1. Introduction
WHO pioneered the concept of essential medicines in 1977 when it introduced the model essential medicine list (EML) [
1]. According to WHO, essential medicines are defined as “
those that satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness” [
2]. The whole idea of essential medicines is to ensure their availability within the context of functioning healthcare systems at all times in sufficient quantities, in appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. Various country-specific essential medicine lists have been developed to address the disease burden of respective nations. India produced its first National Essential Drugs List in 1996 which was later revised in 2003, 2011, and 2015 as the National List of Essential Medicines (NLEM) [
3]. The latest revision of NLEM was released in September 2022 [
4]. In line with NLEM, the majority of the states maintain their state-specific EMLs also which focus on commonly used medicines at different healthcare levels in their respective areas.
One of the major public health concerns identified in recent times is the lack of regular access to essential medicines which in turn has significant implications on the prescribing behavior of clinicians urging them to prescribe lesser effective, more toxic, and/or more expensive agents leading to increased healthcare costs and poor treatment outcomes [
5]. In the case of essential antimicrobials, an additional issue of concern with their restricted availability is the emergence of antimicrobial resistance (AMR), a rapidly emerging global threat [
5]. Poor access to essential antimicrobials is also a driver of high mortality especially in low-middle-income countries [
6]. Besides compromised health outcomes, the economic consequences of such limited access are enormous with an estimated cost of €20-30 million linked to a shortage of just one anti-microbial as per the WHO report [
7]. Hence, there is a need to address the issue of restricted availability of essential antimicrobials which poses a serious threat to rational antimicrobial use and hinders the attainment of successful antimicrobial stewardship.
Extensive data from middle to high-income countries report sub-optimal availability of essential antibiotics [
8,
9,
10,
11]. In a survey conducted by the European Society of Clinical Microbiology and Infectious Diseases Study Group for Antimicrobial Stewardship (ESGAP), the researchers assessed the availability of a list of antibiotics across 38 countries in Europe, US, Canada, and Australia. Systemic antibiotics approved by US Food and Drug Administration (FDA) and/or European Medicine Agency (EMA) and/or in Europe (35 countries), Canada and Australia were enlisted as antibiotics to be surveyed. It was reported that 22 out of the 33 selected antibiotics were available in less than 20 included countries [
8]. Later in 2015, the updated survey reported an even worse situation with 25 of 36 selected antibiotics marketed in 20 of 39 countries or less [
9]. Quadri et al reported a shortage of 148 antibacterial drugs in the USA between 2001 to 2013 with 22% of drugs experiencing multiple shortage periods [
10]. Another study from Japan reported critical shortages in cefazolin supply with resultant many folds increased use of third-generation cephalosporins- cefotaxime and ceftriaxone (both ‘watch’ group antibiotics) [
11].
Data on the availability of antimicrobials from low and middle-income countries (LMICs) is limited and patchy [
12,
13,
14,
15]. In a large survey conducted across 36 developing and middle-income countries by Cameron et al, the mean availability of amoxicillin 250 mg capsule/tablet and ciprofloxacin 500 mg capsule/tablet were reported as 68.7 and 52% in the public sector and 76 and 82.4% in the private sector, respectively [
14]. Knowles et al, in another survey conducted across 20 low- and middle-income countries (LMICs), reported a 48.9% median availability of 27 antibiotics (19 access, 7 watch, 1 unclassified) in all health facilities surveyed [
15].
In a country like India where the drug procurement system in the public sector is quite different from Western countries and the supply of medicines is mainly governed by demand and prescription patterns, an analysis of the status of availability of essential medicines needs to be undertaken. With this background, the present study was planned to generate information on the availability of essential anti-microbials listed in NLEM 2015 in public as well as private sectors in a district of North India and to identify the reasons for their non-availability.
2. Results
A total of 25 pharmacies comprising 13 public (7 primary, 5 secondary, and 1 tertiary healthcare), 10 private, and 2 other sector pharmacies were surveyed in the district.
2.1. Availability of antimicrobials listed in primary (NLEM 2015) and secondary (selected) lists.
2.1.1. Antibiotics
In the public sector, antibiotics with more than 80 percent availability were tablet/ capsule formulations of amoxicillin, amoxicillin-clavulanic acid, cefixime, azithromycin, doxycycline, ciprofloxacin, cotrimoxazole, metronidazole and injection ceftriaxone. Cefazolin, cefuroxime and clarithromycin were not available in public sector. Except cloxacillin and cefazolin, which were available in up to 30 percent of surveyed private retail pharmacies, most of the antibiotics were optimally available in the majority. For most of the antibiotics, availability in other sector pharmacies was parallel to that in the public sector. There was absolute non-availability of benzathine benzyl penicillin and benzyl penicillin across all sectors in the district (
Table 1).
