1. Introduction
The aetiological association between infection with high-risk types of human papillomaviruses (HPV) and cancers of the anogenital area including uterine cervix, anus, penis, vagina and vulva; as well as the head and neck region particularly the lingual and palatine tonsils, is well known [
1]. An alert of cancer epidemic due to HPV-associated oropharyngeal squamous cell carcinoma (OPSCC) has been raised in 2010 [
2]. Reports published over the last decade confirm the increasing burden of OPSCC with high incidence rates observed particularly in Europe, North America, Australia and New Zealand [1, 3, 4]; whereas most parts of Asia are still at a relatively low incidence. The aim of our study was to depict changes in the incidence and the HPV-positive portion of OPSCC over the recent decades in Hong Kong, a metropolitan city in East Asia.
2. Materials and Methods
A retrospective study was conducted to determine changes in the incidence of OPSCC and the proportion infected with HPV in Hong Kong.
The first part of the study was based on the territory-wide cancer statistics captured by the Hong Kong Cancer Registry [
5]. Age-standardized incidence rates of major types of head and neck squamous cell carcinoma in Hong Kong from 1986 to 2020 were analysed. To elucidate any differences between HPV- and non-HPV-associated head and neck cancers, the annual number of new cases of OPSCC and laryngeal SCC were further analysed. Laryngeal SCC was selected as it shares common risk factors, such tobacco, with OPSCC; but not HPV-associated.
The second part of the study was to examine changes in the proportion of OPSCC that were positive for high-risk HPV DNA. To achieve this, we identified histology-confirmed OPSCC cases that were diagnosed between 2010-2020 from four major hospitals. Paraffin-embedded tissues were retrieved for testing. The study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Ethics Committee, and Ethics Committees of the hospitals.
The first and last sections of the formalin-fixed paraffin-embedded tumor tissue blocks were examined to ensure a sufficient tumor mass was obtained. For each sample, 5 sections of 5-µm tissue roll was sectioned for DNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, USA) following the manufacturer's protocol.
The quality of extracted DNA was assessed by a real-time PCR targeting the prostaglandin transporter (PGT) gene. A TaqMan primer/probe set targeting the host prostaglandin transporter (PGT) gene 1 was used to monitor the quality of DNA extracted from FFPE samples. The one-step real-time RT-PCR contained 2 μL of the extracted DNA, 10 μL of 2X TaqMan™ Master Mix (Applied Biosystems, USA), 0.15 µM forward primer (5’-ATC CCC AAA GCA CCT GGT TT-3’), 0.15 µM reverser primer (5’-AGA GGC CAA GAT AGT CCT GGT AA-3’), and 0.25 µM probe (5’-FAM-CC ATC CAT G -ZEN- T CCT CAT CTC -IABkFQ-3’) in a final reaction volume of 20 μL. The cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, and performed with the StepOnePlus Real-Time PCR System (Applied Biosystems, USA). Samples were considered negative if the Ct values exceeded 39.9 cycles. Extracted preparations with sufficient DNA quality were submitted for next-generation sequencing to detect HPV DNA.
HPV detection and genotyping was performed using two PCR-based amplicon sequencing assays targeting the conserved L1 open reading frame (ORF) of HPV as previously described [
6]. In brief, a pair of dual 12-bp barcodes was indexed to the PCR amplicon using forward and reverse primers for demultiplexing. Short reads generated by Illumina MiSeq PE150 were blasted against a comprehensive PV reference database using UPARSE [
7]. An operational taxonomic unit (OTU) count table was created using a 90% identity threshold, assigning each OTU with a PV type. Based on the IARC classification, 12 HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) were ranked as high risk (HR) and considered oncogenic [
8].
Confidence intervals of the observed HPV positive rates were calculated using the Wilson score method without continuity correction [
9]. The trend of change in positive rates was assessed by Chi-square test for tend (Statcalc, Epi Info Version 7.2.5.0, Centre for Disease Control and Prevention, US). P-values less than 0.05 were regarded as statistically significant. Age distribution was assessed by T-test [
10].
