Submitted:
24 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Epileptic (EEs) and Developmental Encephalopathies (DEs)
1.2. DEEs
| DEE | Syndrome or Known Name | Phenotype MIM Number |
Genes | Genetic Loci |
|---|---|---|---|---|
| DEE1 | LISX2; XLAG;Hydranencephaly with abnormal genitalia | 300215 | ARX | Xp21.3 |
| Proud syndrome | 300004 | ARX | Xp21.3 | |
| PRTS; MRX36; MRXS1 | 309510 | ARX | Xp21.3 | |
| XLID29; MRX29;MRX32MRX33;MRX29;MRX38;MRX43;MRX52;MRX54;MRX76; MRX87 | 300419 | ARX | Xp21.3 | |
| DEE2 | EIEE2; ISSX2 | 300672 | CDKL5 | Xp22.13 |
| DEE3 | EIEE3 | 609302 | SLC25A22 | 11p15 |
| DEE4 | EIEE4 | 612164 | STXBP1 | 9q34 |
| DEE5 | EIEE5 | 613477 | SPTAN1 | 9q34 |
| DEE6A | EIEE6; SMEI;DS | 607208 | SCN1A | 2q24.3 |
| DEE6B | Phenotypes caused by heterozygous mutation in the SCN1A gene, not DS | 619317 | SCN1A | 2q24.3 |
| DEE7 | EIEE7 | 613720 | KCNQ2 | 20q13 |
| DEE8 | EIEE8; Hyperkplexia and epilepsy | 300607 | ARHGEF9 | Xq11.1 |
| DEE9 | EIEE9; EFMR; Juberg-Hellman syndrome | 300088 | PCDH19 | Xq22.1 |
| DEE10 | EIEE10; MCSZ | 613402 | PNKP | 19q13 |
| DEE11 | EIEE11 | 613721 | SCN2A | 2q24 |
| DEE12 | EIEE12 | 613722 | PLCB1 | 20p12.3 |
| DEE13 | EIEE13 | 614558 | SCN8A | 12q13 |
| DEE14 | EIEE14 | 614959 | KCNT1 | 9q34 |
| DEE15 | EIEE15 | 615006 | ST3GAL3 | 1p34 |
| DEE16 | EIEE16 | 615338 | TBC1D24 | 16p13 |
| DEE17 | EIEE17 | 615473 | GNAO1 | 16q13 |
| DEE18 | EIEE18 | 615476 | SZT2 | 1p34 |
| DEE19 | EIEE19 | 615744 | GABRA1 | 5q34 |
| DEE20 | EIEE20; GPIBD4MCAHS2 | 300868 | PIGA | Xp22 |
| DEE21 | EIEE21 | 615833 | NECAP1 | 12p13 |
| DEE22 | EIEE22; CDGIIm | 300896 | SLC35A2 | Xp11 |
| DEE23 | EIEE23 | 615859 | DOCK7 | 1p31 |
| DEE24 | EIEE24 | 615871 | HCN1 | 5p12 |
| DEE25 | EIEE25 | 615905 | SLC13A5 | 17p13 |
| DEE26 | EIEE26 | 616056 | KCNB1 | 20q13 |
| DEE27 | EIEE27 | 616139 | GRIN2B | 12p12 |
| DEE28 | EIEE28 | 616211 | WWOX | 16q23 |
| DEE29 | EIEE29 | 616339 | AARS | 16q22 |
| DEE30 | EIEE30 | 616341 | SIK1 | 21q22 |
| DEE31A | EIEE31 | 616346 | DNM1 | 9q34 |
| DEE31B | - | 620352 | DNM1 | 9q34 |
| DEE32 | EIEE32 | 616366 | KCNA2 | 1p13 |
| DEE33 | EIEE33 | 616409 | EEF1A2 | 20q13 |
| DEE34 | EIEE34 | 616645 | SLC12A5 | 20q12 |
| DEE35 | EIEE35 | 616647 | ITPA | 20p13 |
| DEE36 | EIEE36; CDG1s | 300884 | ALG13 | Xq23 |
| DEE37 | EIEE37 | 616981 | FRRS1L | 9q31 |
| DEE38 | EIEE38; GPIBD23 | 617020 | ARV1 | 1q42 |
| DEE39 | EIEE39; AGC1 DEFICIENCY | 612949 | SLC25A12 | 2q31 |
| DEE40 | EIEE40 | 617065 | GUF1 | 4p12 |
| DEE41 | EIEE41 | 617105 | SLC1A2 | 11p13 |
| DEE42 | EIEE42 | 617106 | CACNA1A | 19p13 |
| DEE43 | EIEE43 | 617113 | GABRB3 | 15q11 |
| DEE44 | EIEE44 | 617132 | UBA5 | 3q22 |
| DEE45 | EIEE45 | 617153 | GABRB1 | 4p13 |
| DEE46 | EIEE46 | 617162 | GRIN2D | 19q13 |
| DEE47 | EIEE47 | 617166 | FGF12 | 3q28 |
| DEE48 | EIEE48 | 617276 | AP3B2 | 15q25 |
| DEE49 | EIEE49 | 617281 | DENND5A | 11p15 |
| DEE50 | EIEE50; CDG1Z | 616457 | CAD | 2p23 |
| DEE51 | EIEE51 | 617339 | MDH2 | 7q11 |
| DEE52 | EIEE52 | 617350 | SCN1B | 19q13 |
| DEE53 | EIEE53 | 617389 | SYNJ1 | 21q22 |
| DEE54 | EIEE54 | 617391 | HNRNPU | 1q44. |
| DEE55 | EIEE55; GPIBD14 | 617599 | PIGP | 21q22 |
| DEE56 | EIEE56 | 617665 | YWHAG | 7q11 |
| DEE57 | EIEE57 | 617771 | KCNT2 | 1q31 |
| DEE58 | EIEE58 | 617830 | NTRK2 | 9q21 |
| DEE59 | EIEE59 | 617904 | GABBR2 | 9q22. |
| DEE60 | EIEE60 | 617929 | CNPY3 | 6p21 |
| DEE61 | EIEE61 | 617933 | ADAM22 | 7q21 |
| DEE62 | EIEE62 | 617938 | SCN3A | 2q24 |
| DEE63 | EIEE63 | 617976 | CPLX1 | 4p16 |
| DEE64 | EIEE64 | 618004 | RHOBTB2 | 8p21 |
| DEE65 | EIEE65 | 618008 | CYFIP2 | 5q33 |
| DEE66 | EIEE66 | 618067 | PACS2 | 14q32 |
| DEE67 | EIEE67 | 618141 | CUX2 | 12q23 |
| DEE68 | EIEE68 | 618201 | TRAK1 | 3p25 |
| DEE69 | EIEE69 | 618285 | CACNA1E | 1q25 |
| DEE70 | EIEE70 | 618298 | PHACTR1 | 6p24 |
| DEE71 | EIEE71; Glutaminase deficiency with neonatal epileptic encephalopathy | 618328 | GLS | 2q32 |
| DEE72 | EIEE72 | 618374 | NEUROD2 | 17q12 |
| DEE73 | EIEE73 | 618379 | RNF13 | 3q25 |