2.1.2. Other antimicrobials
Tablet albendazole and ivermectin were optimally available anthelminthics across all sectors. Among anti-virals, acyclovir and oseltamivir were available in majority of the pharmacies while clotrimazole pessary and tablet fluconazole, itraconazole and voriconazole were optimally available anti-fungals. There was, however, poor availability of amphotericin B, anti-malarials, nystatin, other anti-virals and anthelminthics in all surveyed pharmacies (
Table 2).
2.1.3. Pediatric formulations of antimicrobials
Most of the pediatric formulations of antibiotics including amoxicillin, amoxicillin-clavulanic acid, cefadroxil, cefixime, azithromycin, and metronidazole were available in primary and secondary care public pharmacies. Among such formulations available in tertiary care centres were amoxicillin, amoxicillin-clavulanic acid, azithromycin, and cotrimoxazole. The availability of pediatric antibiotics in private and other sector pharmacies was optimal for most of the agents surveyed. Suspension albendazole was available in more than 70% of facilities surveyed across different sectors. There was poor availability of acyclovir pediatric formulation in the desired tertiary care as well as in private and other sector facilities.
2.2. Gap analysis for the availability of antimicrobials in public sector.
According to NLEM 2015, the overall availability of anti-protozoals, anthelminthics and anti-fungals was less than 50 percent across all healthcare levels while anti-virals had sub-optimal overall availability in secondary care facilities (
Table 3).
In contrast, there was good overall availability of all antimicrobial classes across all healthcare levels as per selected list (
Table 4).
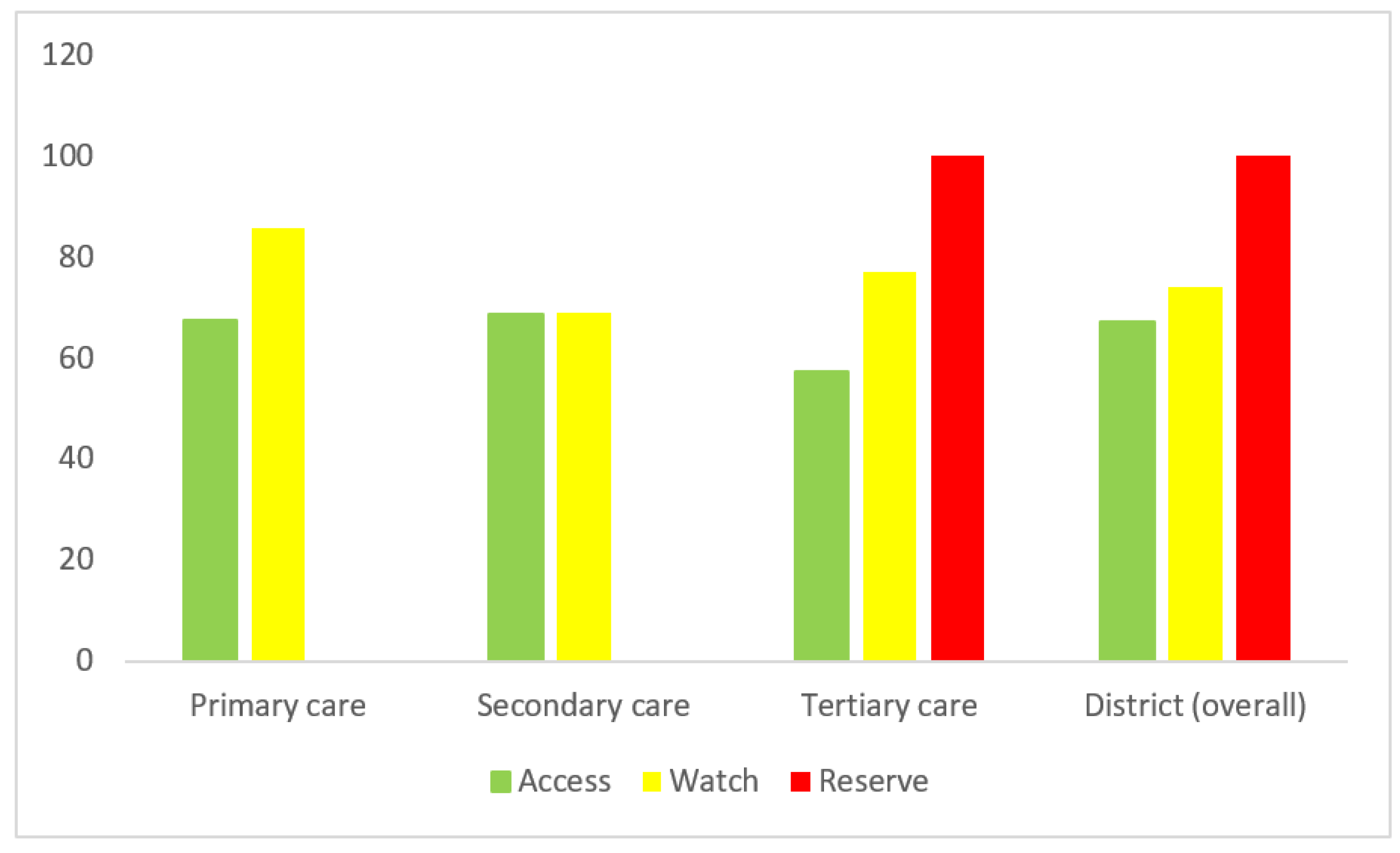
More than 60 percent of the desired ‘Access’ group of antibiotics were available in primary and secondary healthcare facilities, while approximately 70 percent availability of the ‘Watch’ group was observed in either sector. In tertiary care, there was optimal availability (more than 50 percent) for all the AWaRe groups (
Figure 1).
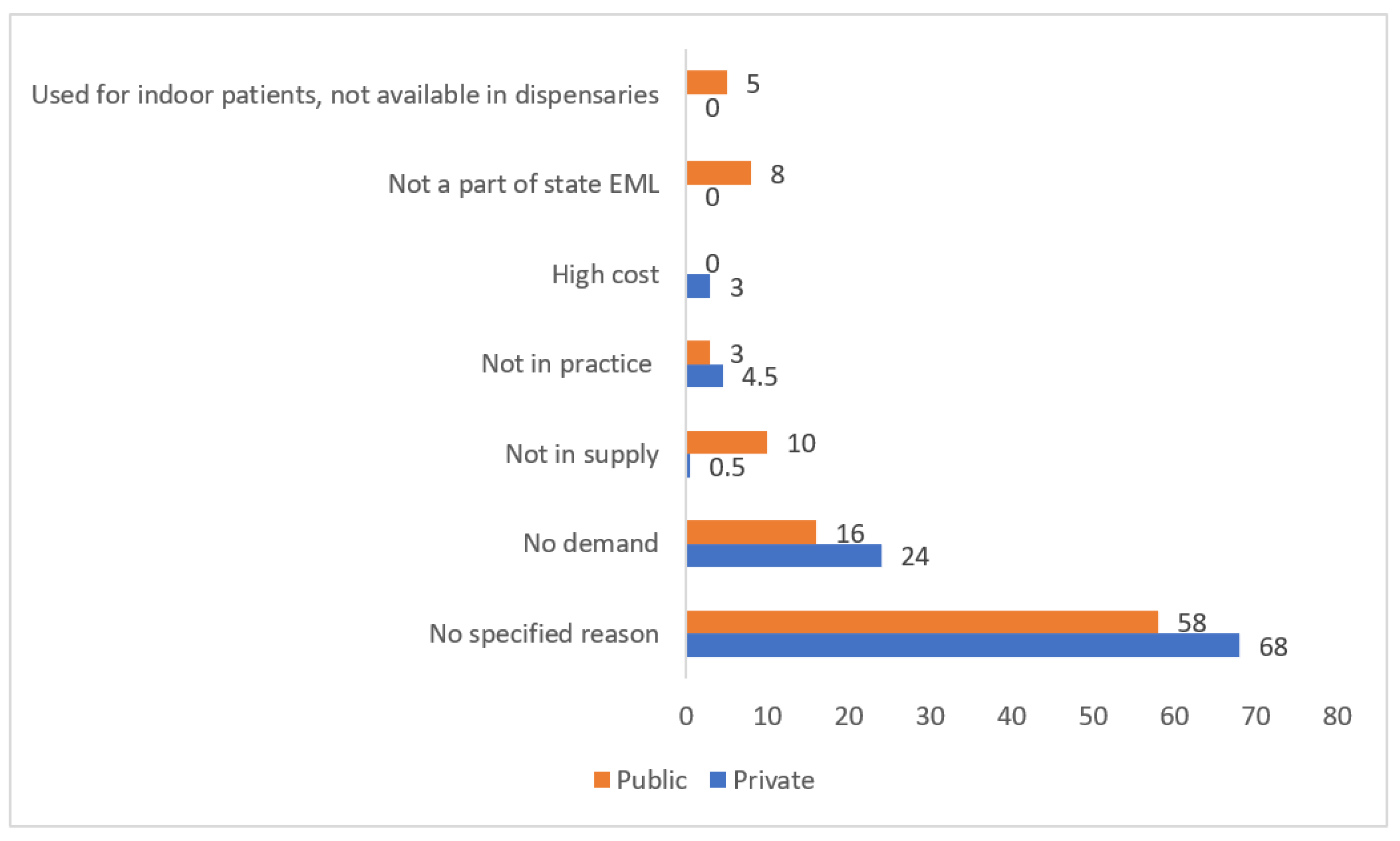
2.3. Reasons for sub-optimal availability
For many antimicrobials, the reasons for their non-availability were not specified. Low demand/ no prescriptions was the most frequently cited reason in either sector while lack of supply and non-listing of the drug/s in the state EML were the other common reasons quoted for their limited availability in public sector (
Figure 2).
