1. Introduction to Elastography
Elastography is a relatively new ultrasonographic technology, created in the 1990s [
1], non-invasive and used to measure the stiffness or elasticity of tissues [
2]. There are two main forms of elastography used as a diagnostic method, static elastography, and dynamic elastography [
2]. The authors described that the static elastography or Strain wave modality involves the manual pressure of the transducer on the area under study, compressing the tissue to measure relative tissue displacement. For this modality, qualitative and semi-quantitative assessments can be performed to estimate the elasticity index by evaluating a color graph (elastogram).
Dynamic elastography encompasses sonoelastography and shear wave elastography. Sonoelastography (Fibroscan) involves low-frequency vibration (20 Hz to 1000 Hz) externally applied to produce internal vibrations in the tissue under study. It allows quantifying the propagation and speed of the shear wave (expressed in kilopascals—kPa). Shear wave speed is directly related to tissue stiffness. Therefore, the stiffer the tissue, the faster the shear wave propagation (higher kPa) [
3].
Shear wave elastography evaluates tissue displacement from a force caused by a focused, high-intensity sound beam that produces shear waves [
2]. These waves laterally pass through the tissue at a speed between 1 to 10 m/s and are rapidly attenuated by organic tissues. This method has lower inter-observer variability compared to manual compression elastography [
1] and can be performed using commercially available equipment such as Acoustic Radiation Force Impulse (ARFI) (Siemens) or SuperSonic Shear Wave Imaging (SuperSonic Imagine) [
4]. Shear wave elastography allows both qualitative evaluations, through the elastogram, representing the degree of tissue elasticity or stiffness in a color graph, and quantitative evaluation, measuring the shear wave speed. Like sonoelastography, tissue elasticity correlates with the speed of sound wave propagation, measured in m/s (meters per second) or kPa (kilopascal), with tissues showing higher stiffness presenting higher shear wave propagation speeds) [
2]. Shear wave elastography generates greater penetration of the mechanical was in a way that obesity or ascites represent no limitation for this exam [
5].
Several studies have used elastography for assessing hepatic fibrosis in humans as a preliminary assessment before tissue biopsies or for predicting and detecting malignancy [
4,
6], as well as for assessing acute and chronic kidney diseases [
7]. In veterinary medicine, recent studies have explored elastography for evaluating the prostate [
8,
9,
10], liver [
11], and kidneys in dogs and cats [
2,
8], as well as for assessing hepatic fibrosis [
12], lymph nodes [
2,
13,
14,
15,
16,
17,
18,
19,
20,
21], and studies in mammary neoplasms [
1,
3,
22,
23,
24]. Elastography has shown to be a promising diagnostic method in the evaluation and prediction of neoplasm malignancy, mainly due to its safety and low invasiveness [
1].
2. Applicability of Elastography
2.1. Mammary Glands
Mammary neoplasia is highly prevalent in dogs and can cause severe consequences to the animals. For that reason, its diagnosis and treatment must be very assertive and effective. Mammary neoplasia presents several molecular and clinicopathological similarities with mammary tumors in women [
25]. For that reason, several studies have attempted to differentiate benign and malignant mammary tumors in dogs [
1,
3].
A study with elastography of canine mammary tumors was able to differentiate benign nodules (such as mammary hyperplasia, adenoma, fibroadenoma and mixed benign tumors) and malignant tumors (such as tubular carcinoma, complex tubular papilliferous carcinoma, mixed carcinoma, simple solid carcinoma, and complex carcinoma) [
1]. This study reported that malignant mammary nodules were more rigid and, therefore, presented higher mean shear wave velocity (3,33 m/s), when compared to benign nodules (1,28 m/s).
ARFI elastography, as well as other imaging modalities, were used to investigate 300 mammary masses in dogs [
22]. The authors described that shear wave velocity higher than 2,57m/s presented 94,7% sensitivity, 97,2% specificity and high accuracy for the detection of malignancy. Researchers clarified that the highest tissue stiffness and higher shear wave velocity of malignant mammary tumors can be explained by the stromal reaction induced by the carcinoma. They said that this event is associated with the increased amount of collagen fibers in the mammary tissue. Similarly, a study with Shear wave elastography reported lower shear wave velocity values for benign mammary nodules when compared to mammary tumors in dogs [
23]. However, these authors highlighted that some benign and mixed malignant tumors can present ossified (more rigid) or cartilaginous tissues (less rigid), which could lead to a misdiagnosis.
Elastographic studies with domestic felines are scarce. Research with ARFI elastography of the mammary tissue of two female cats reported high shear wave velocity (cat 1 = 4,07m/s and cat 2 = 4,54m/s and 6,58m/s). The qualitative evaluation revealed rigid and non-deformable tissues. Those findings suggested the presence of malignant mammary tumors, confirmed by histopathological analysis as tubular carcinoma and cribriform mammary carcinoma [
24].
2.2. Lymph Nodes
Lymph node evaluation is paramount to staging oncological patients since the presence of metastatic lymph nodes indicates negative prognostics. The diagnosis of lymphadenopathies is routinely performed with fine needle aspiration cytology. Some limitations of this method include the (frequent) insufficient amount of tumoral cells obtained in the samples and high possibility of sample contamination, producing false negative results. B-mode ultrasonography is the first-choice imaging modality used to screen for lymph nodes metastasis. However, just like cytology, the ultrasound has some limitations due to the overlap of findings between benign inflammatory or neoplastic etiologies [
13].
ARFI elastography was reported to be more sensitive and specific than the short/long axis ratio (evaluated with B-mode ultrasound) for the detection of axillary and inguinal metastatic lymph nodes in bitches with mammary neoplasia. Metastatic lymph nodes of bitches with mammary tumors were more rigid than reactive or normal lymph nodes [
14], with an accuracy higher than 95% for the detection of malignant lymphoid tissues.
A recent study utilized ARFI elastography to evaluate dogs with tumors in the head or cervical region. It was observed that mandibular or medial retropharyngeal sentinel lymph nodes showed a higher shear wave propagation velocity, indicating greater tissue stiffness. These values were statistically different in cases of metastasis compared to unaffected sentinel lymph nodes [
13]. However, the authors reported low diagnostic sensitivity, supporting studies on head and neck neoplasms in humans [
15,
16], in which the diagnostic sensitivity of ARFI elastography varied with an increase in the number of included lymph nodes in the assessments. Additionally, focal areas with higher stiffness were observed within the lymph nodes compared to the total area of the lymph node itself, showing that in the early stages of the metastatic process, the lymph node is not diffusely affected, which can be a limiting factor and a source of false-negative results in metastasis research [
13].
Another study described that benign lymph nodes exhibited lower stiffness than malignant ones. The lymph nodes were classified according to stiffness scores to differentiate between malignant and benign ones, although there was an overlap in the classification between these two groups [
17]. The assessment of stiffness scores, performed semi-quantitatively through the elastogram, showed high sensitivity and specificity for detecting malignancy in mandibular lymph nodes of dogs with head and neck tumors [
18].
The combination of qualitative elastography and contrast-enhanced ultrasound with microbubbles (CEUS) contributed to the differentiation of metastatic mandibular lymph nodes. These nodes exhibited a contrast filling defect (CEUS) and a high-grade Strain elastography pattern. The Strain elastography pattern was defined based on the percentage of blue areas (stiff areas), where Grade 1 showed no blue areas, while in Grade 4, the entire lymph node appeared blue [
19]. The association of different imaging modalities was also described in another study, where the parameters with the best accuracy for detecting malignancy in mandibular lymph nodes were resistivity index (Doppler ultrasound), short-axis size (B-mode ultrasound), and elasticity (elastography). Malignant lymph nodes showed larger dimensions when compared to benign ones, a mixed vascular distribution, higher resistivity, pulsatility, and elasticity scores than benign nodes [
20].
