2.1. 3d-3d Complexes
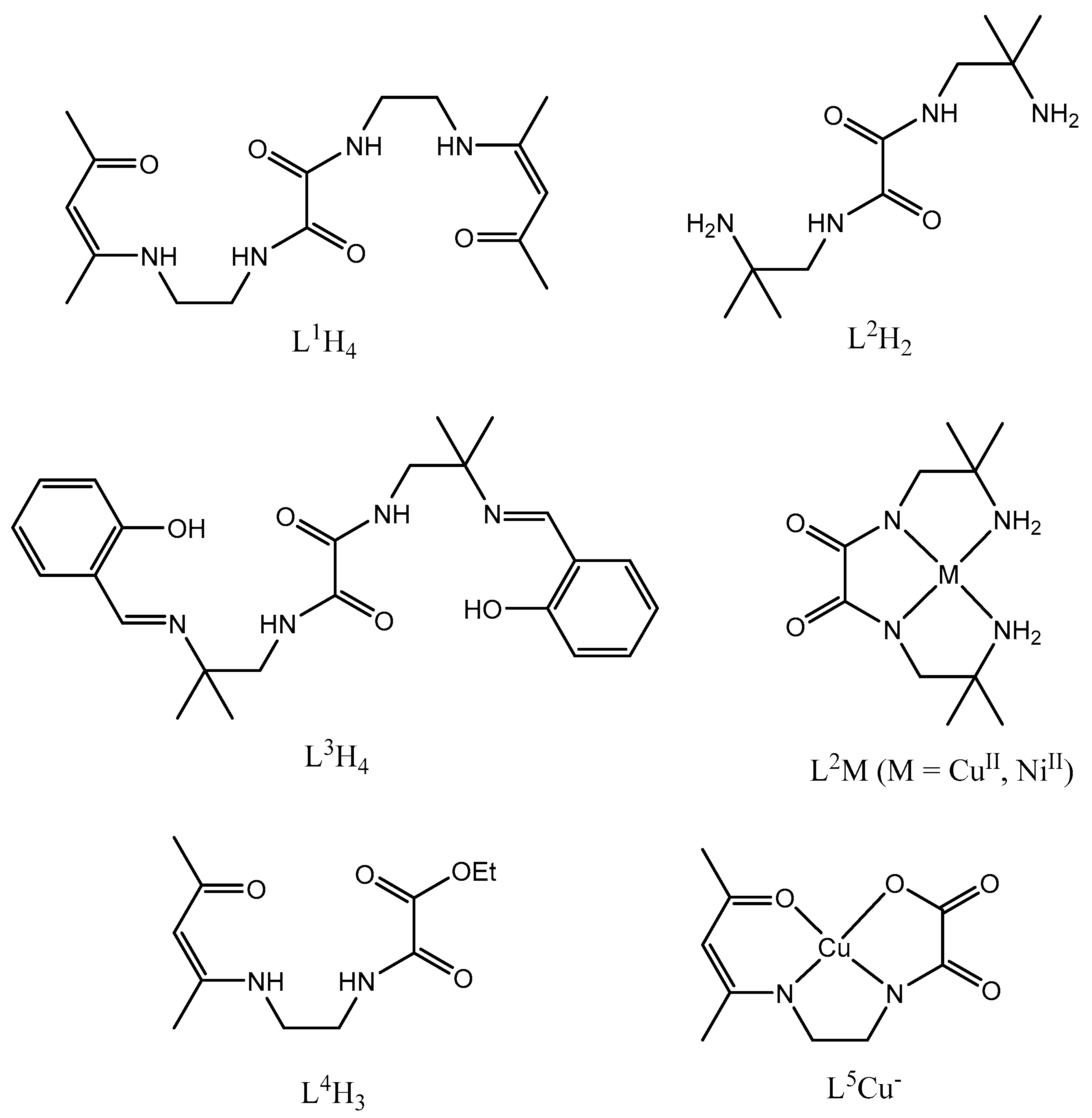
In previous work we showed that symmetrical oxamide ligands such as N-N’-bis(4-methyl-5-aza-3-hepten-2-on-7-yl) oxamide (L
1H
4,
Scheme 1) or N-N’-bis[2,2-dimethyl-4-(2-hydroxyphenyl)-3-aza-3-buten] oxamide (L
3H
4,
Scheme 1) are able to coordinate transition metal ions to yield neutral homodinuclear complexes such as CuL
1Cu, NiL
1Ni [
4] or CuL
3Cu, NiL
3Ni, VOL
3VO [
5], as demonstrated by the structural determination of the [CuL
3Cu](H
2O) complex [
7]. More surprisingly, thanks to the cis-trans isomerization of the oxamide function, the L
1H
4 can react with copper ions to give an anionic species formulated (L
1HCuNa).2H
2O. The EPR spectrum confirms that three nitrogen atoms are involved in the copper coordination sphere. In a following step this species acts as a ligand toward a nickel ion and yields the neutral heterodinuclear CuL
1Ni complex [
4]. Note that the L
1H
4 ligand is prepared by reacting the half-unit ligand 7-amino-4-methyl-5-aza-3-hepten-2-one with diethyl oxalate in a 2:1 ratio while the reaction of this half-unit ligand with diethyl oxalate in a 1/3 ratio allows isolation of the L
4H
3 ligand (
Scheme 1) [
9]. The preparation of this L
3H
4 ligand necessitates a two-step reaction. The reaction of diethyl oxalate with 1,2-diamino-2-methylpropane in a 1:2 ratio first yields the L
2H
2 ligand while addition of salicylaldehyde to the aforementioned L
2H
2 ligand in a 2:1 ratio furnishes the L
3H
4 ligand [