3. Discussion
Access to essential medicines is a crucial element of universal health coverage. The present survey was conducted to evaluate the availability of essential antimicrobials included in NLEM 2015 and a selected/ secondary list for survey.
For both primary and secondary lists of antimicrobials, overall availability at a particular healthcare level and total overall availability across all levels showed comparable results to the study by Prinja et al (total overall availability: 59.6% and 47.2% for antibiotics and antifungals, respectively) [
12] assessing the availability of medicines listed in state EML and provided under National Health programs in addition to NLEM which may explain the discordance seen for antivirals and anthelminthics/ antiprotozoals from the current study. In another survey investigating the availability of a basket of 24 essential and 8 high-end antibiotics across various public and private pharmacies in Delhi, Kotwani et al reported sub-optimal availability of few essential antibiotics such as ampicillin suspension, benzathine penicillin, cefixime, etc. in the public sector pharmacies and reasonably good availability of non-essential and high-end antibiotics in both public and private sectors [
13].
Limited availability of access groups and optimal availability of watch groups are two sides of the same coin and a hindrance not only to the attainment of WHO-defined common global target of access groups to comprise >60% of overall antibiotic use but also effective stewardship interventions [
16]. Of note, there was optimal availability of most access group of antibiotics across public and private sectors. Besides these, antibiotics used to treat common infections encountered in primary or secondary care such as doxycycline, azithromycin, ciprofloxacin, and cefixime were available in most of the respective pharmacies. These findings are in line with an earlier large survey conducted in 20 LMICs [
15].
The issue of poor availability of antibiotics for pediatric use is particularly troublesome owing to the fact that fewer agents are approved by regulatory authorities for use in children as compared to adults and finding safe, alternative therapies for agents with poor availability is therefore quite challenging [
17]. In a survey conducted in Odisha, paediatric formulations of antibiotics included in the state EML (amoxicillin-clavulanic acid, amoxicillin, cotrimoxazole azithromycin) were reported to be available in less than 60 percent of public and private facilities [
18]. In our survey, the availability of desired paediatric antibiotic preparations was though optimal in most primary and secondary care centers, but it was not so in the tertiary care centre. Low demand and less prescriptions were reported as the reason for their non-availability which may partially be explained by the fact that most inpatients in tertiary care referral centres have severe illness demanding parenteral administration of drugs. However, whether it is the true demand-supply relation or vice versa (poor availability affecting the prescribing practices) needs to be further explored. In fact, intravenous to oral switch of antibiotics is a key strategy defined under antibiotic stewardship fruitful implementation of which may be governed by the availability of oral preparations apart from the physicians’ prescribing behaviours. There is limited data regarding impact of antibiotic shortages on paediatric population in India. Few researchers from developed nations have reported a higher incidence of acute kidney injury among children associated with piperacillin-tazobactam and vancomycin combination therapy when used as an alternative to anti-pseudomonal beta-lactam antibiotics due to limited availability of the latter [
19,
20].