The use of multimodal imaging has been suggested by other authors for the differentiation of malignant mesenteric lymph nodes (lymphoma), fibrotic (eosinophilic sclerosing lymphadenitis), and reactive (reactive nodular hyperplasia) lymph nodes in cats. It was possible to differentiate reactive lymph nodes, which obtained lower scores in an ultrasound classification system and lower stiffness in elastographic examination. However, lymph nodes with lymphoma and lymphadenitis exhibited some degree of overlap in the classification. Some fibrotic lymph nodes scored higher in the ultrasound classification and showed greater stiffness in elastography, like lymph nodes affected by lymphoma [
21].
2.3. Spleen
Splenic tumors are quite common in small animal clinics, especially in dogs. Approximately 58% of tumors larger than 1 cm in their largest axis are considered malignant, with hemangiosarcoma being the most frequent [
26]. However, size, shape, and other characteristics from the B-mode ultrasound examination do not safely allow the differentiation between malignant and benign tumors, as benign and malignant neoplasms often share very similar echotexture and echogenicity patterns [
27]. In these cases, elastography can contribute to identifying neoplastic lesions and overcome the limitations of the B-mode, serving as a complementary tool in the study of oncology patients by assessing tissue rigidity.
Strain elastography was used to differentiate malignant and benign hypoechoic splenic lesions smaller than 4 cm in width based on the elasticity index and stiffness value. Malignant lesions presented an elasticity rate equal to or greater than 1.5 and a stiffness value higher than 70% [
28]. These authors explained that stiffness value is calculated as the percentage of the lesion that is encoded as rigid, whereas the elasticity index can be calculated by the ratio between an area of normal parenchyma and the corresponding area of the entire lesion, considering the assessment of areas of the same size and depth.
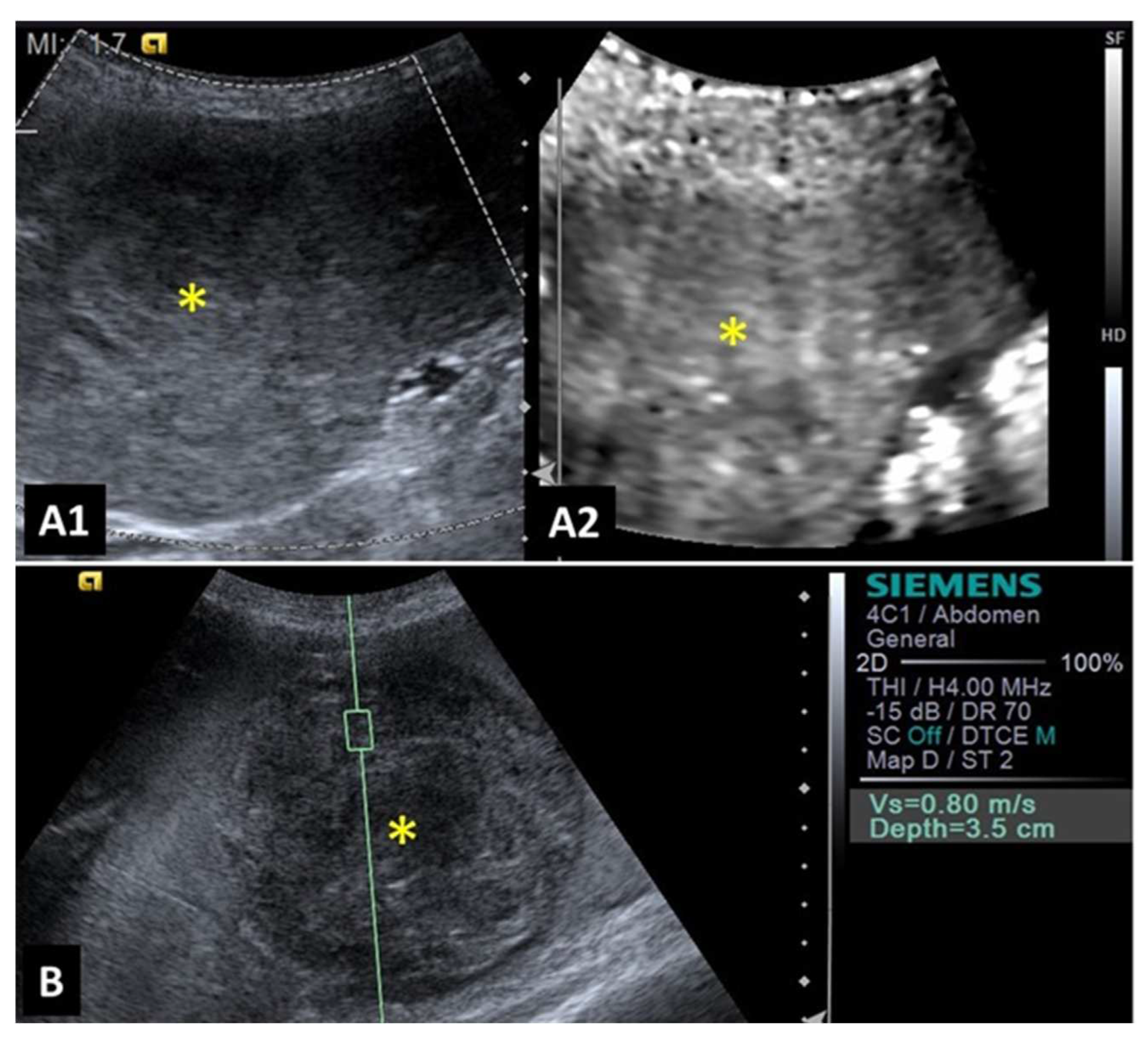
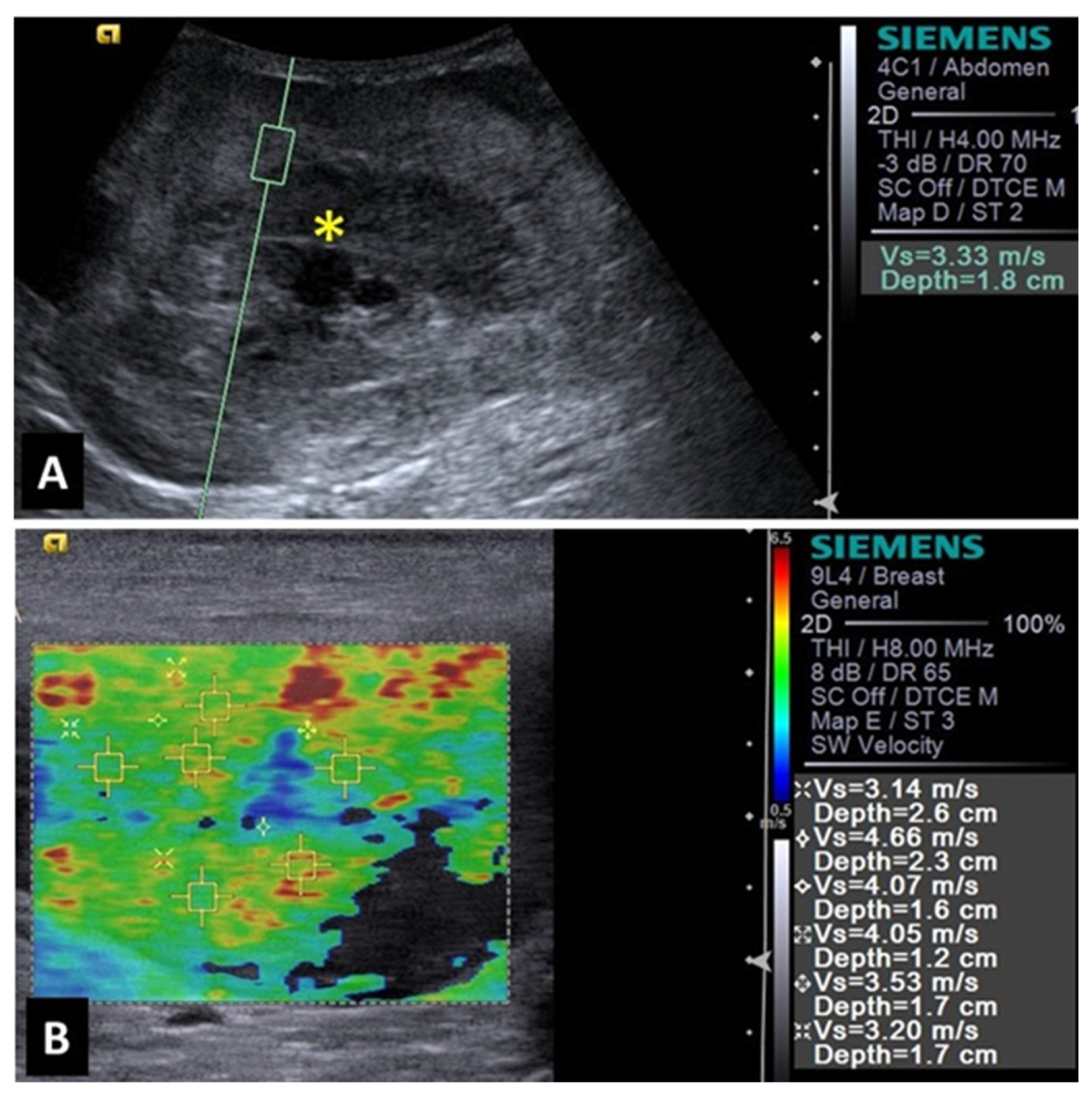
A study evaluating 37 spleens of patients with splenic nodules observed that malignant nodules exhibited higher shear wave velocity compared to benign ones, indicating that malignant lesions are stiffer [
27]. This characteristic demonstrated that shear wave elastography was 97% accurate to detect malignant splenic nodules (considered superior to advanced imaging methods such as magnetic resonance imaging). The authors reported that a shear wave velocity greater than 2.6 m/s was indicative of malignancy in splenic lesions with 95% sensitivity, 100% specificity, and an accuracy of 97%. Examples of elastographic images of splenic nodules can be seen in
Figure 1 and
Figure 2.
