1. Introduction
Sesame (
Sesamum indicum) has long held a distinguished position among oilseeds, celebrated for its culinary and nutritional value and adaptability to diverse environmental conditions [
1]. As a versatile crop, sesame boasts an array of applications, from its seeds that yield high-quality oil and a rich source of protein to the utilisation of its oilcake in animal feed [
2]. Beyond its economic significance, sesame cultivation contributes to sustainable agriculture by improving soil fertility and mitigating the detrimental effects of soil degradation [
3]. While sesame plays a vital role in various agroecosystems, it faces challenges and limitations, particularly in regions characterised by environmental stressors such as drought, salinity, and heat [
4]. These detrimental factors hinder the development of sesame cultivation.
The climate unpredictability associated with global climate change, including the increase in temperature and the intensification of drought, poses a substantial threat to sesame cultivation. Drought stress severely inhibits plant growth and development, impacting crop yield [
5]. In addition to drought, excessive soil salinity poses another formidable obstacle to sesame production. While sesame is known for its tolerance to drought and heat, enabling it to grow in regions with unfavourable conditions, prolonged exposure to elevated salinity and temperature levels can still cause yield loss, affecting both the quantity and quality of sesame production [
6].
Understanding sesame's germination process is paramount, as germination is the initial and most critical stage in the plant's life cycle [
7]. It directly influences stand establishment and, consequently, the overall yield. The impact of salinity and heat stress on sesame seed germination has been a subject of scientific inquiry. Previous studies have shown varying responses of sesame seeds to different salinity and temperature levels, emphasising the need for a comprehensive investigation. For example, Bahrami and Razmjo [
8] reported a substantial inhibition of germination and early seedling growth in sesame cultivars when exposed to water electrical conductivity (ECw) of 12.05 dS m
−1. El Harfi et al. [
9] observed that salinity stress had a less inhibitory effect on germination and seedling growth in comparison to drought stress. Moreover, both stresses had a more significant impact on seedling growth than on seed germination. Suassuna et al. [
10] found that salinity did not affect sesame germination, but seedling growth was impeded at ECw levels of ≥ 1.6 dS m
−1. It has been reported that sesame necessitates an optimal temperature range of 25-35°C throughout its life cycle [
2]. Exposure to temperatures exceeding 45°C, particularly with hot winds, leads to a reduction in oil content [
6]. Additionally, both temperatures surpassing 45°C and falling below 15°C result in a significant decrease in yield [
11]. Nonetheless, there are limited studies examining the impact of temperature on sesame seed germination under different salinity level.
While studies have investigated the effects of salinity on sesame seed germination, there still exists a gap in our understanding of how environmental factors, especially temperature, interact and influence germination processes under salinity stress. The significance of this research lies in its potential to elucidate the combined effects of salinity and temperature on sesame seed germination, which can help develop strategies to enhance sesame production under adverse conditions. Therefore, this study aims to investigate the influence of varying salinity levels and temperature on sesame seed germination, focusing on the germination percentage and mean germination time. The findings will contribute to a deeper comprehension of sesame's resilience and response to environmental stressors, thereby aiding in developing resilient and productive sesame cultivars suitable for challenging agricultural landscapes.
2. Materials and Methods
To investigate the germination response and seedling growth characteristics of sesame (
Sesamum indicum) seeds under different temperature and salinity stress conditions, an experimental study was conducted in 2023. The study was designed in a factorial arrangement as a completely randomised design with six replications at the Physiology Laboratory of the Seed and Plant Improvement Institute in Karaj, Iran. The factors examined in this experiment included two sesame cultivars named Oltan and Darab 1 (details of the cultivars are presented in
Table 1), various salinity levels (0, -3, -6, -9, and -12 bars), and three temperatures (15°C for very early sesame cultivation, 20°C for early sesame cultivation, and 25°C for normal sesame cultivation).
Pure sodium chloride was used in distilled water to impose salinity levels. The amount of salt required to prepare saline solutions, as determined by the Van't Hoff formula using electrical conductivity, followed the equation provided by the International Seed Testing Association [
12].
Where Ψ represents the osmotic potential in terms of pressure, m is the molarity of the solution, i is the ionisation coefficient, R is the gas constant, and T is the temperature in Kelvin.
Before the start of the experiment, healthy seeds of the cultivars were disinfected. For this purpose, the seeds were immersed in a 10% sodium hypochlorite solution for 30 seconds and then thoroughly rinsed with water. Fifty disinfected seeds of sesame cultivars were transferred to sterile containers with smooth filter paper at the bottom. The containers were sealed with parafilm to prevent potential evaporation of the solution. For salinity treatment, 2 millilitres of the prepared solutions were added to the containers containing seeds. Subsequently, the containers were transferred to a germinator (JTGL 400, Jal Tajhiz, IRAN) and placed at different temperatures according to the experimental treatments for 15 days. Seed germination was counted daily (every 24 hours). Seeds were germinated if their radicle length was 2 millimetres or more [
13]. Additionally, 25 seeds from each treatment were evaluated for plant-related traits, including the length and dry weight of both roots and stems, after ten days in the germinator. Furthermore, the germination rate was calculated using the following equation [
14].
Where GR represents the germination rate (number of germinated seeds per day), Si is the number of germinated seeds in each count, Di is the number of days until the nth count, and n is the number of counting times.
In this experiment, the Daily Germination Speed (DGS), which is related to seed structure [
15], was also measured. The Daily Germination Speed, represented by the Mean Daily Germination (MGD), was determined using the following relationships.
Where FGD and d represent the final germination percentage and the number of days to reach maximum final germination, respectively.
Data analysis was conducted using SAS software version 9.4, and for drawing graphs and calculating the Lethal Salinity for 50% Germination, Excel software version 2013 was utilised.
3. Results and discussion
3.1. Germination percentage
The results indicated that temperature, salinity, and cultivar treatments significantly influenced all parameters, except for the effect of cultivar on seed germination speed and plumule dry matter (
Table 2).