3. Results
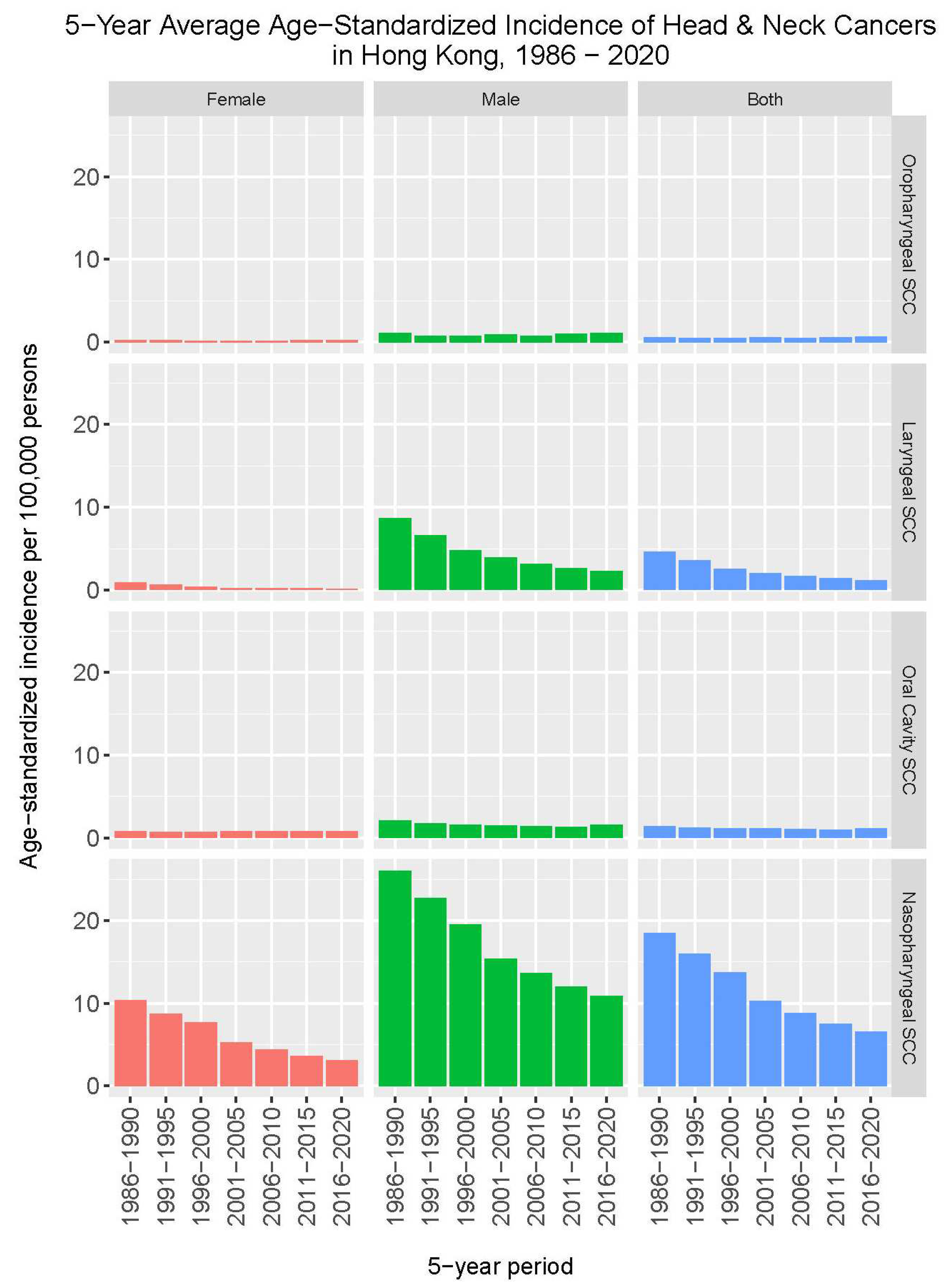
Figure 1 shows changes in the incidence rates of major types of head and neck squamous cell carcinoma from 1986 to 2020 in Hong Kong. In contrast to a marked decrease in the incidence rates of nasopharyngeal cancer and laryngeal cancer, where the 5-year average annual age-standardized incidence rates dropped from 18.5 to 6.6 per 100,000, and 4.6 to 1.2 per 100,000, respectively; such trend of decrease was not observed for cancers of the oropharynx and oral cavity.