2.4. Cutaneous nodules
The cutaneous tissues, as for other tissues and organs previously described benefit from the association of different imaging modalities such as B-mode, Power Doppler and elastography, that contribute to increase the specificity of the evaluations [
29].
Strain elastography was able to differentiate some cutaneous nodules such as mastocytoma and benign follicular tumors. They presented the highest elasticity scores among the neoplastic nodules. Calcified and non-vascularized nodules presented higher elasticity scores and there was a negative correlation between the longitudinal diameter of the cutaneous nodules and qualitative elastographic parameters [
30].
ARFI elastography associated malignant cutaneous and subcutaneous lesions with non-deformable tissues, and shear wave velocity >3,52m/s [
29]. Similarly, another study compared lipomas and malignant cutaneous tumors and attributed the higher stiffness score to malignant lesions [
31].
2.5. Liver
B-mode ultrasonography is the first-choice method for the hepatic evaluation in dogs and cats for its advantages as a non-invasive, quick and low-cost technique, with high sensitivity for the detection of nodular or cystic lesions [
32]. The same authors pointed out that the correlation of ultrasonographic findings with laboratorial exams (such as cytology or histopathology) is mandatory to determine the relevance of the imaging findings or to confirm the diagnosis of tumoral lesions and determine the tumoral cell type. In this way, elastography can provide important information, aiding in the differentiation of tumoral tissue and normal hepatic parenchyma.
Hepatic lesions were submitted to a qualitative evaluation using an elastogram, in which regions in blue represented rigid tissues, green spots were intermediate, and regions in red corresponded to soft tissues. Malignant hepatic lesions were presented in blue indicating rigid tissues. In addition, the average intensity of colors in the elastogram was higher in cases of malignant tumors [
33].
The elastography can present some limitations for the hepatic evaluation as higher frequency transducers are not able to promote the adequate tissue deformation in deeper hepatic regions. The evaluation of deeper lesions or in deep-chested or large breed dogs is limited [
33].
2.6. Prostate and testes
Ultrasound in its various modalities (such as B-mode and Doppler, for example) has limitations in differentiating prostatic and testicular lesions by producing nonspecific information about these lesions [
8]. Therefore, elastography appears as a promising method, providing additional information for distinguishing different types of lesions in the prostate and testicles of dogs and cats or allowing the delineation of the lesion area for puncture and material collection.
A study with dogs assessed tissue homogeneity, deformation capacity, and shear wave velocity, and it described that the shear wave velocity was significantly higher in prostatic alterations when compared to normal prostatic tissue [
9]. The same authors suggested that a velocity greater than 2.35 m/s is potentially associated with malignant lesions.
The number of studies on elastographic evaluation of prostatic and testicular conditions is scarce in domestic cats. Descriptions of normal elastographic parameters of the prostate and testicles of cats can also contribute to the differentiation of benign and malignant tumors, as malignant tumors are typically characterized as rigid and with high shear wave velocity [
34].
A study with healthy cats has enabled the detection of elastographic particularities in this species when compared to dogs [
34]. Testicles of the studied animals exhibited higher shear wave velocity than those of dogs. This fact can be probably justified by the greater amount of fibrous tissue in the canine testicle.
In a pioneering study involving the evaluation of 18 dogs with testicular diseases using ARFI elastography, neoplastic, inflammatory, and degenerative lesions of the testicular parenchyma were classified as non-deformable and heterogeneous, while normal testicles were homogeneous and non-deformable in qualitative assessment [
35]. In quantitative assessment, the authors demonstrated that neoplastic testicles had a shear wave velocity of 3.32±0.65 m/s, 2.99±0.07, and 2.73±0.37 for interstitial cell tumors, Sertolioma, and Leydigoma, respectively. In another study from the same authors [
10], when testicles of healthy dogs were evaluated, the reference values found for shear wave velocity were 1.23 m/s in elderly and young adult patients and 1.28 m/s in juvenile patients (under 1 year of age).
3. Introduction to Contrast-enhanced Ultrasound (CEUS)
Contrast-enhanced ultrasonography (CEUS) was introduced with more confidence and security in medicine during the 90′, to evaluate cardiac perfusion [
36]. Nowadays, this imaging modality is being widely used in medicine and veterinary medicine to evaluate renal perfusion, hepatic, reproductive, and neoplastic tissues, for example [
36,
37,
38,
39,
40,
41].
This imaging modality is based on the use of intravenous injection of contrast media constituted of microbubbles [
42]. The same authors described that more recent products available commercially act exclusively in the intravascular region and are constituted by a lipoprotein capsule containing microbubbles of a gas with high molecular weight and low solubility in water. The researchers reinforced that these characteristics grant higher stability and longer time in the circulation.
One of the main advantages of CEUS is the possibility of real time studies when compared to other contrasted exams such as magnetic resonance imaging (MRI) and computed tomography (CT), which evaluation occurs after the injection of the contrast medium [
39] and the evaluation of non-sedated dogs when compared to CT and MRI [
38].
CEUS is a quali-quantitative evaluation based on the measurement of perfusion rate, time to peak, enhancement pattern, time for wash-in and wash-out and peak of contrast enhancement [
41].
4. Applicability of CEUS
4.1. Male reproductive tract
There are several studies describing the use of contrast-enhanced ultrasound to evaluate the male reproductive tract in dogs. A study described the ultrasonographic aspect of different testicular tumors with CEUS [
37]. These authors observed that testes with interstitial cell tumors presented an inhomogeneous enhancement pattern, with focal hyperechoic lesions, in many of the cases. The same study reported that testes with seminoma presented homogeneous hyperintense sign, persistent intra-tumoral vessels and iso- or hypoechoic parenchyma. Other findings described Sertoli tumors as inhomogeneous, with focal hyperintense homogeneous or heterogeneous lesions, and a hyperintense peripheral rim. In general, this study stated that inhomogeneous testes with hyperintense lesions were associated with malignancy, with 87% sensitivity and 100% positive predictive value.
