Oliferenko S, Blake J, Christie K, Corbani L, Dolan ME, Drabkin HJ, Hill DP, Ni L, Sitnikov D, Smith C, Cuzick A, Seager J, Cooper L, Elser J, Jaiswal P, Gupta P, Jaiswal P, Naithani S, Lera-Ramirez M, Rutherford K, Wood V, De Pons JL, Dwinell MR, Hayman GT, Kaldunski ML, Kwitek AE, Laulederkind SJF, Tutaj MA, Vedi M, Wang SJ, D'Eustachio P, Aimo L, Axelsen K, Bridge A, Hyka-Nouspikel N, Morgat A, Aleksander SA, Cherry JM, Engel SR, Karra K, Miyasato SR, Nash RS, Skrzypek MS, Weng S, Wong ED, Bakker E, Berardini TZ, Reiser L, Auchincloss A, Axelsen K, Argoud-Puy G, Blatter MC, Boutet E, Breuza L, Bridge A, Casals-Casas C, Coudert E, Estreicher A, Livia Famiglietti M, Feuermann M, Gos A, Gruaz-Gumowski N, Hulo C, Hyka-Nouspikel N, Jungo F, Le Mercier P, Lieberherr D, Masson P, Morgat A, Pedruzzi I, Pourcel L, Poux S, Rivoire C, Sundaram S, Bateman A, Bowler-Barnett E, Bye-A-Jee H, Denny P, Ignatchenko A, Ishtiaq R, Lock A, Lussi Y, Magrane M, Martin MJ, Orchard S, Raposo P, Speretta E, Tyagi N, Warner K, Zaru R, Diehl AD, Lee R, Chan J, Diamantakis S, Raciti D, Zarowiecki M, Fisher M, James-Zorn C, Ponferrada V, Zorn A, Ramachandran S, Ruzicka L, Westerfield M. The Gene Ontology knowledgebase in 2023. Genetics. 2023 May 4;224(1):iyad031. PMID: 36866529; PMCID: PMC10158837. [CrossRef]
- 61. Saavedra E, Encalada R, Pineda E, Jasso-Chávez R, Moreno-Sánchez R. Glycolysis in Entamoeba histolytica. Biochemical characterization of recombinant glycolytic enzymes and flux control analysis. FEBS J. 2005 Apr;272(7):1767-83. PMID: 15794763. [CrossRef]
- Higuera, A., X. Villamizar, G. Herrera, J. C. Giraldo, A. L. Vasquez, P. Urbano, O. Villalobos, C. Tovar and J. D. Ramírez (2020). "Molecular detection and genotyping of intestinal protozoa from different biogeographical regions of Colombia." PeerJ 8: e8554. [CrossRef]
- Benchimol, M., I. de Andrade Rosa, R. da Silva Fontes and A. J. Burla Dias (2008). "Trichomonas adhere and phagocytose sperm cells: adhesion seems to be a prominent stage during interaction." Parasitol Res 102(4): 597-604. [CrossRef]
- Chapwanya, A., A. Y. Usman and P. C. Irons (2016). "Comparative aspects of immunity and vaccination in human and bovine trichomoniasis: a review." Trop Anim Health Prod 48(1): 1-7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).