Introduction
A large body of evidence has established incontrovertibly that antimicrobial exposure can result in antimicrobial resistance (AMR) [
1,
2,
3]. In a similar vein, populations with more extensive exposure to an antimicrobial typically have a higher prevalence of resistance to that antimicrobial [
3,
4,
5,
6,
7,
8,
9]. What is less clear is if this effect is saturated at extremely high levels of exposure? In other words, is there an antimicrobial exposure threshold above which the association between consumption and resistance is attenuated?
In the case of macrolides, a number of ecological level studies have found evidence of saturation. For example, once the population level consumption of macrolides increases to approximately 700 defined daily doses per 1000 population per year, the prevalence of macrolide resistance in
Treponema pallidum increases rapidly from less than 10% to over 90% with no evidence of a further increase [
10]. Similar positive associations, although with not as clear evidence for saturation, have been described for macrolides in other species such as
Streptococcus pneumoniae, Mycoplasma genitalium and
Helicobacter pylori [
10,
11,
12,
13,
14,
15,
16,
17].
It is plausible that the individual-level association between macrolide consumption and resistance may be lost in very high consumption populations for a number of reasons as outlined in
Figure 1. Such a saturation effect could have important consequences as it may mean that one may miss the association between antimicrobial use (AMU) and AMR in high consumption populations. This question is particularly prescient in the current era, where a number of countries are considering the roll out of doxycycline post exposure prophylaxis in men who have sex with men (MSM) to reduce the incidence of certain STIs [
18,
19]. This decision has been based on randomized controlled trials showing evidence of reduced STI incidence and little or no risk of AMR emerging [
18,
19]. These trials have, however, been performed in MSM in HIV PrEP cohorts with high consumption of antimicrobials.
In previous studies, we have found that antimicrobial consumption in PrEP cohorts exceeds resistance inducing thresholds for various bacteria-antimicrobial combinations by up to 7-fold [
20,
21]. We have also found evidence compatible with antimicrobial saturation in these cohorts. For example, in one study, we found higher azithromycin, ciprofloxacin and ceftriaxone minimum inhibitory concentrations (MICs) of oral commensal
Neisseria species in MSM on PrEP than the Belgian general population, but no difference between MSM who had and who had not taken antimicrobials in the past 6-months [
22]. We also found that MSM on PrEP had a higher abundance of genes conferring resistance to macrolides, beta-lactams and fluoroquinolones than the general population. However, once again, we found no difference between the MSM who had and who had not taken antimicrobials in the prior 6 months [
23].
The association between AMU and AMR in these three groups of individuals were only tested at the group level, which may obscure individual level associations between the consumption of a particular antimicrobial and resistance to that antimicrobial. The objective of this paper was, therefore, to conduct a secondary data analysis of these three groups to assess if there was an individual level association between the consumption of 4 classes of antimicrobials (macrolides, fluoroquinolones, tetracyclines and beta-lactams) and gen- phenotypic markers of AMR.
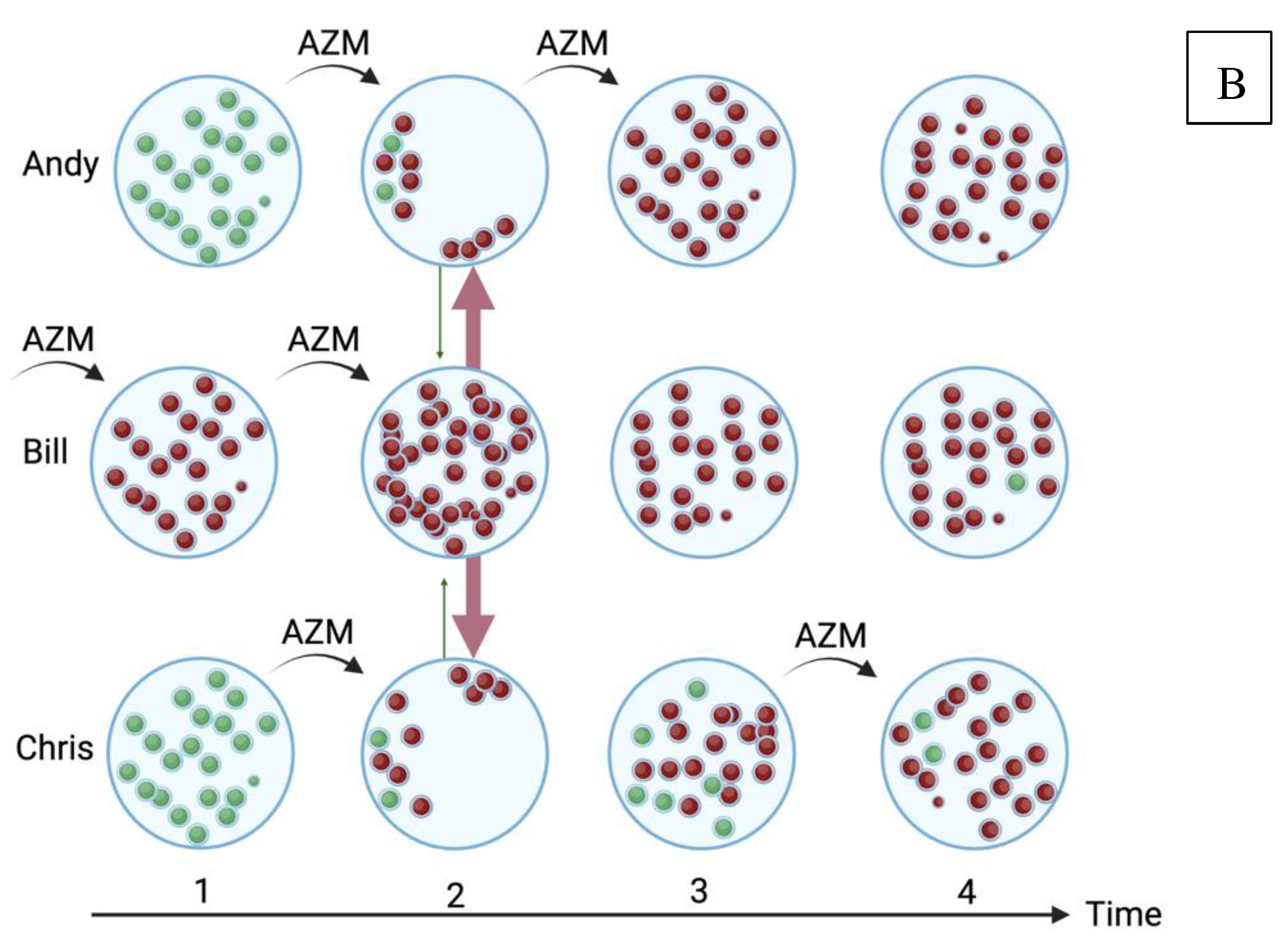
Figure 1.