5]. According to the cis-trans isomerization of the oxamide function, it is possible to isolate L
2M complexes (M = Cu
II, Ni
II). The EPR spectrum of L
2Cu does confirm that the copper ion is bound to two pairs of inequivalent nitrogen atoms (A
N = 15 G and A’
N = 8 G) giving a superhyperfine structure of 13 lines in addition to the hyperfine structure (A
iso = 95 G, g
iso = 2.08) [
8]. Addition of copper ions to this CuL
2 complex induces a trans isomerization of the oxamide ligand and formation of an infinite chain compound in which the copper ions are alternately bridged by the dideprotonated L
2 ligand and two nitrite anions, as demonstrated by the structural determination of the [Cu(NO
2)
2CuL
2]
n compound [
8].
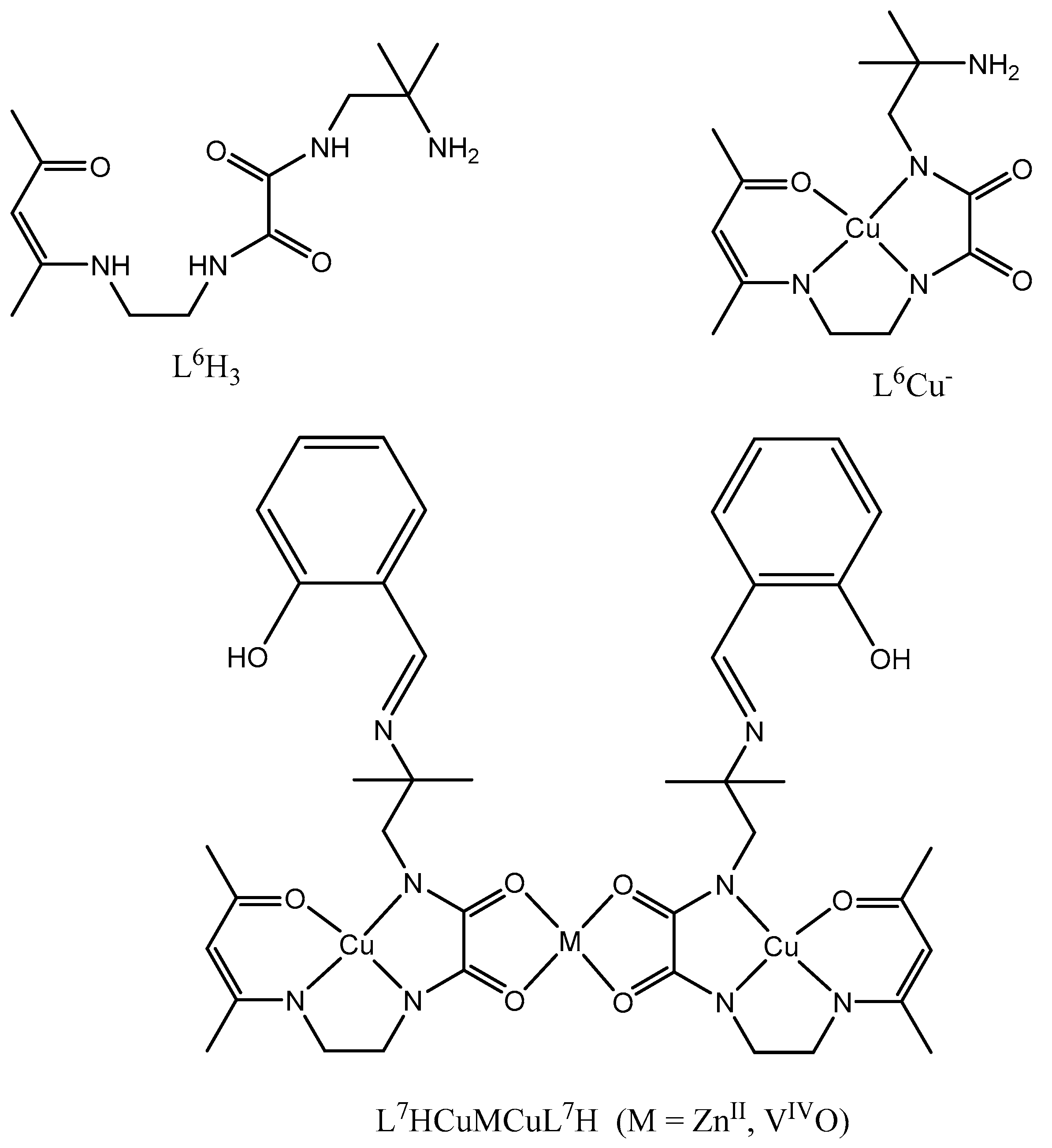
In the present work we have built a new L
6H
3 ligand possessing two different coordination sites. In a first step the L
4H
3 ligand is reacted in a 1:1 ratio with 1,2-diamino-2-methylpropane, thus allowing isolation of the L
6H
3 ligand. Reaction of copper ions in a basic medium gives the anionic L
6Cu
- entity in which the copper ion presents a N
3O coordination sphere. Surprisingly, reaction with Zn(sal)
2.2H
2O or VO(Sal)
2 (sal = deprotonated salicylaldehyde) has not allowed to isolate the expected CuL
7Zn or CuL
7VO neutral complexes. From the analytical, FAB
+, magnetic data, it becomes clear that the isolated complexes correspond to the (L
7HCu)
2Zn·2CH
3OH or (L
7HCu)
2VO·2CH
3OH formulation, the phenol function remaining protonated. As an example the FAB