| DEE74 | EIEE74 | 618396 | GABRG2 | 5q34 |
| DEE75 | EIEE75 | 618437 | PARS2 | 1p32 |
| DEE76 | EIEE76; DECAM | 618468 | ACTL6B | 7q22. |
| DEE77 | EIEE77; GPIBD19 | 618548 | PIGQ | 16p13 |
| DEE78 | EIEE78 | 618557 | GABRA2 | 4p13 |
| DEE79 | EIEE79 | 618559 | GABRA5 | 15q11 |
| DEE80 | EIEE80; GPIBD20 | 618580 | PIGB | 15q21. |
| DEE81 | EIEE81 | 618663 | DMXL2 | 15q21 |
| DEE82 | EIEE82; GOT2 deficiency | 618721 | GOT2 | 16q21 |
| DEE83 | EIEE83; Barakat-Perenthaler syndrome | 618744 | UGP2 | 2p14 |
| DEE84 | EIEE84; Jamuar syndrome | 618792 | UGDH | 4p14 |
| DEE85 | EIEE85 | 301044 | SMC1A | Xp11 |
| DEE86 | EIEE86 | 618910 | DALRD3 | 3p21 |
| DEE87 | EIEE87 | 618916 | CDK19 | 6q21. |
| DEE88 | EIEE88 | 618959 | MDH1 | 2p15 |
| DEE89 | - | 619124 | GAD1 | 2q31 |
| DEE90 | - | 301058 | FGF13 | Xq26 |
| DEE91 | IECEE1 | 617711 | PPP3CA | 4q24 |
| DEE92 | IECEE2 | 617829 | GABRB2 | 5q34 |
| DEE93 | - | 618012 | ATP6V1A | 3q13 |
| DEE94 | EEOC | 615369 | CHD2 | 15q26 |
| DEE95 | GPIBD18 | 618143 | PIGS | 17q11 |
| DEE96 | - | 619340 | NSF | 17q21 |
| DEE97 | - | 619561 | iCELF2 | 10p14. |
| DEE98 | - | 619605 | ATP1A2 | 1q23 |
| DEE99 | 619606 | ATP1A3 | 19q13. | |
| DEE100 | - | 619777 | FBXO28 | 1q42 |
| DEE101 | - | 619814 | GRIN1 | 9q34 |
| DEE102 | - | 619881 | SLC38A3 | 3p21 |
| DEE103 | - | 619913 | KCNC2 | 12q21 |
| DEE104 | - | 619970 | ATP6V0A1 | 17q21 |
| DEE105 | - | 619983 | HID1 | 17q25. |
| DEE106 | - | 620028 | UFSP2 | 4q35 |
| DEE107 | - | 620033 | NAPB | 20p11 |
| DEE108 | - | 620115 | MAST3 | 19p13 |
| DEE109 | - | 620145 | FZR1 | 19p13 |
| DEE110 | - | 620149 | CACNA2D1 | 7q21 |
| DEE111 | - | 62054 | DEPDC5 | 22q12.2-q12.3 |
| DEE112 | - | 620537 | KCNH5 | 14q23.2 |
2. Dravet Syndrome (DS)
2.1. Epidemiology
2.2. Clinical Presentation of DS
2.2.1. Neurodevelopment in DS Starts before the Age of Two Years and Continues to Worsen
2.2.2. Triggering Factors
2.2.3. Seizure Patterns
2.2.4. EEG Evolution in DS
2.2.5. Neuroimaging in DS
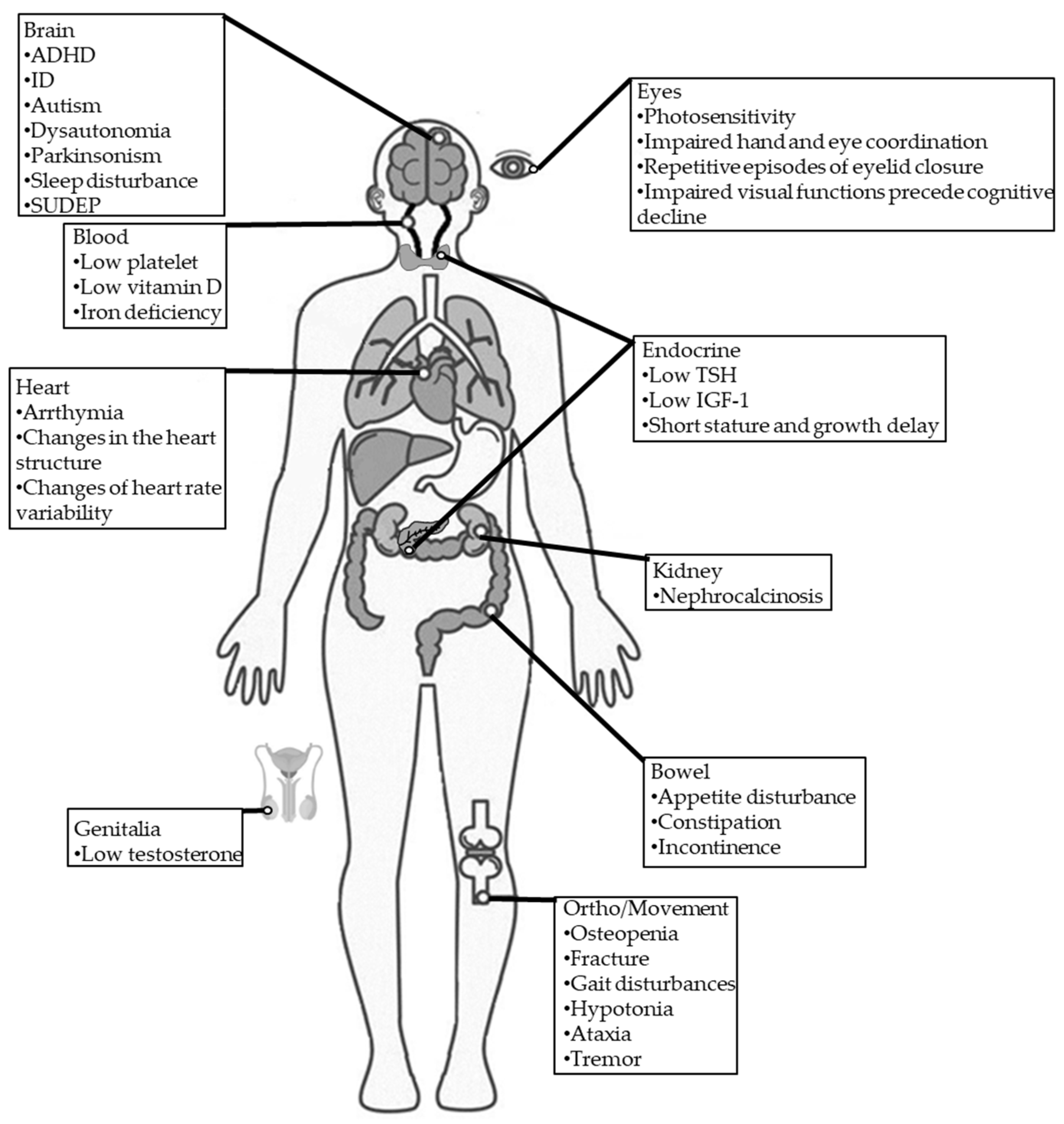
2.2.6. DS Comorbidities
Psychomotor
Blood
Cardiac
Digestive
Endocrine
Infection
Neurodegenerative
Orthopedic/Movement
Sleep
Eyes
2.2.7. SUDEP and Mortality
3. DS Diagnosis and Long-Term Prognosis
3.1. Diagnostic Criteria
- Seizure onset in a previously healthy infant within the first year of life [96].
- A history of febrile and afebrile seizures [97].
- Seizures resistant to conventional AEDs [98].
- Prolonged, recurrent febrile and afebrile seizures [97].
- Appearance of multiple seizure types, including febrile or afebrile generalized/unilateral clonic/tonic seizures, myoclonic, and atypical absence. Focal seizures progress to focal motor or bilateral convulsive seizures [99].
- Children show cognitive and behavioral impairments from the second year of life [97].
- At the onset of the first seizures, neither parents nor physicians notice any developmental delay, which insidiously appears during the second year of life [9].
- A family history of epilepsy. SCN1A gene mutationswere initially identified in cases of genetic epilepsy with febrile seizures plus (GEFS+). A family history of epilepsy or febrile seizures has been noted in 15%–35% of DS cases, with affected family members often showing GEFS+. DS has been documented in identical twins, infrequently in non-identical twins, and occasionally among multiple siblings within a single family. A family history of febrile seizures and epilepsy in individuals with DS and de novo SCN1A mutations implies a polygenic mode of inheritance. Additional modifier genes, such as sodium voltage-gated channel alpha subunit 9 (SCN9A), may contribute to DS manifestations [100,101]. Abnormal EEG findings are characterized by generalized or multifocal epileptiform discharges [9].
- Seizures with photosensitivity are more challanging to control in DS [9].
- Psychomotor slowing followed by crouch gait, pyramidal signs, or interictal myoclonus [9].
- Recent consensus strongly recommended genetic testing for patients with suspected DS [9].