A schematic illustration of how high antimicrobial consumption in a population could obscure the association between azithromycin (AZM) consumption and azithromycin resistance in oral streptococci in 3 individuals, Andy, Bill and Chris. In the low azithromycin consumption population (A), all the streptococci are susceptible (green) to azithromycin at baseline. Bill takes azithromycin at time point 1, which has the twin effect of selecting for resistant streptococci (red) and reducing the abundance of susceptible streptococci [
24]. This niche can be refilled by the growth of endogenous streptococci but also via transmission from Andy and Chris [
24]. If the resistant streptococci have a fitness cost, then the susceptible streptococci should replace the resistant isolates. Azithromycin given at the third time point to any individual would still have a detectable effect on resistance. In the high consumption population (B), Andy, Bill and Chris take azithromycin at time point one, which has the same effect, with the notable difference that due to Bill’s preceding azithromycin exposure the main effect of the azithromycin is to increase the absolute number of resistant streptococci in his oropharynx [
24]. This in turn, increases the probability that transmission of his resistant streptococci to Andy and Bill will take place to fill their oral niches that have been depopulated by azithromycin (red arrows). Continuing exposure to azithromycin leads to all three individuals being colonized predominantly by azithromycin resistant streptococci. In this setting, from timepoint 3 onwards, there would be a high probability of finding no association between azithromycin consumption and resistance. (For a review of all the 5 population-level pathways that link antimicrobial exposure to AMR, please see [
24]; Created with BioRender.com).
Figure 1.
A schematic illustration of how high antimicrobial consumption in a population could obscure the association between azithromycin (AZM) consumption and azithromycin resistance in oral streptococci in 3 individuals, Andy, Bill and Chris. In the low azithromycin consumption population (A), all the streptococci are susceptible (green) to azithromycin at baseline. Bill takes azithromycin at time point 1, which has the twin effect of selecting for resistant streptococci (red) and reducing the abundance of susceptible streptococci [
24]. This niche can be refilled by the growth of endogenous streptococci but also via transmission from Andy and Chris [
24]. If the resistant streptococci have a fitness cost, then the susceptible streptococci should replace the resistant isolates. Azithromycin given at the third time point to any individual would still have a detectable effect on resistance. In the high consumption population (B), Andy, Bill and Chris take azithromycin at time point one, which has the same effect, with the notable difference that due to Bill’s preceding azithromycin exposure the main effect of the azithromycin is to increase the absolute number of resistant streptococci in his oropharynx [
24]. This in turn, increases the probability that transmission of his resistant streptococci to Andy and Bill will take place to fill their oral niches that have been depopulated by azithromycin (red arrows). Continuing exposure to azithromycin leads to all three individuals being colonized predominantly by azithromycin resistant streptococci. In this setting, from timepoint 3 onwards, there would be a high probability of finding no association between azithromycin consumption and resistance. (For a review of all the 5 population-level pathways that link antimicrobial exposure to AMR, please see [
24]; Created with BioRender.com).

