Submitted:
27 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and reagents
2.2. Characterization of biosorbents and wastewater samples
2.3. General batch biosorption procedure
2.4. Data analysis
2.5. Desorption of metal ions from loaded biosorbents
2.6. Wastewater experiments
3. Results and discussion
3.1. Biosorptive potential of algae waste biomass
3.2. Characterization of G-AWB and R-AWB biosorbents
3.3. Biosorption efficiency and isotherm modelling
3.4. Influence of contact time and kinetic modelling
3.5. Desorption of metal ions and biosorbents regeneration
3.6. Evaluation of the applicability in the treatment of industrial effluents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W. Treatment of wastewater from petroleum industry: current practices and perspectives. Environ. Sci. Poll. Res. 2020, 27, 27172–27180. [Google Scholar] [CrossRef]
- Kakade, A.; Sharma, M.; El-Sayed, S.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; Li, X. Heavy metals (HMs) pollution in the aquatic environment: Role of probiotics and gut microbiota in HMs remediation. Environ. Res. 2023, 115186. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.J.; Sillanpaa, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Proc. Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Chen, R.; Chung, T.S.; Ge, Q. Forward osmosis for heavy metal removal: Multi-charged metallic complexes as draw solutes. Desalination 2022, 539, 115924. [Google Scholar] [CrossRef]