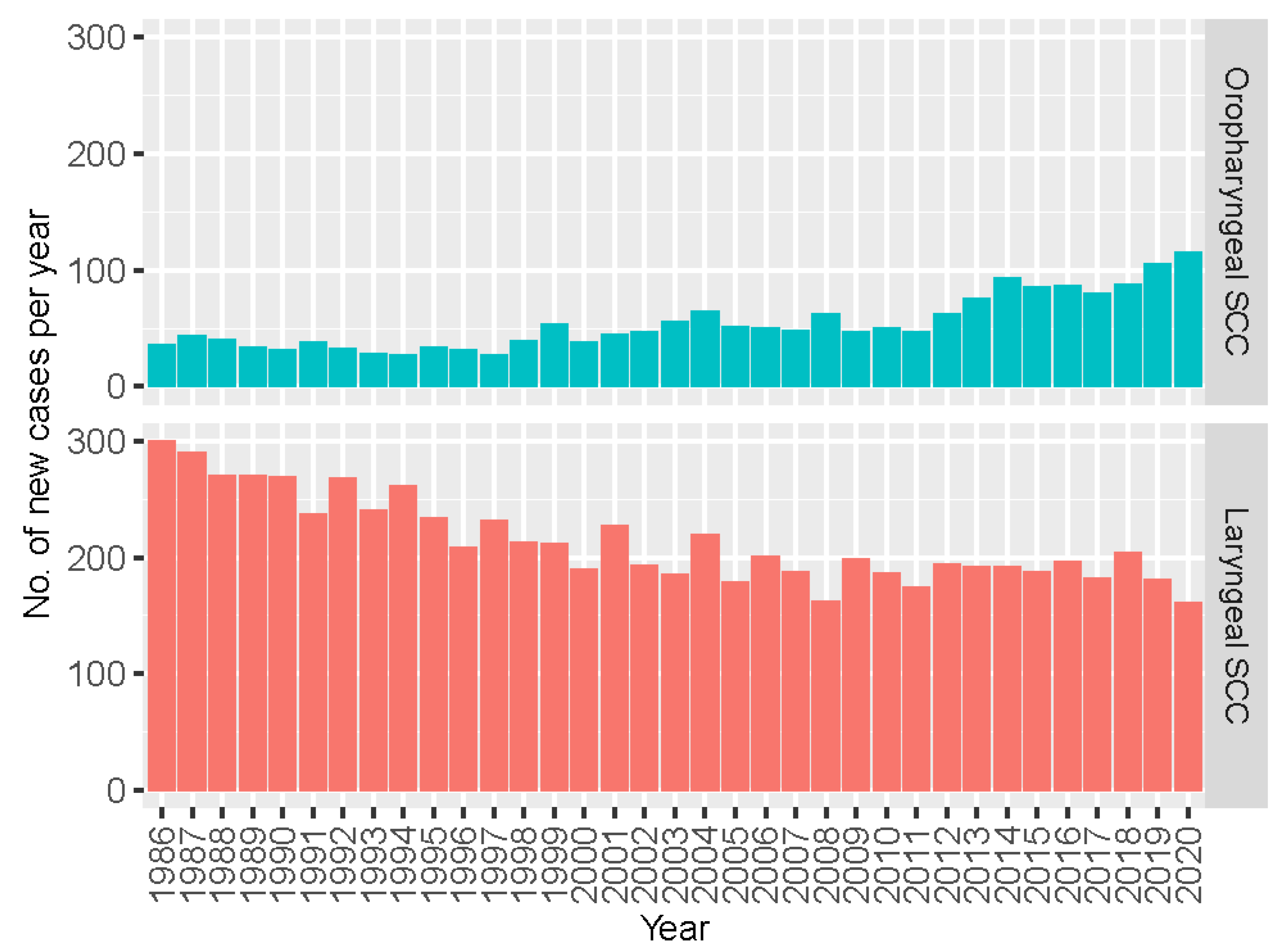
We then took a closer look at OPSCC with reference to laryngeal SCC that serves a proxy of non-HPV associated head and neck cancer. As shown in
Figure 2, a contrasting trend of change between these two types of head and neck cancer was noted. The annual number of new cases of OPSCC increased consistently from 36 cases in 1986 to 116 cases in 2020, whereas those of laryngeal cancer decreased from 300 cases to 162 cases.
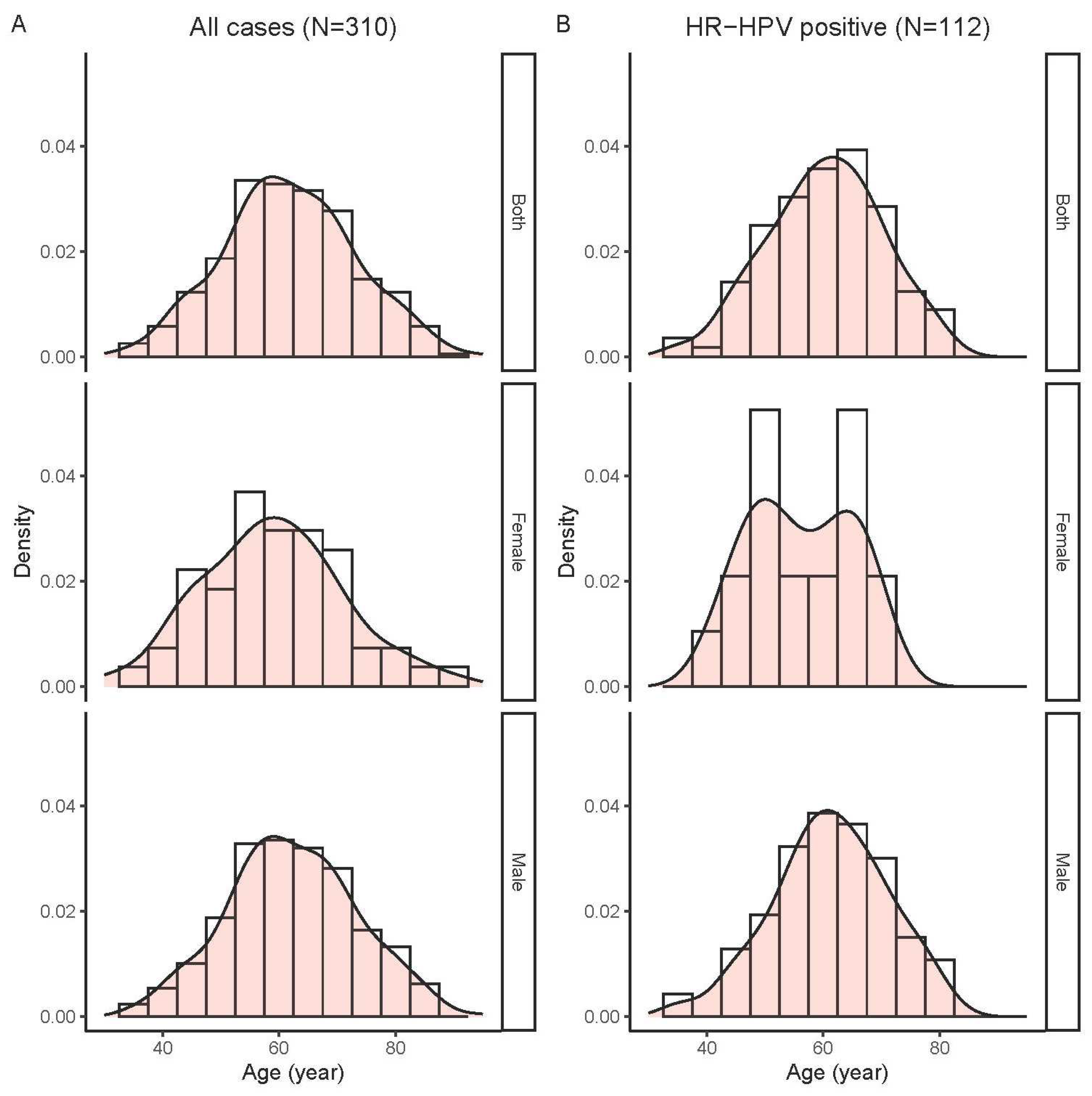
Since infection with high-risk HPV is a known aetiological factor for OPSCC, we further examined changes in the proportion of OPSCC harbouring HPV over the past years. In this regard, 310 cases with adequate DNA quality including 132 tonsil SCC and 178 non-tonsil OPSCC diagnosed between 2010 and 2020 were analysis. These study patients aged 31-94 years (mean, 61.7; standard deviation [SD], 11.4); and the majorities were men (256/310, 82.6%). The age distribution of study cases is shown in