Other study evaluated testicular tumors in non-sedated dogs and reported that interstitial cell tumors were hyperechoic, homogeneous, or inhomogeneous, with peripheral hyperechoic rim and evident intra-tumoral vessels [
38].
There was reported a subtle difference in the vascularization pattern of different testicular tumors in dogs, thus, the use of different imaging modalities was encouraged. CEUS was associated with color or power Doppler ultrasound, allowing the investigation of intra- or perilesional arteries [
40].
To the authors knowledge, there are no studies describing the use of CEUS for the detection of prostatic malignant tumors in veterinary medicine.
4.2. Mammary glands
Physiological alterations in the perfusion, size and the ultrasonographic aspect of mammary glands during the estrous cycle were investigated in bitches and represent the basis for the detection of mammary gland pathologies [
42]. According to this study, during the diestrus, all mammary glands increased in thickness and were presented as heterogeneous (B-mode ultrasonography), with a heterogeneous enhancement pattern (CEUS). The authors pointed out that abdominal cranial mammary glands presented an increase in the average transit time between estrus and late diestrus and a decrease between the end of diestrus and anestrus. Other finding was that inguinal mammary glands presented a higher time to peak during anestrus, when compared to estrus.
A study compared advanced ultrasonography methods for the evaluation of mammary neoplasia, evaluating 300 nodules in dogs [
22]. It stated that CEUS was able to study the tumoral macro- and microcirculation (not correlated with malignancy) and found an 80%-sensitivity and low specificity (16%) for malignancy detection.
CEUS enabled the differentiation and staging of mammary carcinoma in bitches [
43]. Authors described that time of wash-in and time to peak lower than 7.5s and 13.5s, respectively, indicated complex carcinoma (62% sensitivity and 60% specificity). Similarly, grade 1 mammary carcinomas showed low values for the perfusion time in this study. Other important consideration was that the increased perfusion time (wash-in>6.5s, time to peak>12.5s, and wash-out>64,5s) indicated grade 2 or 3 carcinomas, with 68% sensitivity and 62% specificity.
4.3. Kidneys and urinary bladder
Different types of renal tumors presented specific characteristics when evaluated with CEUS [
44]. These authors reported that renal carcinomas presented large tortuous arteries with early contrast-enhancement, when compared to the normal renal parenchyma. Comparatively, in the same study, histiocytic sarcomas and lymphomas were less vascularized, with smaller arteries and early wash-out during the corticomedullary phase.
At the corticomedullary phase (late), renal carcinomas presented homo- or heterogeneous, iso- or hypoechoic enhancement pattern, with progressive wash-out. Metastasis of hemangiosarcoma presented no contrast-enhancement in any phase (neither arterial nor corticomedullary). Besides some particularities of each tumor type, there were some overlapping findings among malignant and benign tumors [
44].
In the urinary bladder, evaluated with CEUS, the faster transit time (wash-in, peak, and wash-out) was associated with malignant lesions, when compared to inflammatory lesions [
45].
Other authors pointed out that ill-defined tumoral margins and poor differentiation between the tumor and the adjacent healthy tissue, if the presence of a vascularized urinary bladder wall, can be associated with infiltrative tumors. Additionally, homo- or heterogeneous hyperenhancement patterns were associated with tumors [
46].
4.4. Lymph nodes
Peripheral lymph nodes were evaluated with CEUS and power Doppler. CEUS was able to detect twice as many blood vessels as did the power Doppler investigation. Lymphomatous nodes presented hilar vessels displacement, neovascularization, and loss of the hyperechoic rim. The majority of the lymph nodes presented moderate to good perfusion with a homogeneous perfusion pattern [
47].
Another study encouraged the association of different imaging modalities for the detection of mandibular lymph node metastasis. CEUS was associated with strain elastography. Contrast-filling defects, detected by CEUS, and high elasticity index (stiffness), obtained with strain elastography, were suggestive of nodal metastasis [
19].
4.5. Spleen
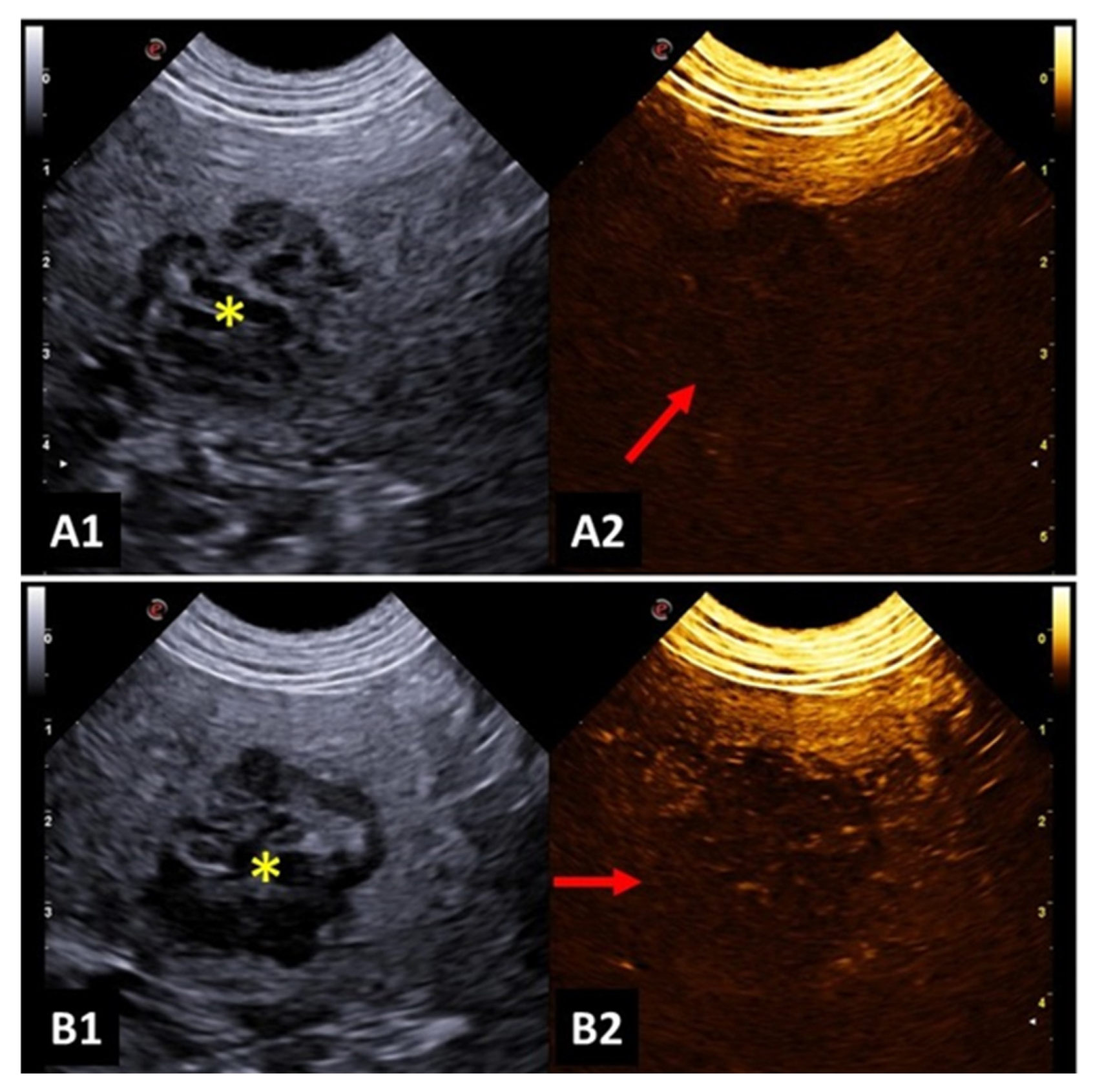
A study investigated focal splenic lesions with CEUS and reported that a hypoechoic lesion during wash-out, associated with tortuous vessels, was suggestive of malignancy (
Figure 3). Meanwhile, benign lesions presented a perfusion pattern like the adjacent splenic parenchyma. The same study described that hemangiosarcoma was presented as a large mass, with no perfusion in any phase, surrounded by a hypervascular splenic parenchyma. Lymphosarcoma presented faster time to peak and early wash-out, with a honeycomb enhancement pattern during wash-out [
48].