3.2. Genetic Testing in DS
4. The Role of SCN1A in DS
4.1. SCN1A Mutations in DS
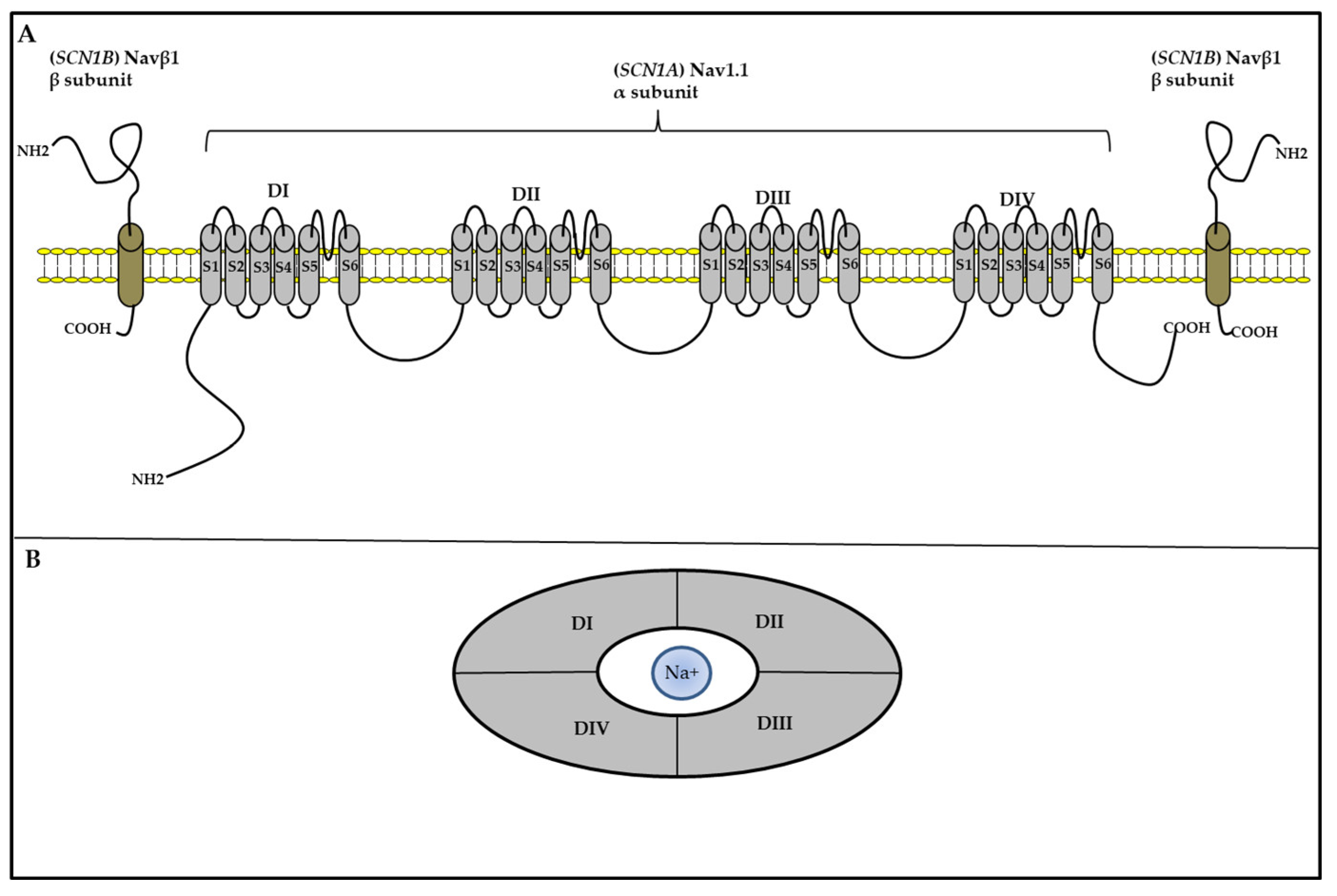
4.2. SCN1A-Mediated Hyperexcitability in DS: The Sodium Channel Interneuronopathy
4.3. Interneuronopathy and Cognition, Developmental Impairment, and Comorbidities
4.3.1. Role of Interneuronopathy in Cognition and Development
5. Novel Pharmacological Approaches for DS Management: What Is the Role of Precision Medicine?
5.1. Standard DS Management
5.2. Novel Pharmacological Approaches
5.2.1. CBD
5.2.2. Atypical Sodium Channel Blocker
5.2.3. Fenfluramine
5.2.4. Other Agents
5.3. Disease-Modifying Therapies
5.3.1. ASO Therapy
5.3.2. AAV-9 Based Gene Therapy
6. Expert Perspectives and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| ACC | agenesis of the corpus callosum |
| ADHD | attention deficit hyperactivity disorder |
| AGC1 | aspartate-glutamate carrier 1 |
| ASO | antisense oligonucleotides |
| AEDs | antiepileptic drugs |
| CBD | cannabidiol |
| CDG | congenital disorder of glycosylation |
| CONTAIN | ClObazam in patieNTs with LennoxGAstaut SyNdrome |
| DEs | developmental encephalopathies |
| DEE | developmental and epileptic encephalopathies |
| DECAM | developmental delay, epileptic encephalopathy, cerebral atrophy, and abnormal myelination |
| DRE | drug-resistant epilepsy |
| DS | Dravet syndrome |
| EEs | epileptic encephalopathies |
| EEG | electroencephalography |
| EFMR | epilepsy and mental retardation restricted to females |
| EEOC | epileptic encephalopathy, childhood-onset |
| FDA | Food and Drug Administration |
| GABA | γ-aminobutyric acid |
| GEFS+ | genetic epilepsy with febrile seizures plus |
| GOF | gain-of-function |
| GOT2 | glutamate oxaloacetate transaminase |
| GPIBD | glycophosphatidylinositol biosynthesis defect |
| IECEE | epileptic encephalopathy, infantile or early childhood |
| IGF-1 | insulin-like growth factor 1 |
| ILAE | International League Against Epilepsy |
| ISSX2 | infantile spasm syndrome, X-linked 2 |
| IQ | intelligence quotient |
| LGS | Lennox Gastaut syndrome |
| LISX2 | lissencephaly, X-linked, 2 |
| LOF | loss-of-function |
| MCAHS | multiple congenital anomalies-hypotonia-seizures syndrome |
| MCSZ | microcephaly, seizures, and developmental delay |
| MRX | mental retardation, X-linked |
| MRXS1 | mental retardation, X-linked, syndromic 1 |
| MRI | magnetic resonance imaging |
| NCSE | non-convulsive status epilepticus |
| PV | parvalbumin |
| PRTS | Partington syndrome |
| SCN1A | voltage-gated channel alpha subunit 1 |
| SCN9A | voltage-gated channel α subunit 9 |
| SE | status epilepticus |
| SMEI | severe myoclonic epilepsy of infancy |
| SST | somatostatin |
| SUDEP | sudden unexpected death in epilepsy |
| TSH | thyroid-stimulating hormone |
| VGSC | voltage-gated sodium channel |
| VNS | vagus nerve stimulation |
| VPA | valproic acid |
| XLAG | X-linked lissencephaly with ambiguous genitalia |
| XLID29 | intellectual developmental disorder, X-linked 29 |
| 5-HT | 5-hydroxytryptamine |
| 5-HT3aR | serotonin receptor 3a |
References
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Engel, J., Jr.; International League Against, E. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001, 42, 796–803. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Liao, J. Deciphering the concepts behind "Epileptic encephalopathy" and "Developmental and epileptic encephalopathy". Eur. J. Paediatr. Neurol. 2020, 24, 11–14. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Palmer, E.E.; Howell, K.; Scheffer, I.E. Natural History Studies and Clinical Trial Readiness for Genetic Developmental and Epileptic Encephalopathies. Neurotherapeutics 2021, 18, 1432–1444. [Google Scholar] [CrossRef]
- Symonds, J.D.; McTague, A. Epilepsy and developmental disorders: Next generation sequencing in the clinic. Eur. J. Paediatr. Neurol. 2020, 24, 15–23. [Google Scholar] [CrossRef]
- Khan, S.; Al Baradie, R. Epileptic encephalopathies: An overview. Epilepsy Res. Treat. 2012, 2012, 403592. [Google Scholar] [CrossRef]
- Sullivan, J.; Wirrell, E.C. Dravet Syndrome as an Example of Precision Medicine in Epilepsy. Epilepsy Curr. 2023, 23, 4–7. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E.; et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1349–1397. [Google Scholar] [CrossRef]
- Cetica, V.; Chiari, S.; Mei, D.; Parrini, E.; Grisotto, L.; Marini, C.; Pucatti, D.; Ferrari, A.; Sicca, F.; Specchio, N.; et al. Clinical and genetic factors predicting Dravet syndrome in infants with SCN1A mutations. Neurology 2017, 88, 1037–1044. [Google Scholar] [CrossRef]
- Connolly, M.B. Dravet Syndrome: Diagnosis and Long-Term Course. Can. J. Neurol. Sci. 2016, 43 (Suppl 3), S3–8. [Google Scholar] [CrossRef]
- Anwar, A.; Saleem, S.; Patel, U.K.; Arumaithurai, K.; Malik, P. Dravet Syndrome: An Overview. Cureus 2019, 11, e5006. [Google Scholar] [CrossRef]
- Villas, N.; Meskis, M.A.; Goodliffe, S. Dravet syndrome: Characteristics, comorbidities, and caregiver concerns. Epilepsy Behav. 2017, 74, 81–86. [Google Scholar] [CrossRef]
- Li, W.; Schneider, A.L.; Scheffer, I.E. Defining Dravet syndrome: An essential pre-requisite for precision medicine trials. Epilepsia 2021, 62, 2205–2217. [Google Scholar] [CrossRef]
- Han, Z.; Chen, C.; Christiansen, A.; Ji, S.; Lin, Q.; Anumonwo, C.; Liu, C.; Leiser, S.C.; Meena; Aznarez, I.; et al. Antisense oligonucleotides increase Scn1a expression and reduce seizures and SUDEP incidence in a mouse model of Dravet syndrome. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Tanenhaus, A.; Stowe, T.; Young, A.; McLaughlin, J.; Aeran, R.; Lin, I.W.; Li, J.; Hosur, R.; Chen, M.; Leedy, J.; et al. Cell-Selective Adeno-Associated Virus-Mediated SCN1A Gene Regulation Therapy Rescues Mortality and Seizure Phenotypes in a Dravet Syndrome Mouse Model and Is Well Tolerated in Nonhuman Primates. Hum. Gene Ther. 2022, 33, 579–597. [Google Scholar] [CrossRef]
- Symonds, J.D.; Elliott, K.S.; Shetty, J.; Armstrong, M.; Brunklaus, A.; Cutcutache, I.; Diver, L.A.; Dorris, L.; Gardiner, S.; Jollands, A.; et al. Early childhood epilepsies: Epidemiology, classification, aetiology, and socio-economic determinants. Brain 2021, 144, 2879–2891. [Google Scholar] [CrossRef]
- Symonds, J.D.; Zuberi, S.M.; Stewart, K.; McLellan, A.; O'Regan, M.; MacLeod, S.; Jollands, A.; Joss, S.; Kirkpatrick, M.; Brunklaus, A.; et al. Incidence and phenotypes of childhood-onset genetic epilepsies: A prospective population-based national cohort. Brain 2019, 142, 2303–2318. [Google Scholar] [CrossRef]
- Wu, Y.W.; Sullivan, J.; McDaniel, S.S.; Meisler, M.H.; Walsh, E.M.; Li, S.X.; Kuzniewicz, M.W. Incidence of Dravet Syndrome in a US Population. Pediatrics 2015, 136, e1310–e1315. [Google Scholar] [CrossRef]