In addition, the results demonstrated that the trend of changes in germination percentage varied with increasing salinity levels at different temperatures (
Figure 1). In both cultivars, at a temperature of 15° C, an increase in salinity by more than -3 bar significantly reduced the germination percentage. Further increases in salinity at this temperature practically halted germination in both cultivars (
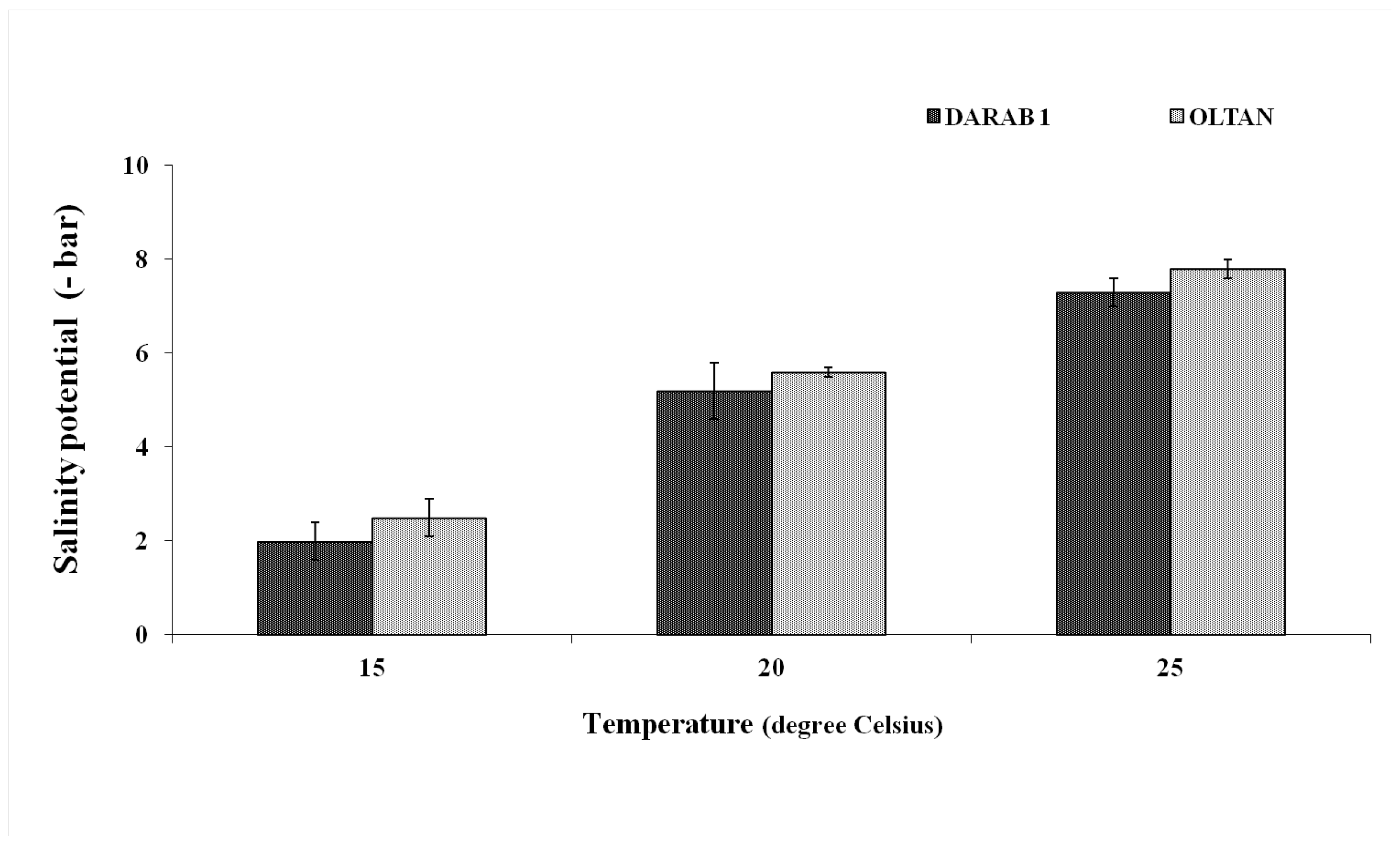
Figure 1). A similar trend was observed at a temperature of 20° C, with the difference that at this temperature and a salinity level of -9 bar, contrary to the 15° C, seed germination was observed in both cultivars. With an increase in temperature to 25° C, not only was the germination percentage higher at all salinity levels compared to other temperatures in both cultivars but even at the highest salinity level (-12 bar), germination rates of 40% and 62% were recorded for Darab 1 and Oltan, respectively. In other words, the results indicated that an increase in temperature in both cultivars reduced the inhibitory effect of salinity. At 25° C, the maximum tolerable salinity potential for germination was achieved at 50% for seeds in both cultivars. At this temperature, the average index for Darab 1 was -7, and Oltan's was -8 bar (
Figure 2). Various studies suggest that under low to moderate salinity (-2 to -6 bar), the reduction in osmotic potential is a limiting factor for germination [
16]. With salt's introduction into the seed's internal structure, the water-holding capacity inside the seed decreases. Even in a moist environment, seed germination is reduced due to the seed's inability to absorb water. Conversely, under saline and highly saline conditions (-6 bar and above), ion toxicity, and consequently, increased absorption of sodium and chloride ions, along with disruption of ion balance, are considered significant factors contributing to a reduction in germination percentage [
17].
In the other hand, because germination is a physiological process dependent on enzymatic activity, an increase in temperature up to an optimal level for germination can enhance the speed of germination processes [
18]. This, in addition to mitigating the adverse effects of salinity, results in an increased germination percentage. Therefore, considering these results, the negative impact of salt stress depends on the ambient temperature. At temperatures lower than 25° C for sesame, the adverse effects of salt stress include water deficiency due to an increase in osmotic potential in the environment, the toxic effect of high ion concentrations, along with a reduction in phosphorus absorption, and consequently, a decrease in ATP production, all of which can contribute to reducing the germination percentage.