- Lumami Kapepula, V.; García Alvarez, M.; Sang Sefidi, V.; Buleng Njoyim Tamungang, E.; Ndikumana, T.; Musibono, D.-D.; Van Der Bruggen, B.; Luis, P. Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technol. 2022, 4, 1300–1316. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Zakir Hossain, S.M.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Biores. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Saravanan, A.; Vo, D.V.N. Advances in biosorbents for removal of environmental pollutants: A review on pretreatment, removal mechanism and future outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef] [PubMed]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.S. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J. Water Proc. Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Karic, N.; Maia, A.S.; Teodorovic, A.; Atanasova, N.; Langergraberf, G.; Crini, G.; Ribeiro, A.R.L.; Dolich, M. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Vasic, V.; Kukic, D.; Sciban, M.; Durisic-Mladenovic, N.; Velic, N.; Pajin, B.; Crespo, J.; Farre, M.; Seres, Z. Lignocellulose-Based Biosorbents for the Removal of Contaminants of Emerging Concern (CECs) from Water: A Review. Water, 2023, 15, 1853. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J.Molec. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Ooi, J.; Yu, K.L.; Tham, T.C.; Chen, W.H.; Ok, Y.S. Engineered macroalgal and microalgal adsorbents: Synthesis routes and adsorptive performance on hazardous water contaminants. J. Hazard. Mater. 2022, 423, 126921. [Google Scholar] [CrossRef]