+ data for (L
7HCu)
2Zn·2CH
3OH gives a first signal corresponding to the [(CuL
7H)
2Zn +1]
+ cation with a maximum peak at m/z = 963 uma and a second one for the [(CuL
7H)
2Zn + Na]
+ cation at m/z = 985 uma. The theoretical and experimental isotopic patterns of these cations are in complete agreement (
Figures S1 and S2). In order to explain such a behavior, we can expect that the free amine function reacts with the aldehyde function of the salicylaldehydato ligand coordinated to the Zn
II ion, which induces a decomplexation of the salicylaldehydato ligand coordinated to the Zn
II ion along with a concomitant protonation of the free phenol function by a proton coming from the reacting nitrogen atom. Then the oxygen atoms of the two oxamide functions that are in the vicinity of the Zn
II ion can enter into coordination with this ion. According to this observation, it appears that the Zn ion is not able to induce the cis-trans isomerization of the oxamide function. A similar observation is made for complex (L
7HCu)
2VO·2CH
3OH. The χ
MT product for this complex is constant and equal to 1.12 cm
3mol
-1K from 100 to 4 K (
Figure S3). Such a value corresponding to three non interacting S = ½ ions does confirm that the vanadyl ion is again not able to induce the cis-trans isomerization of the oxamide function. Contrary to our previous results showing that Cu and Ni ions are able to induce the cis-trans isomerization [
4,
8], it seems that Zn and VO ions are not able to favor such an isomerization.