Figure 3A.
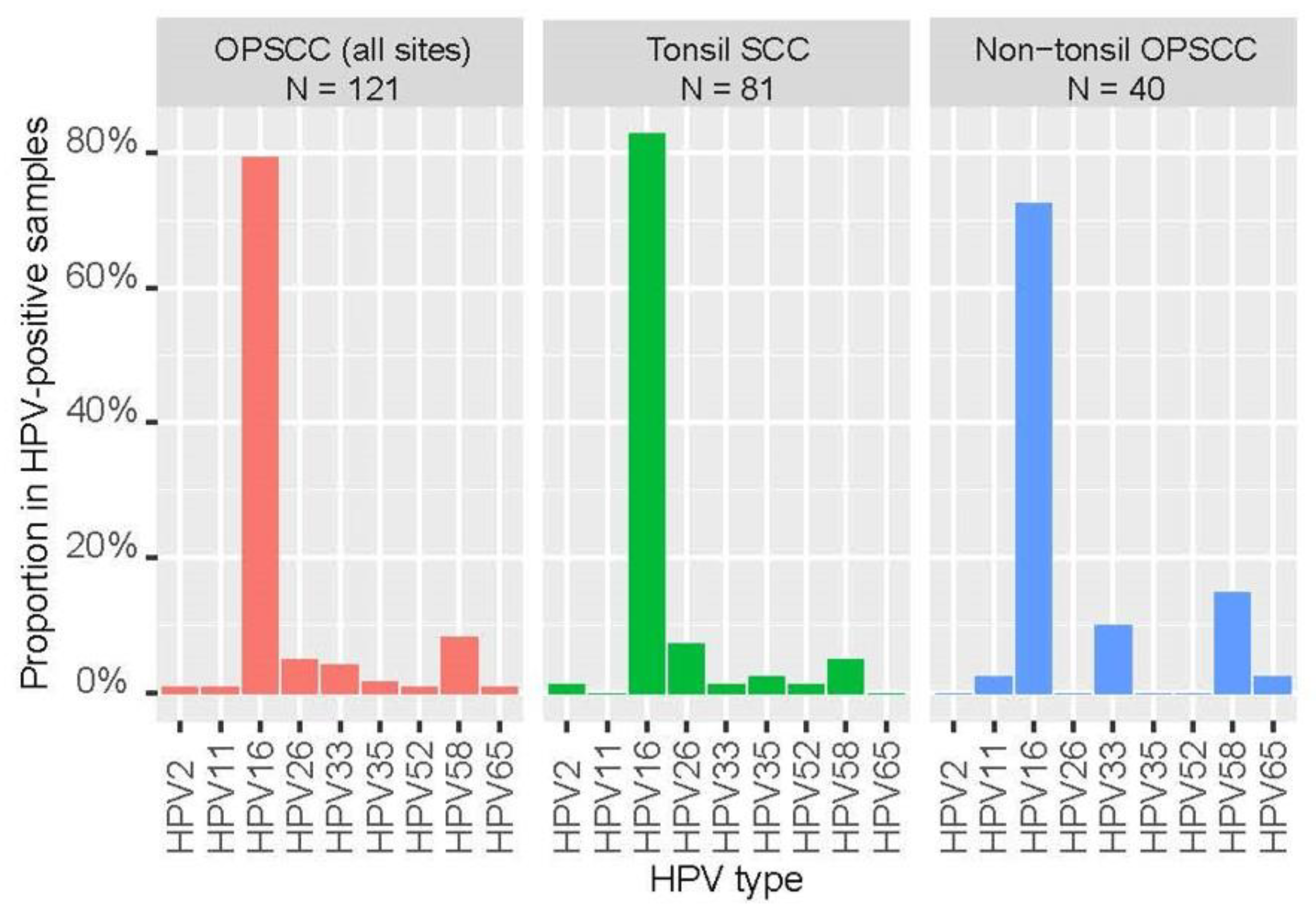
Of the 310 tumour samples, 121 (39.0%) were positive for HPV DNA with a vast majority (92.6%) 112/121) being high-risk types. The positive rates for high-risk HPV were 36.1%, 56.1% and 21.3% for all OPSCC, tonsil SCC, and non-tonsil OPSCC, respectively. Two samples harboured co-infection with both HPV16 and HPV58, others were single-type infections. HPV16 was the most common high-risk type being detected in 79.3% (96/121) of positive samples, followed by HPV58 (10/121, 8.3%) and HPV33 (5/121, 4.1%). Of note, HPV26, a type with uncertain carcinogenicity was detected in 6 cases of tonsil SCC. The distribution of HPV types in tonsil and non-tonsil SCC is shown in
Figure 4.
Further analyses on the correlation between high-risk HPV infection and sex and age were performed. The positive rates for high-risk HPV of all OPSCC cases combined were similar between the two genders (36.3% [93/256] for males, 35.2% [19/54] for females, P = 0.874 by Chi-squared test). There were also no significant difference in age between patient groups positive or negative for high-risk HPV (mean [SD]: 60.3 [9.8] vs. 62.4 [12.1] years, P = 0.118 by T-test). Female, but not male, patients positive for high-risk HPV DNA displayed a bi-modal age distribution (
Figure 3B).
Further subgroup analyses restricted to the 132 patients with tonsil SCC revealed that the age of high-risk HPV positive group was significantly younger than those of high-risk HPV negative group (mean [SD]: 58.9 [9.9] vs. 64.3 [13.3] years, P = 0.006 by T-test), whereas no difference between genders was observed (male/female: 4.7 [61/13] vs 4.3 [47/11] for high-risk HPV positive and negative groups, respectively).