- Alslaibi, T.; Ismail, A.; Mohd, A.; Ahmad, M.A.; Foul, A.A. Heavy metals removal from wastewater using agricultural wastes as adsorbents: A review. Int. J. Chem. Environ. Eng. 2014, 5, 7–10. [Google Scholar]
- Sánchez-Ponce, L.; Díaz-de-Alba, M.; Casanueva-Marenco, M.J.; Gestoso-Rojas, J.; Ortega-Iguña, M.; Galindo-Riaño, M.D.; Granado-Castro, M.D. Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions. Separations 2022, 9, 309. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Nesic, A.; De Bonis, M.V.; Dal Poggetto, G.; Ruocco, G.; Santagata, G. Microwave Assisted Extraction of Raw Alginate as a Sustainable and Cost-Effective Method to Treat Beach-Accumulated Sargassum Algae. Polymers 2023, 15, 2979. [Google Scholar] [CrossRef] [PubMed]
- Kowthaman, C.N.; Selvan, V.A.M.; Kumar, P.S. Optimization strategies of alkaline thermo-chemical pretreatment for the enhancement of biogas production from de-oiled algae. Fuel 2021, 303, 121242. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Mehrez, I.; Kumar, G.; Kim, S.H. Relative evaluation of acid, alkali, and hydrothermal pretreatment influence on biochemical methane potential of date biomass. J. Environ. Chem. Eng. 2021, 9, 106031. [Google Scholar] [CrossRef]
- Ugwu, E.I. , Agunwamba, J.C. A review on the applicability of activated carbon derived from plant biomass in adsorption of chromium, copper, and zinc from industrial wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef] [PubMed]