Sub-optimal availability of some antimicrobials such as penicillin, cefazolin, cloxacillin, antimalarials, antivirals etc. particularly in the public sector seems alarming at first instance, however, a deeper understanding of the associated factors probably throws some insight into the situation. Commonly cited reasons for the poor availability of most surveyed drugs in public pharmacies were poor supply from district warehouses which is mainly governed by demand from various pharmacies and in turn by the prescribing patterns. For example, declining trend in reported cases of syphilis in public tertiary care centres in Rohtak [
21] may partly account for very low prescription rate of benzathine penicillin. This is largely conjectural since the aforementioned data was hospital based and may not be reflective of true prevalence of syphilis in the community where most people may be resorting to private practitioners. Cloxacillin is another beta-lactam antibiotic effective against methicillin-sensitive
staphylococcus aureus whose demand and supply is mainly governed by local resistance patterns, a fact that demands a deeper analysis of the linking between antibiotic resistance and utilization patterns. Cefazolin and cefuroxime, belonging to cephalosporin group of antibiotics and recommended for surgical prophylaxis were not available in public pharmacies with low demand and non-listing of these drugs in Haryana state EML being the quoted reasons for non-availability. Non-availability of antimalarial agents in the public sector was explained by the fact that the district of Rohtak (in addition to six other districts viz. Ambala, Bhiwani, Jind, Kaithal, Karnal, and Kurukshetra in the state of Haryana) was given ‘zero indigenous case’ status in the year 2021 after no case was reported for 1 year.
Extensive use of amphotericin B during the then-recent coronavirus associated mucormycosis outbreak mainly accounted for the limited availability of this anti-fungal at the time of the survey conducted in early 2022. However, there is a recovering trend for its availability and a follow-up survey may provide a better picture regarding this. Lack of supply of anti-leishmanial agents (miltefosine and paromomycin) was ascribed to infrequent reports of leishmaniasis in the district [
22] and the drugs being covered under National Vector Borne Disease Control Programme (NVBDCP) [
23]. Similarly, anti-hepatitis drugs (such as interferons, entecavir, ribavirin, sofosbuvir and tenofovir) are being provided under the National Viral Hepatitis Control Program (NVHCP) [
24] explaining their non-availability in public and private pharmacies.
Non listing of some drugs in state EML was another reason cited for their non-availability, for example, clarithromycin, antivirals except acyclovir and antiprotozoals except diloxanide are not included in Haryana state EML (2013-14), [
25] and thus had poor availability in public sector. On the other hand, availability of some agents used to treat commonly encountered infections such as nitrofurantoin, ivermectin, oseltamivir and itraconazole was reasonably good despite their not being listed in state EML, an observation emphasizing the need to periodically revise the list as per the emerging demands. Ivermectin, cefuroxime, itraconazole, and terbinafine have, however, been included in the latest revision of NLEM released in September 2022.
Few limitations of the study need to be considered. The study was designed to evaluate the availability of antimicrobials on the respective day of survey, however, we calculated the overall availability of antimicrobials for the various levels of facilities using standard mathematical formulae. Also, the conduct of survey across several facilities over a span of 3 months reasonably reflects average availability over time. Secondly, although the survey results give quantitative data regarding availability of antimicrobials but other important factors determining access to medicines such as pitfalls in procurement and/or distribution systems, prescribing practices in different settings, etc. were not taken into account. A concurrent prescription audit might contribute to drawing better conclusions and linking prescribing with local availability, which may be planned in future surveys of such kind. Issues such as the influence of drug pricing and regulation on availability also need to be addressed in future research. Thirdly, availability was assessed as a parameter of the supply of medicines. However, another critical factor influencing availability should be standard treatment guidelines which is a potentially important research question. Lastly, due to feasibility issues, the survey was conducted in a single district of North India and hence the results may not be reflective of the availability patterns in other districts in the state and in the country per se.
Despite the limitations, a few strengths of the study are worth mentioning. The present survey provides a snapshot of the availability of other antimicrobials in addition to antibiotics which were not taken into account in most of the surveys reported earlier. Both public and private sector pharmacies were covered while the earlier surveys from India mainly focussed on the public sector. Generating data on the availability of antimicrobials in private retail pharmacies is important to have a more comprehensive picture of the problem, a fact well recognized previously by researchers.12 Admittedly, a comparative evaluation of the availability of essential versus non-essential/ high-end antibiotics as determinants of prescribing practices is an area demanding further investigation. Besides this, there is a pressing need for periodic review of antibiotic resistance patterns and their correlation with antibiotic availability and prescribing behaviour.
4. Materials and Methods
4.1. Study setting
The present study was conducted in Rohtak, a district in the north Indian state of Haryana, with more than half of the total district population of 1.17 million residing in rural areas [
26]. The district has a 3-tier public healthcare delivery system as in the rest of the country: primary [primary health centers (PHCs) and sub-centers (SCs)], secondary [community health centers (CHCs), civil and district hospitals] and tertiary levels [medical colleges]. Besides the public sector, healthcare is also provided by private clinics, nursing homes, and corporate hospitals. As per the National Sample Survey data (2017-18), around two-thirds of the population depends on private sector for treatment [
27]. Nearly 70 percent expenditure on medicines is borne by patients out-of-pocket; reported as 59 to 86 percent in public sector in the three North Indian states of Haryana, Punjab and Chandigarh [
28].