Methods
Study population
The study population consisted of a cross sectional sample of three groups of 32 individuals: one group from the general population and two groups from MSM attending the HIV preexposure prophylaxis (PrEP) clinic of the Institute of Tropical Medicine (ITM), Antwerp. The 64 MSM were included at the baseline visit of the PReGo study in 2019–2020. PReGo was a randomized placebo controlled trial that assessed the efficacy of an antiseptic mouthwash to prevent STIs among higher risk MSM [
25]. One of the inclusion criteria for PReGo was a documented infection with gonorrhoea, chlamydia or syphilis in the preceding 24 months. Thus, they had consumed at least one course of antimicrobial in the preceding 2 years. The first PReGo participants were enrolled into two groups, depending on if they had (group 1), or had not (group 2) consumed any antimicrobials in the preceding 6 months. Group 3 (general population) was comprised of ITM employees (male or female) who had not used antimicrobials in the preceding 6 months. This group was recruited by posters in June 2020. The first 32 eligible employees who approached the study team were included in this survey.
Data and samples
In brief, an oropharyngeal swab was taken by applying a dry regular flocked swab (COPAN, Brescia, Italy) to both tonsillar pillars and the posterior oropharynx. These were then placed in a cooled transport box and processed as detailed elsewhere [
22].
Taxonomic and resistome characterisation
After read trimmingand filtering of low-quality reads using trimmomatic (v0.39), human reads were removed by mapping the reads against the human reference genome (GRCh38, accession GCF_000001405.26) using Burrows-Wheeler Alignment with default parameters [
26,
27]. Samples with less than 5,500 non-human reads were discarded in order to avoid issues relating to the variation in sequencing depth. The abundance of antimicrobial resistance genes (ARGs) was estimated using MEGARes 2.0 database and BWA-MEM with default settings [
28]. ResistomeAnalyzer (
https://github.com/cdeanj/resistomeanalyzer) was used to classify ARGs with a gene fraction greater than 80% into types, classes, and gene groups for further analyses. ARGs were normalised by the number of bacterial reads per sample (as estimated by Bracken) and multiplied by 10
6 in order to obtain reads per million (RPM). Single nucleotide polymorphisms were not considered for further analysis [
29,
30].
Culture and antimicrobial susceptibility determination of Neisseria species
The ESwabs™ were inoculated onto Modified Thayer-Martin Agar and incubated at 37oC and 5% carbon dioxide. After 48 hours of incubation, Gram negative, oxidase positive colonies were selected, enriched and stored in skim milk at − 80oC.
Isolates were identified to the species level using Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight mass spectrometry (MALDI-TOF MS), on a MALDI Biotyper
® Sirius IVD system using the MBT Compass IVD software and library (Bruker Daltonics, Bremen, Germany). For further details, please see Laumen et al. [
22]. Isolates identified as
N. perflava and
N. flavescens were grouped into one category with
N. subflava [
31].
Minimum inhibitory concentrations (MICs) of Neisseria species to azithromycin, ceftriaxone, and ciprofloxacin were determined on GC agar plates using ETEST® (bioMérieux Marcy-l'Étoile, France) incubated for 24 h at 37 oC and 5% CO2.
N. subflava was detected in 58/96 (60.4%) individuals. The other Neisseria species were detected in less than 15% of individuals and therefore not used for further analyses (N. mucosa (14/96, 14.6%), N. oralis (8/96, 8.3%), N. cinerea (3/96, 3.1%), N. elongata (3/96, 3.1%), N. lactamica (2/96, 2.1%), and N. bacilliformis (1/96, 1.0%)). N. subflava has a number of colony morphologies, and thus in a number of individuals, more than one colony of this species was characterized. For these individuals, we used the median MICs for all isolates for further analyses.
Ethics
This study was approved by ITM’s Institutional Review Board (1276/18 and 1351/20) and the Ethics Committee of the University of Antwerp (19/06/058 and AB/ac/003).