Comparatively, other authors attested that the differentiation of benign and malignant splenic lesions must be based on the vascular tortuosity instead of considering the echogenicity or persistent hypoperfusion. Besides that, fact, hypoperfusion persistent during all contrast phases can suggest malignancy with 40% sensitivity, 80% specificity and 71% accuracy [
49].
4.6. Gastrointestinal tract
In the canine intestine, there was no difference in the perfusion pattern between lymphoma and chronic inflammatory enteropathy or between lymphoma and the control group in dogs [
50].
Gastric neoplasia in dogs can be characterized by B-mode ultrasonography as severe gastric wall thickening (exceeding 1.2cm), marked loss of the wall layering, and involvement of adjacent structures (i.e., regional lymphadenomegaly and steatites). Those features (to a much lower extent) can be seen in cases of inflammatory conditions, with exception of the involvement of lymph nodes and steatites. Malignant gastric tumors presented faster wash-in compared to gastritis. B-mode ultrasound and CEUS were able to distinguish between malignant and benign gastric disorders but the differentiation among several tumor histotypes still relies upon cytological or histopathological exams [
51].
In felines, alimentary lymphoma is the most common malignant neoplasia of the gastrointestinal tract. CEUS and B-mode ultrasound were used to differentiate lymphoma, gastritis, and normal stomach in cats. There were overlapping findings between inflammation and low-grade lymphoma, both in CEUS and B-mode ultrasound evaluations. High-grade lymphoma presented well defined characteristics such as thicker gastric walls with poor layer definition, marked contrast enhancement pattern, regional lymphadenopathy, and local steatitis [
52].
4.7. Liver and biliary system
In the canine liver, a study with hepatocellular carcinoma evaluated with CEUS described some variation in the tumoral presentation (i.e., contrast-enhancement) according to the level of cellular differentiation. The size of the tumor had little influence on the pattern of enhancement, both during wash-in and wash-out [
53]. Different types of hepatic tumors can present variable contrast-enhancement patterns. For that reason, cytology and histopathology are important to confirm the diagnosis. Most sarcomas presented no enhancement during wash-in. Metastasis presented hyper-enhancement during wash-in, with a hypo-enhancement during wash-out [
54]. Well-differentiated hepatocellular carcinoma was characterized with a homogeneous hyper-enhancement during the arterial phase and homogeneous wash-out [
53].
There was an overlapping of qualitative findings with CEUS for different types of hepatobiliary neoplasia in cats. During the peak, biliary duct adenoma, biliary duct carcinoma, and hepatocellular carcinoma presented considerable variation in the echogenicity (hypo- or hyper-enhancement) in comparison to the normal hepatic parenchyma. Some biliary duct adenomas presented inhomogeneous hyper-enhancement during wash-in (a characteristic of malignant tumors in dogs). There was no difference in the wash-out between adenoma and biliary duct adenocarcinoma [
54].
Adenomas, biliary duct carcinomas, and hepatocellular carcinoma shared similar characteristics during the wash-in and wash-out in felines [
55].
A hepatic mass in a cat, diagnosed as hemangiosarcoma (histopathology), was not identified by B-mode ultrasonography. However, CEUS was able to detect a hypoechoic mass during the contrast peak and portal phase [
56].
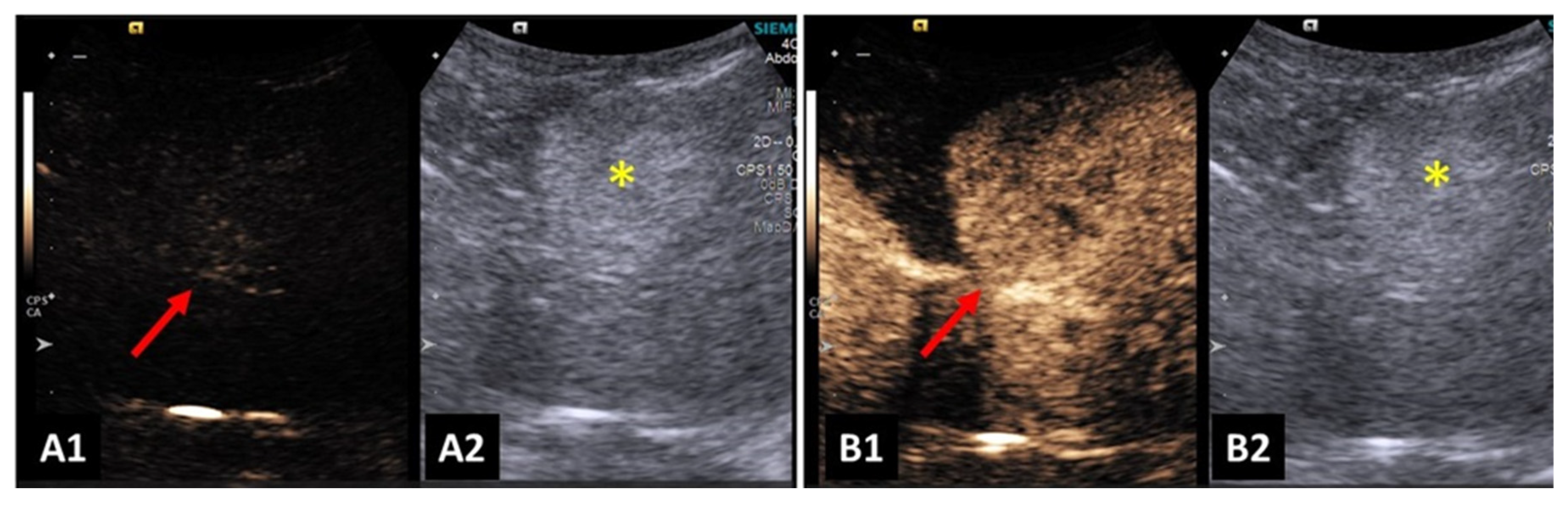
Contrast-enhanced ultrasound enabled an increase in the differentiation of hepatic nodules and normal hepatic parenchyma. There was also higher ability to detect malignant nodules, when compared to B-mode ultrasonography. Benign nodules were less conspicuous and there were no additional nodules detected after contrast-enhancement [
57]. An example can be seen in
Figure 4.
Hypoechoic nodules detected in the hepatic parenchyma during the peak of contrast were highly suggestive of malignancy [
57,
58].
4.8. Adrenal glands
Contrast-enhanced ultrasound studies evaluated adrenal glands neoplasia and established parameters for the differentiation of adenoma, adenocarcinoma, and pheochromocytoma [
59,
60,
61,
62]. Malignant adrenal gland tumors presented a heterogeneous contrast-enhancement pattern. Carcinoma and pheochromocytoma presented lower retinal blood volume when compared to adenoma. Adenocarcinoma presented tortuous feeding vessels during the arterial and venous phases [
59].
The mean transit time (for contrast) was significantly lower for malignant neoplasia than for adenomas [
59]. Pheochromocytoma presented faster time to peak than adenoma and adenocarcinoma, and bigger upslope and downslope than adenocarcinoma [
60].
The level of enhancement combined to the vascularization allowed the differentiation of malignant adrenal tumors (adenocarcinoma and pheochromocytoma) and benign adrenal tumors (adrenocortical adenoma) with 100% sensitivity, 80% specificity and 91.7% accuracy [
61].
According to other authors, wash-out or perfusion patterns can be used to differentiate malignant or benign etiologies. Besides that fact, there was some overlap between those findings. Adenomas, adenocarcinomas, and pheochromocytoma shared similar characteristics, such as intra-lesional microcirculation and regions of hypoperfusion. In this way, cytology and histopathology are gold standard methods to confirm the diagnosis [
62].