4.2. Drug procurement model in the district
The procurement and distribution of medicines in the public sector in the district, Rohtak, Haryana is by “The Medicine Procurement and Management Policy 2012” [
29]. The medicines are procured from funds received from the state government (budget for the purchase of medicines) and the Government of India as part of the National Health Mission (NHM) program. The procurement of medicines is decentralized at district level whereby the district health societies are authorized to procure medicines and consumables and further distribute these to health facilities. Demand for medicines is put up by the health facilities to the local warehouse or supplier of medicine in the form of an indent. Every health facility maintains a record of demands, stockouts and utilization status of medicines.
4.3. Study design
A cross-sectional survey of medicine outlets across various public and private sectors in the district of Rohtak was carried out to gather information relevant to the study objectives. The types of medicine outlets surveyed included: Public sector: Pharmacies in public primary, secondary and tertiary care health centers; Private sector: Private retailers in the community (only licensed pharmacies and drug stores); and Other sector: Private pharmacies in public hospitals, health facilities run by non-governmental organizations such as charitable organizations, health facilities run by religious organizations, private hospitals, etc.
4.4. Survey facilities sampling
The pharmacies of the main public hospital/s in the district viz. Postgraduate Institute of Medical Sciences (PGIMS) and Civil Hospital (Rohtak) were surveyed. Sampling frame was constructed by compiling the lists of facilities in various sectors in the district as: (i)
public sector: all public health facilities (e.g. primary and community health centres, district/ sub-district and civil hospitals) in the district from the website of Health department, Haryana (
www.haryanahealth.gov.in); (ii)
private sector: currently licensed retail pharmacies from the website
www.medicineindia.org; and (iii)
other sector: private pharmacies in public hospitals, health facilities run by non-governmental such as charitable or religious organizations and private hospitals.
The facilities to be surveyed were selected by systematic random sampling. For this, the sampling interval was calculated as the total number of pharmacies (in rural, urban and semi-urban areas in the district) in the sampling frame (N) divided by the sample of interest (n=5 for primary and secondary care public pharmacies; 10 for private pharmacies, and 5 for another sector). If the number of public healthcare facilities at any level was fewer than 5, it was planned that the number selected from another level will be increased accordingly. A random number was generated in Excel and multiplied by the derived sampling interval to yield the sample start number. The number so obtained was (if needed) rounded up to the next integer to obtain the serial number of the facility to begin sampling with. For each facility selected, an additional nearest facility was selected to be used as backup when needed such as in situations where the manager of the facility from the primary sample does not permit data collection even after being shown the required documents.
4.5. Data collection
The data was collected by a team of investigators trained a priori during the period of April to June 2022. Data on the availability of antimicrobials was extracted from stock registers available. Structured and pretested data collection forms were used to record information on the availability/non-availability of listed antimicrobials. A drug was considered available if it was in stock on the day of the survey. Medicines available through vertical health programmes e.g. antitubercular and anti-retroviral drugs were excluded from the current analysis. The data on availability of antimicrobials was collected with respect to antimicrobials listed as essential in NLEM 2015 (primary list) and a selected (secondary) list of antimicrobials which was prepared by consensus among study investigators after reviewing the agents indicated for commonly encountered infectious illnesses in various healthcare settings (by discussion with medical officers and physicians).
4.6. Data analysis
Data was entered into Microsoft Excel and summarized using descriptive statistics. No specific hypothesis was tested. The data was subjected to following analyses:
4.6.1. Availability of antimicrobials listed in primary (NLEM 2015) and secondary (selected) lists.
This was expressed as the number (percentage) of facilities (public/ private/ other) where a particular drug was available on the day of the survey. In the absence of any standard criteria for defining “optimal availability” in published literature, a drug was considered optimally available for this study if at least 50 percent of the surveyed pharmacies in a particular sector had the drug available on the day of survey.
4.6.2. Gap analysis for the availability of antimicrobials in the public sector.
For both primary and secondary lists, gap analyses for availability of antimicrobials in public sector were conducted separately.
Facility-wise availability. Availability of a particular class of antimicrobials (antibiotics, anthelminthics, antifungals, antivirals, and antiprotozoals) in a facility was calculated as n/N*100 where n= number of drugs available within that class on the day of the survey, N= total number of surveyed drugs in that class for the respective sector.