Data analysis
Antimicrobial Resistance Gene (ARG) abundance:
Because of the large number of samples with zero-reads for each class of antimicrobials, we used zero-inflated negative binomial regression models to assess associations between ARG abundance and antimicrobial exposure. The outcome variable was the total count of ARGs per class of antimicrobial, per sample.Study group (coded as a categorical variable - 1,2 or 3) and the logarithm of the count of bacterial reads were included as explanatory and offset variables, respectively.
Antimicrobial susceptibility of N. subflava:
Linear regression was used to assess the association between the azithromycin, ciprofloxacin and ceftriaxone MICs of N. subflava (log transformed) and the consumption of macrolides, fluoroquinolones or ceftriaxone (binary variable) adjusting for the study group (coded 1,2 or 3).
For both these analyses, sensitivity analyses were performed, controlling for time since the last ingestion of the relevant antimicrobial (days) and limiting the study to the two MSM groups.
Statistical analyses and data visualization were conducted using Stata MP V16.1. A p-value <0.05 was considered statistically significant.
Results
All the PrEP participants and 10 of the general population (31.3%) were men. In the 32 PrEP participants who had used antimicrobials in the prior 6 months, 25 (78.1%), 19 (59.4%), 8 (25%) and 2 (6.3%) had used cephalosporins, macrolides, tetracyclines, or fluoroquinolones, respectively (
Table 1).
Antimicrobial susceptibility of N. subflava
In the 58 individuals with
N. subflava identified, the median azithromycin MIC was 2.9 µg/mL (IQR 1.6-4; range 0.125- >256µg/mL), the median ceftriaxone MIC was 0.032 µg/mL (IQR 0.023-0.05; range 0.008- 0.5 µg/mL) and the median ciprofloxacin MIC 0.032 µg/mL (IQR 0.01-0.198; range 0.003- 0.875µg/mL;
Table 1). Using EUCAST definitions of AMR for
N. gonorrhoeae, the prevalence of resistance to azithromycin, ceftriaxone, and ciprofloxacin was 48/58 (82.8%), 4/58 (6.9%) and 24/58 (41.4%), respectively.
Abundance of resistance associated genes
The median abundance of Macrolides, lincosamides and streptogramines (MLS), beta-lactam, fluoroquinolone and tetracycline resistance associated genes was 43.6 RPM (IQR 0-293.9), 0 RPM (IQR 0-39.3), 0 RPM (IQR 0-69.7) and 0 RPM (IQR 0-204.6), respectively (
Table 1).
Table 1.
Demographics, antimicrobial read abundance and MICs of Neisseria subflava in the three study populations.
Table 1.
Demographics, antimicrobial read abundance and MICs of Neisseria subflava in the three study populations.
| |
MSM ABs (Median (IQR)) |
MSM no ABs |
General Population |
| Men |
32 (100) |
32 (100) |
10 (31.3) |
| Age Categories (N persons per category (%) |
|
|
|
| 20–29 |
5 (15.6%) |
4 (12.5%) |
5 (15.6%) |
| 30–39 |
16 (50.0%) |
10 (31.3%) |
9 (28.1%) |
| 40–49 |
6 (18.8%) |
8 (25.0%) |
9 (28.1%) |
| 50–59 |
4 (12.5%) |
7 (21.9%) |
8 (25.0%) |
| 60–69 |
1 (3.1%) |
2 (6.3%) |
1 (3.1%) |
| 70–79 |
0 (0%) |
1 (3.1%) |
0 (0%) |
| Antibiotic exposure in the previous 6 months, n (%) |
|
|
|
| B-lactams |
25 (78.1%) |
|
|
| Macrolides |
19 (59.4%) |
|
|
| Fluoroquinolones |
2 (6.3%) |
|
|
| Others |
8 (25.0%) |
|
|
|
N. subflava median MIC (ug/mL) |
|
|
|
| Ceftriaxone |
0.035 (0.023-0.047) |
0.032 (0.023-0.06) |
0.036 (0.027-0.056) |
| Azithromycin |
3 (1.75-4) |
1.75 (0.38-6) |
3 (2-4) |
| Ciprofloxacin |
0.03 (0.01-0.38) |
0.02 (0.01-0.192) |
0.04 (0.02-0.19) |
| Antimicrobial read abundance (normalized read count) |
|
|
|
| Macrolides |
99 (0-539) |
0 (0-351) |
52 (0-167) |
| Betalactams |
0 (0-48) |
0 (0-65) |
0 (0-66) |
| Fluoroquinolones |
0 (0-83) |
0 (0-101) |
0 (0-63) |
| Tetracyclines |
0 (0-527) |
0 (0-415) |
0 (0-134) |
Association AMU/susceptibility in N. subflava
No association was found between the consumption of macrolides and azithromycin MICs for
N. subflava (
Table 2). The same was true for beta-lactam consumption and ceftriaxone MICs. Both the individuals who had consumed fluoroquinolones did not have
N. subflava cultured from their oropharynx and the association could therefore not be assessed.
Association AMU/gene abundance
There was no association between the consumption of macrolides, betalactams, fluoroquinolones and tetracyclines and the abundance of resistance associated genes to the corresponding class of antimicrobials (
Table 3).