- Bayat, A.; Hjalgrim, H.; Moller, R.S. The incidence of SCN1A-related Dravet syndrome in Denmark is 1:22,000: A population-based study from 2004 to 2009. Epilepsia 2015, 56, e36–e39. [Google Scholar] [CrossRef]
- Schubert-Bast, S.; Kay, L.; Simon, A.; Wyatt, G.; Holland, R.; Rosenow, F.; Strzelczyk, A. Epidemiology, healthcare resource use, and mortality in patients with probable Dravet syndrome: A population-based study on German health insurance data. Epilepsy Behav. 2022, 126, 108442. [Google Scholar] [CrossRef] [PubMed]
- Yakoub, M.; Dulac, O.; Jambaque, I.; Chiron, C.; Plouin, P. Early diagnosis of severe myoclonic epilepsy in infancy. Brain Dev. 1992, 14, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Rosander, C.; Hallbook, T. Dravet syndrome in Sweden: A population-based study. Dev. Med. Child. Neurol. 2015, 57, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Brunklaus, A.; Ellis, R.; Reavey, E.; Forbes, G.H.; Zuberi, S.M. Prognostic, clinical and demographic features in SCN1A mutation-positive Dravet syndrome. Brain 2012, 135, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Brunklaus, A.; Perez-Palma, E.; Ghanty, I.; Xinge, J.; Brilstra, E.; Ceulemans, B.; Chemaly, N.; de Lange, I.; Depienne, C.; Guerrini, R.; et al. Development and Validation of a Prediction Model for Early Diagnosis of SCN1A-Related Epilepsies. Neurology 2022, 98, e1163–e1174. [Google Scholar] [CrossRef] [PubMed]
- Skluzacek, J.V.; Watts, K.P.; Parsy, O.; Wical, B.; Camfield, P. Dravet syndrome and parent associations: The IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia 2011, 52 (Suppl 2), 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bluvstein, J.; Wenniger, S. Two Perspectives on Dravet Syndrome: Viewpoints from the Clinician and the Caregiver. Neurol. Ther. 2023, 12, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.; Chieffo, D.; Siracusano, R.; Waure, C.; Brogna, C.; Ranalli, D.; Contaldo, I.; Tortorella, G.; Dravet, C.; Mercuri, E.; et al. Cognitive decline in Dravet syndrome: Is there a cerebellar role? Epilepsy Res. 2013, 106, 211–221. [Google Scholar] [CrossRef]
- Ragona, F.; Brazzo, D.; De Giorgi, I.; Morbi, M.; Freri, E.; Teutonico, F.; Gennaro, E.; Zara, F.; Binelli, S.; Veggiotti, P.; et al. Dravet syndrome: Early clinical manifestations and cognitive outcome in 37 Italian patients. Brain Dev. 2010, 32, 71–77. [Google Scholar] [CrossRef]
- Riva, D.; Vago, C.; Pantaleoni, C.; Bulgheroni, S.; Mantegazza, M.; Franceschetti, S. Progressive neurocognitive decline in two children with Dravet syndrome, de novo SCN1A truncations and different epileptic phenotypes. Am. J. Med. Genet. A 2009, 149A, 2339–2345. [Google Scholar] [CrossRef]
- Verheyen, K.; Verbecque, E.; Ceulemans, B.; Schoonjans, A.S.; Van De Walle, P.; Hallemans, A. Motor development in children with Dravet syndrome. Dev. Med. Child. Neurol. 2019, 61, 950–956. [Google Scholar] [CrossRef]
- Wolff, M.; Casse-Perrot, C.; Dravet, C. Severe myoclonic epilepsy of infants (Dravet syndrome): Natural history and neuropsychological findings. Epilepsia 2006, 47 Suppl. 2, 45–48. [Google Scholar] [CrossRef]
- Kedem, P.; Scher, D.M. Evaluation and management of crouch gait. Curr. Opin. Pediatr. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Rodda, J.M.; Scheffer, I.E.; McMahon, J.M.; Berkovic, S.F.; Graham, H.K. Progressive gait deterioration in adolescents with Dravet syndrome. Arch. Neurol. 2012, 69, 873–878. [Google Scholar] [CrossRef]
- Chieffo, D.; Battaglia, D.; Lettori, D.; Del Re, M.; Brogna, C.; Dravet, C.; Mercuri, E.; Guzzetta, F. Neuropsychological development in children with Dravet syndrome. Epilepsy Res. 2011, 95, 86–93. [Google Scholar] [CrossRef]
- Guzzetta, F. Cognitive and behavioral characteristics of children with Dravet syndrome: An overview. Epilepsia 2011, 52 Suppl. 2, 35–38. [Google Scholar] [CrossRef]
- Jansson, J.S.; Hallbook, T.; Reilly, C. Intellectual functioning and behavior in Dravet syndrome: A systematic review. Epilepsy Behav. 2020, 108, 107079. [Google Scholar] [CrossRef]
- Takayama, R.; Fujiwara, T.; Shigematsu, H.; Imai, K.; Takahashi, Y.; Yamakawa, K.; Inoue, Y. Long-term course of Dravet syndrome: A study from an epilepsy center in Japan. Epilepsia 2014, 55, 528–538. [Google Scholar] [CrossRef]
- Ragona, F. Cognitive development in children with Dravet syndrome. Epilepsia 2011, 52 (Suppl 2), 39–43. [Google Scholar] [CrossRef]
- Nabbout, R.; Chemaly, N.; Chipaux, M.; Barcia, G.; Bouis, C.; Dubouch, C.; Leunen, D.; Jambaque, I.; Dulac, O.; Dellatolas, G.; et al. Encephalopathy in children with Dravet syndrome is not a pure consequence of epilepsy. Orphanet J. Rare Dis. 2013, 8, 176. [Google Scholar] [CrossRef]
- Dravet, C.; Bureau, M.; Dalla Bernardina, B.; Guerrini, R. Severe myoclonic epilepsy in infancy (Dravet syndrome) 30 years later. Epilepsia 2011, 52 (Suppl 2), 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.C.; Laux, L.; Donner, E.; Jette, N.; Knupp, K.; Meskis, M.A.; Miller, I.; Sullivan, J.; Welborn, M.; Berg, A.T. Optimizing the Diagnosis and Management of Dravet Syndrome: Recommendations from a North American Consensus Panel. Pediatr. Neurol. 2017, 68, 18–34.e13. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, N.; Laguitton, V.; Viellard, M.; Lepine, A.; Chabrol, B.; Dravet, C.; Milh, M. Cognitive and adaptive evaluation of 21 consecutive patients with Dravet syndrome. Epilepsy Behav. 2014, 31, 143–148. [Google Scholar] [CrossRef]
- Gataullina, S.; Dulac, O. From genotype to phenotype in Dravet disease. Seizure 2017, 44, 58–64. [Google Scholar] [CrossRef]
- Han, S.; Tai, C.; Westenbroek, R.E.; Yu, F.H.; Cheah, C.S.; Potter, G.B.; Rubenstein, J.L.; Scheuer, T.; de la Iglesia, H.O.; Catterall, W.A. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012, 489, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.M.; Berg, A.T.; Hood, V.; Knupp, K.G.; Koh, S.; Laux, L.; Meskis, M.A.; Miller, I.; Perry, M.S.; Scheffer, I.E.; et al. Dravet syndrome: A quick transition guide for the adult neurologist. Epilepsy Res. 2021, 177, 106743. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hung, P.L.; Fan, P.C.; Lin, K.L.; Hsu, T.R.; Chou, I.J.; Ho, C.S.; Chou, I.C.; Lin, W.S.; Lee, I.C.; et al. Clinical spectrum and the comorbidities of Dravet syndrome in Taiwan and the possible molecular mechanisms. Sci. Rep. 2021, 11, 20242. [Google Scholar] [CrossRef] [PubMed]
- Dravet, C.; Oguni, H. Dravet syndrome (severe myoclonic epilepsy in infancy). Handb. Clin. Neurol. 2013, 111, 627–633. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Sun, H.; Liu, X.; Yang, X.; Xiong, H.; Jiang, Y.; Bao, X.; Wang, S.; Yang, Z.; et al. Early clinical features and diagnosis of Dravet syndrome in 138 Chinese patients with SCN1A mutations. Brain Dev. 2014, 36, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Hattori, J.; Ouchida, M.; Ono, J.; Miyake, S.; Maniwa, S.; Mimaki, N.; Ohtsuka, Y.; Ohmori, I. A screening test for the prediction of Dravet syndrome before one year of age. Epilepsia 2008, 49, 626–633. [Google Scholar] [CrossRef]