Overall availability of a particular class of antimicrobials for a particular level of facility. This was calculated by the formula: Σ(ni)*100/A*B where n=number of drugs available within a particular class in a facility, A=total number of drugs in that class that are essential for the respective level of the facility, and B=number of facilities surveyed in that level.
Total overall availability of a particular class of antimicrobials across all levels of care. This was obtained using the formula: Σ(ni)*100/ ΣAi*Bi where n=number of drugs available within a particular class in a facility, A=total number of drugs in that class that are essential for the respective level of the facility, and B=number of facilities surveyed in that level.
Overall percentage availability of all drugs in a facility. This was calculated as Σ(ni)*100/ ΣAi where n=total number of antimicrobials available in a facility and A= total number of antimicrobials required for the respective level of the facility.
For gap analysis, a drug was considered “available” at a particular facility if at least one of the dosages was available.
4.6.3. Gap analysis for availability of antibiotics in public sector as per WHO access, watch and reserve (AWaRe) 2021 classification [30].
From the list of overall antibiotics surveyed in different healthcare levels (primary: 17; secondary: 25; tertiary: 29), gap analysis for access, watch and reserve groups of antibiotics was conducted separately in the similar manner as described earlier.
4.6.4. Reasons for sub-optimal availability of antimicrobials.
For non-available agents, the reasons for their non-availability were enquired from the pharmacist/ store-in-charge/ any other person handling procurement and dispensing of medicines at the facility level through open-ended questions. The reasons of non-availability were noted down and expressed as frequency measures for overall antimicrobials.
4.7. Ethical and administrative approvals
The study was conducted after obtaining ethical and administrative approvals from the Institutional Ethics Committee of PGIMS, Rohtak, Haryana (vide letter no. BREC/21/50) and Director General Health Services (DGHS) Haryana (vide letter no. 8/65-HE-2021/805), respectively. Before data collection, written informed consent was taken from the medical officer/ chief pharmacist and manager/owner of medicine outlets for public, other and private sector pharmacies, respectively.
5. Conclusions
Increased awareness of the problem is a crucial step toward addressing the issue of poor availability of essential drugs. The present survey conducted in a district of Haryana, a North Indian state reported optimal availability of most of the surveyed antimicrobials with the exception of a few antibiotics, amphotericin B and antimalarials. A deeper scrutiny of associated factors (no prescriptions/demand, prevailing resistance patterns, dwindling demand-supply chain for amphotericin B, and ‘zero indigenous case’ status of the district for malaria), however, projects that the situation is not as alarming as it seems at first instance. However, enough evidence needs to be generated in this regard from different regions of India as well as other LMICs to devise measures for ascertaining better availability of antimicrobial agents, especially antibiotics at regional, national, and global scales. Notwithstanding the fact that innovation, access to existing and time-tested drugs, and judicious usage should go hand in hand to facilitate the rational use of drugs and achieve better clinical and economic outcomes.
Supplementary Materials
The following supporting information can be downloaded at: at the website of this paper posted on Preprints.org, Table S1: Selected (secondary) list of antibiotics for survey; Table S2: Selected (secondary) list of other antimicrobials for survey.
Author Contributions
Conceptualization, N.M., R.M., Methodology, N.M., R.M., Formal analysis, N.M., S.S., Investigation, S.S., S.G., Data curation, N.M., S.S., S.G., Writing—original draft preparation, N.M., Writing—review and editing, N.M., R.M., S.S., S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Biomedical Research Ethics Committee of Pt. B.D. Sharma Postgraduate Institute of Medical Sciences, University of Health Sciences, Rohtak (vide letter no. BREC/21/50).
Informed Consent Statement
A written informed consent was taken from the medical officer/ chief pharmacist and manager/owner of medicine outlets for public, other and private sector pharmacies, respectively prior to data collection.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO Model Lists of Essential Medicines. Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists.
- Report of a WHO Expert Committee. Geneva: World Health Organization; 2002. The Selection and Use of Essential Medicines. (WHO Technical Report Series, No. 914).
- National List of Essential Medicines of India. Available online: https://pharmaceuticals.gov.in/sites/default/files/NLEM.pdf.
- National List of Essential Medicines 2022. Available online: https://main.mohfw.gov.in/sites/default/files/Notification%20and%20Report%20on%20National%20List%20of%20Essential%20Medicines%2C%202022.pdf.