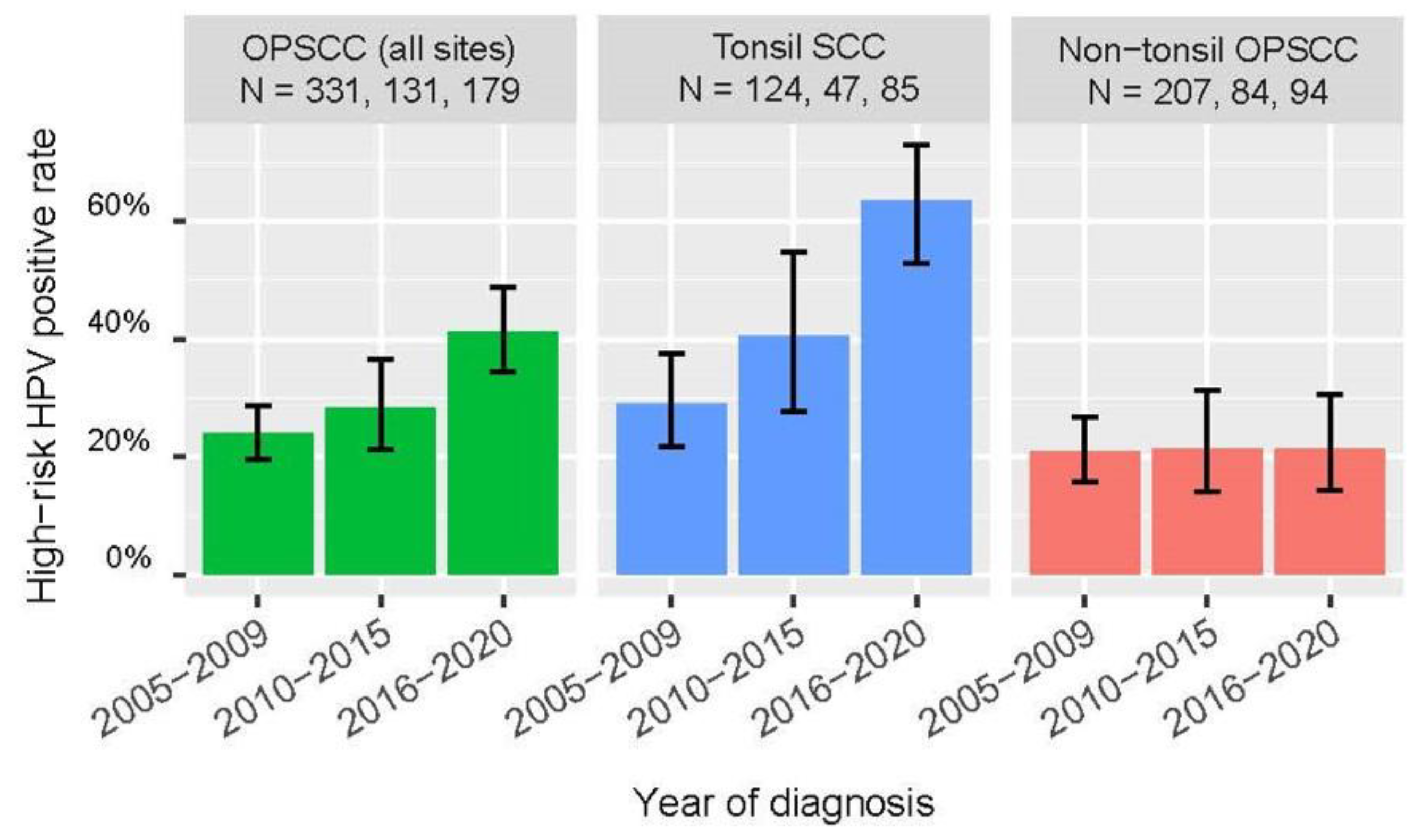
We observed a significantly higher positive rate for high-risk HPV among OPSCC (all sites combined) and tonsil SCC diagnosed between 2016-2020 compared to those diagnosed between 2010-2015 (all-site OPSCC: 41.9% [95% CI: 34.9-49.2] vs 28.2% [21.2-36.5], P = 0.013 by Chi-squared test; tonsil SCC: 64.7% [54.1-74.0] vs. 40.4 [27.6-54.7], P = 0.007 by Chi-squared test; whereas no significant increase was observed for non-tonsil SCC (
Table 1).
Figure 5 incorporated findings of a previous local study based on similar methodologies to exhibit the trend of change in high-risk HPV positive rates [
11]. Of note, there was a significant linear trend of increase in the proportion of OPSCC (all-site combined) and tonsil SCC positive for high-risk HPV from 2005 to 2020 (P < 0.001 for both by Chi-squared test for linear trend).
4. Discussion
There is a good body of evidence to demonstrate the success of HPV vaccine in preventing cervical cancer. However, similar efficacy data for oropharyngeal cancer is not yet available. The lack of a readily detectable pre-cancerous stage as an efficacy trial end-point, and the long lag time between oral infection and cancer development are the key bottlenecks [
12]. Nevertheless, current circumstantial evidence suggests HPV vaccine may play a role in preventing oropharyngeal and other HPV-associated head and neck cancers. While several health authorities have included the prevention of HPV-associated head and neck cancers as part of the indication for HPV vaccination, a gender-neutral vaccination program is still not widely available, including Hong Kong. In this context, solid data on the local trend of change in the incidence of HPV-associated head and neck cancers can support an informed public health decision as whether the HPV vaccination program should be expanded.