- Akpor, O.B.; Muchie, M. Environmental and public health implications of wastewater quality. Afr. J. Biotechnol. 2011, 10, 2379–2387. [Google Scholar]
- Fresenius, W.; Quentin, K.E.; Schneider, W. Water Analysis. A Practical Guide to Physico-Chemical, Chemical and Microbiological Water Examination and Quality Assurance; Springer: Berlin, Germany, 1988. [Google Scholar]
- Lucaci, A.R. Bădescu, I.S., Bulgariu, L. Optimization of biosorption parameters for Cu(II) ions removal by red and green marine algae biomass. Sci. Pap. J. 2018, 61, 329–334. [Google Scholar]
- Rangabhashiyam, S.; Anu, N.; Nandagopal Giri, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural by-products. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Sahmoune, M.N. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ. Chem. Lett. 2019, 17, 697–704. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J.Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef] [PubMed]
- Demiral, I.; Samdan, C.; Demiral, H. Enrichment of the surface functional groups of activated carbon by modification method. Surf. Interfaces 2021, 22, 100873. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Tang, J.; Yang, Z.; Zhang, L.; Huang, X. New insights into the interactions between Pb(II) and fruit waste biosorbent. Chemosphere 2022, 303, 135048. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Usman, M.U.; Akhtar, A.; Heydari, W.; Shazia, S.; Malik, T.; Mosavat, A.; Hamdollah, S.; Sergey, P.; Ali, S.M. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Complement. Integr. Med. 2020, 17, 20190203. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Alotaibi, B.S.; EL-Sheekh, M.M. Therapeutic uses of red macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.A. Handbook of Analytical Chemistry; Mc-Grow Hill Inc.: New York, NY, USA, 1995. [Google Scholar]
- Feng, N.C.; Guo, X.Y. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar] [CrossRef]
- Yulianti, A.; Lestari, D.; Chafidz, A.; Hapsari, A.R.; Denly, W. Magnetically modified corn cob as a new low-cost biosorbent for removal of Cu (II) and Zn (II) from wastewater. J. Bahan Alam Terbarukan 2021, 9, 96–102. [Google Scholar]
- Tokay, B.; Akpınar, I. A comparative study of heavy metals removal using agricultural waste biosorbents. Biores. Technol. Rep. 2021, 15, 100719. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef] [PubMed]
- Lucaci, A.R. Bulgariu, D.; Ahmad, I.; Bulgariu, L. In situ functionalization of iron oxide particles with alginate: A promising biosorbent for retention of metal ions. Polymers, 2020, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.S. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J. Water. Proc. Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Chatterjee, A. , Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef] [PubMed]
- NTPA 001/2005. Available online: https://wordpress.com/2015/07/ntpa-002-28-02-2002.pdf (accessed on 15 June 2023).
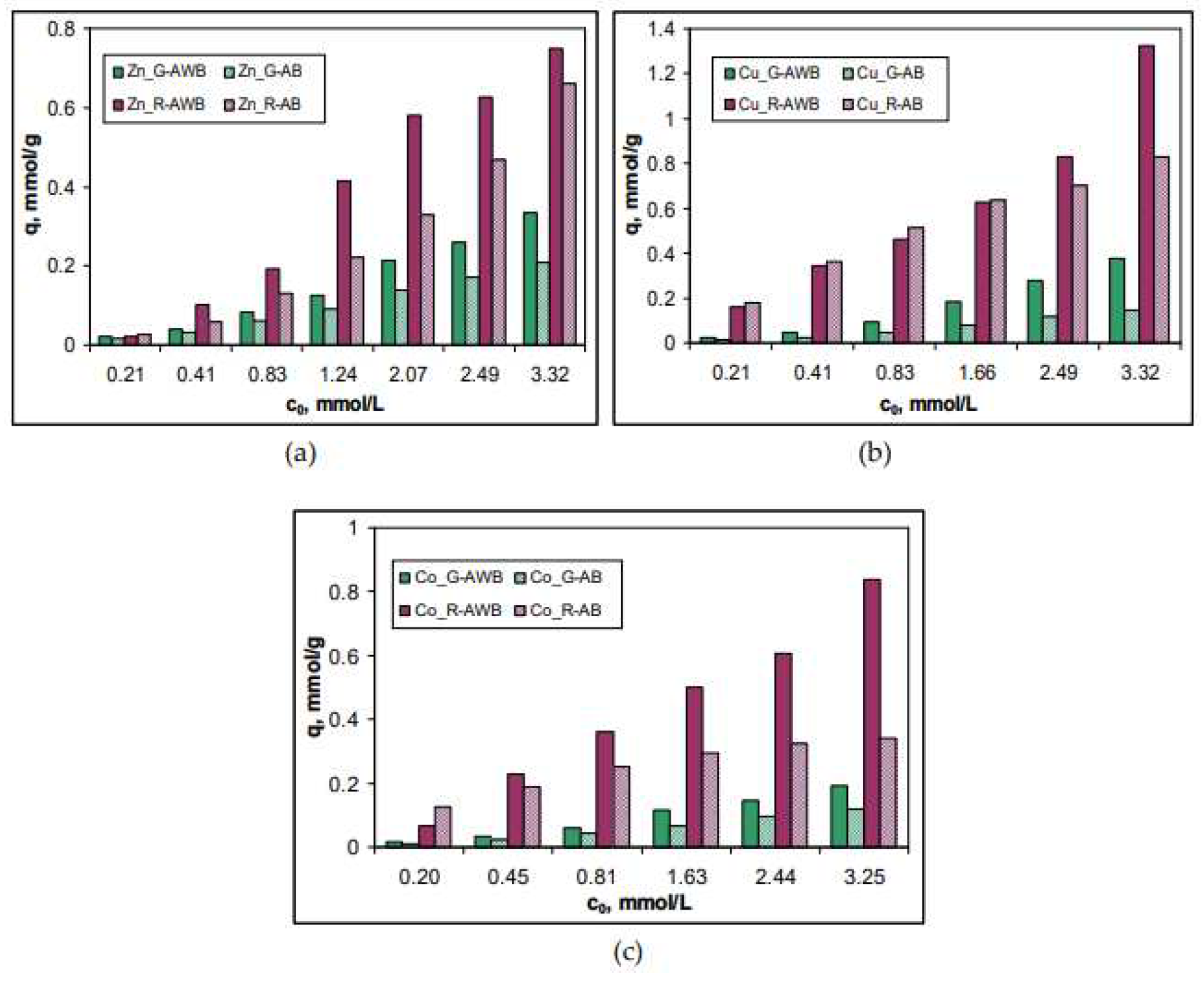
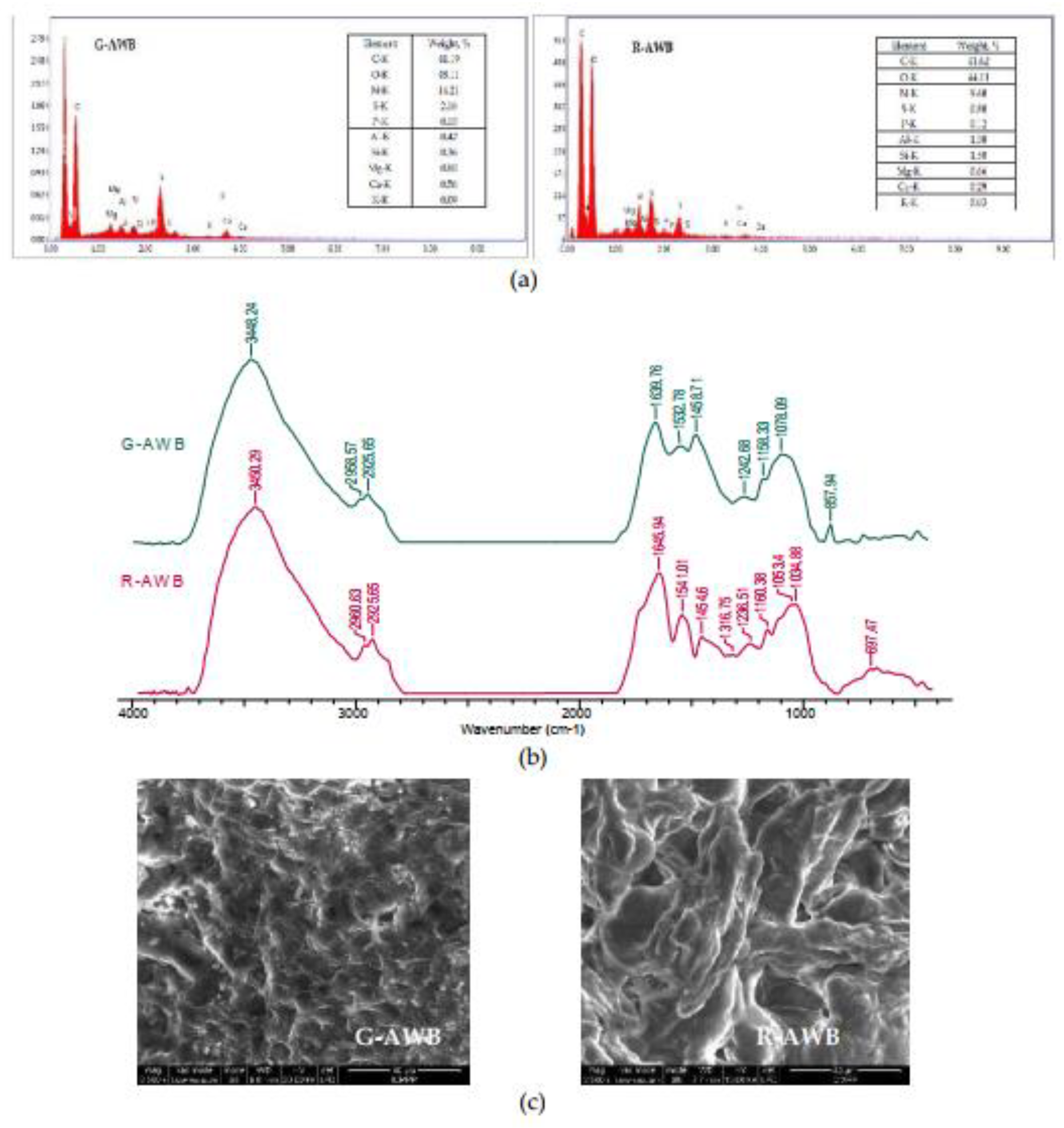
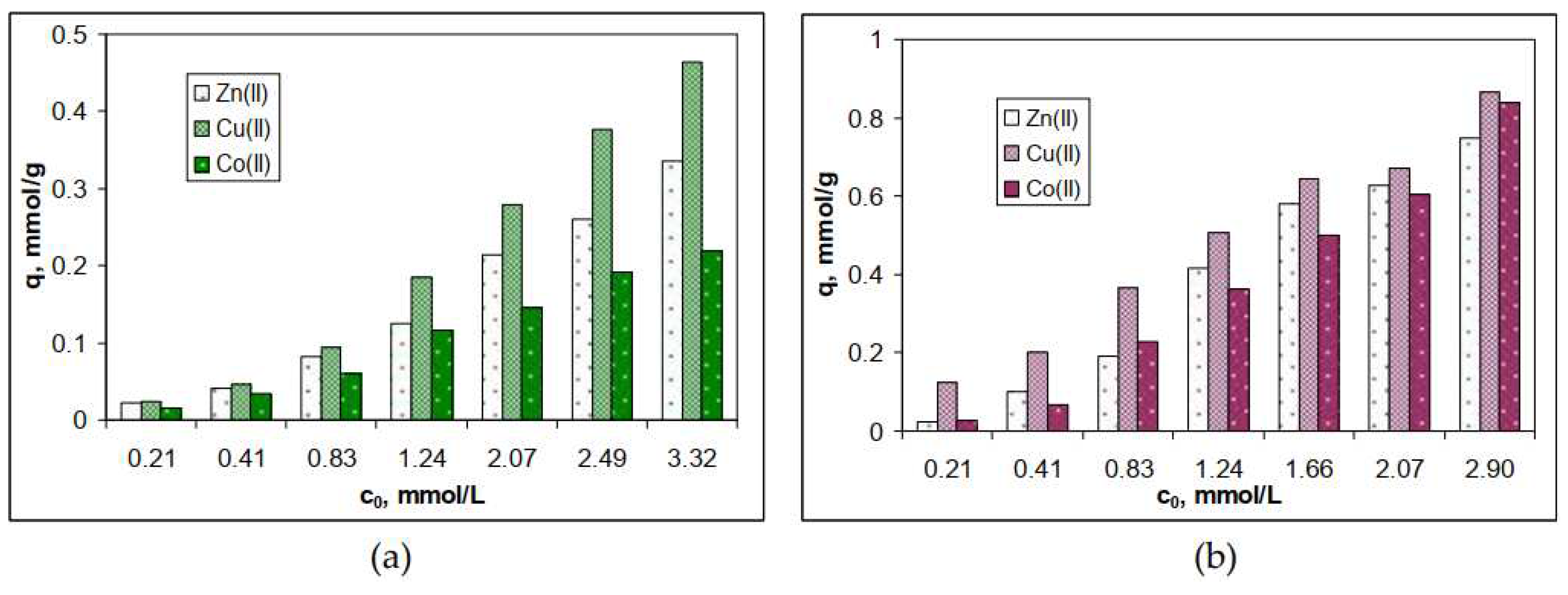