- Oguni, H.; Hayashi, K.; Awaya, Y.; Fukuyama, Y.; Osawa, M. Severe myoclonic epilepsy in infants--a review based on the Tokyo Women's Medical University series of 84 cases. Brain Dev. 2001, 23, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Bureau, M.; Dalla Bernardina, B. Electroencephalographic characteristics of Dravet syndrome. Epilepsia 2011, 52 Suppl. 2, 13–23. [Google Scholar] [CrossRef]
- Genton, P.; Velizarova, R.; Dravet, C. Dravet syndrome: The long-term outcome. Epilepsia 2011, 52 (Suppl 2), 44–49. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, R.H.; Fejerman, N. Dravet syndrome: A study of 53 patients. Epilepsy Res. 2006, 70 (Suppl 1), S231–238. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kobayashi, K.; Yoshinaga, H.; Ohtsuka, Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia 2010, 51, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Dravet, C. The core Dravet syndrome phenotype. Epilepsia 2011, 52 (Suppl 2), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.E.; Sadleir, L.G.; Harkin, L.A.; Vadlamudi, L.; McMahon, J.M.; Mulley, J.C.; Scheffer, I.E.; Berkovic, S.F. Severe myoclonic epilepsy of infancy (Dravet syndrome): Recognition and diagnosis in adults. Neurology 2006, 67, 2224–2226. [Google Scholar] [CrossRef] [PubMed]
- Rilstone, J.J.; Coelho, F.M.; Minassian, B.A.; Andrade, D.M. Dravet syndrome: Seizure control and gait in adults with different SCN1A mutations. Epilepsia 2012, 53, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, A.; Zulfiqar-Ali, Q.; Marques, P.; Rong, M.; Andrade, D.M. A systematic review of adults with Dravet syndrome. Seizure 2021, 87, 39–45. [Google Scholar] [CrossRef]
- Specchio, N.; Balestri, M.; Trivisano, M.; Japaridze, N.; Striano, P.; Carotenuto, A.; Cappelletti, S.; Specchio, L.M.; Fusco, L.; Vigevano, F. Electroencephalographic features in dravet syndrome: Five-year follow-up study in 22 patients. J. Child. Neurol. 2012, 27, 439–444. [Google Scholar] [CrossRef]
- Korff, C.; Laux, L.; Kelley, K.; Goldstein, J.; Koh, S.; Nordli, D., Jr. Dravet syndrome (severe myoclonic epilepsy in infancy): A retrospective study of 16 patients. J. Child. Neurol. 2007, 22, 185–194. [Google Scholar] [CrossRef]
- Kim, S.H.; Nordli, D.R., Jr.; Berg, A.T.; Koh, S.; Laux, L. Ictal ontogeny in Dravet syndrome. Clin. Neurophysiol. 2015, 126, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Belin, J.; Harris, A.; Chen, B.; Zhou, J.; Ciulla, T.; Verticchio, A.; Antman, G.; Chang, M.; Siesky, B. Aging Effects on Optic Nerve Neurodegeneration. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Barba, C.; Parrini, E.; Coras, R.; Galuppi, A.; Craiu, D.; Kluger, G.; Parmeggiani, A.; Pieper, T.; Schmitt-Mechelke, T.; Striano, P.; et al. Co-occurring malformations of cortical development and SCN1A gene mutations. Epilepsia 2014, 55, 1009–1019. [Google Scholar] [CrossRef]
- Gaily, E.; Anttonen, A.K.; Valanne, L.; Liukkonen, E.; Traskelin, A.L.; Polvi, A.; Lommi, M.; Muona, M.; Eriksson, K.; Lehesjoki, A.E. Dravet syndrome: New potential genetic modifiers, imaging abnormalities, and ictal findings. Epilepsia 2013, 54, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Garcia-Penton, L.; Canales-Rodriguez, E.J.; Lerma-Usabiaga, G.; Iturria-Medina, Y.; Roman, F.J.; Davidson, D.; Aleman-Gomez, Y.; Acha, J.; Carreiras, M. Brain morphometry of Dravet syndrome. Epilepsy Res. 2014, 108, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Bureau, M.G.P.; Dravet, C.; Delgado-Escueta, A.V.; Tassinari, C.A.; Thomas, P.; Wolff, P. Epileptic Syndromes in Infancy, Childhood and Adolescence, 5th ed.; John Libbey Eurotext: France, 2005. [Google Scholar]
- Berkvens, J.J.; Veugen, I.; Veendrick-Meekes, M.J.; Snoeijen-Schouwenaars, F.M.; Schelhaas, H.J.; Willemsen, M.H.; Tan, I.Y.; Aldenkamp, A.P. Autism and behavior in adult patients with Dravet syndrome (DS). Epilepsy Behav. 2015, 47, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Boel, M.J. Behavioural and neuropsychological problems in refractory paediatric epilepsies. Eur. J. Paediatr. Neurol. 2004, 8, 291–297. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Li, B.M.; Li, Z.X.; Wang, J.; Liu, X.R.; Meng, H.; Tang, B.; Bian, W.J.; Shi, Y.W.; Liao, W.P. Few individuals with Lennox-Gastaut syndrome have autism spectrum disorder: A comparison with Dravet syndrome. J. Neurodev. Disord. 2018, 10, 10. [Google Scholar] [CrossRef]
- Kwong, A.K.; Fung, C.W.; Chan, S.Y.; Wong, V.C. Identification of SCN1A and PCDH19 mutations in Chinese children with Dravet syndrome. PLoS ONE 2012, 7, e41802. [Google Scholar] [CrossRef]
- Li, B.M.; Liu, X.R.; Yi, Y.H.; Deng, Y.H.; Su, T.; Zou, X.; Liao, W.P. Autism in Dravet syndrome: Prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy Behav. 2011, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ouss, L.; Leunen, D.; Laschet, J.; Chemaly, N.; Barcia, G.; Losito, E.M.; Aouidad, A.; Barrault, Z.; Desguerre, I.; Breuillard, D.; et al. Autism spectrum disorder and cognitive profile in children with Dravet syndrome: Delineation of a specific phenotype. Epilepsia Open 2019, 4, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Lagae, L.; Irwin, J.; Gibson, E.; Battersby, A. Caregiver impact and health service use in high and low severity Dravet syndrome: A multinational cohort study. Seizure 2019, 65, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Nabbout, R. SCN1A-related phenotypes: Epilepsy and beyond. Epilepsia 2019, 60 (Suppl 3), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Perulli, M.; Battista, A.; Sivo, S.; Turrini, I.; Musto, E.; Quintiliani, M.; Gambardella, M.L.; Contaldo, I.; Veredice, C.; Mercuri, E.M.; et al. Heart rate variability alterations in Dravet Syndrome: The role of status epilepticus and a possible association with mortality risk. Seizure 2022, 94, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Daverio, M.; Ciccone, O.; Boniver, C.; De Palma, L.; Corrado, D.; Vecchi, M. Supraventricular Tachycardia During Status Epilepticus in Dravet Syndrome: A Link Between Brain and Heart? Pediatr. Neurol. 2016, 56, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Ergul, Y.; Ekici, B.; Tatli, B.; Nisli, K.; Ozmen, M. QT and P wave dispersion and heart rate variability in patients with Dravet syndrome. Acta Neurol. Belg. 2013, 113, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Minderhoud, C.A.; Postma, A.; Jansen, F.E.; Verhoeven, J.S.; Schrijver, J.J.; Goudswaard, J.; Andreae, G.; Otte, W.M.; Braun, K.P.J.; Brilstra, E.H. Gastrointestinal and eating problems in SCN1A-related seizure disorders. Epilepsy Behav. 2023, 146, 109361. [Google Scholar] [CrossRef] [PubMed]