- Shafiq N, Pandey AK, Malhotra S, et al. Shortage of essential antimicrobials: a major challenge to global health security. BMJ Global. Health. 2021, 6, e006961. [Google Scholar] [CrossRef]
- Mendelson M, Røttingen J-A, Gopinathan U, et al. Maximizing access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet. 2016, 387, 188–98. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Directorate of Health, Oslo, Norway, 10-11 December 2018. Meeting report: antibiotic shortages: magnitude, causes, and possible solutions. Available online: https://www.who.int/publications/i/item/meeting-report-antibiotic-shortages-magnitude-causes-and-possible-solutions (accessed on 15 March 2023).
- Pulcini C, Bush K, Craig WA, et al. Forgotten antibiotics: an inventory in Europe, the United States, Canada, and Australia. Clin. Infect. Dis. 2012, 54, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Pulcini C, Mohrs S, Beovic B, et al. Forgotten antibiotics: a follow-up inventory study in Europe, the USA, Canada, and Australia. Int. J. Antimicrob. Agents. 2017, 49, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Quadri F, Mazer-Amirshahi M, Fox ER, et al. Antibacterial drug shortages from 2001 to 2013: implications for clinical practice. Clin. Infect. Dis. 2015, 60, 1737–42. [Google Scholar] [CrossRef] [PubMed]
- Honda H, Murakami S, Tokuda Y, Tagashira Y, Takamatsu A. Critical National Shortage of Cefazolin in Japan: Management Strategies. Clin. Infect. Dis. 2020, 71, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Prinja S, Bahuguna P, Tripathy JP, Kumar R. Availability of medicines in public sector health facilities of two North Indian States. BMC Pharmacol. Toxicol. 2015, 16, 43. [Google Scholar] [CrossRef]
- Kotwani A, Holloway K. Access to antibiotics in New Delhi, India: implications for antibiotic policy. J. Pharm. Policy. Pract. 2013, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009, 373, 240–9. [Google Scholar] [CrossRef] [PubMed]
- Knowles R, Sharland M, Hsia Y, Magrini N, Moja L, Siyam A, Tayler E. Measuring antibiotic availability and use in 20 low- and middle-income countries. Bull. World. Health. Organ. 2020, 98, 177–187C. [Google Scholar] [CrossRef] [PubMed]
- AWaRe policy brief. Available online https://adoptaware.org/assets/pdf/aware_policy_brief.pdf (accessed March 2022).
- Banerjee R, Thurm CW, Fox ER, Hersh AL. Antibiotic Shortages in Pediatrics. Pediatrics. 2018, 142, e20180858. [Google Scholar] [CrossRef] [PubMed]
- Swain TR, Rath B, Dehury S, et al. Pricing and availability of some essential child-specific medicines in Odisha. Indian. J. Pharmacol. 2015, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Downes KJ, Cowden C, Laskin BL, et al. Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr. 2017, 171, e173219. [Google Scholar] [CrossRef]
- Cook KM, Gillon J, Grisso AG, et al. Incidence of Nephrotoxicity Among Pediatric Patients Receiving Vancomycin With Either Piperacillin-Tazobactam or Cefepime: A Cohort Study. J. Pediatric. Infect. Dis. Soc. 2019, 8, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Nishal PK, Kapoor A, Jain VK, Dayal S, Aggarwal K. Changing trends in acquired syphilis at a Tertiary Care Center of North India. Indian. J. Sex. Transm. Dis. 2015, 36, 149–53. [Google Scholar] [CrossRef] [PubMed]
- Kaushal K, Veena M. Two cases of Kala-azar in Haryana with no evidence of local transmission. J Commun Dis. 2008, 40(1): 87-8.
- https://ncvbdc.mohfw.gov.in/index1.php?lang=1&level=1&sublinkid=5899&lid=3686.
- National Viral Hepatitis Control Program. Available online: https://nvhcp.mohfw.gov.in/.
- Essential Medicine List (2013-14). Available online: http://nhmharyana.gov.in/files/essentialdruglist2013.pdf.
- Rohtak district population. Available online: https://www.indiagrowing.com/Haryana/Rohtak_District. Accessed 16 November 2022.
- India Health system review. Health systems in transition 2022; 11 (1). Available online: https://apo.who.int/publications/i/item/india-health-system-review (accessed on 10 September 2023).
- Prinja S, Kanavos P, Kumar R. Health care inequities in north India: role of public sector in universalizing health care. Indian. J. Med. Res. 2012, 136, 421–31. [Google Scholar]
- The Medicine procurement and management policy 2012. Available online: http://www.nhmharyana.gov.in/files/drugpolicyfinal.pdf.
- WHO 2021 Aware Classification. (accessed September 2022). Available online: https://www.who.int/publications/i/item/2021-aware-classification.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).