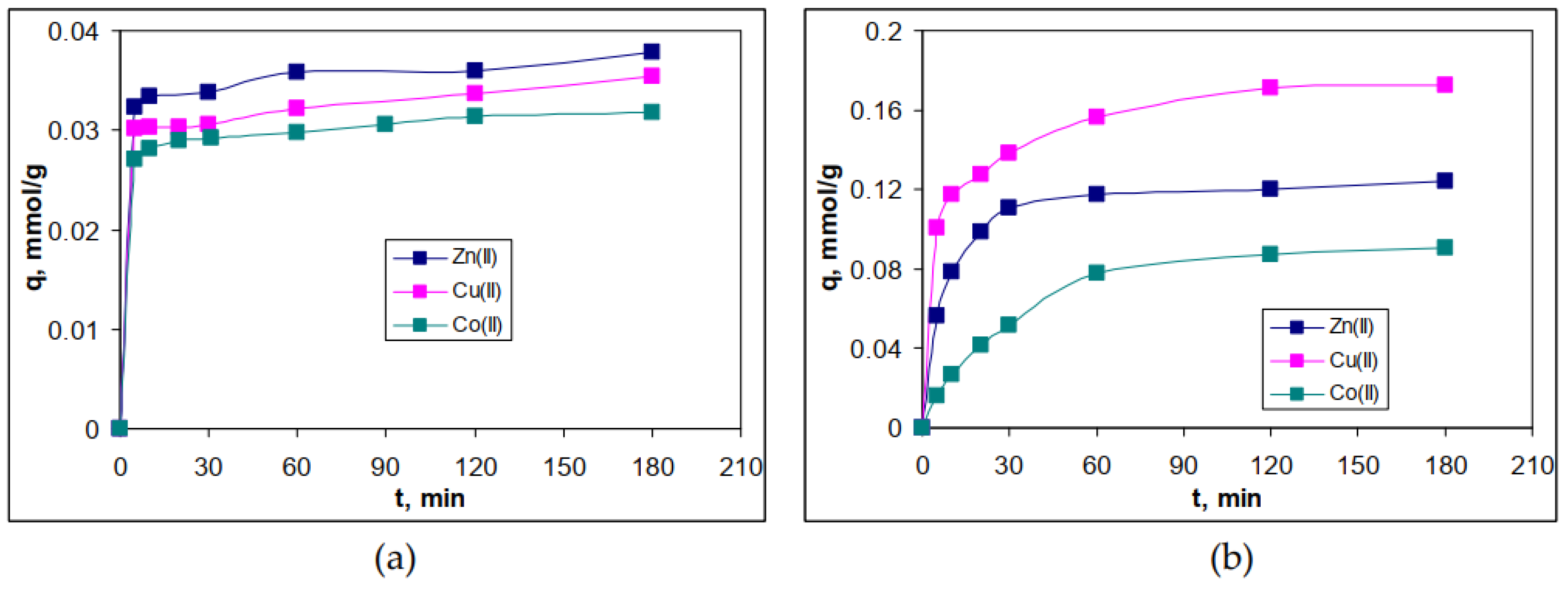
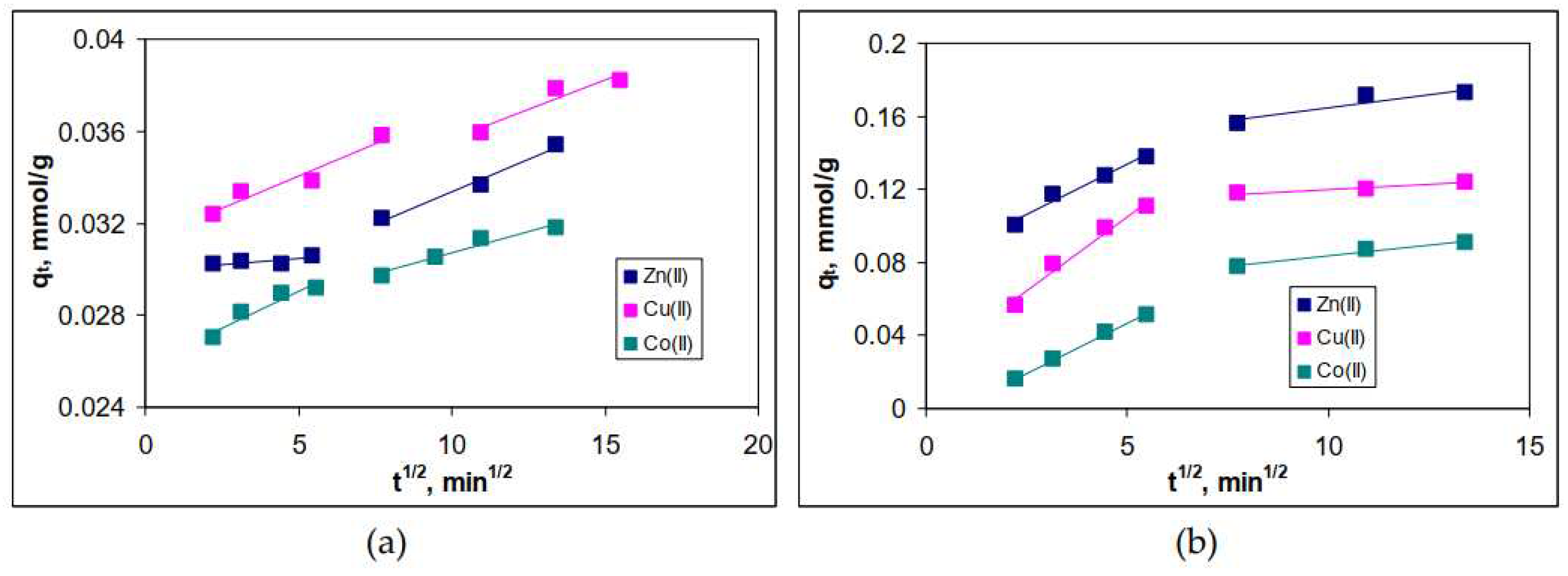
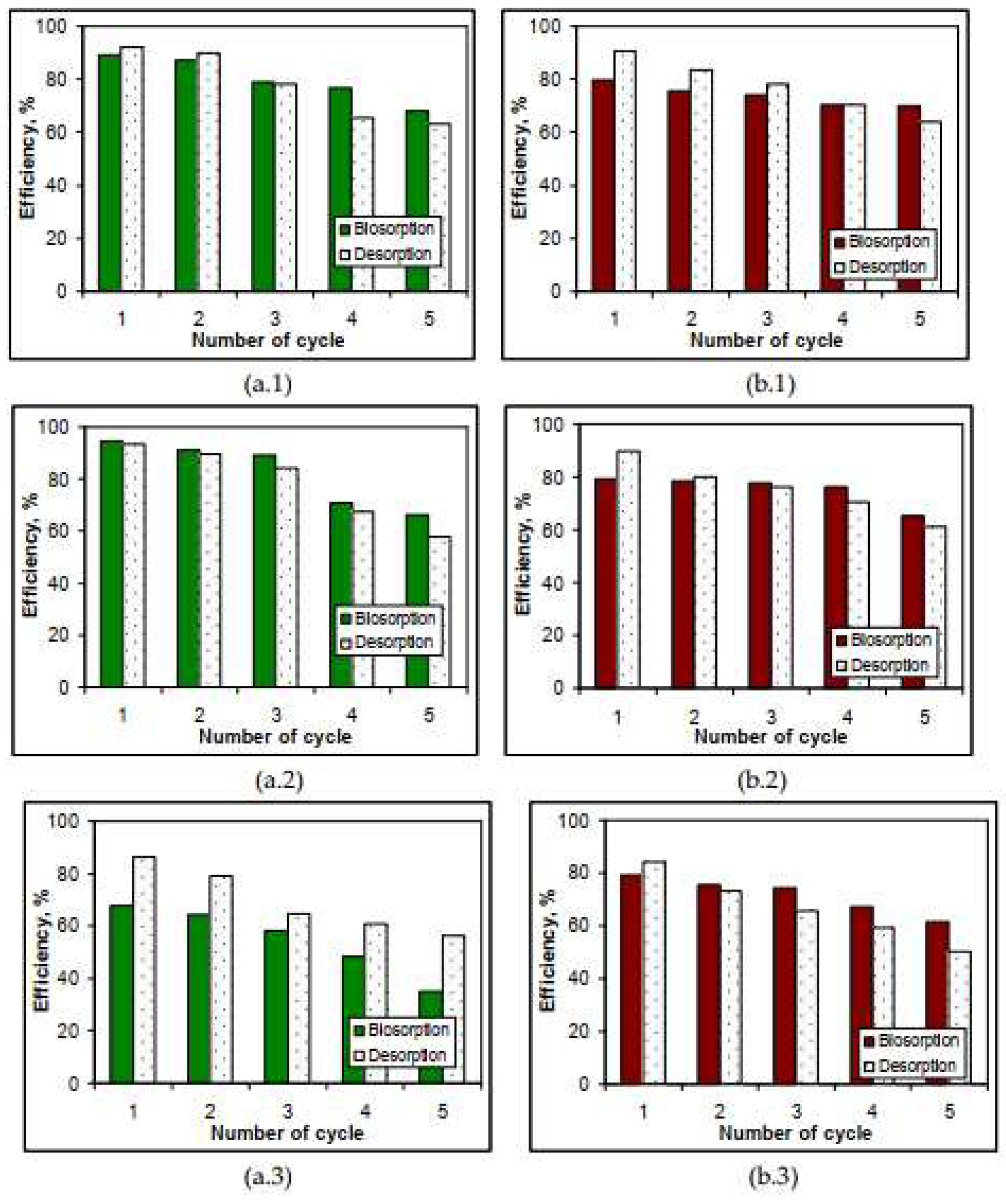
| Metal ion | q, mmol/g | Δq, % | q, mmol/g | Δq, % | ||
| G-AWB | G-AB | R-AWB | R-AB | |||
| Zn(II) | 0.34 | 0.21 | 61.90 | 1.05 | 0.49 | 114.28 |
| Cu(II) | 0.38 | 0.15 | 153.33 | 1.33 | 0.83 | 60.24 |
| Co(II) | 0.19 | 0.12 | 58.33 | 0.83 | 0.34 | 144.12 |
| Isotherm parameter |
G-AWB | R-AWB | ||||
| Zn(II) | Cu(II) | Co(II) | Zn(II) | Cu(II) | Co(II) | |
| Langmuir model | ||||||
| R2 | 0.9856 | 0.9934 | 0.9993 | 0.9791 | 0.9789 | 0.9813 |
| RMSD | 1.04 | 1.56 | 2.11 | 1.16 | 2.01 | 2.12 |
| qmax, mmol/g | 0.41 | 0.52 | 0.39 | 1.72 | 1.78 | 1.66 |
| KL, L/mmol | 1.87 | 4.87 | 1.55 | 1.56 | 1.67 | 1.44 |
| RL | 0.04 | 0.05 | 0.09 | 0.18 | 0.17 | 0.19 |
| ΔG, kJ/mol | -14.35 | -13.88 | -10.75 | -10.91 | -12.58 | -10.08 |
| Freundlich model | ||||||
| R2 | 0.9778 | 0.9722 | 0.9687 | 0.9597 | 0.9222 | 0.9073 |
| RMSD | 2.03 | 1.87 | 1.98 | 1.89 | 2.01 | 2.08 |
| 1/n | 0.95 | 0.88 | 0.70 | 0.52 | 0.61 | 0.81 |
| KF, L/mmol | 0.54 | 0.99 | 0.12 | 3.42 | 1.37 | 1.18 |
| Temkin model | ||||||
| R2 | 0.9317 | 0.9082 | 0.9641 | 0.9112 | 0.9508 | 0.9190 |
| RMSD | 1.76 | 1.59 | 2.03 | 3.02 | 2.76 | 2.59 |
| AT, L/mol | 0.18 | 0.21 | 0.16 | 0.65 | 0.26 | 0.53 |
| BT, J/mol | 12.93 | 18.99 | 12.10 | 14.93 | 25.03 | 14.68 |
| Biosorbent | Zn(II) | Cu(II) | Co(II) | References |
| Orange peel | 1.22 | 1.12 | - | 40 |
| Corn Cob | 1.16 | 1.01 | - | 41 |
| Rice husk | 0.01 | 0.02 | 0.01 | 42 |