The following variables were controlled for in these analyses: study group (1,2 or 3), the abundance of bacterial reads.
Sensitivity analyses, including the time since ingestion of antimicrobials and restricted to the two groups of MSM, produced similar results for both ARG abundance and antimicrobial susceptibility (S
Table 1 and
Table 2).
Discussion
We found no association between the use of antimicrobials in the prior 6 months and geno- and phenotypic markers of resistance in our study population. There are a number of possible explanations for our findings.
We may have missed an association between AMR and AMU.
Our sample sizes were small, and our look back period on antimicrobial use (AMU) was only 6 months. Larger sample sizes and longer look back periods may have revealed a positive association. The longer look back period may be particularly important for antimicrobials such as azithromycin, whose effect on pheno- and genotypic resistance can last for up to 6 months [
32,
33,
34]. The abundance of bacteria and hence ARGs is higher in the gut than in the oropharynx. Although studies have typically found that the effect of antimicrobials on the resistome is similar in these two sites [
33], it is possible that our results may have differed if we included the gut resistome. In a similar vein, we only included one species,
Neisseria subflava, as our indicator organism for phenotypic resistance. Furthermore, we used the median MICs of the one to three
N. subflava colonies isolated per individual. Alternative strategies would include testing a broader range of bacterial species or measuring the proportion of each type of bacteria resistant to each antimicrobial. To characterize azithromycin susceptibility of commensal
Neisseria spp., this method could use selective agar plates with 2 µg/ml azithromycin and without azithromycin to provide the proportion of all commensal
Neisseria spp. with resistance at this threshold. By assessing the antimicrobial susceptibilities of closer to 100 colonies than 1 colony, this method may be more likely to provide a more representative view of the susceptibility of the target bacteria. In addition, our sequencing depth may not have been deep enough to provide an optimal resolution of bacterial reads. This limitation could have been influenced by the abundance of human reads in samples. Additionally, the exclusion of SNP confirmation or the insufficiency of our ARG bioinformatics pipeline may have hindered the ability to accurately detect differences in ARGs. As a secondary analysis, we assessed for associations between AMU and relevant individual resistance genes but found no associations (data not shown).
There may be no association between AMU and AMR in this population
As mentioned above, a number of studies have found that our PrEP cohort is exposed to high levels of antimicrobials with consequently high levels of AMR [
10,
20]. Previous analyses found that both pheno- and genotypic resistance were more prevalent in the two populations of MSM than in the general population, but there was no difference between the two groups of MSM [
22,
23]. In a recent randomized controlled trial of ceftriaxone versus ceftriaxone plus azithromycin 2g PO for infection with
N. gonorrhoeae in this same PrEP cohort, we found that the additional azithromycin had no detectable effect on the abundance of macrolide resistance genes in the gut or the prevalence of azithromycin resistant oral streptococci or commensal
Neisseria spp [
35]. A plausible explanation for these findings was the high levels of macrolide resistance found in the baseline specimens - 100%/92.5% had azithromycin resistant streptococci/
Neisseria spp.
These findings would imply that a degree of AMR saturation has been attained in this population, whereby additional antimicrobial exposure has little detectable effect on AMR. If this were to be the case, this would be an important finding for a number of reasons. Firstly, it would mean we need to be careful about using these populations to evaluate the relationship between AMU and AMR. A contemporaneous example here would be the studies that have concluded that doxycycline PEP is safe as it does not result in much appreciable AMR in MSM PrEP cohorts [
18,
19]. Secondly, it suggests the need for enhanced antimicrobial stewardship in these populations to bring AMU down to safer limits. Of note, excessive AMU has been linked not only to AMR but also to reductions in microbial diversity with the associated adverse clinical outcomes [
22,
23,
36].
The association between AMU and AMR is complex, with pathways operating at population, individual human and lower levels [
24,
37]. One of these pathways is the transmission of resistance between individuals, particularly in populations with dense sexual networks, such as PrEP cohorts [
38]. Another pathway is the cross-selection of resistance. Baquero et al., for example, have found evidence that the consumption of macrolides is a more important driver of penicillin resistance in
Streptococcus pneumoniae than beta-lactam consumption [
39]. We were unable to evaluate these factors.