Scheme 2.
Schematic representation of genuine ligands and complexes.
Scheme 2.
Schematic representation of genuine ligands and complexes.
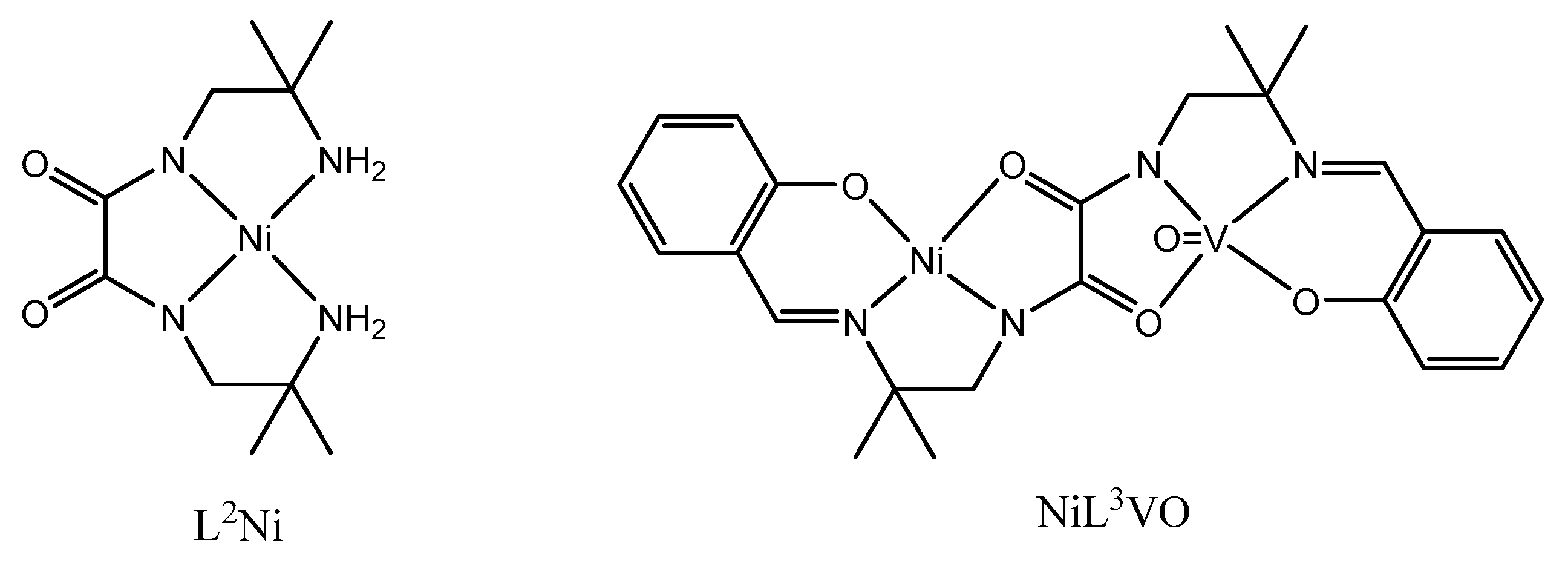
Reacting first the NiL
2 complex with salicylaldehyde in a basic medium, followed by addition of vanadyl ions allows isolation of the NiL
3VO complex in which the two 3d ions are coordinated to the symmetrical ligand possessing two N
2O
2 coordination sites. As the Ni ion is diamagnetic, we observe a nice EPR spectrum with 8 lines at room temperature and in solution (CH
2Cl
2), characteristic of the vanadyl ion (
Figure S4). In the present case we must conclude that the reaction of the amine functions with salicylaldehyde induces the cis-trans isomerization, thus yielding the L
3 ligand in which only one N
2O
2 coordination site is occupied by the Ni ion while the vanadyl ion is introduced in the other N
2O
2 coordination site to give the NiL
3VO compound. The eight lines EPR spectrum (
Figure S4) confirms the isolation of a heterodinuclear complex, with absence of scrambling of the Ni and VO ions. Its magnetic behavior follows a Curie law with C equal to 0.40 cm
3mol
-1K. In order to understand the absence of scrambling, the reaction of the primary amine functions with the aldehyde functions must yield a [NiL
3H]
- anionic species in a first stage, in which the Ni
II ion is coordinated to three nitrogen atoms and to the oxygen atom of a deprotonated phenol function. Then the cis-trans isomerization is favored by the basic medium, that allows the VO ion to enter into coordination in the newly formed N
2O
2 site, as in the case of the CuL
1Ni complex [
4]. This result is surprising in view of our previous assertion in the above paragraph, where we were telling that the VO ion was not able to favor the cis-trans isomerization. We must conclude that the cis-trans isomerization is possible in a basic medium and is not observed in a neutral medium, whatever the concerned ions. So we again show that a symmetrical ligand possessing two identical N
2O
2 coordination sites allows formation of heterodinuclear complexes, what is quite surprising and original.
Scheme 3.
Schematic representation of the starting and final complexes.
Scheme 3.
Schematic representation of the starting and final complexes.