- Beck, V.C.; Isom, L.L.; Berg, A.T. Gastrointestinal Symptoms and Channelopathy-Associated Epilepsy. J. Pediatr. 2021, 237, 41–49 e41. [Google Scholar] [CrossRef]
- Eschbach, K.; Scarbro, S.; Juarez-Colunga, E.; Allen, V.; Hsu, S.; Knupp, K. Growth and endocrine function in children with Dravet syndrome. Seizure 2017, 52, 117–122. [Google Scholar] [CrossRef]
- Delogu, A.B.; Spinelli, A.; Battaglia, D.; Dravet, C.; De Nisco, A.; Saracino, A.; Romagnoli, C.; Lanza, G.A.; Crea, F. Electrical and autonomic cardiac function in patients with Dravet syndrome. Epilepsia 2011, 52 (Suppl 2), 55–58. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Borlot, F.; Lang, A.E.; Andrade, D.M. Antecollis and levodopa-responsive parkinsonism are late features of Dravet syndrome. Neurology 2014, 82, 2250–2251. [Google Scholar] [CrossRef] [PubMed]
- Andress, D.L.; Ozuna, J.; Tirschwell, D.; Grande, L.; Johnson, M.; Jacobson, A.F.; Spain, W. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch. Neurol. 2002, 59, 781–786. [Google Scholar] [CrossRef]
- Bjurulf, B.; Reilly, C.; Sigurdsson, G.V.; Thunstrom, S.; Kolbjer, S.; Hallbook, T. Dravet syndrome in children-A population-based study. Epilepsy Res. 2022, 182, 106922. [Google Scholar] [CrossRef]
- Licheni, S.H.; McMahon, J.M.; Schneider, A.L.; Davey, M.J.; Scheffer, I.E. Sleep problems in Dravet syndrome: A modifiable comorbidity. Dev. Med. Child. Neurol. 2018, 60, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kalume, F.; Oakley, J.C.; Westenbroek, R.E.; Gile, J.; de la Iglesia, H.O.; Scheuer, T.; Catterall, W.A. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol. Dis. 2015, 77, 141–154. [Google Scholar] [CrossRef]
- Van Nuland, A.; Ivanenko, A.; Meskis, M.A.; Villas, N.; Knupp, K.G.; Berg, A.T. Sleep in Dravet syndrome: A parent-driven survey. Seizure 2021, 85, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Chieffo, D.; Battaglia, D.; Brogna, C.; Contaldo, I.; De Clemente, V.; Losito, E.; Dravet, C.; Mercuri, E.; Guzzetta, F. A prospective longitudinal study on visuo-cognitive development in Dravet syndrome: Is there a "dorsal stream vulnerability"? Epilepsy Res. 2015, 109, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sala-Coromina, J.; Raspall-Chaure, M.; Marce-Grau, A.; de la Ossa, A.M.; Macaya, A. Early-onset eyelid stereotypies are a frequent and distinctive feature in Dravet syndrome. Seizure 2021, 92, 155–157. [Google Scholar] [CrossRef]
- Cooper, M.S.; McIntosh, A.; Crompton, D.E.; McMahon, J.M.; Schneider, A.; Farrell, K.; Ganesan, V.; Gill, D.; Kivity, S.; Lerman-Sagie, T.; et al. Mortality in Dravet syndrome. Epilepsy Res. 2016, 128, 43–47. [Google Scholar] [CrossRef]
- Kearney, J. Sudden unexpected death in dravet syndrome. Epilepsy Curr. 2013, 13, 264–265. [Google Scholar] [CrossRef]
- Auerbach, D.S.; Jones, J.; Clawson, B.C.; Offord, J.; Lenk, G.M.; Ogiwara, I.; Yamakawa, K.; Meisler, M.H.; Parent, J.M.; Isom, L.L. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS ONE 2013, 8, e77843. [Google Scholar] [CrossRef]
- Cheah, C.S.; Westenbroek, R.E.; Roden, W.H.; Kalume, F.; Oakley, J.C.; Jansen, L.A.; Catterall, W.A. Correlations in timing of sodium channel expression, epilepsy, and sudden death in Dravet syndrome. Channels 2013, 7, 468–472. [Google Scholar] [CrossRef]
- de Lange, I.M.; Gunning, B.; Sonsma, A.C.M.; van Gemert, L.; van Kempen, M.; Verbeek, N.E.; Nicolai, J.; Knoers, N.; Koeleman, B.P.C.; Brilstra, E.H. Influence of contraindicated medication use on cognitive outcome in Dravet syndrome and age at first afebrile seizure as a clinical predictor in SCN1A-related seizure phenotypes. Epilepsia 2018, 59, 1154–1165. [Google Scholar] [CrossRef]
- Dravet, C.B.M.; Oguni, H.; Cokar, O.; Guerrini, R.; Wolf, P. Epileptic Syndromes in Infancy, Childhood and Adolescence, 6th ed.; John Libbey Eurotext: Arcueil, France, 2019. [Google Scholar]
- Dravet, C. Severe myoclonic epilepsy in infants and its related syndromes. Epilepsia 2000, 41 (Suppl 9), 7. [Google Scholar] [CrossRef]
- Ohki, T.; Watanabe, K.; Negoro, T.; Aso, K.; Haga, Y.; Kasai, K.; Kito, M.; Maeda, N. Severe myoclonic epilepsy in infancy: Evolution of seizures. Seizure 1997, 6, 219–224. [Google Scholar] [CrossRef]
- Ozmen, M.; Dilber, C.; Tatli, B.; Aydinli, N.; Caliskan, M.; Ekici, B. Severe myoclonic epilepsy of infancy (Dravet syndrome): Clinical and genetic features of nine Turkish patients. Ann. Indian. Acad. Neurol. 2011, 14, 178–181. [Google Scholar] [CrossRef]
- Depienne, C.; Trouillard, O.; Saint-Martin, C.; Gourfinkel-An, I.; Bouteiller, D.; Carpentier, W.; Keren, B.; Abert, B.; Gautier, A.; Baulac, S.; et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: Analysis of 333 patients. J. Med. Genet. 2009, 46, 183–191. [Google Scholar] [CrossRef]
- Singh, R.; Andermann, E.; Whitehouse, W.P.; Harvey, A.S.; Keene, D.L.; Seni, M.H.; Crossland, K.M.; Andermann, F.; Berkovic, S.F.; Scheffer, I.E. Severe myoclonic epilepsy of infancy: Extended spectrum of GEFS+? Epilepsia 2001, 42, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Dravet Syndrome: A Sodium Channel Interneuronopathy. Curr. Opin. Physiol. 2018, 2, 42–50. [Google Scholar] [CrossRef]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.M.; Isom, L.L. Voltage-gated sodium channel beta subunits: The power outside the pore in brain development and disease. Neuropharmacology 2018, 132, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Plummer, N.W.; Meisler, M.H. Evolution and diversity of mammalian sodium channel genes. Genomics 1999, 57, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Isom, L.L.; De Jongh, K.S.; Catterall, W.A. Auxiliary subunits of voltage-gated ion channels. Neuron 1994, 12, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Kalume, F.; Oakley, J.C. NaV1.1 channels and epilepsy. J. Physiol. 2010, 588, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, E.; Santorelli, F.M.; Bertini, E.; Buti, D.; Gaggero, R.; Gobbi, G.; Lini, M.; Granata, T.; Freri, E.; Parmeggiani, A.; et al. Somatic and germline mosaicisms in severe myoclonic epilepsy of infancy. Biochem. Biophys. Res. Commun. 2006, 341, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Mei, D.; Helen Cross, J.; Guerrini, R. Mosaic SCN1A mutation in familial severe myoclonic epilepsy of infancy. Epilepsia 2006, 47, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.C.; Morse, R.P.; Scott, R.C.; Holmes, G.L.; Lenck-Santini, P.P. SCN1A mutations in Dravet syndrome: Impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy Behav. 2012, 23, 177–186. [Google Scholar] [CrossRef]