Despite the numerous limitations of our study, our findings serve as a reminder to include populations with low AMU in studies evaluating the risk of AMR in interventions involving antimicrobials. As noted above, a number of countries are considering the roll out of doxycycline PEP in MSM to reduce the incidence of certain STIs [
18,
19,
40]. The most recent doxycycline PEP RCTs performed in MSM have found little or no risk of AMR emerging [
18,
19]. These trials have, however, been performed in MSM in HIV PrEP cohorts with high consumption of antimicrobials. It may be prudent to repeat these studies in populations with lower consumption of antimicrobials before concluding that the risk of inducing AMR is low. Of note in this regard is an RCT of minocycline PEP from 1979 in a group of sailors in the US navy on shore leave. This population presumably had a low antimicrobial consumption and unlike the more recent studies in cohorts of MSM this study found that the use of minocycline was associated with selecting for tetracycline resistance in
N. gonorrhoeae [
41].
If other studies were to confirm the existence of such a saturation effect, then it would be useful to assess the optimal methods to measure the effects of excessive antimicrobial exposure and establish safe thresholds of antimicrobial exposure. These could be measured at individual and population levels. One option would be to perform surveillance of oral commensal
Neisseria and Streptococcal species in key populations such as those taking PrEP [
42,
43,
44]. Once resistance exceeds particular thresholds antimicrobial stewardship interventions could then be introduced.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., STables 1 and 2.
Funding
This study was funded by the Belgian Research Foundation - Flanders (FWO 121.00). The funder had no role in the design and conduct of the study nor the decision to prepare and submit the manuscript for publication.
Authors Contributions
Conceptualization, Thibaut Vanbaelen and Chris Kenyon; Methodology, Jolein Laument, Tessa De Block, Christophe Van Dijck and Sheeba Santhini Manoharan-Basil; Software, Chris Kenyon; Formal analysis, Chris Kenyon; Writing – original draft, Chris Kenyon; Writing – review & editing, Thibaut Vanbaelen and Tessa De Block; Visualization, Chris Kenyon. All authors read, contributed to and approved the final draft.
Consent for publication
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- J. M. Bahn, H. Ackerman, and C. M. Carpenter. Development in vitro of Penicillin-Resistant Strains of the Gonococcus. Proceedings of the Society for Experimental Biology and Medicine 1945, 58, 21–24. [Google Scholar] [CrossRef]
- C. M. Carpenter, J. M. C. M. Carpenter, J. M. Bahn, and et al., "Adaptability of gonococcus to four bacteriostatic agents, sodium sulfathiazole, rivanol lactate, promin, and penicillin," Proc Soc Exp Biol Med, vol. 60, pp. 168-71, Oct 1945. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/21004069.
- B. G. Bell, F. B. G. Bell, F. Schellevis, E. Stobberingh, H. Goossens, and M. Pringle, "A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance," BMC Infect Dis, vol. 14, p. 13, Jan 9 2014. [CrossRef]
- R. Bruyndonckx, N. R. Bruyndonckx, N. Hens, M. Aerts, H. Goossens, J. Cortinas Abrahantes, and S. Coenen, "Exploring the association between resistance and outpatient antibiotic use expressed as DDDs or packages," J Antimicrob Chemother, vol. 70, no. 4, pp. 1241-4, Apr 2015. [CrossRef]
- C. Silva, P. J. Nogueira, and J.-A. Paiva. Determinants of antimicrobial resistance among the different European countries: more than human and animal antimicrobial consumption. Antibiotics 2021, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- S. Bungau, D. M. S. Bungau, D. M. Tit, T. Behl, L. Aleya, and D. C. Zaha, "Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents," Current Opinion in Environmental Science & Health 2021, 19, 100224. ,.
- M. Cižman and T. P. Srovin, "Antibiotic consumption and resistance of gram-negative pathogens (collateral damage)," GMS infectious diseases, vol. 6, 2018.
- L. Baditoiu et al., "Intensive care antibiotic consumption and resistance patterns: a cross-correlation analysis," Annals of Clinical Microbiology and Antimicrobials 2017, 16, 1-10. ,.
- C.-C. Lai et al., "Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009," Journal of antimicrobial chemotherapy 2011, 66, 1374-1382, ,.
- C. Kenyon, S. S. C. Kenyon, S. S. Manoharan-Basil, and C. Van Dijck, "Is there a resistance threshold for macrolide consumption? Positive evidence from an ecological analysis of resistance data from streptococcus pneumoniae, treponema pallidum, and mycoplasma genitalium," Microbial Drug Resistance, 2021.
- F. Baquero, G. F. Baquero, G. Baquero-Artigao, R. Cantón, and C. García-Rey, "Antibiotic consumption and resistance selection in Streptococcus pneumoniae," Journal of Antimicrobial Chemotherapy 2002, 50, 27-38. ,.