We have previously shown that the free L
3H
4 ligand reacts with vanadyl ions in basic medium to yield the VOL
3VO complex [
5]. We have also demonstrated that the thermal dependence of the magnetic susceptibility follows a Curie law, with C = 0.69 cm
3mol
-1K, so that the magnetic interaction of the two vanadyl ions is absent or very weak. The EPR spectra (CH
2Cl
2 solution, 295 K) are more informative but they can be slightly different on going from a sample to another one, depending on the reaction time. It appears that two spectra are superimposed with a g parameter equal to 1.98, one with 8 lines and the other one with 15 lines, the 8 lines species appearing as a minor species giving shoulders in some lines of the main EPR spectrum after a prolongated heating, while the 8 lines species is favored before the thermal equilibrium is reached. (
Figure S5). Such a result can be explained by the presence of two isomers, with a cis or trans arrangement of the V=O groups. The hyperfine splittings A are respectively equal to 94.6 x 10
-4 cm
-1 (8 lines spectrum) and 47.3 x 10
-4 cm
-1 (15 lines spectrum). From these data we can estimate the interaction parameters
J. For the eight lines spectrum
J must be lower than A, so that a
J value lower than 10
-3 cm
-1 is expected, while for the fifteen lines spectrum the
J parameter must be higher than A. It is expected to be comprised in between 10
-3 and 10
-2 cm
-1. These
J values are very weak, in complete agreement with previous literature data [
12]. In absence of structural determination, we can expect that the main compound should be the most symmetrical complex, with the apical VO groups in a trans arrangement. The previously published structural determination of the [(terpy)Cu(ox)VO(ox)(H2O)]H2O complex indicates that the Cu and VO ions are linked by an oxalate bridge while a very weak Cu-VO magnetic interaction, expected to be ferromagnetic, is observed thanks to the magnetic study. Furthermore it is told that the magnetic susceptibility of the trinuclear [(terpy)Cu(ox)VO(ox)(H
2O)Cu(terpy](ClO
4) ·2H
2O complex follows a Curie law in the 4-100 K temperature domain, with a χ
MT value of 1.20 cm
3mol
-1K [
12]. This result does agree with our observation for the complex (L
7HCu)
2VO·2CH
3OH described above.