- Gambardella, A.; Marini, C. Clinical spectrum of SCN1A mutations. Epilepsia 2009, 50 (Suppl 5), 20–23. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Brunklaus, A.; Birch, R.; Reavey, E.; Duncan, J.; Forbes, G.H. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology 2011, 76, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Beckh, S.; Noda, M.; Lubbert, H.; Numa, S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 1989, 8, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Ohmori, I.; Ouchida, M.; Ohno, Y.; Tsurumi, T.; Miki, T.; Wakamori, M.; Ishihara, S.; Yoshida, T.; Takizawa, A.; et al. A missense mutation of the gene encoding voltage-dependent sodium channel (Nav1.1) confers susceptibility to febrile seizures in rats. J. Neurosci. 2010, 30, 5744–5753. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.S.; Grunnet, M.; Bastlund, J.F. Therapeutic potential of Na(V)1.1 activators. Trends Pharmacol. Sci. 2014, 35, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.S.; Dutt, K.; Papale, L.A.; Dube, C.M.; Dutton, S.B.; de Haan, G.; Shankar, A.; Tufik, S.; Meisler, M.H.; Baram, T.Z.; et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J. Biol. Chem. 2010, 285, 9823–9834. [Google Scholar] [CrossRef]
- Hayashi, K.; Ueshima, S.; Ouchida, M.; Mashimo, T.; Nishiki, T.; Sendo, T.; Serikawa, T.; Matsui, H.; Ohmori, I. Therapy for hyperthermia-induced seizures in Scn1a mutant rats. Epilepsia 2011, 52, 1010–1017. [Google Scholar] [CrossRef]
- Rhodes, T.H.; Lossin, C.; Vanoye, C.G.; Wang, D.W.; George, A.L., Jr. Noninactivating voltage-gated sodium channels in severe myoclonic epilepsy of infancy. Proc. Natl. Acad. Sci. U S A 2004, 101, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Oakley, J.C.; Kalume, F.; Yu, F.H.; Scheuer, T.; Catterall, W.A. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc. Natl. Acad. Sci. USA 2009, 106, 3994–3999. [Google Scholar] [CrossRef]
- Kepecs, A.; Fishell, G. Interneuron cell types are fit to function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kondo, S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J. Neurocytol. 2002, 31, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G.; Wang, X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.H.; Vaiana, M.; Nakuci, J.; Somarowthu, A.; Goff, K.M.; Goldstein, N.; Murthy, P.; Muldoon, S.F.; Goldberg, E.M. Interneuron Desynchronization Precedes Seizures in a Mouse Model of Dravet Syndrome. J. Neurosci. 2020, 40, 2764–2775. [Google Scholar] [CrossRef]
- Freund, T.F. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003, 26, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kalume, F.; Westenbroek, R.E.; Cheah, C.S.; Yu, F.H.; Oakley, J.C.; Scheuer, T.; Catterall, W.A. Sudden unexpected death in a mouse model of Dravet syndrome. J. Clin. Invest. 2013, 123, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.B.; Madsen, J.R.; Schulze-Bonhage, A.; Aschenbrenner-Scheibe, R.; Newman, E.L.; Kahana, M.J. Human theta oscillations related to sensorimotor integration and spatial learning. J. Neurosci. 2003, 23, 4726–4736. [Google Scholar] [CrossRef]
- Cobb, S.R.; Buhl, E.H.; Halasy, K.; Paulsen, O.; Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 1995, 378, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.C.; Jiao, X.; Sinha, S.; Beck, K.D.; Servatius, R.J. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus 2011, 21, 835–846. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Jeong, H.Y.; Kruglikov, I.; Tremblay, R.; Lazarenko, R.M.; Rudy, B. Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cereb. Cortex 2011, 21, 666–682. [Google Scholar] [CrossRef]
- Chiron, C.; Dulac, O. The pharmacologic treatment of Dravet syndrome. Epilepsia 2011, 52 Suppl. 2, 72–75. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Hood, V.; Knupp, K.G.; Meskis, M.A.; Nabbout, R.; Scheffer, I.E.; Wilmshurst, J.; Sullivan, J. International consensus on diagnosis and management of Dravet syndrome. Epilepsia 2022, 63, 1761–1777. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.C.; Lee, H.S.; Chang, K.P.; Lee, Y.Y.; Lai, H.C.; Hung, P.L.; Lee, H.F.; Chi, C.S. The Impact of Anti-Epileptic Drugs on Growth and Bone Metabolism. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef]
- Dressler, A.; Trimmel-Schwahofer, P.; Reithofer, E.; Muhlebner, A.; Groppel, G.; Reiter-Fink, E.; Benninger, F.; Grassl, R.; Feucht, M. Efficacy and tolerability of the ketogenic diet in Dravet syndrome - Comparison with various standard antiepileptic drug regimen. Epilepsy Res. 2015, 109, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Ohtsuka, Y.; Oguni, H.; Tohyama, J.; Baba, H.; Fukushima, K.; Ohtani, H.; Takahashi, Y.; Ikeda, S. Stiripentol open study in Japanese patients with Dravet syndrome. Epilepsia 2009, 50, 2362–2368. [Google Scholar] [CrossRef]
- Shi, X.Y.; Tomonoh, Y.; Wang, W.Z.; Ishii, A.; Higurashi, N.; Kurahashi, H.; Kaneko, S.; Hirose, S.; Epilepsy Genetic Study Group, J. Efficacy of antiepileptic drugs for the treatment of Dravet syndrome with different genotypes. Brain Dev. 2016, 38, 40–46. [Google Scholar] [CrossRef]
- Freche, C. [Study of an anxiolytic, clobazam, in otorhinolaryngology in psychosomatic pharyngeal manifestations]. Sem. Hop. Ther. 1975, 51, 261–263. [Google Scholar] [PubMed]
- Group, C.C.C. Clobazam in treatment of refractory epilepsy: The Canadian experience. A retrospective study. Epilepsia 1991, 32, 407–416. [Google Scholar]
- Gauthier, A.C.; Mattson, R.H. Clobazam: A Safe, Efficacious, and Newly Rediscovered Therapeutic for Epilepsy. CNS Neurosci. Ther. 2015, 21, 543–548. [Google Scholar] [CrossRef]
- Pernea, M.; Sutcliffe, A.G. Clobazam and Its Use in Epilepsy. Pediatr. Rep. 2016, 8, 6516. [Google Scholar] [CrossRef]
- Ng, Y.T.; Conry, J.A.; Drummond, R.; Stolle, J.; Weinberg, M.A.; Investigators, O.V.S. Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. Neurology 2011, 77, 1473–1481. [Google Scholar] [CrossRef]
- Conry, J.A.; Ng, Y.T.; Kernitsky, L.; Mitchell, W.G.; Veidemanis, R.; Drummond, R.; Isojarvi, J.; Lee, D.; Paolicchi, J.M.; Investigators, O.V.S. Stable dosages of clobazam for Lennox-Gastaut syndrome are associated with sustained drop-seizure and total-seizure improvements over 3 years. Epilepsia 2014, 55, 558–567. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Laux, L.; Franz, D.N.; Sullivan, J.; Saneto, R.P.; Morse, R.P.; Devinsky, O.; Chugani, H.; Hernandez, A.; Hamiwka, L.; et al. Stiripentol in Dravet syndrome: Results of a retrospective U.S. study. Epilepsia 2013, 54, 1595–1604. [Google Scholar] [CrossRef]
- Giraud, C.; Treluyer, J.M.; Rey, E.; Chiron, C.; Vincent, J.; Pons, G.; Tran, A. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab. Dispos. 2006, 34, 608–611. [Google Scholar] [CrossRef]
- Frisium (clobazam): Summary of product characteristics. Available online: https://www.medicines.org.uk/emc/product/1574/smpc#gref (accessed on 15 November 2021).