While the overall trend of changes in germination percentage was nearly similar for both cultivars across different temperatures and salinity levels, differences in the response of the cultivars were observed. For instance, in conditions without salt stress, a decrease in temperature from 25 to 15° C reduced the germination percentage of Darab 1 by 5%, while Oltan showed a decrease of up to 10%. Furthermore, with increased salinity, Darab 1 exhibited greater sensitivity to a decrease in temperature compared to Oltan. For example, at a temperature of 20° C and a salinity level of -6 bar, the germination percentages for Oltan and Darab 1 were 80% and 70%, respectively. Additionally, at a temperature of 25° C, where the highest germination percentage was observed for both cultivars, the germination percentage for Oltan was higher than that of Darab 1 at each salinity level. This difference increased with the intensity of the stress.
It has been documented that the joint impact of salt stress and temperature on seed germination may differ among various crops [
17]. However, our findings indicate that this variability extends to different cultivars within the same plant species. This research underscores that the interaction effect is contingent upon factors such as cultivar type, salinity levels, temperature, and the combined influence of salinity and temperature. Sesame exhibits high polymorphism and is recognised for possessing the greatest genetic diversity among crops [
19], with its diverse cultivars exhibiting variations in stress tolerance during the germination phase [
1]. The unequal response of cultivars' seed germination to increasing salinity under varying temperatures supports the proposition that the Oltan cultivar likely demonstrates salt tolerance at this stage.
3.2. Germination speed
Success in crop production depends not only on a high seed germination rate but also on uniformity in seedling growth and the speed of plant establishment in the soil, which directly correlates with germination speed [
14]. Germination speed is a crucial concept in seed structural features [
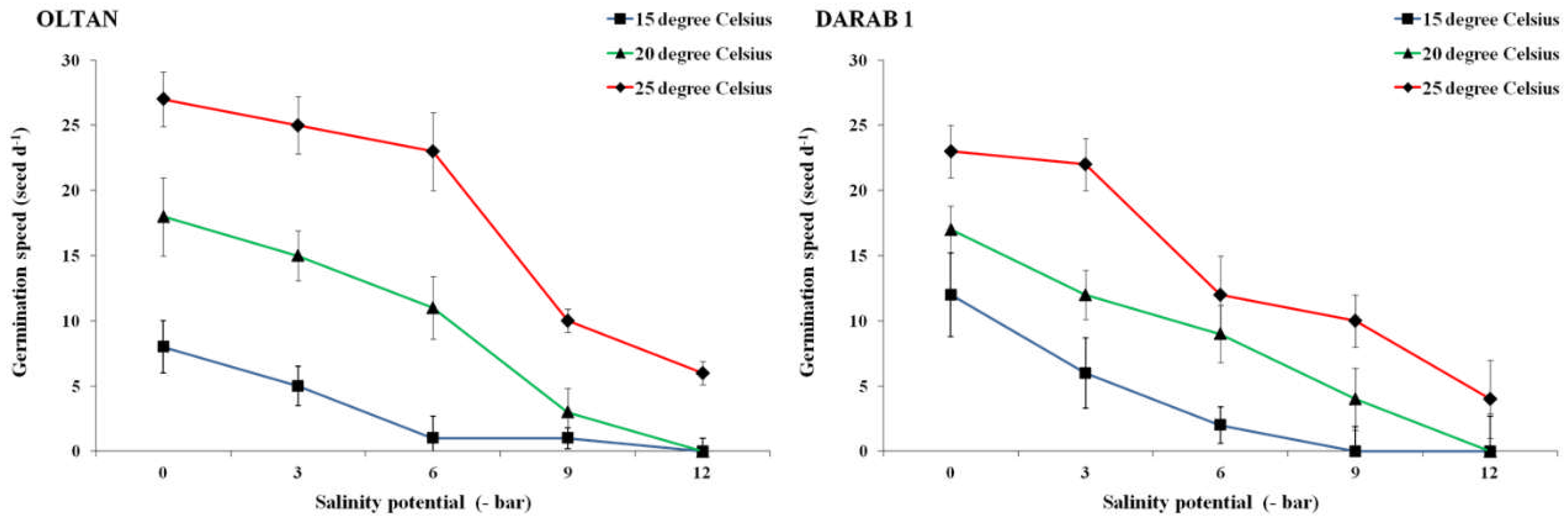
20], and its assessment can be influential in predicting effective plant establishment. This is particularly relevant to sesame plants due to the small size of their seeds, which adds special significance to this aspect. Results showed that the interactive effect of salinity and temperature also impacted the germination speed in both cultivars (
Figure 3). The results indicated that an increase in the intensity of salt stress was associated with a reduction in germination speed in both cultivars. This effect was particularly pronounced at temperatures of 15 and 20° C; even at a temperature of 25° C, an escalation in salinity led to a decrease in germination speed in both cultivars. However, the difference was notable, as the increase in salinity from -3 to -6 bar had a more substantial impact on the germination speed of the Darab 1 variety. In comparison, Oltan exhibited a significant decline in germination speed with an increase in salinity from -6 to -9 bar (
Figure 3).
As expected, the highest germination speed in both cultivars was observed under non-saline conditions and at 25° C. It is evident that for vital seed activities and subsequent germination, the seed must absorb an adequate amount of water. If water absorption is disrupted due to low temperatures [
21] or environmental salinity [
22], or if it occurs slowly, physiological activities within the seed also proceed gradually. The duration of root emergence from the seed increases, and in other words, germination speed decreases. However, the results demonstrated that the optimal temperature for germination could mitigate the detrimental effects of salinity on germination speed. This effect was particularly prominent in the Oltan variety. The germination of higher plants, including sesame, is influenced by temperature in two distinct ways: firstly, it affects the speed of the process, also known as the germination rate, which is expressed as the relative number of seeds germinating per unit of time; and secondly, it influences the total fraction of seeds in a lot that undergoes germination, termed germinability, expressed as a percentage. In this scenario, it is anticipated that the germinating Oltan seedlings under a temperature of 25° C activate various tolerance mechanisms to alleviate salt stress. These mechanisms may include the exclusion of excessive Na
+ [
23] or its compartmentalisation into vacuoles [
24], as well as the upregulation of defence genes and β-expansin proteins [
25] to sustain growth.