4.9. Pancreas
Contrast-enhanced ultrasound was used to investigate canine pancreatic neoplasia. This imaging modality allowed the differentiation of adenocarcinomas, insulinomas, and benign nodules. Adenocarcinomas presented hypoechoic contrast-enhancement [
63,
64] and hypoperfusion [
64], whereas insulinomas were presented as solid lesions, with a homogeneous and hyperechoic contrast-enhancement [
63], and uniform hyperperfusion [
64].
Comparatively, nodular hyperplasia was isoattenuation to the surrounding pancreatic parenchyma, whereas cystic formations presented no contrast-enhancement [
64].
A study with three dogs described an increased conspicuity and better differentiation of pancreatic nodules (of insulinoma) after contrast injection. The enhancement pattern was very variable among the evaluated animals [
65]. CEUS contributed to the detection of pancreatic nodules (of insulinoma) that were not detected by B-mode ultrasound [
66].
Contrast-enhanced ultrasound of benign and malignant pancreatic nodules in cats reported that nodular hyperplasia was presented as small, hypoechoic nodules, isoechoic to the surrounding pancreatic parenchyma, with no wash-out phase. Cysts were anechoic, with thin layers and acoustic enhancement in B-mode ultrasonography. Those structures presented no enhancement on CEUS. Other benign lesions were like pseudocysts, with no intralesional vascularization. Pseudocyst-like lesions with intralesional vascularization were classified as adenocarcinomas. Adenocarcinomas and lymphomas presented large nodules with mixed echogenicity and hyper- or hypo-enhancement pattern [
67]. The same study proved that contrast-enhanced ultrasound was sensitive and specific to differentiate nodular hyperplasia (100 e 94%), adenocarcinoma (85 e 77%) and other benign lesions (70 e 93%). However, this imaging modality was not able to differentiate lymphoma. The authors concluded that associating B-mode ultrasonography and CEUS can increase the accuracy to determine the etiology of the focal pancreatic lesions in cats. However, cytology and histopathology are paramount to confirm the diagnosis.
5. Conclusions
Elastography and contrast-enhanced ultrasonography provide important data on the differentiation of benign and malignant tumors in dogs and cats. These non-invasive imaging modalities are safe, and can be easily performed in non-sedated animals, constituting interesting techniques for the investigation of neoplasia and metastasis in different tissues and organs. Their efficiency can be increased by the association with other imaging modalities such as B-mode or Doppler ultrasonography. Cytology and histopathology are the gold-standard methods to classify benign and malignant nodules and masses and determine the cellular type of a given neoplasm. However, they require invasive methods for tissue sampling, such as fine-needle aspiration or biopsy. In this way, elastography and CEUS, as well as other imaging modalities, can be used as screening tests, and can potentially represent an alternative to those invasive sampling methods.
Author Contributions
Writing—original draft preparation: A.C.M.E., A.S.U., L.P.N.A., D.R.G., S.T.T., G.S.M.F., M.A.R.F.; writing—review, editing and visualization: A.C.M.E., A.S.U., L.P.N.A., D.R.G., S.T.T., G.S.M.F., M.A.R.F.; supervision, M.A.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author for scientific purposes.
Acknowledgments
The authors would like to thank CNPq for the productivity scholarship (process 305182/2020-0) and FAPESP for the financial support to the research group (process 2022/07366-0). We appreciate the support of FEALQ—Fundação de Estudos Agrários Luiz de Queiroz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feliciano, M.A.R.; Maronezi, M.C.; Pavan, L.; Castanheira, T.L.; Simões, A.P.; Carvalho, C.; Canola, J.C.; Vicente, W.R. ARFI elastography as a complementary diagnostic method for mammary neoplasia in female dogs—preliminary results. J Small Anim Pract 2014, 55, 10, 504-508. [CrossRef]
- Appleby, R.B.; Vaden, S. L.; Monteith, G.; Seiler, G.S. Shear wave elastography evaluation of cats with chronic kidney disease. Vet Radiol Ultrasound 2023, 64, 2, 330-336. [CrossRef]
- Massimini, M.; Della Salda, L.; Di Francesco, L.; Contri, A. Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Vet Sci 2022, 9, 9, 506-520. [CrossRef]
- Sporea, I.; Bota, S.; Jurchis, A.; Sirli, R.; Gradinaru-Tascau, O.; Popescu, A.; Ratiu, I.; Szilaski, M. Acoustic Radiation force impulse and supersonic shear imaging versus transient elastography for liver fibrosis assessment. Ultras Med Biol 2013, 39, 11, 1933-1941. [CrossRef]
- Carvalho, C.F.; Cintra, T.C.F.; Chammas, M.C. Elastography: principles and considerations for clinical research in veterinary medicine. JVMAH 2015, 7, 3, 99-110. [CrossRef]
- Boozari, B.; Potthoff, A.; Mederacke, I.; Hahn, A.; Reising, A.; Rifai, K.; Wedemeyer, H.; Bahr, M.; Kubicka, S.; Manns, M.; Gebel, M. Evaluation of sound speed for detection of liver fibrosis. Prospective comparison with transient elastography and histology. J Ultrasound Med 2010, 29, 1581-1588. [CrossRef]
- Menzilcioglu, M.S.; Duymus, M.; Citil, S.; Avcu, S.; Gungor, G.; Sahin, T.; Boysan, S.N.; Altunoren,O.; Sarica, A. Strain wave elastography for evaluation of renal parenchyma in chronic kidney disease. Br J Radiol 2015, 88, 1050, 1-6. [CrossRef]
- Domoslawska, A.; Zdunczyk, S.; Jurczak, A.; Janowski, T. Elastography as a diagnostic tool in the prostate tumour detection in Labrador retriever. Androl 2018, 50, 10, e13139. [CrossRef]
- Cintra, C.A.; Feliciano, M.A.R.; Santos, V.J.C.; Maronezi, M.C.; Cruz, I.K.; Gasser, B.; Silva, P.; Crivellenti, L.Z.; Uscategui, R.A.R. Applicability of ARFI elastography on the evaluation of canine prostatic alterations detected by B-mode and Doppler ultrasonography. Arq Bras Med Vet Zootec 2020, 72, 6, 2135-2140. [CrossRef]
- Feliciano, M.A.R., Maronezi, M.C.; Simões, A.P.R.; Uscategui, R.R.; Maciel, G.S.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Acoustic radiation force impulse elastography of prostate and testes of healthy dogs: preliminary results. J Small Anim Pract 2015, 56, 320-324. [CrossRef]
- Assawarachan, S.N.; Chuchalermporn, P.; Maneesaay, P.; Thengchaisri, N. Evaluation of hepatobiliary ultrasound scores in healthy dogs and dogs with liver diseases. Vet World 2019, 12, 8, 1266-1272. [CrossRef]
- Tamura, M.; Ohta, H.; Shimbo, G.; Osuga, T.; Sasaki, N.; Morishita, K.; Kagawa, Y.; Takiguchi, M. Usefulness of noninvasive shear wave elastography for the assessment of hepatic fibrosis in dogs with hepatic disease. J Vet Intern Med 2019, 33, 5, 2067-2074. [CrossRef]
- Favril, S.; Stock, E.; Broeckx, B.J.G.; Devriendt, N.; de Rooster, H.; Vanderperren, K. Shear wave elastography of lymph nodes in dogs with head and neck cancer: A pilot study. Vet Comp Oncol 2022, 20, 2, 521-528. [CrossRef]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci Rep 2018, 8, 17708. [CrossRef]
- Azizi, G.; Keller, J.M.; Mayo, M.L.; Piper, K.; Puett, D.; Earp, K.M.; Malchoff, C.D. Shear Wave Elastography and Cervical Lymph Nodes: Predicting Malignancy. Ultrasound Med Biol 2016, 42, 6, 1273-1281. [CrossRef]
- Chen, B.B.; Li, J.; Xiao, W.W.; Zhao, C.; Lu, T.X.; Han, F. The value of shear wave elastography in predicting undiagnosed small cervical lymph node metastasis in nasopharyngeal carcinoma: A preliminary study. Eur J Radiol 2018, 103, 19-24. [CrossRef]
- Seiler, G.S.; Griffith, E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet Radiol Ultrasound 2018, 59, 79-88. [CrossRef]
- Choi, M.; Yoon, J.; Choi, M. Semi-quantitative strain elastography may facilitate pre-surgical prediction of mandibular lymph nodes malignancy in dogs. J Vet Sci 2019, 20, 6, e62. [CrossRef]
- Choi, M.; Yoon, J.; Choi, M. Contrast-enhanced ultrasound sonography combined with strain elastography to evaluate mandibular lymph nodes in clinically healthy dogs and those with head and neck tumors. Vet J 2020, 257, 105447.
