1. Introduction
Beta-secretase 1 (BACE1), also known as beta-site amyloid precursor protein cleavage enzyme 1, is an enzyme encoded by the BACE1 gene in humans. This enzyme is mainly expressed in neurons. Its importance is evident in the formation of myelin sheaths in peripheral nerve cells, with high expression during postnatal stages in mice, related to myelination[
1,
2,
3,
4].
The structure of BACE1 is characterized by two aspartate residues in the active site of its extracellular protein domain. It can function as a dimer, and its cytoplasmic tail is crucial for proper maturation and efficient intracellular trafficking, although it does not directly influence enzymatic activity. It is produced as a proenzyme, undergoing endoproteolysis after leaving the endoplasmic reticulum and reaching the Golgi apparatus. During this process, the pro-peptide receives additional sugars to increase its molecular mass, and the tail becomes palmitoylated[
1,
2,
3,
4].
BACE1 expression is subject to the influence of the inflammatory state. During Alzheimer's disease (AD), cytokines downregulate PPAR1, an inhibitor of BACE1 mRNA, thus contributing to the regulation of BACE1 expression during inflammatory conditions[
1,
2,
3,
4].The present study aims to investigate this protein with a natural molecule called Hypericin known for its anti-inflammator [
5], anti-viral and anti-tumor properties [
6].Several pharmaceutical companies are studying different BACE1 inhibitors [
7]. However, the priority need is to discover a natural molecule with low side effects that has the ability to behave as a selective inhibitor of this protein, implicated in Alzheimer's disease, a progressive and irreversible neurodegenerative disease that affects the brain. It characterized by a gradual loss of memory and cognitive function, Alzheimer's is the most common form of dementia [
8].The present study is based on computational methods based on Molecular Docking[
9], a highly precise in-Silico technique that aims to automatically calculate the energetic affinities between Hypericin and BACE-1. Furthermore, the amino acids involved in the active site of the protein involved with Hypericin are also investigated.
2. Material and Methods
Crystal Structure of Beta-secretase 1 (BACE-1) was taken from Protein Data Bank and Molecular Docking investigation [
9] was performed by Mcule Database[
10]. Binding site center of BACE-1 protein ( PDB Code 3cic) was X( 19,4438), Y( 32,672) Z( 57,4953). Hypericin was taked from Drug Bank Database.
3. Results and Discussion
This brief communication presents, for the first time, an exploration of the interaction between Hypericin and BACE-1. Hypericin, derived from anthraquinone, is naturally found in the yellow flowers of Hypericum perforatum (St. John's wort). It demonstrates antidepressant properties, as well as potential antiviral, antineoplastic, and immunostimulating activities [
5,
6].
The selected approach was Molecular Docking[
9], a well-established and potent method commonly employed in drug design, typically employed prior to conducting in vitro and in vivo studies.
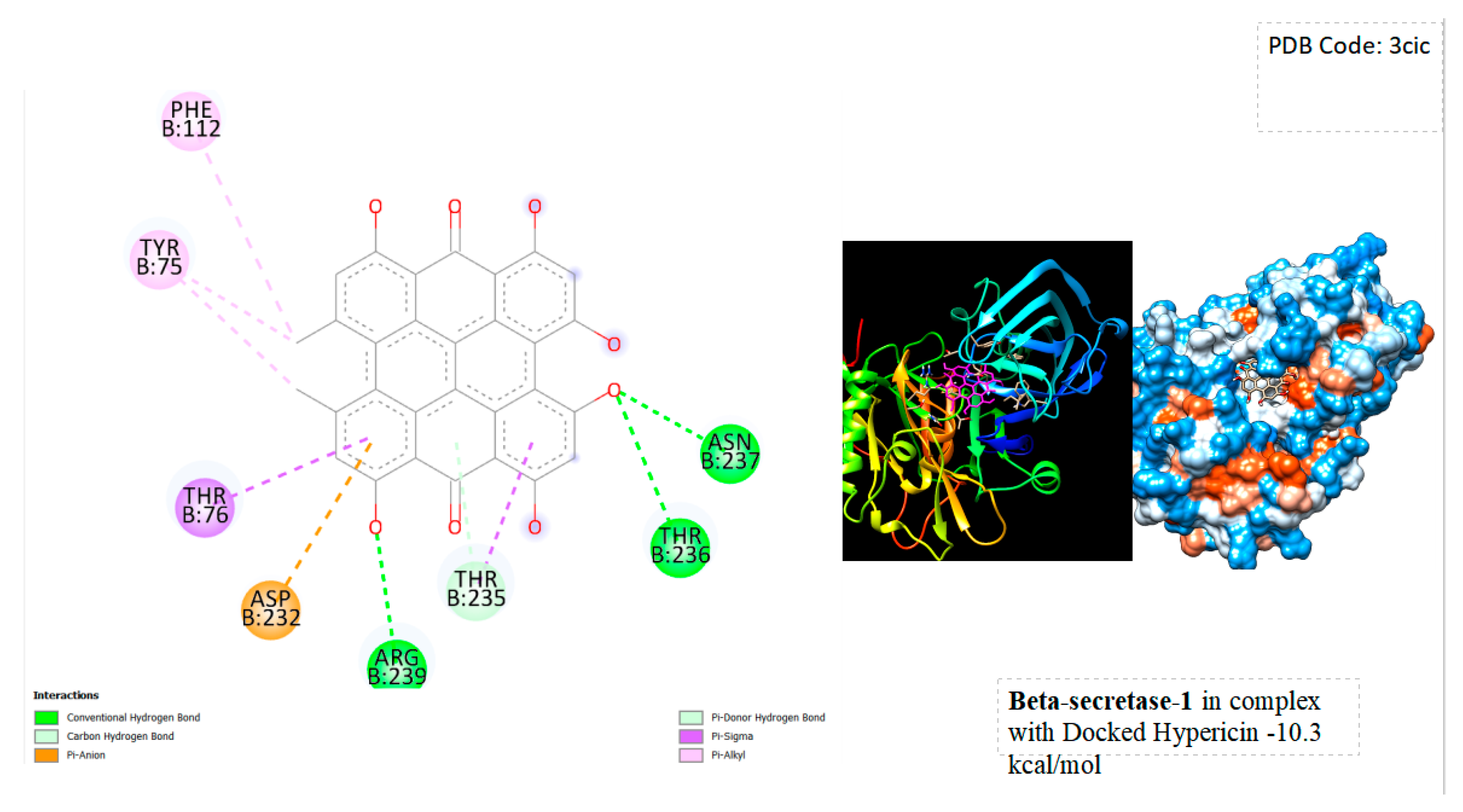
In
Figure 1 below, the outcomes of Molecular Docking and associated characterizations between BACE-1 and hypericin are depicted. The results are notably intriguing, revealing a remarkably high binding energy affinity of hypericin with the protein, registering a score of -10.3 kcal/mol. This suggests that, theoretically, this natural substance can effectively bind to the active site of the protein. Furthermore, hypericin seems to establish several chemical bonds with the protein, including hydrogen bonds with ASN 237, THR 236, and ARG 239, as well as hydrophobic bonds with ASP 232, THR 76, TYR 75, and PHE 112.