- Frisium (clobazam): Summary of product characteristics. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203993s005lbl.pdf (accessed on 15 November 2021).
- Chiron, C.; Marchand, M.C.; Tran, A.; Rey, E.; d'Athis, P.; Vincent, J.; Dulac, O.; Pons, G. Stiripentol in severe myoclonic epilepsy in infancy: A randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet 2000, 356, 1638–1642. [Google Scholar] [CrossRef]
- Nickels, K.C.; Wirrell, E.C. Stiripentol in the Management of Epilepsy. CNS Drugs 2017, 31, 405–416. [Google Scholar] [CrossRef]
- Caraballo, R.H.; Cersosimo, R.O.; Sakr, D.; Cresta, A.; Escobal, N.; Fejerman, N. Ketogenic diet in patients with Dravet syndrome. Epilepsia 2005, 46, 1539–1544. [Google Scholar] [CrossRef]
- Frampton, J.E. Stiripentol: A Review in Dravet Syndrome. Drugs 2019, 79, 1785–1796. [Google Scholar] [CrossRef]
- Yamada, M.; Suzuki, K.; Matsui, D.; Inoue, Y.; Ohtsuka, Y. Long-term safety and effectiveness of stiripentol in patients with Dravet syndrome: Interim report of a post-marketing surveillance study in Japan. Epilepsy Res. 2021, 170, 106535. [Google Scholar] [CrossRef]
- Na, J.H.; Shin, S.; Yang, D.; Kim, B.; Kim, H.D.; Kim, S.; Lee, J.S.; Choi, J.R.; Lee, S.T.; Kang, H.C. Targeted gene panel sequencing in early infantile onset developmental and epileptic encephalopathy. Brain Dev. 2020, 42, 438–448. [Google Scholar] [CrossRef]
- Dibue-Adjei, M.; Fischer, I.; Steiger, H.J.; Kamp, M.A. Efficacy of adjunctive vagus nerve stimulation in patients with Dravet syndrome: A meta-analysis of 68 patients. Seizure 2017, 50, 147–152. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Miller, B.; Jeong, A.; Bertrand, M.E.; Limbrick, D.D., Jr.; Smyth, M.D. Palliative epilepsy surgery in Dravet syndrome-case series and review of the literature. Childs Nerv. Syst. 2016, 32, 1703–1708. [Google Scholar] [CrossRef]
- Andrade, D.M.; Hamani, C.; Lozano, A.M.; Wennberg, R.A. Dravet syndrome and deep brain stimulation: Seizure control after 10 years of treatment. Epilepsia 2010, 51, 1314–1316. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V.; et al. Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef]
- Anderson, L.L.; Hawkins, N.A.; Thompson, C.H.; Kearney, J.A.; George, A.L., Jr. Unexpected Efficacy of a Novel Sodium Channel Modulator in Dravet Syndrome. Sci. Rep. 2017, 7, 1682. [Google Scholar] [CrossRef]
- Schoonjans, A.S.; Ceulemans, B. An Old Drug for a New Indication: Repurposing Fenfluramine from an Anorexigen to an Antiepileptic Drug. Clin. Pharmacol. Ther. 2019, 106, 929–932. [Google Scholar] [CrossRef]
- Schoonjans, A.S.; Ceulemans, B. A critical evaluation of fenfluramine hydrochloride for the treatment of Dravet syndrome. Expert. Rev. Neurother. 2022, 22, 351–364. [Google Scholar] [CrossRef]
- Martin, P.; de Witte, P.A.M.; Maurice, T.; Gammaitoni, A.; Farfel, G.; Galer, B. Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav. 2020, 105, 106989. [Google Scholar] [CrossRef]
- Ceulemans, B.; Boel, M.; Leyssens, K.; Van Rossem, C.; Neels, P.; Jorens, P.G.; Lagae, L. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 2012, 53, 1131–1139. [Google Scholar] [CrossRef]
- Ceulemans, B.; Schoonjans, A.S.; Marchau, F.; Paelinck, B.P.; Lagae, L. Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia 2016, 57, e129–e134. [Google Scholar] [CrossRef]
- Lagae, L.; Sullivan, J.; Knupp, K.; Laux, L.; Polster, T.; Nikanorova, M.; Devinsky, O.; Cross, J.H.; Guerrini, R.; Talwar, D.; et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: A randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 2243–2254. [Google Scholar] [CrossRef]
- Nabbout, R.; Mistry, A.; Zuberi, S.; Villeneuve, N.; Gil-Nagel, A.; Sanchez-Carpintero, R.; Stephani, U.; Laux, L.; Wirrell, E.; Knupp, K.; et al. Fenfluramine for Treatment-Resistant Seizures in Patients With Dravet Syndrome Receiving Stiripentol-Inclusive Regimens: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 300–308. [Google Scholar] [CrossRef]
- Sullivan, J.; Specchio, N.; Devinsky, O.; Auvin, S.; Perry, M.S.; Strzelczyk, A.; Gil-Nagel, A.; Dai, D.; Galer, B.S.; Gammaitoni, A.R. Fenfluramine significantly reduces day-to-day seizure burden by increasing number of seizure-free days and time between seizures in patients with Dravet syndrome: A time-to-event analysis. Epilepsia 2022, 63, 130–138. [Google Scholar] [CrossRef]
- Specchio, N.; Pietrafusa, N.; Doccini, V.; Trivisano, M.; Darra, F.; Ragona, F.; Cossu, A.; Spolverato, S.; Battaglia, D.; Quintiliani, M.; et al. Efficacy and safety of Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: A real-world study. Epilepsia 2020, 61, 2405–2414. [Google Scholar] [CrossRef]
- Specchio, N.; Pietrafusa, N.; Ferretti, A.; Trivisano, M.; Vigevano, F. Successful use of fenfluramine in nonconvulsive status epilepticus of Dravet syndrome. Epilepsia 2020, 61, 831–833. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Pringsheim, M.; Mayer, T.; Polster, T.; Klotz, K.A.; Muhle, H.; Alber, M.; Trollmann, R.; Spors, H.; Kluger, G.; et al. Efficacy, tolerability, and retention of fenfluramine for the treatment of seizures in patients with Dravet syndrome: Compassionate use program in Germany. Epilepsia 2021, 62, 2518–2527. [Google Scholar] [CrossRef]
- Trowbridge, S.; Poduri, A.; Olson, H. Early diagnosis and experimental treatment with fenfluramine via the Investigational New Drug mechanism in a boy with Dravet syndrome and recurrent status epilepticus. Epileptic Disord. 2021, 23, 954–956. [Google Scholar] [CrossRef]
- Cross, J.H.; Galer, B.S.; Gil-Nagel, A.; Devinsky, O.; Ceulemans, B.; Lagae, L.; Schoonjans, A.S.; Donner, E.; Wirrell, E.; Kothare, S.; et al. Impact of fenfluramine on the expected SUDEP mortality rates in patients with Dravet syndrome. Seizure 2021, 93, 154–159. [Google Scholar] [CrossRef]
- Fintepla: Prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212102s000lbl.pdf (accessed on 15 November 2021).
- Fintepla: Summary of product characteristics. Available online: https://www.medicines.org.uk/emc/product/11998/smpc#gref (accessed on 15 November 2021).
- Schoonjans, A.; Maes, P.; Ceulemans, B. Aggravation of valproic acid induced thrombocytopenia after the introduction of fenfluramine, a case report. Seizure 2021, 93, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Oakley, J.C.; Cho, A.R.; Cheah, C.S.; Scheuer, T.; Catterall, W.A. Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet syndrome. J. Pharmacol. Exp. Ther. 2013, 345, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Laux, L.R.C.; Knupp, K.G.; Schreiber, J.M.; Lallas, M.; Wirrell, E.; Devinsky, O.; Stutely, J.; Brathwaite, C.; Avendaño, J.; Parkerson, K.; Meena, M.; Wyant, N.; Ticho, B.; Sullivan, J. Positive Interim Safety, PK, and CSF Exposure Data from the Phase 1/2a MONARCH Study of STK-001, an Antisense Oligonucleotide (ASO), in Children and Adolescents with Dravet Syndrome (DS). In Proceedings of the American Epilepsy Society Annual meeting McCormick Place West, Chicago, IL, USA; 2021. [Google Scholar]
| Features | Children | Older children & Adults |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