| Neochloris sp.(green microalgae) | 1.20 | 1.23 | - | 43 |
| Alginate | 0.57 | 1.01 | 0.32 | 44 |
| Ulva lactuca (green algae) | 0.34 | 0.47 | 0.22 | This study |
| Callithamnion sp. (red algae) | 0.75 | 0.87 | 0.64 | This study |
| G-AWB | 0.41 | 0.52 | 0.39 | This study |
| R-AWB | 1.72 | 1.78 | 1.68 | This study |
| Kinetic parameter | G-AWB | R-AWB | |||||
| Zn(II) | Cu(II) | Co(II) | Zn(II) | Cu(II) | Co(II) | ||
| qeexp, mmol/g | 0.035 | 0.038 | 0.032 | 0.124 | 0.173 | 0.091 | |
| Pseudo-first order model | |||||||
| R2 | 0.9765 | 0.8231 | 0.9421 | 0.8517 | 0.9854 | 0.9916 | |
| qecalc, mmol/g | 0.059 | 0.053 | 0.043 | 0.045 | 0.089 | 0.0832 | |
| k1⋅102, 1/min | 0.440 | 0.360 | 0.590 | 1.061 | 1.473 | 1.192 | |
| Pseudo-second order model | |||||||
| R2 | 0.9986 | 0.9989 | 0.9995 | 0.9998 | 0.9991 | 0.9992 | |
| qecalc, mmol/g | 0.035 | 0.038 | 0.032 | 0.127 | 0.178 | 0.106 | |
| k2, g/mmol min | 8.326 | 10.753 | 13.158 | 1.365 | 0.847 | 0.324 | |
| Intra-particle diffusion model | |||||||
| I | R2 | 0.6256 | 0.9348 | 0.9242 | 0.9696 | 0.9771 | 0.9987 |
| c, mmol/L | 0.030 | 0.031 | 0.026 | 0.079 | 0.023 | 0.008 | |
| kIdiff, mmol/g min1/2 | 9⋅10-5 | 6⋅10-4 | 6⋅10-4 | 0.017 | 0.017 | 0.011 | |
| II | R2 | 0.9863 | 0.8976 | 0.9431 | 0.8657 | 0.9793 | 0.9626 |
| c, mmol/L | 0.028 | 0.031 | 0.027 | 0.135 | 0.109 | 0.061 | |
| kIIdiff, mmol/g min1/2 | 6⋅10-4 | 6⋅10-4 | 6⋅10-4 | 0.003 | 0.001 | 0.002 | |
| Parameter | Recommended values [47] | Before biosorption | After biosorption | |