- Belotta, A.F.; Gomes, M.C.; Rocha, N.S.; Melchert, A.; Giuffrida, R.; Silva, J.P.; Mamprim, M.J. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J Vet Intern Med 2019, 33, 3, 1403-1413. [CrossRef]
- Febo, E.; Del Signore, F.; Bernabo, N.; Paolini, A.; Simeoni, F.; De Bonis, A.; Rosto, M.; Canal, S.; Vignoli, M. Ultrasonography and Sonoelastography Characteristics of Benign vs. Malignant Mesenteric Lymph Nodes in Cats: An Update. Anim 2023, 13, 16, 2664. [CrossRef]
- Feliciano, M.A.R; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS One 2017, 12, 5, e0178143. [CrossRef]
- Glinska-Suchocka, K.; Jankowski, M.; Kubiak, K.; Spuzak, J.; Dzimira, S.; Nicpon, J. Application of shear wave elastography in the diagnosis of mammary gland neoplasm in dogs. Pol J Vet Sci 2013, 16, 3, 477-82. [CrossRef]
- Feliciano, M.A.R.; Maronezi, M.C.; Brito, M.B.S.; Simões, A.P.R.; Maciel, G.S.; Castanheira, T.L.L.; Garrudo, E.; Uscategui, R.R.; Miceli, N.G.; Vicente, W.R.R. Doppler and Elastography as complementary diagnostic methods for mammary neoplasms in female cats. Arq Bras Med Vet Zootec 2015, 67, 3, 935-839. [CrossRef]
- Holen, I.; Speirs, V.; Morrissey, B.; Blyth, K. In vivo models in breast cancer research: progress, challenges and future directions. Dis Model Mech 2017, 10, 4, 359-371. [CrossRef]
- Sherwood, J.M.; Haynes, A.M.; Klocke, E.; Higginbotham, M.L.; Thomson, E.M.; Weng, H.Y.; Millard, H.A.T. Occurrence and clinicopathologic features of splenic neoplasia based on body weight: 325 dogs (2003-2013). J Am Anim Hosp Assoc 2016, 52, 4, 220-226. [CrossRef]
- Maronezi, M.C.; Carneiro, R.K.; Cruz, I.C.K.; Oliveira, A,P.L.; De Nardi, A.B.; Pavan, L.; Silva, P.A.; Uscategui, R.A.R.; Feliciano, M.A.R. Accuracy of B-mode ultrasound and ARFI elastography in predicting malignancy of canine splenic lesions. Sci Rep 2022, 12, 4252. [CrossRef]
- Barella, G., Lodi, M.; Faverzani, S. Role of strain elastography in differentiating malignant hypoechoic splenic lesions in dogs: Preliminary results. Bulg. J Vet Med 2017, 20, 3, 255-263. [CrossRef]
- Cruz, I.C.K.; Carneiro, R.K.; de Nardi, A.B.; Uscategui, R.A.R.; Bortoluzzi, E.M.; Feliciano, M.A.R. Malignancy prediction of cutaneous and subcutaneous neoplasms in canines using B-mode ultrasonography, Doppler, and ARFI elastography. BMC Vet Res 2022, 18, 10. [CrossRef]
- Brizzi, G.; Crepaldi, P.; Roccabianca, P.; Morabito, S.; Zini, E.; Auriemma, E.; Zanna, G. Strain elastography for the assessment of skin nodules in dogs. Vet Dermatol 2021, 32, 3, 272-e75. [CrossRef]
- Longo, M.; Bavcar, S.; Handel, I.; Smith, S.; Liuti, T. Real-time elastosonography of lipomatous vs. malignant subcutaneous neoplasms in dogs: Preliminary results. Vet Radiol Ultrasound 2018, 59, 2, 198-202. [CrossRef]
- Assawarachan, S.N.; Chuchalermporn, P.; Maneesaay, P.; Thengchaisri, N. Evaluation of hepatobiliary ultrasound scores in healthy dogs and dogs with liver diseases. Vet World 2019, 12, 8, 1266-1272. [CrossRef]
- Huaijantug, S.; Yatmark, P.; Phophug, P.; Worapakdee, M.; Phutrakul, A.; Julapanthong, P.; Chuaychoo, K. Quantitative ultrasound elastography and serum ferritin level in dogs with liver tumors. J Adv Vet Anim Res 2020, 7, 4, 575-584. [CrossRef]
- Brito, M.B.S.; Feliciano, M.A.R.; Coutinho, L.N.; Simões, A.P.R.; Maronezi, M.C.; Garcia, P.H.S.; Uscategui, R.R.; de Almeida, V.T.; Crivelaro, R.M.; Vicente, W.R.R. ARFI Elastography of healthy adult felines testes. Acta Scientiae Veterinariae 2015, 43, 1303-1307.