3.3. Radicle and plumule length
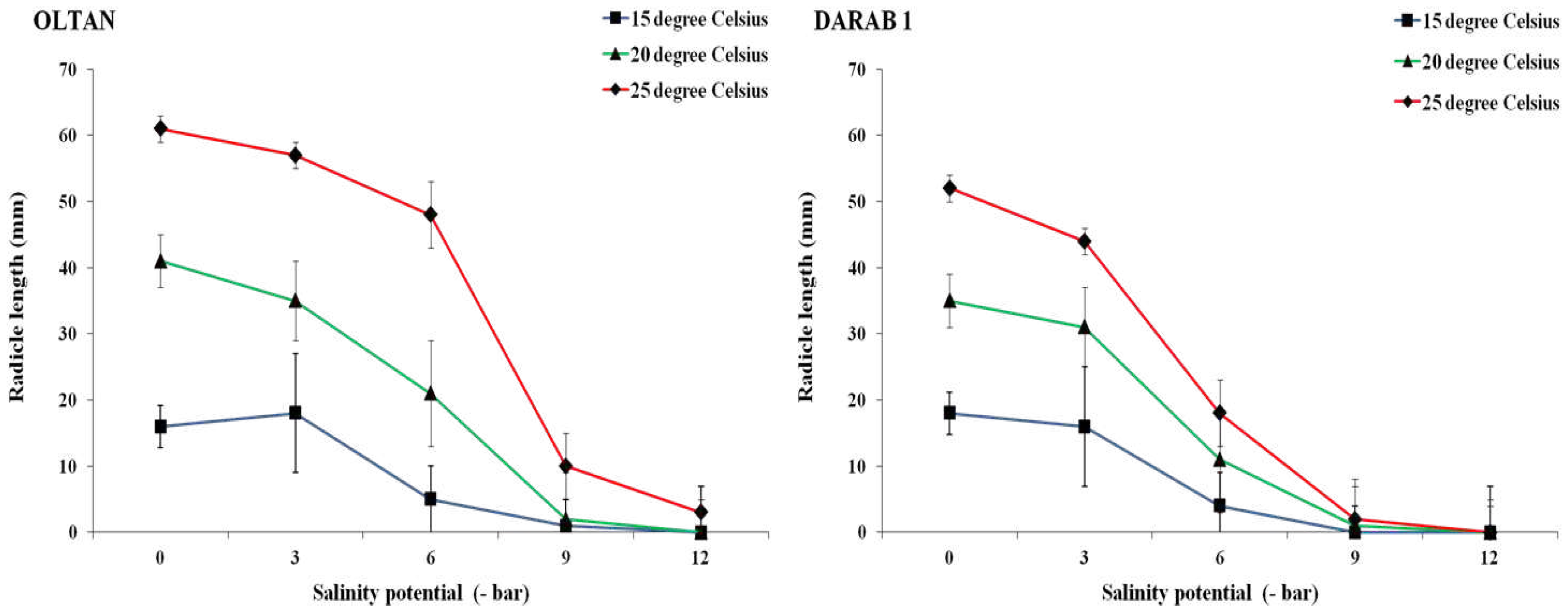
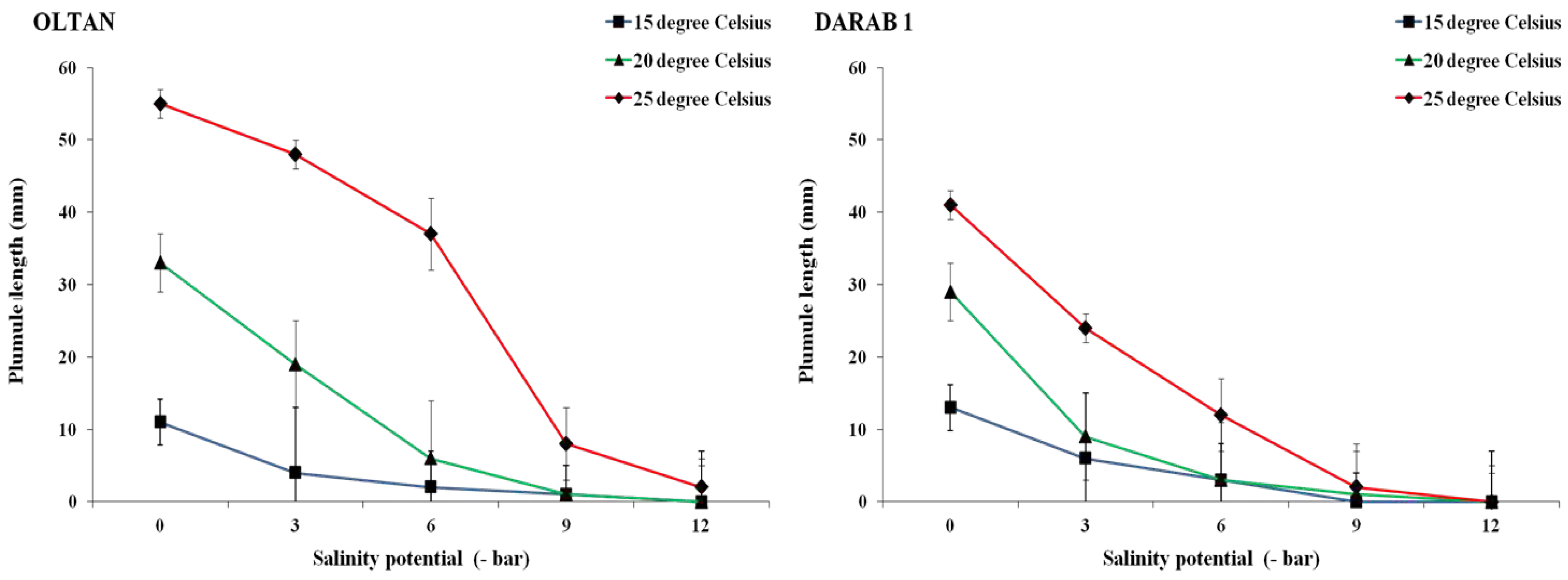
While an increase in salinity intensity in different temperatures led to a reduction in both radicle and plumule lengths in both cultivars, the negative impact of salinity on these traits varied depending on the cultivar and temperature treatment (
Figure 4 and
Figure 5). As anticipated, the maximum radicle and plumule lengths of both cultivars were achieved under non-saline conditions and at a temperature of 25° C. However, even under these conditions, the Oltan variety exhibited greater radicle and plumule lengths than Darab 1. Ghasemi Hamedani et al. [
4] also highlighted the longer root length and faster establishment of the Oltan cultivar than other Iranian sesame cultivars, including Darab 1. The results indicated that an increase in salinity up to -6 bar resulted in a respective decrease of 78%, 69%, and 65% in the radicle length of Darab 1 at temperatures of 15, 20, and 25° C. For the Oltan cultivar, these values were 69%, 49%, and 21%. In both cultivars, an increase in salinity intensity up to -9 bar at temperatures of 15 and 20° C minimised both radicle and plumule lengths to their minimum values (1 to 1.5 millimetres). This trend was also observed for the Darab 1 variety at a temperature of 25° C. However, the Oltan cultivar, under a salinity level of -9 bar at a temperature of 25° C, exhibited some tolerance to salinity stress, producing radicle and plumule lengths of 10 millimetres and 8 millimetres, respectively. Regardless of temperature, an increase in salinity intensity up to -12 bar (the highest salinity level) completely halted germination and, subsequently, the growth of radicle and plumule in the Darab 1 cultivar. A similar pattern was observed for the Oltan variety, with the difference that under the highest salinity level, at a temperature of 25° C, minimal radicle length (3 millimetres) and plumule length (2 millimetres) were observed in this cultivar. The varying reactions of sesame cultivars to salinity can be attributed to the genetic characteristics inherent to each cultivar, their origins and the conditions in which they are cultivated.