While a notable trend of increase in the incidence of HPV-associated oropharyngeal cancer has been reported across North America and Europe, the findings from Asia are more varied. A report from Taiwan showed that both the HPV-related and -unrelated head and neck cancers increased during 1995-2009, with the fastest increase in tonsil cancer, particularly among men. The incidence of HPV-related head and neck cancers among Taiwanese men increased from 2.24 per 100,000 in 1995 to 6.15 per 100,000 in 2009 that was similar to those of Western countries [
13]. A study from Korea based on Health Insurance Review and Assessment (HIRA) database showed an increase in male oropharyngeal cancer from 2.7 to 3.1 per 100,000 from 2013 to 2016 [
14]. While studies from mainland China and Japan also reported an increase in incidence, the magnitude of change were relatively small. A study from mainland China based on the Chinese Cancer Registry Annual Report covering 2007-2015 observed an increase in male oropharyngeal cancer incidence by 3.1% annually [
15]. In Japan, between 1993 and 2015, an annual 5% increase in incidence of male oropharyngeal cancer was reported [
16].
The current study represents the first of its kind in Hong Kong where changes in the incidence rate as well as the HPV-attributed proportion of oropharyngeal cancer were examined. The 310 cases available for HPV test in this study accounted for 34.7% of all OPSCC cases recorded by the Hong Kong Cancer Registry during the study period 2010-2020 [
5]. Our findings should be representative of the whole Hong Kong population. We found a remarkable increase in the proportion of tonsil cancers that were positive for high-risk HPV, whereas the positive rate among OPSCC of non-tonsil sites remained unchanged over the years. This is in line with the current understanding that tonsils are the most preferred site within the head and neck region for HPV-mediated carcinogenesis [
17].
Oropharyngeal cancer and laryngeal cancer share common risk factors such as tobacco, and yet the former but not the latter has a strong aetiological association with HPV. Our finding of an opposite direction of change in incidence of these two cancers further support that HPV is the driving force for the consistent increase in incidence of oropharyngeal cancer over the last few decades in Hong Kong. Although a separate data for tonsil and non-tonsil OPSCC were not available from the Hong Kong Cancer Registry, our results suggest that HPV-associated tonsil cancer was the main driving force for the overall increase in oropharyngeal cancer in Hong Kong. Given the strong carcinogenicity of HPV, tobacco control alone is unlikely to achieve satisfactory effect on combating oropharyngeal cancer. While the current number of new cases of oropharyngeal cancer in Hong Kong is relatively low, at around a hundred cases per year; actions should be taken early to prevent further upsurge in the coming decades. A gender-neutral HPV vaccination program in Hong Kong is worth considering.
Our study revealed a difference in the distribution of HPV types in oropharyngeal cancer compared to those reported from the West. While HPV16 was the most common type found in our locality and the West [
12,
17]; HPV58 being rarely detected in the West ranked the second in our locality. Such ranking of high-risk HPV types in oropharyngeal cancer in Hong Kong is similar to those of cervical cancer [
18]. It would be worthwhile to investigate whether HPV58 is also more prevalent in oropharyngeal cancer in other cities in East Asia and Latin America where HPV58 also ranks high in cervical cancer [
19,
20].
HPV26 is an HPV type with uncertain carcinogenicity. For the purpose of analysis in this study, we classified the 6 tonsil SCC harbouring HPV26 into the non-high-risk HPV group. Of note, HPV26 was reported to be the third most common type, following HPV16 and HPV33, detected from 1090 oropharyngeal cancer cases collected from 29 countries in Europe, Africa, Asia and America [
21]. The true oncogenicity of HPV26 in oropharyngeal cancer deserves further investigation, particularly in view of the lack of coverage of current vaccines for this type.
Our study has limitations. Firstly, there was underestimation in the incidence of oropharyngeal cancer in Hong Kong since those cancers originated from the base, i.e. posterior one-third, of tongue could be counted under “tongue” rather “oropharyngeal” squamous cell carcinoma in the Hong Kong Cancer Registry. Secondly, we used a sensitive method to detect HPV DNA that may reveal by-stander infections that were not the culprit of carcinogenesis. Our study did not include testing for HPV E6/E7 mRNA and p16 to verify the role of HPV detected. Nevertheless, the trend of increase in HPV positivity observed in this study is likely a reflection of a genuine increase in HPV attribution in oropharyngeal cancer in Hong Kong over the last few decades.
5. Conclusions
The burden of HPV-associated oropharyngeal cancer is escalating worldwide, and Hong Kong is not an exception. While a great effort has been focused on the control of tobacco consumption, the other important carcinogen, HPV infection, should not be neglected. Research studies to generate more direct and indirect evidence on the protection offered by HPV vaccination would enhance an evidence-based decision of policy makers to broaden the scope of vaccination program against other health needs. Development on HPV-based and other biomarkers for early cancer screening is an urgent priority.