- Feliciano, M.A.R.; Maronezi, M.C.; Simões, A.P.R.; Maciel, G.S.; Pavan, L.; Gasser, B.; Silva, P.; Uscategui, R.R.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Acoustic radiation force impulse (ARFI) elastography of testicular disorders in dogs: preliminary results. Arq Bras Med Vet Zootec 2016, 68, 2, 283-291. [CrossRef]
- Patton, H.M.; Johnson, B.F.; Smorodinsky, E.; Sirlin, C.B. Imaging and Noninvasive Diagnosis of Liver Disease: Computerized Tomography, Ultrasound, Magnetic Resonance Imaging, and Emerging Techniques. Zakim and Boyer’s Hepatology 2012, 216-254. [CrossRef]
- Volta, A.; Manfredi, S.; Vignoli, M.; Russo, M.; England, G.C.; Rossi, F.; Bigliardi, E.; Di Ianni, F.; Parmigiani, E.; Bresciani, C.; Gnudi, G. Use of contrast-enhanced ultrasonography in chronic pathologic canine testes. Reprod Domest Anim 2014, 49, 2, 202-9. [CrossRef]
- Quartuccio, M.; Mangano, C.; Macri, F.; Rizzo, M.; Di Pietro, S.; Pugliese, M.; Mazzullo, G.; Cristarella, S.; De Majo, M. Contrast-enhanced ultrasound evaluation of testicular interstitial cell tumors in conscious non-sedated dogs. Veterinarni Medicina 2018, 63, 3, 125-130. [CrossRef]
- Hillaert, A.; Stock, E.; Duchateau, L.; de Rooster, H.; Devriendt, N.; Vanderperren, K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Anim 2022, 12, 20, 2765. [CrossRef]
- Orlandi, R.; Vallesi, E.; Boiti, C.; Polisca, A.; Bargellini, P.; Troisi, A. Characterization of Testicular Tumor Lesions in Dogs by Different Ultrasound Techniques. Anim 2022, 12, 210. [CrossRef]
- Sinagra, L.; Orlandi, R.; Caspanello, T.; Troisi, A.; Iannelli, N.M.; Vallesi, E.; Pettina, G.; Bargellini, P.; de Majo, M.; Boiti, C.; Cristarella, S.; Quartuccio, M.; Polisca, A. Contrast-Enhanced Ultrasonography (CEUS) in Imaging of the Reproductive System in Dogs: A Literature Review. Anim 2023, 13, 10, 1615. [CrossRef]
- Vanderperren, K.; Saunders, J.H.; Van der Vekens, E.; Wydooghe, E.; de Rooster, H.; Duchateau, L.; Stock, E. B-mode and contrast-enhanced ultrasonography of the mammary gland during the estrous cycle of dogs. Anim Reprod Sci 2018, 199, 15-23. [CrossRef]
- Feliciano, M.A.R.; Ramirez, R.A.U.; Maronezi, M.C.; Maciel, G.S.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; GAsser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of four ultrasonography techniques in predicting histopathological classification of canine mammary carcinomas. Vet Radiol Ultrasound 2018, 59, 4, 444-452. [CrossRef]
- Haers, H.; Vignoli, M.; Paes, G.; Rossi, F.; Taeymans, O.; Daminet, S.; Saunders, J.H. Contrast harmonic ultrasonographic appearance of focal space-occupying renal lesions. Vet Radiol Ultrasound 2010, 51, 5, 516-22. [CrossRef]
- Spediacci, C.; Manfredi, M.; Sala, G.; Liuti, T.; Israeliantz, N.; Zani, D.D.; Di Giancamillo, M.; Longo, M. Fall time may be a reliable discriminator between neoplastic and non-neoplastic urinary bladder lesions in dogs undergoing contrast-enhanced ultrasound: a pilot study. Vet Radiol Ultrasound 2022, 63, 5, 609-619. [CrossRef]
- Macrì, F.; Di Pietro, S.; Mangano, C.; Pugliese, M.; Mazzullo, G.; Iannelli, N.M.; Angileri, V.; Morabito, S.; De Majo, M. Quantitative evaluation of canine urinary bladder transitional cell carcinoma using contrast-enhanced ultrasonography. BMC Vet Res 2018, 14, 84. [CrossRef]
- Salwei, R.M.; O’Brien, R.T.; Matheson, J.S. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and Power Doppler ultrasound. Vet Radiol Ultrasound 2005, 46, 5, 411-6. [CrossRef]
- Rossi, F.; Leone, V.F.; Vignoli, M.; Laddaga, E.; Terragni, R. Use of contrast-enhanced ultrasound for characterization of focal splenic lesions. Vet Radiol Ultrasound 2008, 49, 2, 154-64. [CrossRef]
- Taeymans, O.; Penninck, D. Contrast enhanced sonographic assessment of feeding vessels as a discriminator between malignant vs. benign focal splenic lesions. Vet Radiol Ultrasound 2011, 52, 4, 457-61. [CrossRef]
- Nisa, K.; Lim, S.Y.; Shinohara, M.; Osuga, T.; Yokoyama, N.; Tamura, M.; Nagata, N.; Sasaoka, K.; Dermlim, A.; Leela-Arporn, R.; Morita, T.; Sasaki, N.; Morishita, K.; Nakamura, K.; Ohta, H.; Takiguchi, M. Evaluation of duodenal perfusion by contrast-enhanced ultrasonography in dogs with chronic inflammatory enteropathy and intestinal lymphoma. J Vet Intern Med 2019, 33, 2, 559-568. [CrossRef]
- Simeoni F, Del Signore F, Aste G, Bargellini P, Rubini G, Terragni R, Tamburro R, Falerno I, de Pasquale F, Russo M, Vignoli M. B-Mode and Contrast Enhanced Ultrasonography Features of Gastric Inflammatory and Neoplastic Diseases in Dogs. Anim 2021, 11, 3, 670. [CrossRef]
- Simeoni, F.; Terragni, R.; Rubini, G.; Tamburro, R.; Del Signore, F.; Falerno, I.; Aste, G.; Russo, M.; Mastromatteo, G.; Vignoli, M. B-Mode and Contrast Enhanced Ultrasonography Features of Gastric Inflammatory and Neoplastic Diseases in Cats. Anim 2020, 10, 8, 1444. [CrossRef]
- Banzato, T.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Zotti, A. Contrast-enhanced ultrasound features of hepatocellular carcinoma in dogs. Vet Rec 2020, 186, 6, 187. [CrossRef]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Banzato, T. Contrast-enhanced ultrasound features of malignant focal hepatic masses in dogs. Scientific Reports 2020, 10, 6076. [CrossRef]
- Banzato, T.; Burti, S.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Zotti, A. Contrast-enhanced ultrasonography features of hepatobiliary neoplasms in cats. Vet Rec 2020, 186, 10, 320. [CrossRef]
- Webster, N.; Holloway, A. Use of contrast ultrasonography in the diagnosis of metastatic feline visceral hemangiosarcoma. Feline Med Surg 2008, 10, 4, 388-394. [CrossRef]
- O’Brien, R.T.; Iani, M.; Matheson, J.; Delaney, F.; Young, K. Contrast harmonic ultrasound of spontaneous liver nodules in 32 dogs. Vet Radiol Ultrasound 2004, 45,6, 547-53. [CrossRef]
- Nakamura, K.; Takagi, S.; Sasaki, N.; Kumara, W.R.B.; Murakami, M.; Ohta, H.; Yamasaki, M.; Takiguchi, M. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet Radiol Ultrasound 2010, 51, 79-85. [CrossRef]
- Pey, P.; Rossi, F.; Vignoli, M.; Duchateau, L.; Marescaux, L.; Saunders, J.H. Use of contrast-enhanced ultrasonography to characterize adrenal gland tumors in dogs. Am J Vet Res 2014, 75, 10, 886-92. [CrossRef]
- Nagumo, T.; Ishigaki, K.; Yoshida, O.; Iizuka, K.; Tamura, K.; Sakurai, N.; Terai, K.; Seki, M.; Edamura, K.; Asano, K. Utility of contrast-enhanced ultrasound in differential diagnosis of adrenal tumors in dogs. J Vet Med Sci 2020, 12, 82, 11, 1594-1601. [CrossRef]
- Bargellini, P.; Orlandi, R.; Dentini, A.; Paloni, C.; Rubioni, G.; Fonti, P.; Diana, A.; Peterson, M.E.; Boiti, C. Use of contrast-enhanced ultrasound in the diagnosis of adrenal tumors in dogs. J Am Anim Hosp Assoc 2016, 52, 3, 132-143. [CrossRef]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Contiero, B.; Bendazzoli, M.; Banzato, T. Contrast-enhanced ultrasound features of adrenal lesions in dogs. Vet Rec 2023, 193, 3, e2949. [CrossRef]
- Burti, S.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Contiero, B.; Banzato, T. Contrast-enhanced ultrasound features of focal pancreatic lesions in dogs. Vet Rec 2022, 191, 8, e2080. [CrossRef]
- Vanderperren, K.; Haers, H.; Van der Vekens, E.; Stock, E.; Paepe, D.; Daminet, S.; Saunders, J.H. Description of the use of contrast-enhanced ultrasonography in four dogs with pancreatic tumors. J Small Anim Pract 2014, 55, 3, 164-9. [CrossRef]
- Nakamura, K.; Lim, S.Y.; Ochiai, K.; Yamasaki, M.; Ohta, H.; Morishita, K.; Takagi, S.; Takiguchi, M. Contrast-enhanced ultrasonographic findings in three dogs with pancreatic insulinoma. Vet Radiol Ultrasound 2015, 56, 55-62. [CrossRef]
- Cervone, M.; Harel, M.; Segard-Weisse, E.; Krafft, E. Use of contrast-enhanced ultrasonography for the detection of a feline insulinoma. JFMS Open Rep 2019, 5, 2, 2055116919876140. [CrossRef]
- Burti, A.; Zotti, A.; Rubini, G.; Orlandi, R.; Bargellini, P.; Bonsembiante, F.; Contiero, B.; Marcuzzi, M.; Banzato, T. Contrast-enhanced ultrasound features of focal pancreatic lesions in cats. Front Vet Sci 2022, 28, 9, 986948. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).