With an increase in osmotic potential due to salinity, the water potential decreases, and less water becomes available to the seed. With a decrease in available water to the seed and consequently a reduction in imbibition, the growth of plant organs, including root and shoot, is compromised [
26]. Additionally, a decrease or impaired transport of nutrients from the endosperm to the embryo has been reported as one of the reasons for reduced shoot length under saline conditions [
22]. Besides reducing shoot length, an increase in salinity may also cause increased physical damage to seedlings during germination through a decrease in coleoptile length [
23]. In other studies, growth indices in sesame plants have been reported to decrease due to salinity stress [
27]. It has been reported that the optimal temperature can influence shoot and root lengths by affecting seed decay, reducing seed dormancy, and other germination processes [
28]. In other words, it can be stated that both sesame cultivars, especially under saline conditions, exhibited acceptable germination and growth only at a temperature of 25° C, and temperatures lower than 25° C were unable to mitigate the significant effects of salinity. Therefore, understanding germination processes, including the intensity of environmental salinity and its interaction with other environmental factors such as temperature, is crucial in making informed decisions about the precise timing of sesame cultivation and selecting the appropriate variety.
3.4. Radicle and plumule dry weight
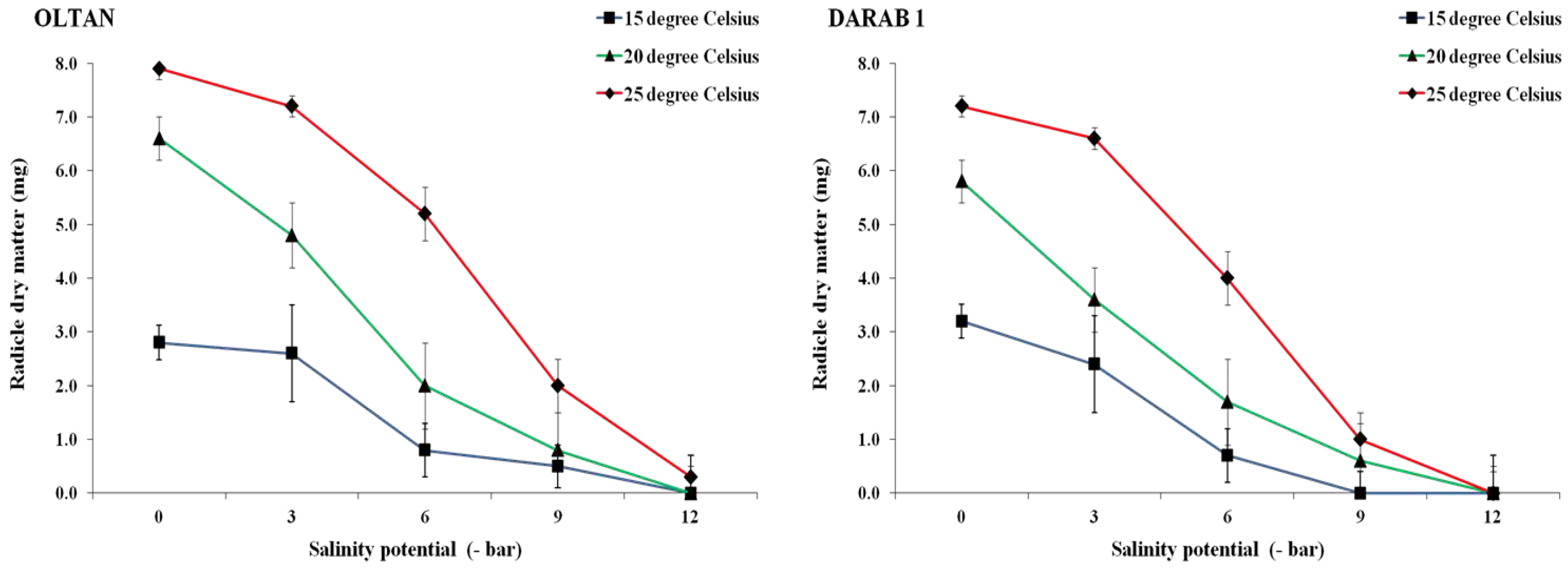
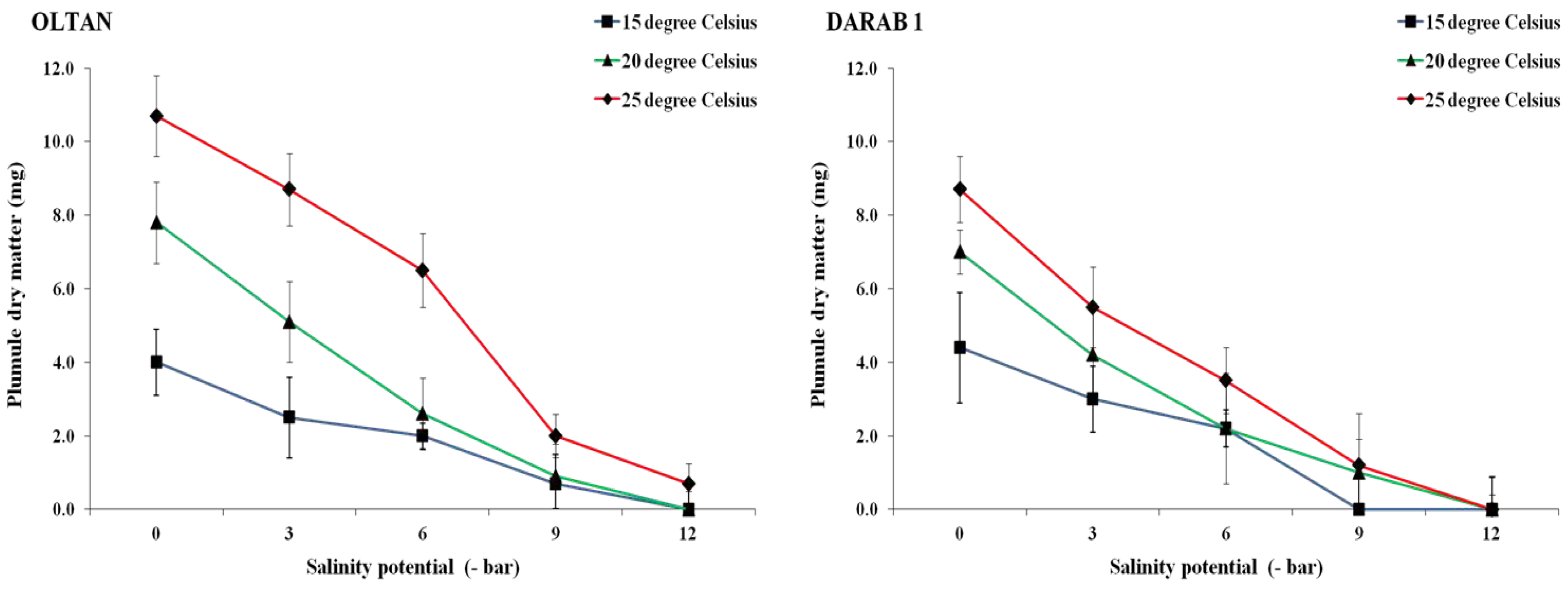
The results indicated that different salinity levels and temperatures influenced the radicle and plumule dry weight in both sesame cultivars (
Figure 6 and
Figure 7). In both cultivars, the maximum dry weight of radicle (2.7 mg in Darab 1 and 9.7 mg in Oltan) and plumule (0.8 mg in Darab 1 and 1.0 mg in Oltan) was obtained at zero salinity potential and a temperature of 25° C. With the increase in salinity intensity from 0 to -6 bar, the dry weight of Darab 1 radicle decreased by 78%, 70%, and 