Author Contributions
Conceptualization, PKSC, EWHL; methodology, ZC, SSB, WCSH; resources, ABWC, LSK, MHC, WTL; formal analysis, JYKC, SSB; investigation, CC, CX; writing—original draft preparation, PKSC, ZC; writing—review and editing, all authors; supervision, RWYN, CKCL; project administration, WCSH; funding acquisition, PKSC. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Investigator Studies Program (MISP59351) from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., 35 Rahway, NJ, USA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Joint Chinese University of Hong Kong-New Territories East Cluster Ethics Committee (Ref. No. 2020.481, date of approval: 14 December 2020), and the Ethics Committees of Tuen Mun Hospital (Ref. No. NTWC/REC/21047, date of approval 17 June 2021), Yan Chai Hospital (KWC-REC Ref. No.: KW/EX-21-164(166-06), date of approval: 5 January 2022) and Queen Elizabeth Hospital (KC/KE-21-0281/ER-4, date of approval: 13 January 2022).
Informed Consent Statement
The ethics committees approved to waive consent from individual patients.
Data Availability Statement
Conflicts of Interest
PKSC received honorarium from Merck Sharp and Dohme, GlaxoSmithKline, Moderna and Pfizer for serving as speaker, advisor or consultant. JYKC and EWHL received honorarium from Merck Sharp and Dohme for serving as a speaker, advisor or consultant. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D'Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Lorenzoni, V.; Chaturvedi, A.K.; Vignat, J.; Laversanne, M.; Bray, F.; Vaccarella, S. The Current Burden of Oropharyngeal Cancer: A Global Assessment Based on GLOBOCAN 2020. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 2054–2062. [Google Scholar] [CrossRef]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Hong Kong Cancer Statistics. Hong Kong Cancer Registry, Hospital Authority. Available online: https://www3.ha.org.hk/cancereg/default.asp. (accessed on 1 Sep 2023).
- Wong, M.C.S.; Vlantis, A.C.; Liang, M.; Wong, P.Y.; Ho, W.C.S.; Boon, S.S.; Sze, R.K.H.; Leung, C.; Chan, P.K.S.; et al. Prevalence and Epidemiologic Profile of Oral Infection with Alpha, Beta, and Gamma Papillomaviruses in an Asian Chinese Population. J. Infect. Dis. 2018, 218, 388–397. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–441. [Google Scholar]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Statistics in Medicine 1998, 17, 857–872. [Google Scholar] [CrossRef]
- T-test Calculator. Social Science Statistics. Available online: https://www.socscistatistics.com/tests/studentttest/default2.aspx. (accessed on 30 August 2023).
- Lam, E.W.; Chan, J.Y.; Chan, A.B.; Ng, C.S.; Lo, S.T.; Lam, V.S.; Chan, M.M.; Ngai, C.M.; Vlantis, A.C.; Ma, R.K.; et al. Prevalence, Clinicopathological Characteristics, and Outcome of Human Papillomavirus-Associated Oropharyngeal Cancer in Southern Chinese Patients. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 165–173. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–42. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.Z.; Hsiao, J.R.; Tsai, C.R.; Chang, J.S. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995-2009. Int. J. Cancer 2015, 137, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, D.; Son, K.B.; Bae, S. Incidence, cost and gender differences of oropharyngeal and noncervical anogenital cancers in South Korea. BMC Public Health 2020, 20, 1035. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Xu, K.; Huang, L.; Yuan, M.; Wang, H.; Qiao, Y.; Zhao, F. Temporal Trends and Projection of Cancer Attributable to Human Papillomavirus Infection in China, 2007-2030. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 1130–1136. [Google Scholar] [CrossRef]
- Kawakita, D.; Oze, I.; Iwasaki, S.; Matsuda, T.; Matsuo, K.; Ito, H. Trends in the incidence of head and neck cancer by subsite between 1993 and 2015 in Japan. Cancer Med. 2022, 11, 1553–1560. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Chan, P. K.; Cheung, T. H.; Tam, A. O.; Lo, K. W.; Yim, S. F.; Yu, M. M.; To, K. F.; Wong, Y. F.; Cheung, J. L.; Chan, D. P.; et al. Biases in human papillomavirus genotype prevalence assessment associated with commonly used consensus primers. Int. J. Cancer 2006, 118, 243–5. [Google Scholar] [CrossRef]
- Chan, P. K.; Ho, W. C.; Chan, M. C.; Wong, M. C.; Yeung, A. C.; Chor, J. S.; Hui, M. Meta-Analysis on Prevalence and Attribution of Human Papillomavirus Types 52 and 58 in Cervical Neoplasia Worldwide. PLoS ONE 2014, 9, e107573. [Google Scholar] [CrossRef]
- Chan, P. K.; Zhang, C.; Park, J. S.; Smith-McCune, K. K.; Palefsky, J. M.; Giovannelli, L.; Coutlée, F.; Hibbitts, S.; Konno, R.; Settheetham-Ishida, W.; et al. Geographical distribution and oncogenic risk association of human papillomavirus type 58 E6 and E7 sequence variations. Int. J. Cancer 2013, 132, 2528–36. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).