| G-AWB | R-AWB | |||
| pH* | 6.5 – 8.5 | 5.00 | 5.78 | 6.14 |
| Zn(II), mg/L | 1.0 | 50.00 | 2.36 | 1.85 |
| Cu(II), mg/L | 0.2 | 50.00 | 0.64 | 0.53 |
| Co(II), mg/L | 1.0 | 50.00 | 4.01 | 2.79 |
| Ca(II)*, mg/L | 300 | 91.40 | 92.03 | 92.38 |
| Mg(II)*, mg/L | 100 | 32.20 | 31.89 | 32.03 |
| Chloride*, mg/L | 500 | 127.21 | 131.14 | 129.02 |
| Sulphate*, mg/L | 600 | 567.25 | 548.31 | 550.87 |
| CCO-Cr*, mg O2/L | 125 | 41.57 | 43.58 | 42.93 |
| TSS*, mg/L | - | 873.11 | 881.04 | 883.32 |
| Criteria | Ideal | G-AWB | R-AWB | |||||
| Zn(II) | Cu(II) | Co(II) | Zn(II) | Cu(II) | Co(II) | |||
| Biosorbent obtaining | Biomass purchase | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| Preparation steps | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Stability over time | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Technical performances | Ease of achieving optimal conditions | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| Biosorption efficiency | 3 | 2 | 3 | 1 | 2 | 3 | 1 | |
| Desorption efficiency | 3 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Number of usage cycles | 3 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Recovery/recycling costs | 3 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Quality of treated effluents | Final content of metal ions | 3 | 2 | 2 | 1 | 2 | 2 | 1 |
| Secondary pollution | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Total score | 30 | 24 | 25 | 21 | 24 | 25 | 21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