45% at temperatures of 15, 20, and 25° C, respectively. The corresponding values for Oltan were 71%, 60%, and 34%. This trend was also observed in the dry weight of plumule, wherein initially, with an increase in salinity, especially at temperatures of 15 and 20° C, the dry weight of plumule decreased more steeply compared to the temperature of 25° C. Secondly, the resistance of the Oltan cultivar to prevent a reduction in the dry weight of radicle and plumule at a temperature of 25° C was higher in comparison to the Darab 1 cultivar in saline treatments.
Investigation of saline soils has shown that the highest accumulation of salts occurs in the surface layer of the soil profile [
29]. Therefore, seeds planted in soil are in a place with a high concentration of salts in the soil profile. In these conditions, seeds capable of producing longer roots and expanding their root system are likely more successful than seeds lacking this capability. Hence, the hypothesis is suggested that seeds producing longer root and shoot lengths with greater weight in laboratory experiments will likely have higher salt tolerance during the germination and initial establishment stages in natural conditions. One of the reasons for the reduction in the dry weight of roots and shoots in saline treatments is the disruption of ion and osmotic balance in the plant, which is a detrimental effect of salt stress [
30]. The root is the first organ to face stress due to the absorption of elements directly [
13]. After absorbing water and germination, before the emergence of primary leaves and the onset of photosynthesis utilising stored nutrients, the seed uses a series of hormones and essential enzymes produced within the seed, including lipases, proteases, and amylases. This leads to the breakdown of stored nutrients in the seed, such as starch, and their dissolution in water, providing the necessary energy for the emergence and growth of roots and shoots. Therefore, reduced water absorption by seeds in saline environments leads to a decrease in the growth and development of seedlings, which can be examined by reducing the length of roots, shoots, and their dry weight.