2.2. 3d-4f Complexes
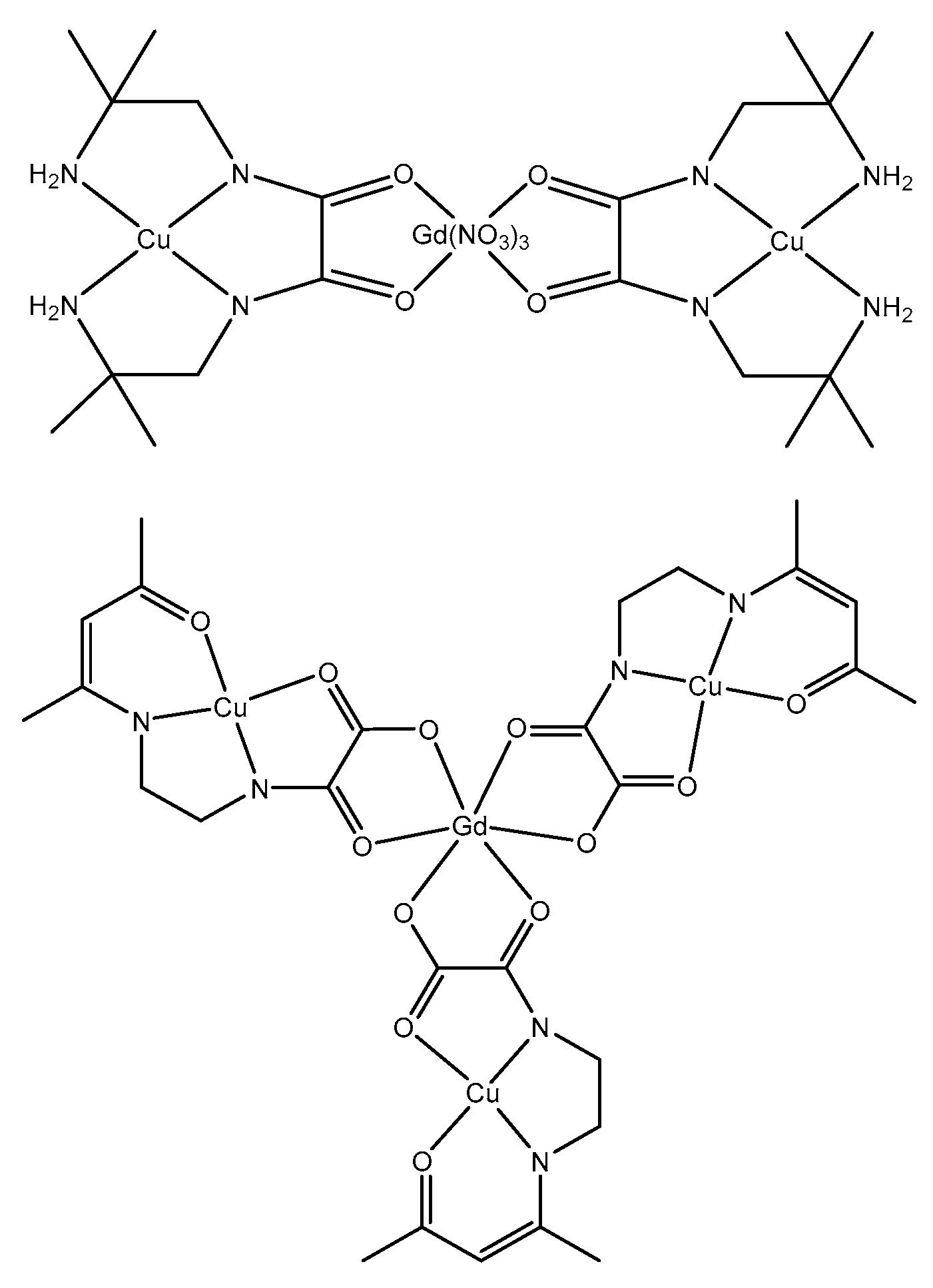
It is possible to prepare 3d-4f entities by reaction of some of these mononuclear 3d complexes with lanthanide ions. So the L
2Cu complex reacts with gadolinium nitrate to yield a compound formulated (L
2Cu)
2Gd(NO
3)
3·5H
2O while the [L
5Cu]
- anionic species gives a neutral complex formulated (L
5Cu)
3Gd·5H
2O. In these compounds, the oxophilic character of the Ln ions impedes any cis-trans isomerization. A look at the magnetic data is of interest. In the first complex the Gd ion is surrounded by two Cu ions and the χ
MT product is equal to 8.30 cm
3mol
-1K at 300 K. It remains constant till 10 K and then slightly decreases to 8.14 cm
3mol
-1K at 2K (
Figure S6). In the second compound the Gd ion is surrounded by three Cu ions. The χ
MT product is equal to 9.15 cm
3mol
-1K at 300 K, does not change in the 20-300 K temperature domain while a slight increase at 9.80 cm
3mol
-1K is observed at very low temperature (
Figure S7). We have previously shown that spin polarization is the mechanism that allows to understand the strength and sign of the magnetic Cu–Gd interactions in complexes assembling Cu and Gd ions [
13]. When spin polarization is active an odd number of bridging atoms in between the ions bearing the spins favors a parallel alignment of the spins, whereas an even number of bridging atoms favors an antiparallel alignment. In the present complexes we have a mixture of even and odd numbers of atoms in the ligand bridging the metal ions, so that the resulting magnetic interaction is expected to be very weak. Furthermore we have to remember that the presence of singly occupied 3d