4. Conclusion
BACE1's role in beta-amyloid peptide production makes it a target for Alzheimer's disease treatment, aiming to mitigate the associated beta-amyloid accumulation. Computational analyses suggest hypericin's potential as a BACE1 inhibitor. Molecular Docking reveals a robust binding affinity with a significant energy score of -10.3 kcal/mol, supported by the formation of chemical bonds (hydrogen and hydrophobic) indicating effective interaction at BACE1's active site. While promising, in silico results require validation through in vitro and in vivo studies to deepen our understanding of hypericin's inhibitory capabilities against BACE1. Additionally, practical use as a BACE1 inhibitor mandates careful consideration of factors such as bioavailability, safety, and efficacy in a therapeutic context. Hypericin's capacity to modulate BACE1 presents a potential avenue for Alzheimer's disease intervention, though further research is vital to confirm its efficacy and safety in more complex biological settings.
References
- Venugopal, C., Demos, C. M., Jagannatha Rao, K. S., Pappolla, M. A., & Sambamurti, K. (2008). Beta-secretase: structure, function, and evolution. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 7(3), 278-294. [CrossRef]
- Shen, Y., Wang, H., Sun, Q., Yao, H., Keegan, A. P., Mullan, M., ... & Hampel, H. (2018). Increased plasma beta-secretase 1 may predict conversion to Alzheimer’s disease dementia in individuals with mild cognitive impairment. Biological psychiatry, 83(5), 447-455. [CrossRef]
- Cervellati, C., Trentini, A., Rosta, V., Passaro, A., Bosi, C., Sanz, J. M., ... & Zuliani, G. (2020). Serum beta-secretase 1 (BACE1) activity as candidate biomarker for late-onset Alzheimer’s disease. GeroScience, 42, 159-167. [CrossRef]
- Chacón-Quintero, M. V., Pineda-López, L. G., Villegas-Lanau, C. A., Posada-Duque, R., & Cardona-Gómez, G. P. (2021). Beta-secretase 1 underlies reactive astrocytes and endothelial disruption in neurodegeneration. Frontiers in Cellular Neuroscience, 15, 656832. [CrossRef]
- Dellafiora, L., Galaverna, G., Cruciani, G., Dall’Asta, C., & Bruni, R. (2018). On the mechanism of action of anti-inflammatory activity of hypericin: An in silico study pointing to the relevance of Janus kinases inhibition. Molecules, 23(12), 3058. [CrossRef]
- Miskovsky, P. (2002). Hypericin-a new antiviral and antitumor photosensitizer: mechanism of action and interaction with biological macromolecules. Current drug targets, 3(1), 55-84. [CrossRef]
- Moussa-Pacha, N. M., Abdin, S. M., Omar, H. A., Alniss, H., & Al-Tel, T. H. (2020). BACE1 inhibitors: Current status and future directions in treating Alzheimer's disease. Medicinal research reviews, 40(1), 339-384. [CrossRef]
- Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., ... & van der Flier, W. M. (2021). Alzheimer's disease. The Lancet, 397(10284), 1577-1590. [CrossRef]
- Fan, J., Fu, A., & Zhang, L. (2019). Progress in molecular docking. Quantitative Biology, 7, 83-89. [CrossRef]
- Odhar, H. A., Rayshan, A. M., Ahjel, S. W., Hashim, A. A., & Albeer, A. A. M. A. (2019). Molecular docking enabled updated screening of the matrix protein VP40 from Ebola virus with millions of compounds in the MCULE database for potential inhibitors. Bioinformation, 15(9), 627. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).