4. Conclusion
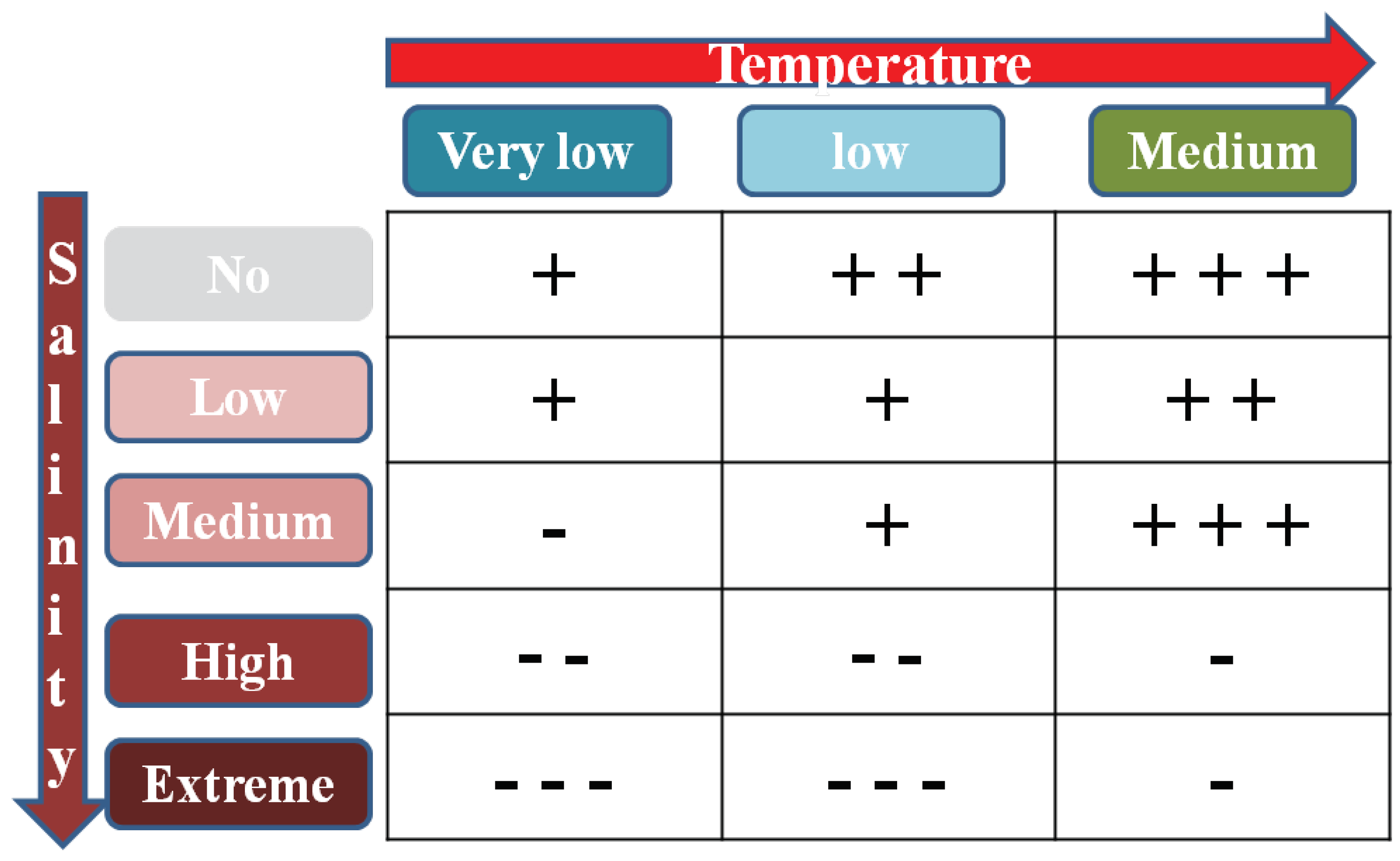
In this experiment, our hypothesis centred on examining the interaction effect of salinity and temperature on the germination of different sesame cultivars. The results indicated that the inhibitory effect of salinity on seed germination was mitigated when the temperature was at its optimal level (
Figure 8). Notably, our observations in one of the two cultivars, Oltan, revealed that the germination percentage and associated traits were higher at the optimal temperature of 25°C compared to the other two temperature levels. Additionally, the germination percentage exhibited a slower decline with increasing salinity at the optimal temperature, in contrast to the other temperature levels. Our study underscores the significant role of temperature in influencing how salinity stress impacts germination and early seedling growth. Moreover, it emphasises that the temperature-salinity interaction's effects are species-specific, aligning with the expected high intraspecific variability. Intriguingly, specific cultivars, such as Oltan, commonly found in arid regions with elevated temperatures, demonstrate increased tolerance to salinity stress. These adaptations may contribute to sesame's ability to overcome the adverse effects of salinity stress.