x2-y2 orbitals is needed for the observation of ferromagnetic 3d-Gd interactions, the 3d ions with unoccupied 3d
x2-y2 orbitals yielding antiferromagnetic 3d-Gd interactions [
14]. In the trinuclear CuGdCu complex (L
2Cu)
2Gd(NO
3)
3·5H
2O, the Cu and Gd ions are interacting through two three-atom OCN bridges and two four-atom OCCN bridges. It has been previously shown that such OCN bridges [
13] and OCO bridges [
15] give weak ferromagnetic interactions. Observation of a weak antiferromagnetic interaction in the trinuclear complex means that the interaction through the four-atom OCCN bridge overcomes the one coming from the three-atom OCN bridge. In the tetranuclear complex (L
5Cu)
3Gd·5H
2O, the Cu and Gd ions are interacting through two three-atom OCO and OCN bridges, and two four-atom OCCO and OCCN bridges. Observation of a weak ferromagnetic interaction implies that, according to the magnetic behavior of the trinuclear (L
2Cu)
2Gd(NO
3)
3·5H
2O complex, the magnetic interaction through the three-atom OCO bridges is slightly larger than the one coming from the four-atom bridges and becomes the predominant magnetic interaction. Eventually we can conclude that the magnetic behavior of these two complexes assembling Cu and Gd ions can be understood by the presence of an active spin polarization interaction. But we cannot forget that these interactions are very weak. Structural determinations of similar complexes along with theoretical calculations will be necessary to confirm such an assertion.
Scheme 4.
Schematic representation of trinuclear and tetranuclear Cu-Gd complexes.
Scheme 4.
Schematic representation of trinuclear and tetranuclear Cu-Gd complexes.