- R. Bruyndonckx, N. R. Bruyndonckx, N. Hens, M. Aerts, H. Goossens, J. Cortiñas Abrahantes, and S. Coenen, "Exploring the association between resistance and outpatient antibiotic use expressed as DDDs or packages," Journal of Antimicrobial Chemotherapy 2015, 70, 1241-1244.
- H. Goossens, M. H. Goossens, M. Ferech, R. Vander Stichele, and M. Elseviers, "Outpatient antibiotic use in Europe and association with resistance: a cross-national database study," The Lancet 2005, 365, 579-587.
- D. A. Machalek et al., "Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis," Lancet Infect Dis, Jul 2 2020. [CrossRef]
- H. Seppala, "The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland: Finnish Study Group for Antimicrobial Resistance. Engl. J. Med. 1997, 337, 441–446.
- M. Bergman, S. M. Bergman, S. Huikko, P. Huovinen, P. Paakkari, H. Seppälä, and F. S. G. f. A. R. †, "Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae," Antimicrobial agents and chemotherapy 2006, 50, 3646-3650.
- M. Čižman, M. M. Čižman, M. Pokorn, K. Seme, A. Oražem, and M. Paragi, "The relationship between trends in macrolide use and resistance to macrolides of common respiratory pathogens," Journal of Antimicrobial Chemotherapy 2001, 47, 475-477.
- J.-M. Molina et al., "Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial," The Lancet Infectious Diseases 2018, 18, 308-317. ,.
- F. Luetkemeyer et al., "Postexposure doxycycline to prevent bacterial sexually transmitted infections," New England Journal of Medicine 2023, 388, 1296-1306. ,.
- T. Vanbaelen et al., "Screening for STIs is one of the main drivers of macrolide consumption in PrEP users," International journal of STD & AIDS, p. 09564624211025940, 2021.
- C. Kenyon, "Dual azithromycin/ceftriaxone therapy for gonorrhoea in PrEP cohorts results in levels of macrolide consumption that exceed resistance thresholds by up to 7-fold," J Infect Dis, Apr 2 2021. [CrossRef]
- J. G. E. Laumen et al., "Antimicrobial susceptibility of commensal Neisseria in the general population and men who have sex with men in Belgium," Scientific Reports, 2022. [CrossRef]
- C. Van Dijck et al., "The oropharynx of men using HIV pre-exposure prophylaxis is enriched with antibiotic resistance genes: A cross-sectional observational metagenomic study," Journal of Infection, vol. 86, no. 4, pp. 329-337, 2023.
- M. Lipsitch and M. H. Samore, "Antimicrobial use and antimicrobial resistance: a population perspective," Emerg Infect Dis, vol. 8, no. 4, pp. 347-54, Apr 2002. [CrossRef]
- C. Van Dijck et al., "Antibacterial mouthwash to prevent sexually transmitted infections in men who have sex with men taking HIV pre-exposure prophylaxis (PReGo): a randomised, placebo-controlled, crossover trial," The Lancet Infectious Diseases, vol. 21, no. 5, pp. 657-667, 2021.
- M. Bolger, M. M. Bolger, M. Lohse, and B. Usadel, "Trimmomatic: a flexible trimmer for Illumina sequence data," Bioinformatics, vol. 30, no. 15, pp. 2114-20, Aug 1 2014. [CrossRef]
- H. Li and R. Durbin, "Fast and accurate short read alignment with Burrows–Wheeler transform," bioinformatics, vol. 25, no. 14, pp. 1754-1760, 2009.
- E. Doster et al., "MEGARes 2.0: a database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data," Nucleic acids research, vol. 48, no. D1, pp. D561-D569, 2020.
- J. Lu, F. P. J. Lu, F. P. Breitwieser, P. Thielen, and S. L. Salzberg, "Bracken: estimating species abundance in metagenomics data," PeerJ Computer Science, vol. 3, p. e104, 2017.
- B. Slizovskiy, K. B. Slizovskiy, K. Mukherjee, C. J. Dean, C. Boucher, and N. R. Noyes, "Mobilization of antibiotic resistance: are current approaches for colocalizing resistomes and mobilomes useful?," Frontiers in Microbiology, vol. 11, p. 1376, 2020.
- S. Bennett et al., "A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria," Microbiology, vol. 158, no. Pt 6, p. 1570, 2012.
- S. Malhotra-Kumar, C. S. Malhotra-Kumar, C. Lammens, S. Coenen, K. Van Herck, and H. Goossens, "Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study," The Lancet, vol. 369, no. 9560, pp. 482-490, 2007.