Author Contributions
Conceptualization and methodology, M.G. and A.D.; writing—original draft preparation, M.G.; writing—review and editing, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Iran National Science Foundation (INSF), project No. 4021780.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balouchi, H.; Soltani Khankahdani, V.; Moradi, A.; Gholamhoseini, M.; Piri, R.; Heydari, S.Z.; Dedicova, B. Seed fatty acid changes germination response to temperature and water potentials in six sesame (Sesamum indicum L.) cultivars: Estimating the cardinal temperatures. Agriculture. 2023, 13, 1936. [Google Scholar] [CrossRef]
- Gholamhoseini, M. Optimizing irrigation and nitrogen fertilization of Iranian sesame cultivars for grain yield and oil quality. J. Food Compos. Anal. 2022, 108, 104448. [Google Scholar] [CrossRef]
- Ghasemi Hamedani, N.; Gholamhoseini, M.; Bazrafshan, F.; Habibzadeh, F.; Amiri, B. Yield, irrigation water productivity and nutrient uptake of arbuscular mycorrhiza inoculated sesame under drought stress conditions. Agric. Water Manage. 2022, 266, 107569. [Google Scholar] [CrossRef]
- Ghasemi Hamedani, N.; Gholamhoseini, M.; Bazrafshan, F.; Amiri, B.; Habibzadeh, F. Variability of root traits in sesame genotypes under different irrigation regimes. Rhizosphere. 2020, 13, 100190. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Habibzadeh, F.; Ataei, R.; Hemmati, P.; Ebrahimian, E. Zeolite and hydrogel improve yield of greenhouse cucumber in soil-less medium under water limitation. Rhizosphere. 2018, 6, 7–10. [Google Scholar] [CrossRef]
- Karimzadeh, H.; Bahador, M.; Tadayon, M.R.; Dehkordi, A.G. Modelling seed germination and seedling emergence of flax and sesame as affected by temperature, soil bulk density, and sowing depth. Ind. Crops Prod. 2019, 141, 111770. [Google Scholar] [CrossRef]
- Yadav, R.; Kalia, S.; Rangan, P.; Pradheep, K.; Rao, G.P.; Kaur, V.; Pandey, R.; Rai, V.; Vasimalla, C.C.; Langyan, S.; Sharma, S.; Thangavel, B.; Rana, V.S.; Vishwakarma, H.; Shah, A.; Saxena, A.; Kumar, A.; Singh, K.; Siddique, K.H.M. Current research trends and prospects for yield and quality improvement in sesame, an important oilseed crop. Front. Plant Sci. 2022, 13, 863521. [Google Scholar] [CrossRef]
- Bahrami, H.; Razmjoo, J. Effect of salinity stress (NaCl) on germination and early seedling growth of ten sesame cultivars (Sesamum indicum L.). Int. J. AgriSci. 2012, 2, 529–537. [Google Scholar]
- El Harfi, M.; Hanine, H.; Rizki, H.; Latrache, H.; Nabloussi, A. Effect of drought and salt stresses on germination and early seedling growth of different color-seeds of sesame (Sesamum indicum). Int. J. Agric. Biol. 2016, 18, 1088–1094. [Google Scholar] [CrossRef]
- Suassuna, J.F.; Fernandes, P.D.; Brito, M.E.B.; Arriel, N.H.C.; de Melo, A.S.; Fernandes, J.D. Tolerance to salinity of sesame genotypes in different phenological stages. Am. J. Plant Sci. 2017, 8, 1904–1920. [Google Scholar] [CrossRef]
- Ranganatha. A.R.G. Improved technology for maximizing production of sesame [Revied Ed.,] Project coordinator, AICRP on sesame and Niger, ICAR, JNKVV Campus, Jabalpur. 2013, pp. 1 -17.
- ISTA. Rules for seed testing; The international seed testing association (ISTA), Zurich, Switzerland. 2015.
- Guo, X.; Zhou, G.; Zhu, G.; Jiao, X. Effects of calcium on emergence and seedling growth of castor bean under salinity stress. Curr. Sci. 2019, 116, 2028–2035. [Google Scholar] [CrossRef]
- Ghaleb, W.; Ahmed, L.Q.; Wagner, M.-H.; Eprinchard-Ciesla, A.; Olivares-Rodríguez, W.E.; Perrot, C.; Chenu, K.; Norton, M.; Escobar- Gutiérrez, A.J. The concepts of seed germination rate and germinability: A re-evaluation for cool-season grasses. Agronomy, 2022, 12, 1291. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Tall fescue (Festuca arundinacea Schreb.) shows intraspecific variability in response to temperature during germination. Agronomy, 2022, 12, 1245. [Google Scholar] [CrossRef]
- Nedjimi, B. Effect of salinity and temperature on germination of lygeum spartum. Agric. Res. 2013, 2, 340–345. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; De Boeck, H.J.; Hou, F. Effects of temperature and salinity on seed germination of three common grass species. Front. Plant Sci. 2021, 12, 731433. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.B.; Xu, L.Y.; Jin, X.Q.; Chen, J.H.; Lu, H.F. Effect of temperature regime on germination of seed of perennial ryegrass (Lolium Perenne). Grass Forage Sci. 2008, 63, 249–256. [Google Scholar] [CrossRef]
- Gholamhoseini, M. Evaluation of sesame genotypes for agronomic traits and stress indices grown under different irrigation treatments. Agronomy J. 2020, 112, 1794–1804. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.B. Principles of seed science and technology, 4th ed.; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Fernandez, I.C.D.; Luque, E.G.; Mercado, F.G.; Marrero, J.M. Germination responses of Limonium insigne (Coss.) Kuntze to salinity and temperature. Pak. J. Bot. 2015, 47, 807–812. [Google Scholar]
- Gorai, M.; Neffati, M. Germination responses of Reaumuria vermiulata to salinity and temperature. Ann. Appl. Biol. 2007, 151, 53–59. [Google Scholar] [CrossRef]
- Gu, R.; Zhou, Y.; Song, X.; Xu, S.; Zhang, X.; Lin, H. Effects of temperature and salinity on Ruppia sinensis seed germination, seedling establishment and seedling growth. Mar. Pollut. Bull. 2018, 134, 177–185. [Google Scholar] [CrossRef]
- Hakim, M.A.; Juraimi, A.S.; Begum, M.; Hanafi, M.M.; Ismail, M.R.; Selamat, A. Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L). Afr. J. Biotechnol. 2010, 5, 1911–1918. [Google Scholar] [CrossRef]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Bhatt, A.; Gairola, S.; Caron, M.M.; Santo, A.; Murru, V.; El-Keblawy, A. Effects of light, temperature, salinity, and maternal habitat on seed germination of Aeluropus lagopoides (Poaceae): an economically important halophyte of arid Arabian deserts. Botany, 2020, 98, 117–125. [Google Scholar] [CrossRef]
- Shahbazi, N.; Kazemitabar, S.K.; Kiani, G.; Pakdin Parizi, A.; Mehraban Joubani, P. Evaluating the germination indices of different genotypes of sesame Plant (Sesamum indicum) under salinity stress. Iranian J. Seed Res. 2022, 8, 10–20. [Google Scholar] [CrossRef]
- Nikolić, N.; Ghirardelli, A.; Schiavon, M.; Masin, R. Effects of the salinity-temperature interaction on seed germination and early seedling development: a comparative study of crop and weed species. BMC Plant Biol. 2023, 23, 446. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Irrigation systems and zones of salinity development. In: guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Springer, Cham. 2018.
- Khan, M.A.; Gul, B.; Weber, D. J. Seed germination in relation to salinity and temperature in Sarcobatus vermiculatus. Biol. Plant. 2001, 45, 133–135. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).