- H. E. Jakobsson, C. H. E. Jakobsson, C. Jernberg, A. F. Andersson, M. Sjolund-Karlsson, J. K. Jansson, and L. Engstrand, "Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome," PLoS One, vol. 5, no. 3, p. e9836, 2010. [CrossRef]
- M. Choo et al., "Impact of long-term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non-cystic fibrosis bronchiectasis," Msphere, vol. 3, no. 2, pp. e00103-18, 2018.
- T. Vanbaelen et al., "Effect on the resistome of dual- vs monotherapy for the treatment of Neisseria gonorrhoeae: results from a randomized controlled trial (ResistAZM Trial)," Open Forum Infectious Diseases, 2023. [CrossRef]
- Z. Aversa et al., "Association of Infant Antibiotic Exposure With Childhood Health Outcomes," Mayo Clin Proc, vol. 96, no. 1, pp. 66-77, Jan 2021. [CrossRef]
- F. Baquero, A. P. F. Baquero, A. P. Tedim, and T. M. Coque, "Antibiotic resistance shaping multi-level population biology of bacteria," (in eng), Front Microbiol, vol. 4, p. 15, 2013. [CrossRef]
- J. C. Kwong et al., "Whole-genome sequencing reveals transmission of gonococcal antibiotic resistance among men who have sex with men: an observational study," Sexually transmitted infections, vol. 94, no. 2, pp. 151-157, 2018.
- C. Garcia-Rey, L. C. Garcia-Rey, L. Aguilar, F. Baquero, J. Casal, and R. Dal-Re, "Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae," J Clin Microbiol, vol. 40, no. 1, pp. 159-64, Jan 2002. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/11773111.
- Mårdh and, D. Plachouras, "Using doxycycline for prophylaxis of bacterial sexually transmitted infections: considerations for the European Union and European Economic Area," Eurosurveillance, vol. 28, no. 46, p. 2300621, 2023.
- W. O. Harrison et al., "A trial of minocycline given after exposure to prevent gonorrhea," New England Journal of Medicine, vol. 300, no. 19, pp. 1074-1078, 1979.
- Goytia and C., B. Wadsworth, "Canary in the Coal Mine: How Resistance Surveillance in Commensals Could Help Curb the Spread of AMR in Pathogenic Neisseria," Mbio, vol. 13, no. 5, pp. e01991-22, 2022.
- A. Fiore, J. C. A. Fiore, J. C. Raisman, N. R. H. Wong, A. O. Hudson, and C. B. Wadsworth, "Exploration of the Neisseria Resistome Reveals Resistance Mechanisms in Commensals That May Be Acquired by N. gonorrhoeae through Horizontal Gene Transfer," (in English), Antibiotics-Basel, vol. 9, no. 10, Oct 2020. [CrossRef]
- C. B. Wadsworth, B. J. C. B. Wadsworth, B. J. Arnold, M. R. A. Sater, and Y. H. Grad, "Azithromycin Resistance through Interspecific Acquisition of an Epistasis-Dependent Efflux Pump Component and Transcriptional Regulator in Neisseria gonorrhoeae," (in English), Mbio, vol. 9, no. 4, Jul-Aug 2018. [CrossRef]
Table 2.
Linear regression of association between Neisseria subflava MICs for azithromycin and ceftriaxone (log values) and consumption of macrolides and beta-lactams, respectively.
Table 2.
Linear regression of association between Neisseria subflava MICs for azithromycin and ceftriaxone (log values) and consumption of macrolides and beta-lactams, respectively.
| |
Coef. (95% CI) |
P-value |
| Azithromycin/macrolides |
0.19 (-1.06- 1.45) |
0.759 |
| Ceftriaxone/betalactams |
-0.03 (-0.66- 0.37) |
0.926 |
Table 3.
Zero inflated negative binomial regression of association between the abundance of macrolide, beta-lactam, fluoroquinolone and tetracycline resistance associated genes and the consumption of these classes of antimicrobials.
Table 3.
Zero inflated negative binomial regression of association between the abundance of macrolide, beta-lactam, fluoroquinolone and tetracycline resistance associated genes and the consumption of these classes of antimicrobials.
| Antimicrobial consumption/gene abundance |
Coef. (95% CI) |
P-value |
| Azithromycin/macrolides |
0.23 (-0.30- 0.77) |
0.393 |
| Ceftriaxone/betalactams |
-0.03 (-0.66- 0.37) |
0.926 |
| Fluoroquinolone/fluoroquinolone |
-0.35 (-1.67 -0.96) |
0.598 |
| Tetracycline/tetracycline |
0.03 (-0.60- 0.67) |
0.916 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







